Mitigation of Karenia brevis Cells and Brevetoxins Using Curcumin, a Natural Supplement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Analyses
2.3. Data Reduction and Statistical Analyses
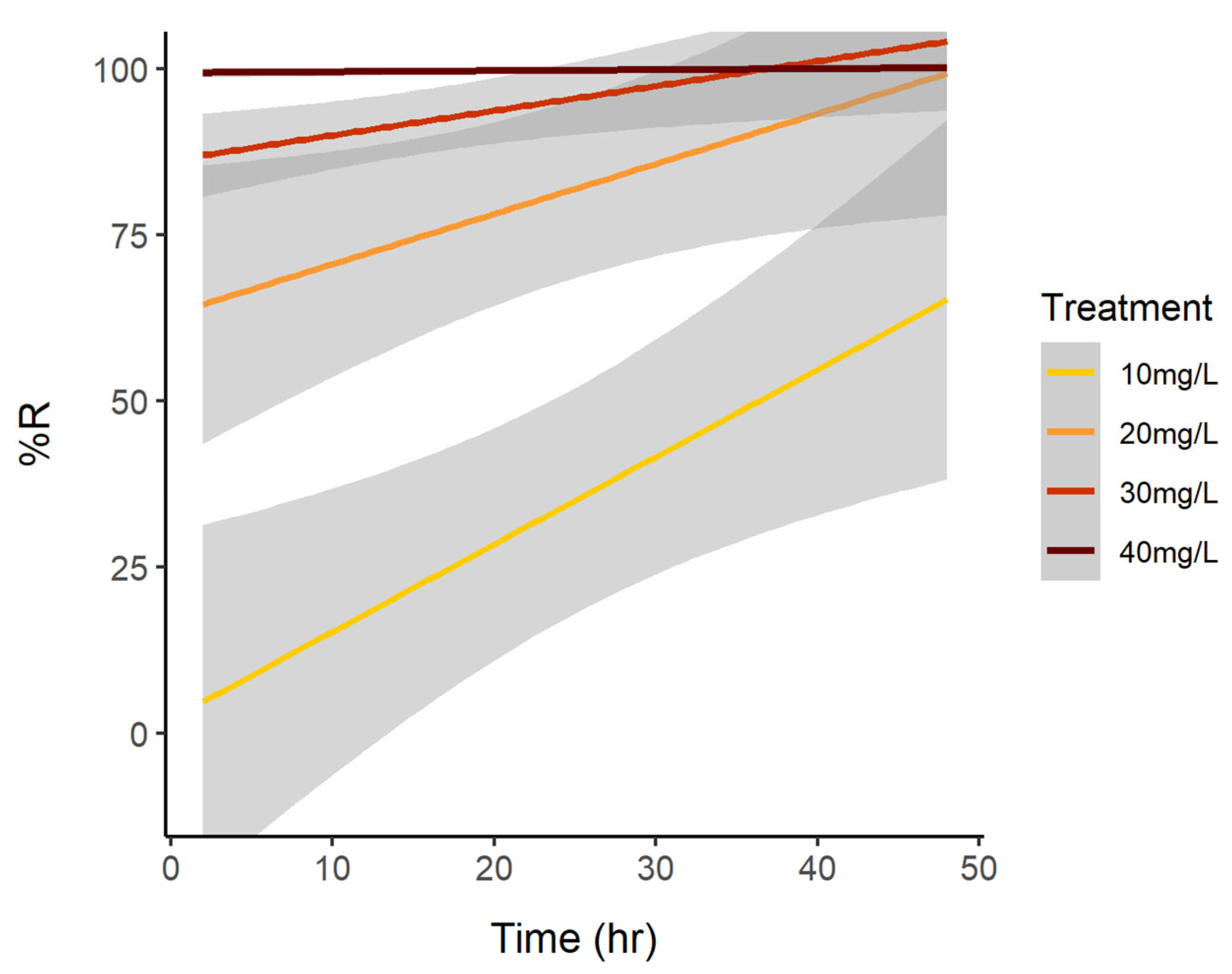
3. Results
3.1. Cell Reduction
3.2. Reduction of Brevetoxins
3.3. Water Quality and Nutrients
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heil, C.A.; Muni-Morgan, A.L. Florida’s Harmful Algal Bloom (HAB) Problem: Escalating Risks to Human, Environmental and Economic Health with Climate Change. Front. Ecol. Evol. 2021, 9, 646080. [Google Scholar] [CrossRef]
- Steidinger, K.A. Historical perspective on Karenia brevis red tide research in the Gulf of Mexico. Harmful Algae 2009, 8, 549–561. [Google Scholar] [CrossRef]
- Davis, C.C. Gymnodinium brevis, sp. nov., a cause of discolored water and animal mortality in the Gulf of Mexico. Bot. Gaz. 1948, 109, 358–360. [Google Scholar] [CrossRef]
- Anderson, D.M.; Fensin, E.; Gobler, C.J.; Hoeglund, A.E.; Hubbard, K.A.; Kulis, D.M.; Landsberg, J.H.; Lefebvre, K.A.; Provoost, P.; Richlen, M.L.; et al. Marine harmful algal blooms (HABs) in the United States: History, current status and future trends. Harmful Algae 2021, 102, 101975. [Google Scholar] [CrossRef] [PubMed]
- Heil, C.A.; Steidinger, K.A. Monitoring, management, and mitigation of Karenia blooms in the eastern Gulf of Mexico. Harmful Algae 2009, 8, 611–617. [Google Scholar] [CrossRef]
- Pierce, R.H.; Henry, M.S. Harmful algal toxins of the Florida red tide (Karenia brevis): Natural chemical stressors in South Florida coastal ecosystems. Ecotoxicology 2008, 17, 623–631. [Google Scholar] [CrossRef]
- Fleming, L.E.; Kirkpatrick, B.; Backer, L.C.; Walsh, C.J.; Nierenberg, K.; Clark, J.; Reich, A.; Hollenbeck, J.; Benson, J.; Cheng, Y.S.; et al. Review of Florida red tide and human health effects. Harmful Algae 2011, 10, 224–233. [Google Scholar] [CrossRef]
- Pierce, R.H.; Henry, M.S.; Blum, P.C.; Osborn, S.E.; Cheng, Y.S.; Zhou, Y.; Irvin, C.M.; Bourdelais, A.J.; Naar, J.; Baden, D.G. Compositional changes in neurotoxins and their oxidative derivatives from the dinoflagellate, Karenia brevis, in seawater and marine aerosol. J. Plankton Res. 2011, 33, 343–347. [Google Scholar] [CrossRef]
- Stumpf, R.P.; Li, Y.; Kirkpatrick, B.; Litaker, R.W.; Hubbard, K.A.; Currier, R.D.; Kohler Harrison, K.; Tomlinson, M.C. Quantifying Karenia brevis bloom severity and respiratory irritation impact along the shoreline of Southwest Florida. PLoS ONE 2022, 17, e0260755. [Google Scholar] [CrossRef]
- Watkins, S.M.; Reich, A.; Fleming, L.E.; Hammond, R. Neurotoxic shellfish poisoning. Mar. Drugs 2008, 6, 431–455. [Google Scholar] [CrossRef]
- Heil, D.C. Karenia brevis monitoring, management, and mitigation for Florida molluscan shellfish harvesting areas. Harmful Algae 2009, 8, 608–610. [Google Scholar] [CrossRef]
- Flewelling, L.J.; Naar, J.P.; Abbott, J.P.; Baden, D.G.; Barros, N.B.; Bossart, G.D.; Bottein, M.Y.D.; Hammond, D.G.; Haubold, E.M.; Heil, C.A.; et al. Red tides and marine mammal mortalities. Nature 2005, 435, 755–756. [Google Scholar] [CrossRef]
- Sagarese, S.R.; Harford, W.J. Evaluating the risks of red tide mortality misspecification when modeling stock dynamics. Fish. Res. 2022, 250, 106271. [Google Scholar] [CrossRef]
- Hoagland, P.; Scatasta, S. The economic effects of harmful algal blooms. In Ecology of Harmful Algae; Granéli, E., Turner, J.T., Eds.; Springer: Berlin, Germany, 2006; Volume 189, pp. 391–402. [Google Scholar] [CrossRef]
- Bechard, A. Red tide at morning, tourists take warning? County-level economic effects of HABS on tourism dependent sectors. Harmful Algae 2019, 85, 101689. [Google Scholar] [CrossRef]
- Kirkpatrick, B.; Kohler, K.; Byrne, M.M.; Studts, J. Florida Red Tide Knowledge and Risk Perception: Is there a need for tailored messaging? Harmful Algae 2014, 32, 27–32. [Google Scholar] [CrossRef]
- Kirkpatrick, B.; Currier, R.; Nierenberg, K.; Reich, A.; Backer, L.C.; Stumpf, R.; Fleming, L.; Kirkpatrick, G. Florida red tide and human health: A pilot beach conditions reporting system to minimize human exposure. Sci. Total Environ. 2008, 402, 1–8. [Google Scholar] [CrossRef]
- Rounsefell, G.A.; Evans, J.E. Large-scale experimental test of copper sulfate as a control for the Florida red tide. In United States Fish and Wildlife Service Special Science Report—Fisheries No.270; US Dept. of the Interior, Fish and Wildlife Service: Washington, DC, USA, 1958. [Google Scholar]
- Kim, H.G. Mitigation and controls of HABs. In Ecology of Harmful Algae; Springer: Berlin, Germany, 2006; Volume 189, pp. 327–338. [Google Scholar] [CrossRef]
- Sengco, M. Mitigation of effects of harmful algal blooms. In Food Science, Technology and Nutrition: Shellfish Safety and Quality; Woodhead Publishing: Sawston, UK, 2006; pp. 175–199. [Google Scholar] [CrossRef]
- Florida House of Representatives. Available online: https://www.myfloridahouse.gov/Sections/Bills/billsdetail.aspx?BillId=65923&SessionId=87 (accessed on 25 April 2024).
- Roy, G.C.; Chakraborty, K.; Nandy, P.; Moitra, M.N. Pros and cons of curcumin as bioactive phytocompound for effective management of insect pests. Am. Sci. Res. J. Eng. Technol. Sci. 2014, 7, 31–43. Available online: https://asrjetsjournal.org/index.php/American_Scientific_Journal/article/view/697 (accessed on 25 April 2024).
- Zhou, L.H.; Zheng, T.L.; Wang, X.; Ye, J.L.; Tian, Y.; Hong, H.S. Effect of five Chinese traditional medicines on the biological activity of a red-tide causing alga—Alexandrium tamarense. Harmful Algae 2007, 6, 354–360. [Google Scholar] [CrossRef]
- Zhou, L.H.; Zheng, T.L.; Chen, X.H.; Wang, X.; Chen, S.B.; Tian, Y.; Hong, H.S. The Inhibitory effects of garlic (Allium sativum) and Diallyl Trisulfide on Alexandrium tamarense and other Harmful Algal Species. J. Appl. Phycol. 2008, 20, 349–358. [Google Scholar] [CrossRef]
- Wang, S.B.; Xu, B.; Yang, T.X.; Chen, Y.B.; Fan, Z.Q. Effect of extracts from nine traditional Chinese medicines on the growth of Microcystis aeruginosa. J. Fudan Univ. 2011, 50, 667–669. [Google Scholar]
- Yi, Y.-L.; Lei, Y.; Yin, Y.-B.; Zhang, H.-Y.; Wang, G.-X. The antialgal activity of 40 medicinal plants against Microcystis aeruginosa. J. Appl. Phycol. 2012, 24, 847–856. [Google Scholar] [CrossRef]
- Li, Y.-H.; Wu, T.; Yang, W.-D.; Li, H.-Y.; Liu, J.-S. The effectiveness of five natural products against three species of harmful algae. Water Environ. J. 2014, 28, 270–276. [Google Scholar] [CrossRef]
- Liu, F.; He, Z.-B.; Li, H.-Y.; Liu, J.-S.; Yang, W.-D. Inhibition of five natural products from Chinese herbs on growth of Chattonella marina. Environ. Sci. Pollut. Res. 2016, 23, 17793–17800. [Google Scholar] [CrossRef]
- Venturini, F.P.; de Souza, L.M.; Garbuio, M.; Inada, N.M.; de Souza, J.P.; Kurachi, C.; de Oliveira, K.T.; Bagnato, V.S. Environmental safety and mode of action of a novel curcumin-based photolarvicide. Environ. Sci. Pollut. Res. 2020, 27, 29204–29217. [Google Scholar] [CrossRef]
- Yuan, K.-K.; Duan, G.-F.; Liu, Q.-Y.; Li, H.-Y.; Yang, W.-D. Inhibition of Diarrheal Shellfish Toxins Accumulation in the Mussel Perna viridis by Curcumin and Underlying Mechanisms. Toxins 2021, 13, 578. [Google Scholar] [CrossRef]
- Xavier, M.J.; Dardengo, G.M.; Navarro-Guillén, C.; Lopes, A.; Colen, R.; Valente, L.M.P.; Conceição, L.E.C.; Engrola, S. Dietary Curcumin Promotes Gilthead Seabream Larvae digestive capacity and modulates oxidative status. Animals 2021, 11, 1667. [Google Scholar] [CrossRef]
- Shah, B.R.; Mraz, J. Advances in nanotechnology for sustainable aquaculture and fisheries. Rev. Aquac. 2020, 12, 925–942. [Google Scholar] [CrossRef]
- Liu, Y.-M.; Zhang, Q.-Z.; Xu, D.-H.; Fu, Y.-W.; Lin, D.-J.; Zhou, S.-Y. Antiparasitic efficacy of commercial curcumin against Ichthyophthirius multifiliis in grass carp (Ctenopharyngodon idellus). Aquaculture 2017, 480, 65–70. [Google Scholar] [CrossRef]
- Stanić, Z. Curcumin, a compound from natural sources, a true scientific challenge—A review. Plant Foods Hum. Nutr. 2017, 72, 1–12. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A review of its effects on human health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Jacob, J.; Amalraj, A.; Raj, K.J.; Divya, C.; Kunnumakkara, A.B.; Gopi, S. A novel bioavailable hydrogenated curcuminoids formulation (CuroWhite™) improves symptoms and diagnostic indicators in rheumatoid arthritis patients—A randomized, double blind and placebo controlled study. J. Tradit. Complement. Med. 2018, 9, 346–352. [Google Scholar] [CrossRef]
- Lide, D.R.; Milne, G.W.A. (Eds.) Handbook of Data on Organic Compounds, 3rd ed.; CRC Press, Inc.: Boca Raton, FL, USA, 1994. [Google Scholar]
- Carvalho, D.D.M.; Takeuchi, K.P.; Geraldine, R.M.; Moura, C.J.D.; Torres, M.C.L. Production solubility and antioxidant activity of curcumin nanosuspension. Food Sci. Technol. 2015, 35, 115–119. [Google Scholar] [CrossRef]
- Crippen, R.W.; Perrier, J.L. The use of neutral red and Evans blue for live-dead determinations of marine plankton (with comments on the use of rotenone for inhibition of grazing). Stain. Technol. 1974, 49, 97–104. [Google Scholar] [CrossRef]
- Dixon, L.K.; Kirkpatrick, G.K.; Hall, E.R.; Nissanka, A. Nitrogen, phosphorus and silica on the West Florida Shelf: Patterns and relationships with Karenia spp. occurrence. Harmful Algae 2014, 38, 8–19. [Google Scholar] [CrossRef]
- Huang, M.-T.; Newmark, H.L.; Frenkel, K. Inhibitory effects of curcumin on tumorigenesis in mice. J. Cell. Biochem. 1997, 67, 26–34. [Google Scholar] [CrossRef]
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as “Curecumin”: From kitchen to clinic. Biochem. Pharmacol. 2008, 75, 787–809. [Google Scholar] [CrossRef]
- Zhao, G.; Hong, Y.; Li, L.; Zhang, H.; Xu, R.; Hao, Y. Selection and characterization of plant-derived alkaloids with strong antialgal inhibition: Growth inhibition selectivity and inhibitory mechanism. Harmful Algae 2022, 117, 102272. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. The chemistry of curcumin: From extraction to therapeutic agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef]
- Thayyullathil, F.; Chathoth, S.; Hago, A.; Patel, M.; Galadari, S. Rapid reactive oxygen species (ROS) generation induced by curcumin leads to caspase-dependent and-independent apoptosis in L929 cells. Free. Radic. Biol. Med. 2008, 45, 1403–1412. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, S.; Peng, Y.; Li, Y.; Chen, Z.; Xu, H.; Yi, Z.; Zheng, W.; Zheng, T. Effects of marine actinomycete on the removal of a toxicity alga Phaeocystis globose in eutrophication waters. Front. Microbiol. 2015, 6, 474. [Google Scholar] [CrossRef]
- Bidle, K.D. Programmed cell death in unicellular phytoplankton. Curr. Biol. 2016, 26, R594–R607. [Google Scholar] [CrossRef] [PubMed]
- Li, F.-M.; Wu, M.; Yao, Y.; Zheng, X.; Zhao, J.; Wang, Z.-Y.; Xing, B.-S. Inhibitory effects and oxidative target site of dibutyl phthalate on Karenia brevis. Chemosphere 2015, 132, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.R.; Silva, C.M.; da Silva, L.M.; Machulek, A., Jr.; De Souza, A.P.; de Oliveira, K.T.; Souza, L.M.; Inada, N.M.; Bagnato, V.S.; Oliveira, S.L.; et al. Environmentally safe photodynamic control of Aedes aegypti using sunlight-activated synthetic curcumin: Photodegradation, aquatic ecotoxicity, and field trial. Molecules 2022, 27, 5699. [Google Scholar] [CrossRef]
- Poli, M.A.; Mende, T.J.; Baden, D.G. Brevetoxins, unique activators of voltage-sensitive sodium channels, bind to specific sites in rat brain synaptosomes. Mol. Pharmacol. 1986, 30, 129–135. [Google Scholar] [PubMed]
- Landsberg, J.H. The effects of harmful algal blooms on aquatic organisms. Rev. Fish. Sci. 2002, 10, 113–390. [Google Scholar] [CrossRef]
- Bourdelais, A.J.; Jacocks, H.M.; Wright, J.L.; Bigwarfe, P.M., Jr.; Baden, D.G. A New Polyether Ladder Compound Produced by the Dinoflagellate Karenia brevis. J. Nat. Prod. 2005, 68, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.; Plakas, S.M.; Wang, Z.; Jester, E.L.; El Said, K.R.; Granade, H.R.; Henry, M.S.; Blum, P.C.; Pierce, R.H.; Dickey, R.W. Characterization of polar brevetoxin derivatives isolated from Karenia brevis cultures and natural blooms. Toxicon 2006, 48, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Devillier, V.M.; Hall, E.R.; Lovko, V.; Pierce, R.; Anderson, D.M.; Lewis, K.A. Mesocosm study of PAC-modified clay effects on Karenia brevis cells and toxins, chemical dynamics, and benthic invertebrate physiology. Harmful Algae 2024, 134, 102609. [Google Scholar] [CrossRef] [PubMed]
- Tsaplev, Y.B.; Lapina, V.A.; Trofimov, A.V. Curcumin in dimethyl sulfoxide: Stability, spectral, luminescent and acid-base properties. Dye. Pigment. 2020, 177, 108327. [Google Scholar] [CrossRef]
- Pranata, A.; Surya, R. Effects of The Addition of Complexing Agents on Curcumin Stability Using Accelerated Shelf Life Testing. J. Phys. Conf. Ser. 2021, 2049, 012034. [Google Scholar] [CrossRef]
- Zang, X.; Yu, Z.; Jiang, W.; Song, X.; Cao, X. Dosage-effectiveness of modified clay flocculating red tide organisms: Mechanical mechanism and mathematical model. Sep. Purif. Technol. 2023, 305, 122422. [Google Scholar] [CrossRef]
- Sheng, Y.P.; Peene, S.; Yassuda, E. Circulation and transport in Sarasota Bay, Florida: The effect of tidal inlets on estuarine circulation and flushing quality. In Mixing in Estuaries and Coastal Seas; Pattiaratchi, C., Ed.; American Geophysical Union: Washington, DC, USA, 1996; Volume 50, pp. 184–210. [Google Scholar] [CrossRef]




| Experiment Treatment | Time (h) | N | Mean | SE |
|---|---|---|---|---|
| 0.1 mg/L | 2 | 3 | −4.09253 | 13.73799 |
| 0.1 mg/L | 4 | 3 | −12.6984 | 28.23137 |
| 0.1 mg/L | 24 | 6 | 2.045177 | 10.18821 |
| 0.1 mg/L | 48 | 6 | −14.3294 | 9.434375 |
| 0.1 mg/L | 72 | 6 | −3.76942 | 11.52276 |
| 1 mg/L | 2 | 3 | −34.0451 | 38.45247 |
| 1 mg/L | 4 | 3 | −10.582 | 13.60313 |
| 1 mg/L | 6 | 3 | −1.96078 | 2.995148 |
| 1 mg/L | 24 | 9 | 17.14217 | 5.885734 |
| 1 mg/L | 48 | 9 | 11.12028 | 14.83291 |
| 1 mg/L | 72 | 9 | 31.97017 | 8.466663 |
| 1 mg/L | 96 | 3 | 12.67123 | 17.26313 |
| 2 mg/L | 6 | 3 | 23.85621 | 3.682819 |
| 2 mg/L | 24 | 3 | 35.51724 | 6.792316 |
| 2 mg/L | 48 | 3 | 26.47059 | 6.878833 |
| 2 mg/L | 72 | 3 | 43.24324 | 5.479966 |
| 2 mg/L | 96 | 3 | 42.46575 | 6.840748 |
| 3 mg/L | 2 | 3 | 8.695652 | 9.584471 |
| 3 mg/L | 4 | 3 | −2.80899 | 21.91011 |
| 3 mg/L | 6 | 6 | 6.194942 | 5.37714 |
| 3 mg/L | 24 | 9 | 44.62133 | 10.11696 |
| 3 mg/L | 48 | 9 | 27.89827 | 7.832955 |
| 3 mg/L | 72 | 6 | 54.69643 | 3.472442 |
| 3 mg/L | 96 | 6 | 64.72603 | 4.073759 |
| 5 mg/L | 2 | 14 | 34.36232 | 14.05441 |
| 5 mg/L | 4 | 11 | 16.17168 | 16.11522 |
| 5 mg/L | 6 | 5 | 22.53342 | 7.324052 |
| 5 mg/L | 24 | 20 | 89.2621 | 3.573754 |
| 5 mg/L | 48 | 9 | 66.93636 | 5.136131 |
| 5 mg/L | 72 | 12 | 87.41292 | 4.515164 |
| 10 mg/L | 2 | 2 | 2.486679 | 1.598579 |
| 10 mg/L | 24 | 2 | 38.1068 | 29.36893 |
| 10 mg/L | 48 | 2 | 63.14433 | 2.319588 |
| 20 mg/L | 2 | 8 | 63.28393 | 15.23073 |
| 20 mg/L | 24 | 8 | 83.63228 | 7.094545 |
| 20 mg/L | 48 | 5 | 98.14433 | 1.147993 |
| 30 mg/L | 2 | 16 | 75.38678 | 9.131826 |
| 30 mg/L | 4 | 13 | 91.74382 | 3.06602 |
| 30 mg/L | 6 | 10 | 99.42712 | 0.324572 |
| 30 mg/L | 24 | 16 | 99.59807 | 0.232054 |
| 30 mg/L | 48 | 11 | 100 | 0 |
| 40 mg/L | 2 | 3 | 99.26471 | 0.735294 |
| 40 mg/L | 24 | 3 | 100 | 0 |
| 40 mg/L | 48 | 3 | 100 | 0 |
| Ethanol + KB Control | 2 | 3 | −5.32787 | 4.420407 |
| Ethanol + KB Control | 4 | 3 | 22.80072 | 19.76823 |
| Ethanol + KB Control | 24 | 3 | 5.766871 | 10.12772 |
| Ethanol + KB Control | 48 | 3 | −19.0566 | 1.799885 |
| Experiment Treatment | Time (h) | N | Mean | SE |
|---|---|---|---|---|
| 5 mg/L | 2 | 6 | 21.02426 | 11.18452 |
| 5 mg/L | 24 | 6 | 44.03432 | 9.778153 |
| 5 mg/L | 48 | 6 | 40.92657 | 14.96852 |
| 10 mg/L | 2 | 3 | 7.412399 | 2.139422 |
| 10 mg/L | 24 | 3 | 1.276935 | 3.959779 |
| 10 mg/L | 48 | 3 | 10.26528 | 7.934202 |
| 25 mg/L | 2 | 3 | 31.57895 | 2.905701 |
| 25 mg/L | 24 | 3 | −2.59259 | 2.592593 |
| 25 mg/L | 48 | 3 | −0.21834 | 3.647007 |
| 50 mg/L | 2 | 3 | 29.40109 | 0.654365 |
| 50 mg/L | 24 | 3 | −11.5556 | 3.449817 |
| 50 mg/L | 48 | 3 | 15.93886 | 3.86284 |
| Experiment Treatment | Time(h) | N | Mean | SE |
|---|---|---|---|---|
| 1 mg/L | 6 | 3 | 38.66509 | 3.967214 |
| 1 mg/L | 24 | 3 | 2.670066 | 16.2927 |
| 1 mg/L | 48 | 3 | −34.2765 | 17.4238 |
| 1 mg/L | 72 | 3 | −9.9279 | 14.43228 |
| 1 mg/L | 96 | 3 | 34.27588 | 6.965364 |
| 2 mg/L | 6 | 3 | 43.86169 | 4.237249 |
| 2 mg/L | 24 | 3 | −4.73139 | 19.13836 |
| 2 mg/L | 48 | 3 | 6.412479 | 14.36292 |
| 2 mg/L | 72 | 3 | 41.94564 | 1.712205 |
| 2 mg/L | 96 | 3 | 46.85426 | 1.304109 |
| 3 mg/L | 4 | 3 | 19.36946 | 20.81235 |
| 3 mg/L | 6 | 3 | 52.50545 | 3.286115 |
| 3 mg/L | 24 | 6 | 43.53052 | 8.099593 |
| 3 mg/L | 48 | 3 | 53.82285 | 1.938302 |
| 3 mg/L | 72 | 6 | 73.78873 | 2.462797 |
| 3 mg/L | 96 | 3 | 79.09634 | 0.540372 |
| 5 mg/L | 4 | 3 | 38.29573 | 23.27433 |
| 5 mg/L | 24 | 3 | 60.23022 | 15.44914 |
| 5 mg/L | 72 | 3 | 79.28445 | 2.337412 |
| 10 mg/L | 24 | 2 | 21.31284 | 0.902916 |
| 10 mg/L | 48 | 2 | 22.50616 | 8.595048 |
| 20 mg/L | 24 | 5 | 33.99884 | 3.749756 |
| 20 mg/L | 48 | 5 | 51.37654 | 9.761752 |
| 30 mg/L | 24 | 3 | 28.41301 | 2.527997 |
| 30 mg/L | 48 | 3 | 69.53011 | 5.037381 |
| 40 mg/L | 24 | 3 | 48.46884 | 10.62812 |
| 40 mg/L | 48 | 3 | 64.35353 | 1.435036 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hall, E.R.; Heil, C.A.; Frankle, J.D.; Klass, S.; Devillier, V.; Lovko, V.; Toyoda, J.H.; Pierce, R. Mitigation of Karenia brevis Cells and Brevetoxins Using Curcumin, a Natural Supplement. Water 2024, 16, 1458. https://doi.org/10.3390/w16101458
Hall ER, Heil CA, Frankle JD, Klass S, Devillier V, Lovko V, Toyoda JH, Pierce R. Mitigation of Karenia brevis Cells and Brevetoxins Using Curcumin, a Natural Supplement. Water. 2024; 16(10):1458. https://doi.org/10.3390/w16101458
Chicago/Turabian StyleHall, Emily R., Cynthia A. Heil, Jessica D. Frankle, Sarah Klass, Victoria Devillier, Vincent Lovko, Jennifer H. Toyoda, and Richard Pierce. 2024. "Mitigation of Karenia brevis Cells and Brevetoxins Using Curcumin, a Natural Supplement" Water 16, no. 10: 1458. https://doi.org/10.3390/w16101458






