Abstract
Sanitary landfilling is the predominant process for solid urban waste disposal, but it generates leachate that poses environmental, economic, and social concerns. Landfill leachate (LL) contains complex and refractory pollutants and toxic compounds that can vary depending on landfill maturity, age, and biochemical reactions, making its treatment challenging. Due to its unique characteristics and occurrence in remote locations, LL requires separate treatment from wastewater. Various conventional treatment processes involving biological, chemical, and physical processes have been used for LL treatment, but a single treatment process is insufficient to meet environmental standards. This review demonstrates that combined treatment processes are more effective and efficient for LL treatment compared to single processes. Among the various combinations, chemical–chemical and chemical–biological treatments are the most commonly used. Specifically, the integration of Fenton with adsorption and a membrane bioreactor (MBR) with nanofiltration (NF) processes shows promising results. The combined processes of MBR with NF, Fenton with adsorption, and PF with biological treatment show maximum removal efficiencies for COD, reaching 99 ± 1%, 99%, 98%, and 97%, respectively. Additionally, the combined Fenton with adsorption process and EC with SPF process enhance biodegradability as indicated by increased BOD5/COD ratios, from 0.084 to 0.82 and 0.35 to 0.75, respectively. The findings emphasize the importance of developing and implementing enhanced combined treatment processes for LL, with the aim of achieving efficient and comprehensive pollutant mineralization. Such processes have the potential to address the environmental concerns associated with LL and contribute to sustainable waste management practices.
1. Introduction
The increasing industrialization, urbanization, and global population have led to a greater emphasis on achieving a high quality of life and well-being for the population. As a consequence, there has been a significant increase in the generation of municipal solid waste (MSW). The sanitary landfill process has been used and will continue to be the most popular choice for disposing of MSW due to its economic benefits [1]. Usually, at the landfill site, the waste is placed in thin layers and compressed to reduce its size as much as possible. Additionally, it is covered periodically with suitable material. During that, many chemical, physical, and biological reactions take place, causing organic compounds to become immersed and undergo decomposition. As rainwater infiltrates the waste, it combines with these decomposed materials, resulting in the formation of a highly contaminated liquid known as “leachate” [2,3,4]. This leachate may eventually seep into the soil and then into the groundwater as it drains to bodies of surface water [5,6]. Leachates may include high concentrations of organic matter (OM), ammonium nitrogen [7], heavy metals [8], and chlorinated inorganic and organic salts [9]. Organic pollutants in leachate are characterized using global parameters, namely total organic carbon (TOC) [10], 5-day biochemical oxygen demand (BOD5) [8], and chemical oxygen demand (COD) [11].
The characteristics of leachate can vary depending on parameters such as climate changes, temperature, precipitation, waste composition, and moisture content of buried solid waste [12,13]. These variations may occur from site to site and from time to time [13]. Landfill leachate (LL) is characterized by its composition and generation rate, both of which are affected by the age of the landfill site. Based on the age of the landfill, leachate can be classified into three categories: young (acid-phase, <5 years), intermediate (5–10 years), and mature or “stabilized” (methanogenic-phase, >10 years) [14]. In young landfills, leachate exhibits high levels of COD (>10,000 mg/L) and a high ratio of BOD5/COD (ranging from 0.5 to 1). On the other hand, leachate from stabilized landfills is characterized by relatively lower COD levels (less than 4000) and a low BOD5/COD ratio (less than 0.1) [14]. Improper disposal of leachate from landfills represents a significant contamination source that can result in severe risks to soil and water ecosystems. Additionally, it poses a substantial risk to the health of residents, disrupts ecological balance, and disrupts regional element cycles [14,15,16]. Reports by Foo and Hameed [14], SQ et al. [17], and Bashir et al. [18] have highlighted the potential risk of highly polluted leachate seeping into the earth and polluting groundwater, surface water, and soils. Therefore, it is of utmost importance to ensure proper treatment of generated leachate before its release into the environment.
Different treatment processes have been used for the treatment of LL, with varying degrees of success [6]. Conventional processes, including physical, biological, and chemical treatment processes, have been used for LL treatment [19]. Chemical treatments, including reverse osmosis (RO) [20] and coagulation–flocculation (C/F) [21], and electrochemical treatments such as electro-Fenton (EF), electro-oxidation (EO), and electro-coagulation (EC) [22,23,24], have also been effective. Physical treatment processes such as adsorption [25] and filtration [25] are commonly used to treat LL. Additionally, various biological treatment processes have been investigated successfully under both aerobic and anaerobic conditions. For instance, Contrera et al. [26] implemented the sequencing batch reactor (SBR) process to effectively treat LL. However, each treatment process has its own set of advantages and disadvantages. After increasing the age of LL, the effluent becomes more complex, the biodegradability (BOD5/COD) decreases, and bio-refractory pollutants emerge, presenting challenges for the aforementioned treatment processes when used individually [27,28]. Frequently, a single treatment process fails to treat leachate from landfills to a level that meets the necessary standards for environmental release [19,29,30]. Therefore, Mojiri et al. [19] and Jamrah et al. [29] strongly recommend the implementation of a combination of chemical, biological, and physical treatment processes to achieve effective treatment outcomes.
Recently, there has been a growing recognition of the significance of utilizing combined treatment processes that harness the synergistic efficiency of biological, physical, and chemical approaches. Numerous integrated processes have also been put into full practice and tested in laboratories. Up to 85–90% of the pollutants can be reduced by employing two or more of the aforementioned treatment processes [31,32]. In this sense, Jegadeesan et al. [33] showed successful results in eliminating color and COD from stabilized LL (for more than 15 years) by using the combined EC and solar photo Fenton (SPF) processes. Under optimal conditions, the sole employment of the EC process resulted in a removal efficiency of 76% for color and 75% for COD. However, when the leachate was subjected to the SPF process after EC treatment, notable enhancements were observed. The combined treatment approach led to a significant improvement, with color and COD removal efficiencies reaching 91% and 90%, respectively. Additionally, the biodegradability of the leachate increased from 0.35 to 0.73. Tałałaj et al. [34] utilized a combined SBR and RO to eliminate ammonia nitrogen, total organic carbon (TOC), BOD, Cl−, and Fe from two types of LL (stabilized and young leachates). The implementation of SBR as a pre-treatment process proved to be highly effective, achieving ammonia nitrogen removal rates exceeding 98% for both leachates. The study has demonstrated that the combined SBR and RO process has a high removal efficiency (more than 80%) for all parameters examined. By combining various treatment processes, the limitations associated with relying solely on a single treatment approach can be overcome, leading to enhanced treatment efficiency and improved outcomes [29,35].
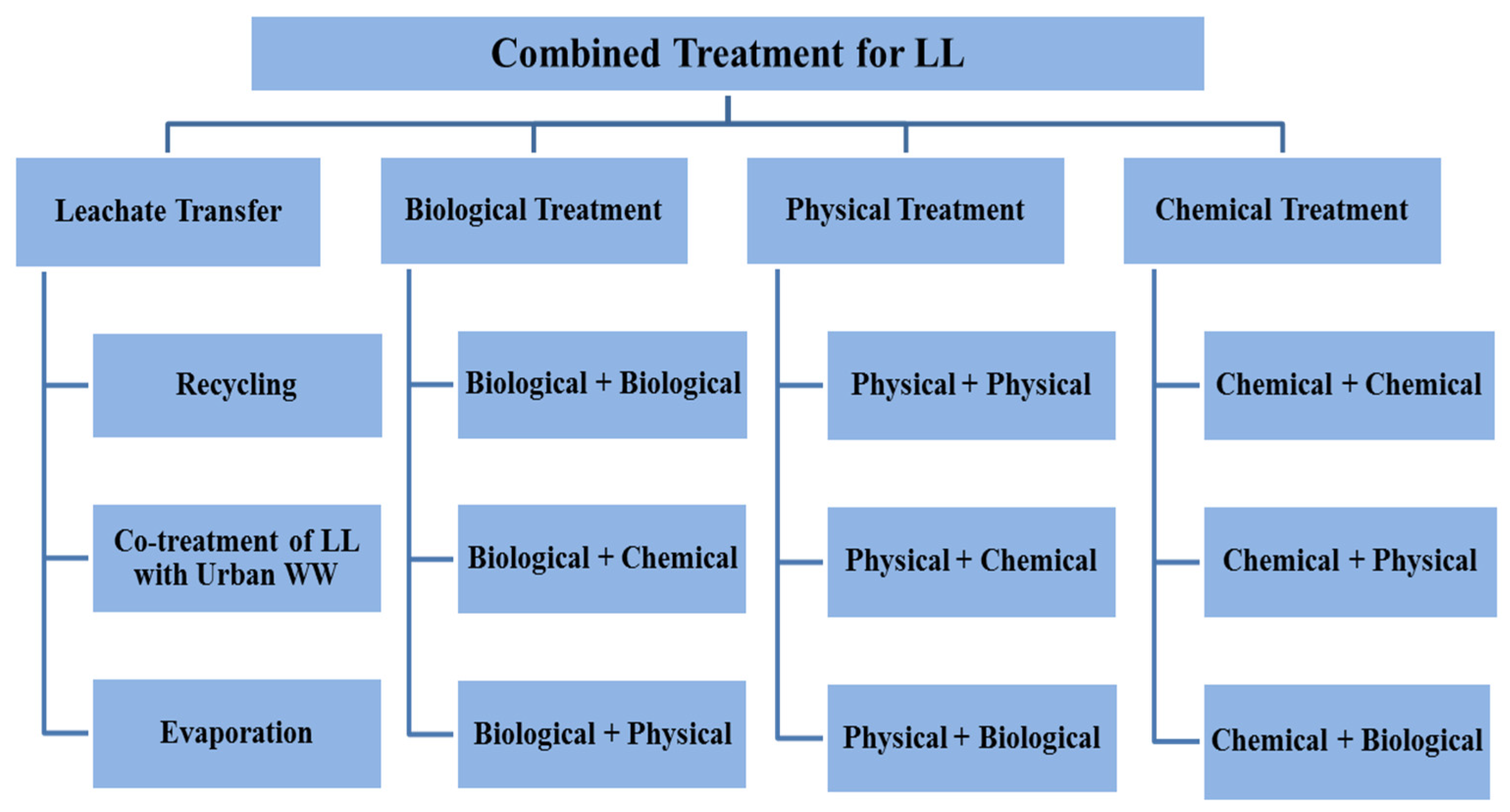
Numerous research and review studies have been conducted, examining both single treatment processes and combined treatment processes for different types of LL treatment. Notably, these studies have primarily focused on the characterization, composition, and generation of LL [6,36]. However, none of these reviews have specifically addressed the need for a comprehensive analysis of the existing literature solely pertaining to integrated treatment processes for LL treatment. The aim of this review is to evaluate the efficacy of different combined treatment processes and to discuss limitations and future perspectives for each process, in addition to discussing its mechanism of action. This manuscript aims to enhance researchers’ understanding of the characteristics of various combined treatment processes, their combinations, and the rationale behind arranging each process as pre- or post-treatment based on leachate characteristics. Additionally, it addresses the identified gaps encountered by many researchers in previous studies. The authors conclude the manuscript by offering recommendations for the effective implementation of combined treatment for LL. The major findings and conditions of the available research will be summarized for each combined process in a table to make comparisons easier. The suggested treatment process for LL is shown schematically in Figure 1.
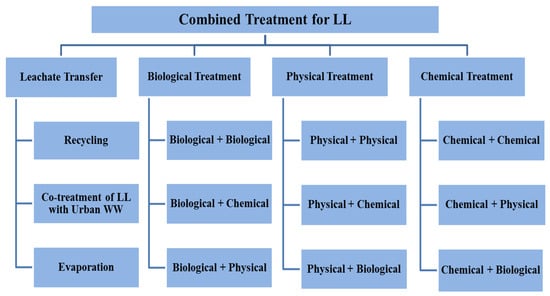
Figure 1.
Proposed combined treatment processes for LL.
As depicted in Figure 1, the suggested approach for treating LL is illustrated, which is based on previous research studies conducted by scholars and involves a combination of treatment processes.
2. Methodology
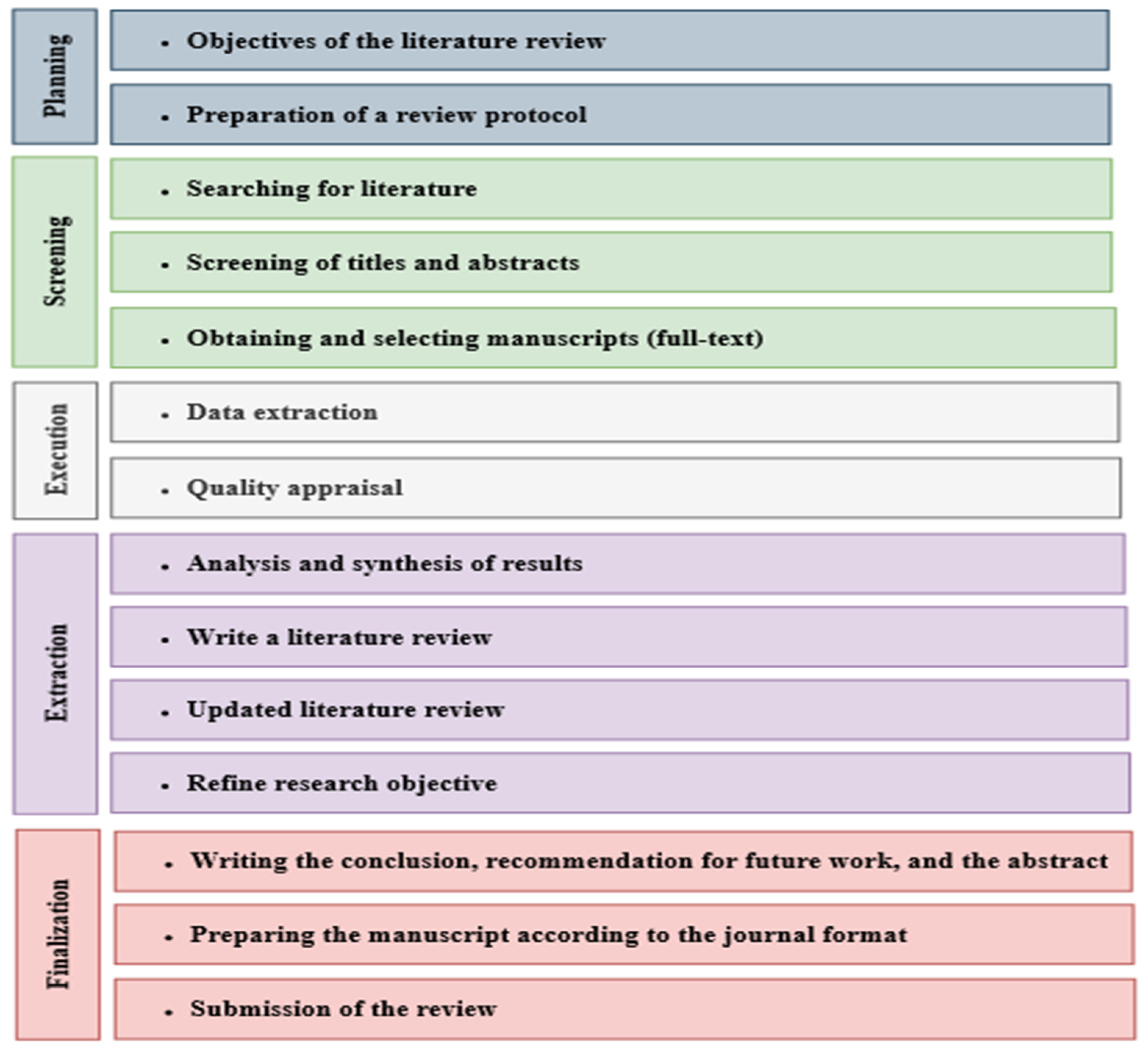
In this review study, a systematic technique was employed to assess the efficacy and efficiency of integrated treatment processes for LL. The initial stage involved identifying relevant, high-quality research articles discussing integrated treatment processes in LL. Subsequently, studies that utilized appropriate analysis processes to ensure the applicability and reliability of their findings were selected. This review encompasses a comprehensive compilation of papers published between 2011 and November 2023, which was then screened to include any research that could be pertinent. A filtering process was applied to exclusively include articles from scholarly journals with a high impact factor that are indexed in the Web of Science and Scopus databases. According to the type of applied process, the relevant, high-quality research articles were categorized, and their key findings were compared and discussed (see Figure 2). Through this analysis, several conclusions were drawn regarding the main objectives of integrated treatment processes. These objectives include achieving high efficiency in treating LL and attaining a level of wastewater discharge that complies with specific standards and requirements. This review also highlights the importance of implementing integrated treatment processes to achieve these objectives effectively.
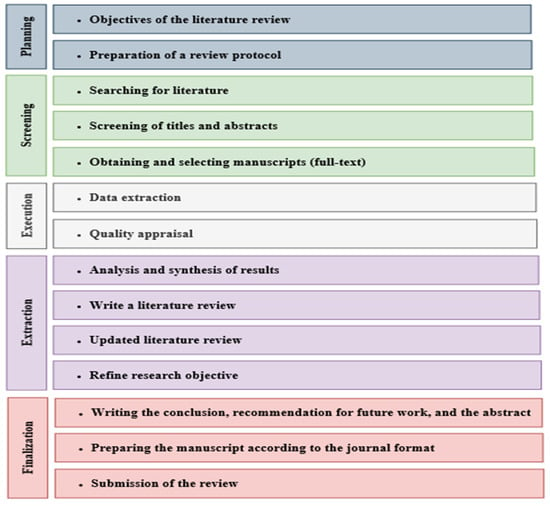
Figure 2.
The methodology of the systematic review.
3. Leachate from Landfill
The quality of leachates is influenced by various factors, including their waste type, seasonal weather variations, chemical composition, age, and precipitation. Particularly, the composition of LL is significantly influenced by landfill age [37]. The fundamental metrics of heavy metals, total Kjeldahl nitrogen (TKN), suspended solids (SSs), COD, BOD5, pH, the BOD5/COD ratio, and ammonia nitrogen (NH3-N) are often utilized to characterize the properties of leachate. According to the age of the landfill, leachate can be categorized into three different categories, as illustrated in Table 1 [14].

Table 1.
Summary of the relationship between landfill age and LL characteristics (NA: not available, VFAs: volatile fatty acids, HA: humic acid, and FA: fulvic acid) adapted from [14]. (CCC License Number 5751491181329.)
As shown in Table 1, it can be seen that the water quality of stabilized LL is characterized by alkaline with pH > 7.5, low COD below 4000 mg/L, high contamination with NH3-N content over 400 mg/L, and poor biodegradability (BOD5/COD). The water quality of fresh LL is characterized by being acidogenic with a pH < 6.5, high COD over 10,000 mg/L, low ammonia nitrogen concentration below 400 mg/L, and strong biodegradability. The water quality of intermediate LL is between the fresh phase and the stabilized phase.
Precipitation plays a significant role in leachate production. Waste is typically layered, compressed, and periodically covered. Within waste cells, various chemical, physical, and biological processes occur, transforming waste materials into pollutants. Rainfall, along with the moisture present in the waste and cover material, leads to the generation of leachate (Figure 3) [38,39]. Leachate generation is also influenced by factors such as surface runoff, groundwater infiltration, evapotranspiration, oxidation, and microbial degradation [40,41,42].
As shown in Figure 3, rainfall contributes to the distribution of water within the waste and thus distributes the polluted water to the soil and in a lateral direction. Throughout the landfill’s operational lifespan, the quantity and composition of leachate vary based on rainwater percolation, waste moisture content, landfill compaction, and biological/chemical processes [43,44]. Less compacted waste generally results in higher leachate production due to reduced filtration rates [44].
The biological and chemical reactions that occur within the landfill were divided into four stages, as illustrated in Figure 4 [45]:
- Aerobic stage;
- Anaerobic and acidogenic stage;
- Unstable methanogenic stage;
- Stable methanogenic stage.
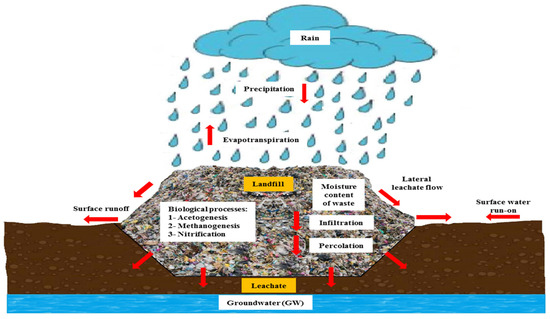
Figure 3.
Generation of leachate in landfill adapted from [6,19].
Figure 3.
Generation of leachate in landfill adapted from [6,19].
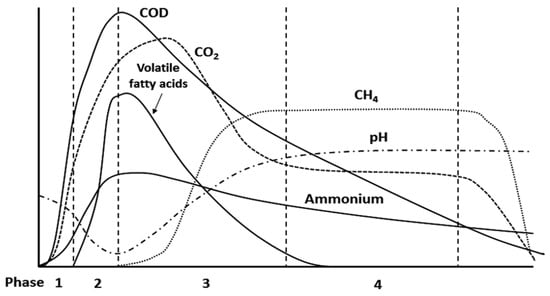
Figure 4.
Scheme of biological treatment of LL. 1 = aerobic stage; 2 = anaerobic acidogenic stage; 3 = unstable methanogenic stage; 4 = stable methanogenic stage [45]. (CCC License Number 5751390347312).
Figure 4.
Scheme of biological treatment of LL. 1 = aerobic stage; 2 = anaerobic acidogenic stage; 3 = unstable methanogenic stage; 4 = stable methanogenic stage [45]. (CCC License Number 5751390347312).
According to Figure 4, decomposition takes place under the influence of aerobic bacteria during the aerobic stage of the biochemical activities that take place inside the landfill. When there is a solid–liquid contact between the waste and the surrounding liquids, this process occurs quickly. This process, which ultimately decomposes organic matter into sulfate, nitrate, H2O, CO2, and other biodegradable chemicals, is catalyzed by aerobic bacteria. This occurs quite quickly because the O2 is consumed within a few days [46]. In the anaerobic stage, a series of reactions occur that take place in the absence of O2. Acid fermentation takes place, bringing the pH down to 5.5–5.6, mostly because CO2 and VFA are produced. This fermentation is the consequence of anaerobic bacteria interacting with organic waste during its degradation [45,46]. The initial disruption of the unstable methanogenic stage occurs due to the accumulation of a significant quantity of VFA produced during the anaerobic stage. In this stage, both CO2 and VFA are converted into methane. The duration of this stage can range from a few months to approximately two years, and it leads to a gradual increase in pH levels [45]. Finally, in the stable methanogenic stage, methane production continues until all of the biodegradable organic matter is completely consumed. This process, which takes 15 to 20 years to complete, marks the end of the biodegradation of organic matter in landfills [46]. The methane created during this stage can be utilized as fuel or to produce electricity.
The assessment of the landfill’s projected production of leachate is another important factor. Traditionally, the amount of LL has been estimated based on the concepts of hydrological balance. Equation (1) demonstrates the utilization of the hydrologic evaluation of landfill performance (HELP) model, as recommended by the EPA, to determine the quantities of LL [47,48,49]. This model takes into account various factors, such as the total water volume entering the landfill, including infiltration and percolation, and subtracts it from the water volumes consumed during biological and chemical decomposition, as well as those lost through water evapotranspiration [50,51].
The hydrologic evaluation of landfill performance involves several components. These components include the amount of leachate (L), surface runoff from outside areas (R*), runoff (R), precipitation (P), evapotranspiration (ET), variations in water content of the capping material (∆US), variation in the water content of the disposed waste volume (∆UW), irrigation and leachate recirculation (J), infiltration water from groundwater (IG), infiltration water from surface water bodies (IS), and the consumption or production of water related to the various anaerobic and aerobic biochemical degradation reactions of organic substances (b). These factors are considered in the evaluation process, and their interactions play a crucial role in determining the overall performance of the landfill [48,49]. The amount of waste generated in many countries ends up in landfills, especially in developing countries, which contributes significantly to the generation of a large amount of leachate. Several studies have detailed the amount of leachate generation and waste disposed of at various landfill locations globally as shown in Table 2.

Table 2.
Information of waste generation, landfill deposits, and climate conditions in different countries.
Data on the waste generation and the amount of waste deposited in landfills, as indicated in Table 2, are an important and crucial element in leachate management and should not be disregarded easily. Climate conditions greatly affect the amount of waste generated from landfills. During the rainy season, there is a lot of rain, which increases the moisture content in landfills and thus increases the amount of waste generated from landfills. Based on Table 2, it was found that countries with climate conditions tropical Savanna, continental, and humid subtropical, which are characterized by heavy rain, generate more waste compared to climate conditions humid continental, Mediterranean, and semi-arid. Croatia had the highest amount of waste generation, ranging from 1000 to 21,000 mg/L, due to continental climate conditions.
The composition of LL varies in different regions around the world based on local factors related to waste management, the conversion process used, and the age of the landfills. The classification of LL in various countries around the world is shown in Table 3, Table 4 and Table 5 based on the age of the landfill: young, medium, and mature, respectively.

Table 3.
Composition of young leachate from some significant landfills.

Table 4.
Composition of intermediate leachates from some significant landfills.

Table 5.
Composition of stabilized leachates from some significant landfills.
As summarized in Table 3, Table 4 and Table 5, the leachate compositions reported in the literature from different sites show a wide range of variation. In contrast to stabilized LL, young LL processes extremely high concentrations of all chemical parameters, with the exception of pH, as seen in Table 3 and Table 4. The range of COD concentration is from 10,193 mg/L [95] to 68,250 mg/L [93] for young LL and 1150 [111] to 4505 [107] for stabilized LL samples. The biodegradability (BOD5/COD ratio) of young LL varied from 0.0845 [95] to 0.582 [56]. The biodegradability (BOD5/COD ratio) of stabilized LL varied from 0.0234 [106] to 0.11 [110]. The pH value of the young LL was observed in the range of 5.1 [98] to 8.9 [100], but the stabilized LLs were alkaline with a pH range of 7 to 9 [82]. This might be explained by the fact that the quantity of free volatile acids in the anaerobic component has decreased, as fatty acids can partially ionize and raise pH levels. Based on Table 3, Table 4 and Table 5, most of the organic LLs referred to as COD were located in Morocco (COD = 22,000 mg/L), China (COD = 6880 ± 180 mg/L), and Algeria (COD = 3847.7 mg/L) for young, intermediate, and stabilized LL.
4. Leachate Transfer
4.1. CO-Treating Landfill Leachate and Urban Wastewater
Due to the high ammonium concentration, high COD/BOD5 ratio, low biodegradability, and presence of heavy metal ions in LL, the biological treatment of leachate is more challenging than that of treating industrial and municipal wastes [54,112]. LLs are currently commonly treated in urban wastewater treatment plants with urban wastewater. However, the need for separate treatment and disposal of LL has grown as a result of tighter regulations on nitrogen discharge and issues with the possible impact of resistant leachate elements on the biological treatment phase [113]. Researchers have also suggested treating wastewater and LL together [112].
Co-treatment of LL with urban wastewater sources at wastewater treatment plants has emerged as a cost-effective, easy maintenance, and viable solution [114]. Co-treatment of LL with wastewater has the advantage that neither of the nutrients has to be added at the wastewater treatment plant because the former often includes surplus nitrogen and the latter excess phosphorous (whereas leachates typically have low phosphorous levels). Table 6 summarizes the merits and demerits of co-treating LL with wastewater.

Table 6.
Merits and demerits of co-treatment of LL with wastewater.
However, due to the demerits in Table 6, efforts were required to optimize the volume distribution of LL and wastewater in order to mitigate the coexistence of nitrogen in LL and phosphorous in wastewater [118,121]. Few studies have been recently conducted on the feasibility of co-treatment and the ideal ratio of leachate to wastewater that ensures optimal treatment performance and preserves the quality of the final effluent [118]. A summary of some co-treatments of LL with wastewater and their efficiency are listed in Table 7.

Table 7.
A summary of co-treatment of LL with wastewater processes and their results.
As indicated in Table 7, two studies conducted by Mojiri [116,124] examined the co-treatment of LL with different wastewater sources and evaluated the efficiency of various treatment processes. In the first study conducted by Mojiri [116], the researchers compared the effectiveness of an SBR system with and without the use of ZELIAC powder (PZ) in removing heavy metals from LL and household wastewater. The results showed that the use of PZ-SBR was more effective in removing heavy metals compared to SBR alone. The study highlights the effectiveness of integrating different treatment processes for treating LL and household wastewater and improving pollutant removal and treatment efficiency.
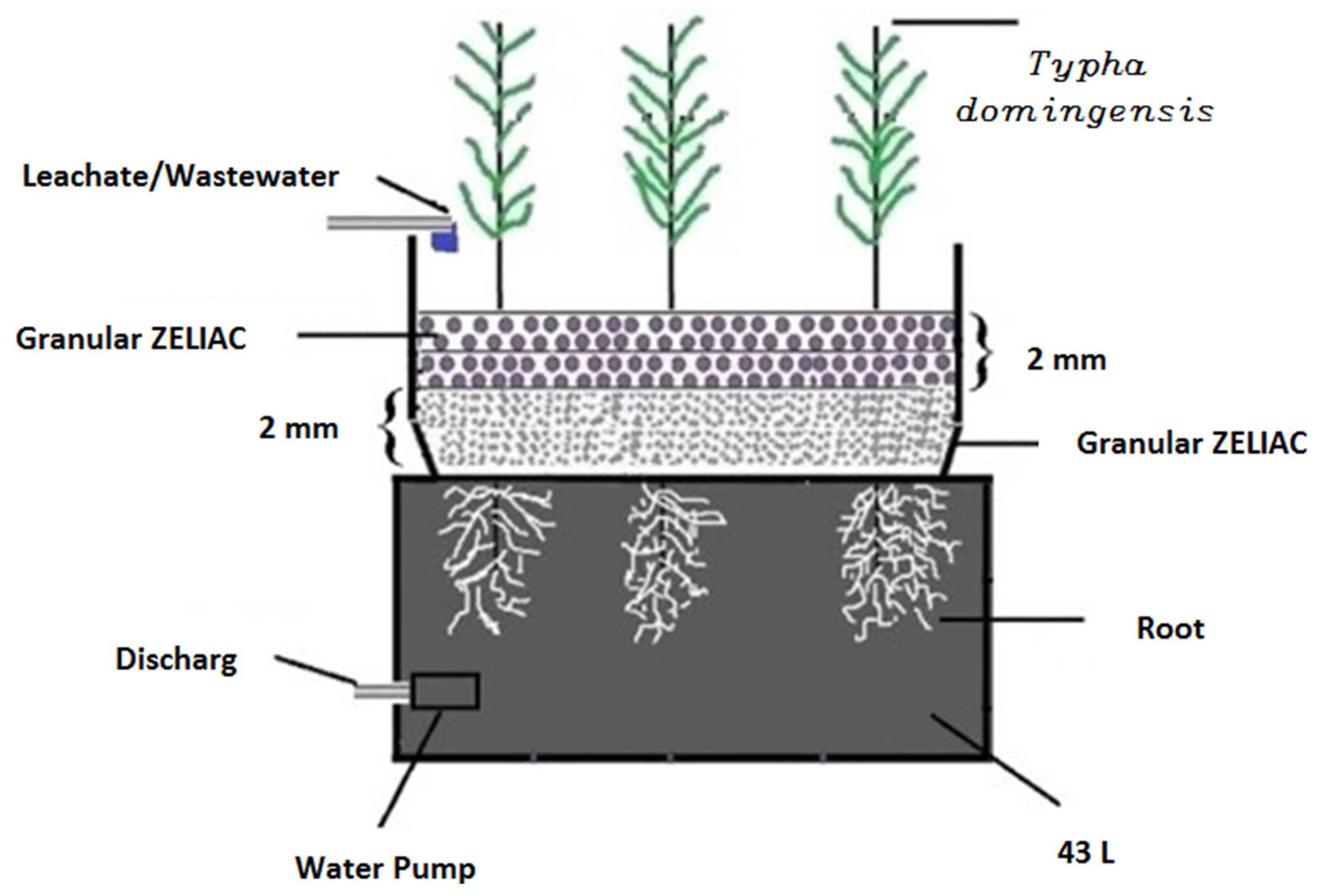
The second study by Mojiri [124] focused on co-treating LL and municipal wastewater (MWW) using a constructed wetland (CW) and an adsorption process, as illustrated in Figure 5.
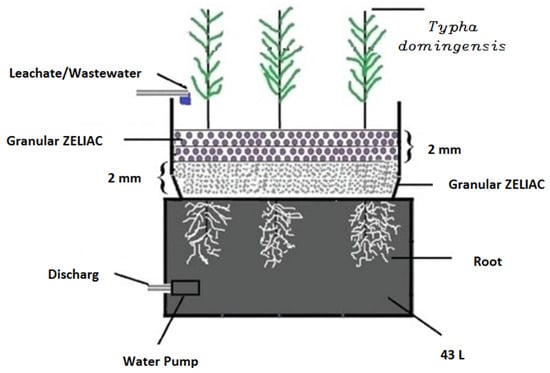
Figure 5.
Schematic diagram of co-treated LL and MWW treatment in the CW + adsorption process [124]. (CCC License Number 5751390880495).
The study showed that using a CW consisting of two layers of adsorbent materials (zeolite and ZELIAC) resulted in effective pollutant removal, as shown in Figure 5. The accumulation of nickel and cadmium in the roots and shoots of Typha domingensis plants cultivated in the CW was also monitored. These studies shed light on the effectiveness of integrating different treatment processes for treating wastewater and dairy wastewater and improving pollutant removal and treatment efficiency.
Chakraborty [119] conducted an assessment of a combined process involving SBR and EC for the co-treatment of LL and MWW. In the experiment, SBR was initially used to co-treat a mixture of 20% (v/v) LL and MWW. The effluent from the SBR was then treated further using EC for post-treatment. When subjected to post-treatment by EC, the overall reduction efficiencies reached 98% for COD, 98% for TSS, and 99% for ammonia, nitrate, and phosphate. During the EC process, the pH increased over time, resulting in a decline in EC efficiency, leading to the removal of COD, particulate matter, and nutrients. Based on these findings, the study suggests that using SBR followed by EC as a post-treatment option can be an effective approach for treating a mixture of LL and MWW. However, the study indicates that the efficiency of the EC process decreases over time due to an increase in pH, which may affect the removal of COD and other organic matter.
In a study by Kumar [91], innovative flocculants combined with an SBR were found to be effective in treating LL and MWW to reduce pollution. The SBR treatment efficiency decreased as leachate concentration increased, with COD removal rates ranging from 58% to 70%, phosphate removal rates from 69% to 95%, ammonia removal rates from 86% to 93%, and nitrate removal rates from 76% to 83%. C/F treatment using ferric chloride and alum combined with a novel flocculant (GGI-g-PAM) achieved COD removal rates of 77% and 74%, respectively, at a leachate concentration of 20%. The combined SBR and C/F treatment at a 20% LL-to-MWW ratio resulted in total removal rates of 93% for TSS, 89% for COD, 98% for turbidity, 82% for nitrate, and 83% for ammonia using alum. The study concludes that using novel flocculants with SBR offers an efficient approach for treating LL and MWW, meeting international standards for water discharge.
Verma [122] conducted a study using granular activated carbon–SBR (GAC-SBR) to co-treat LL with MWW. The researchers examined the GAC-SBR system at different mixing ratios of LL and MWW and various HRTs. The treatment efficiency of the GAC-SBR was evaluated based on parameters such as COD, phosphate, nitrate, ammonia, turbidity, mixed liquor suspended solids (MLSSs), and mixed liquor volatile suspended solids (MLVSSs) removal. A univariate analysis of variance (ANOVA) was employed to determine the statistical significance of the treatment at different LL-to-MWW ratios. The findings indicated that increasing the GAC concentration resulted in enhanced removal of ammonia and COD from stabilized LL. However, the adsorption efficiency either decreased or remained constant after reaching a GAC concentration of 15 g/L.
The combination of SBR with other techniques has been demonstrated to be a highly efficient approach compared to alternative methods. Scientific studies have provided evidence that incorporating SBR with techniques such as adsorption or chemical processes like C/F can significantly enhance the removal efficiency of various pollutants. Specifically, the combined method of SBR with adsorption has shown promising results in eliminating contaminants such as ammonia, nickel, and phosphate. To achieve high removal efficiency of these pollutants, adsorbent materials like zeolites and ZELIAC materials can be employed. Furthermore, experimental findings have indicated that integrating adsorption followed by SBR can improve the removal efficiency of food and organic substances. The use of GAC in this process can effectively remove COD. However, it is important to consider additional factors in order to further enhance the method. One potential factor worth exploring is the impact of temperature on removal efficiency. Research has shown that higher temperatures can enhance adsorption processes and improve overall efficiency. Therefore, investigating the relationship between temperature and removal efficiency could be beneficial in optimizing the combined SBR with the adsorption process.
4.2. Recycling
Leachate recycling is a process used in landfills to control and promote physical, biological, and chemical processes. The aim is to use the substances and resources found in decomposed solid waste that collect in landfills, add them again to the landfill, and create a procedure known as a bioreactor landfill [115,126,127]. This process has been shown to have several benefits. The recycling landfill eliminates the need for treatment plants, and thus the costs of the process become low [115,128]. Studies have demonstrated that leachate recirculation increases moisture content and facilitates the distribution of nutrients and enzymes, leading to improved leachate quality [129]. It also accelerates landfill stabilization, which leads to reducing land use, reducing the required time from several decades to 2–3 years, and reducing environmental pollution [130]. Leachate recycling can decrease the COD and BOD5 concentrations of leachate over time, but it may also result in a low BOD5/COD ratio and an increase in nitrogen concentration [131,132]. Leachate recirculation enhances waste decomposition, landfill gas production, settlement, and leachate treatment. It also provides conditions for advanced leachate treatment. Long-term recirculation, however, may result in a buildup of ammonia nitrogen, which prevents waste from decomposing [133].
Micro-aeration can mitigate this issue by promoting in situ nitrification and denitrification [134]. Overall, leachate recycling is an effective technique for landfill management, but careful management is required to avoid potential drawbacks and optimize results [127].
5. Combined Treatment Processes
Previous research has proven the effectiveness of applying integrated treatment processes to treat LL and has shown that a variety of combined processes are quite successful at eliminating specific contaminants from LL. The treatment processes of LL are classified into four categories, including (1) chemical processes; (2) physical processes; (3) biological processes (anaerobic or aerobic); and (4) leachate transfer [135]. Chemical treatment processes, such as C/F [65], precipitation [136], electrocoagulation (EC) [7], and Fenton [137,138], are applied to enhance biodegradability and remove metals, colloids, and organic compounds from LL [38]. Physical treatment processes, such as reverse osmosis (RO) [34], adsorption [82], filtration (nanofiltration (NF) [139], ultrafiltration (UF) [140], and microfiltration (MF)) [141], agitation [62], air stripping [142], and sedimentation [143], are used to remove metals, organic compounds, and ammonia [144]. Biological treatment processes, such as sequencing batch reactors (SBRs) [37], air lagoons [71], trickling filters [145], and membrane bioreactors (MBRs) [146], are utilized to remove biodegradable total nitrogen and organics due to their reliability, operational cost, and suitability. The evaluation of the biological technique operating circumstances is made possible by control relationship conditions such as the sludge retention time (SRT), food–microorganism ratio (F/M), and hydraulic retention time (HRT) and cell residence time (sludge age) [1]. Leachate transfer processes, such as co-treatment of leachate with wastewater [91], evaporation [11], and recycling of nutrients from wastewater, thus save energy consumption or produce biofuels [135]. The final waste of treatment (such as sludge and biomass) can be used as agricultural fertilizers [144]. Due to the large quantity of OM, chlorinated inorganic and organic salts, and heavy metals present in LL [7,8], none of these treatment processes can successfully decrease contaminants to an acceptable level on their own [27,147,148].
Therefore, combining treatment processes can leverage the advantages of each process to overcome challenges and limitations, resulting in treated wastewater that meets environmental regulations and can be safely discharged into water bodies. The following sections provide a breakdown of the different treatment processes categorized by the type of integrated treatment processes.
5.1. Chemical + Chemical Integrated Processes
The treatment of LL is based on the chemical composition of the leachate. LL typically contains various challenging components such as recalcitrant and heat-resistant organic matter, toxic substances, suspended and colloidal solids, heavy metals, and humic substances [64,105]. Humic substances are large anionic molecules with different molecular weights, and LL often has a low ratio of BOD5/COD < 0.1 [149]. The presence of humic substances and non-degradable organic matter makes biological treatment processes less effective [149]. Therefore, a combination of chemical processes is employed for the treatment of LL. Chemical processes, including Fenton, C/F, and EC, have been successfully used for LL treatment. These processes are often used in combination with each other. Other AOPs such as EF, PF, SPF, oxidation, ozonation, and photocatalysis are also used. While individual chemical processes have limited effectiveness in treating LL, their efficiency can be enhanced by employing pre- and post-treatment steps [58]. Pre-treatment steps are employed to remove suspended and colloidal solids from LL, while post-treatment steps focus on decomposing soluble organic compounds and removing refractory organic matter, including heavy metals, pesticides, humic and fulvic acids, and non-biodegradable substances [64]. These steps are necessary due to the presence of these substances in LL. The sequential execution of combined chemical treatment processes enables the efficient removal of COD from LL.
The combination of chemical processes is particularly advantageous for treating organic compounds that are difficult to biodegrade and have high toxicity. Table 8 provides an overview of a few chemical + chemical combination treatment processes along with an analysis of their effectiveness.

Table 8.
A summary of the reviewed chemical + chemical integrated treatment processes and their results.
As indicated in Table 8, Cortez [147] examined several pre-treatment processes for stabilized LL to enhance the organic matter biodegradability in preparation for subsequent biological treatment. They tested the Fenton process (Fe2+/H2O2) and various ozone-based AOPs such as O3/H2O2, O3/OH−, and O3. Ozone demonstrated the highest levels of biodegradability and removal effectiveness, particularly when used in combination with H2O2 and at higher pH values. Overall, the study found that, at natural and neutral pH levels, Fenton treatment, O3/OH−, and O3/H2O2 processes were more effective in removing TOC and COD compared to O3 treatment. The results presented in Table 8 clearly demonstrate that the Fe2+/H2O2 + O3 process significantly enhanced the removal of TOC and COD compared to the individual process (Fe2+/H2O2). Despite the leachate quality improvements, a biodegradability of 0.4 was reached, which is thought to be required for efficient biological treatment. Further research should focus on improving pre-treatment techniques such as ozonation and Fenton treatment at an alkaline pH or in conjunction with H2O2 to obtain a leachate that is more biodegradable. A cost analysis revealed that the Fe2+/H2O2 system was the most economical option for treating the LL.
In a study conducted by Orkun and Kuleyin [56], the researchers examined the effectiveness of using an EC process to remove COD from young LL. They found that the EC process alone achieved a certain level of COD removal efficiency. Furthermore, the researchers explored the combined application of EC and EF processes for COD removal from LL. They observed that the EF process effectively removed COD from LL, and the combined process resulted in an improvement in COD removal efficiency compared to the EC process alone.
In studies conducted by Moradi and Ghanbari [64], Cheibub [108], and Amor [58], they focused on the removal of COD from LL using the combined C/F and Fenton or photo-Fenton treatment processes. Moradi and Ghanbari [64] employed the combined C/F and Fenton processes for young LL treatment. In the study, the C/F process using FeCl3 was employed as a pre-treatment process for young LL. After that, the leachate was subjected to the Fenton process to degrade it. The research employed RSM for the experiment design, modeling, and data analysis. Based on the germination index, which significantly increased following the C/F and Fenton processes, a phytotoxicity test was also carried out.
Similarly, Cheibub [108] studied the removal of COD from stabilized LL using the combined C/F followed by Fenton and photo-Fenton (PF) treatment processes. Treatment with FeCl3 in the C/F removed approximately 53% of COD. After C/F, the remaining Fe3+ ions were unable to catalyze H2O2 breakdown into hydroxyl radicals, which enhanced COD elimination. The LL was first treated by the C/F process, and the addition of Fe2+ ions and subsequent interaction with H2O2 in the Fenton step enhanced the COD removal to 83.3%. A 75% decrease in COD was observed, demonstrating the effectiveness of the PF process when used directly on the raw effluent. The COD of the LL was significantly decreased by the AOPs. However, the COD of the raw LL was dramatically lowered by combining the C/F process with the Fenton or PF process.
Amor [58] investigated the treatment of stabilized LL with a focus on meeting discharge limits. They first investigated the effect of initial pH and different coagulants on the C/F process. They then evaluated the efficiency of two AOPs, namely Fenton and solar photo-Fenton (SPF), for leachate remediation. The C/F pre-treatment step removed a substantial amount of polyphenols, turbidity, and COD. Combining C/F with the Fenton reagent achieved a COD removal rate of 89% in 96 h. However, this combined treatment required several days for effective results. Furthermore, combining C/F with SPF yielded higher reductions in dissolved organic carbon (DOC) (75%), compared to single SPF (54%). The treated LL exhibited increased biodegradability and showed no toxicity in biodegradability and toxicity tests.
In summary, these three studies [58,64,108] focused on the treatment of LL using the integration of chemical-based processes such as C/F and AOPs (Fenton and SPF). The studies demonstrated the effectiveness of these processes in removing pollutants from the leachate. The combined treatments resulted in improved biodegradability and reduced toxicity of the treated leachate.
Luo [101] used flocculation with PAC as a flocculant followed by microelectrolysis-Fenton (MEF) to effectively remove COD and humic acids (HAs) from LL. Superior decontamination performance was achieved by combining the PAC coagulation with MEF processes; the expected clearance rates of HA and COD were 93.79% and 90.27%, respectively. Whereas MEF was more successful in destructing organic contaminants that were resistant to removal, particularly those that have humic-like and fulvic-like substances, PAC coagulation was more successful in eliminating compounds that resembled protein-like substances.
Ishak [148] investigated the combination of C/F and UV-based sulfate radical advanced oxidation (UV/SRAOP) for COD removal from stabilized LL. C/F treatment achieved 76.9% COD removal, and subsequent UV/SRAOP treatment using UV/PMS or UV/PS resulted in approximately 91.5% and 90.9% COD removal, respectively. The presence of sulfate radical ions in the treated effluent maintained a stable toxicity level after UV/PS treatment, but it slightly increased after UV/PMS treatment. These findings suggest that UV/SRAOP could be a viable alternative for LL treatment.
Rebolledo [149] conducted experiments to treat stabilized LL using two different combination treatments. The first treatment involved coagulation/sedimentation (C/S) with polyaluminum chloride (PAC), followed by the SPF process. The second treatment involved C/S with FeCl3:6H2O, followed by SPF induced by ferrioxalate. The objective was efficient COD removal. The researchers suggested exploring solar radiation as a sustainable and renewable energy source in LL treatment. These alternative treatments offer potential for efficient COD reduction in LL while considering cost and sustainability factors.
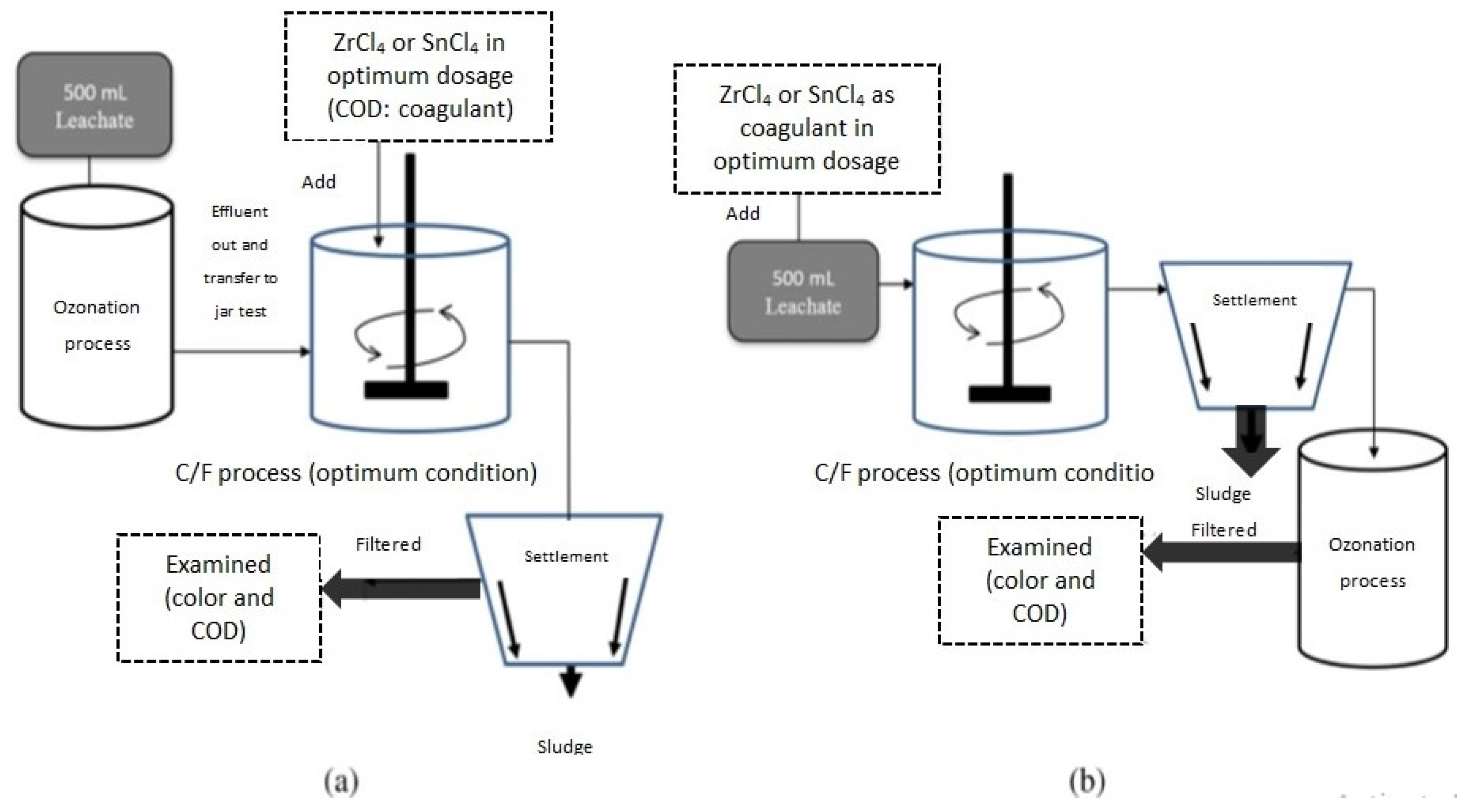
Zakaria [88] investigated the use of tin tetrachloride (SnCl4) and zirconium tetrachloride (ZrCl4) as coagulants for treating stabilized LL, as illustrated in Figure 6. SnCl4 showed favorable performance, removing 77% of COD and 97% of color. The researchers also explored combined treatments using ozonation (O3) and C/F processes.
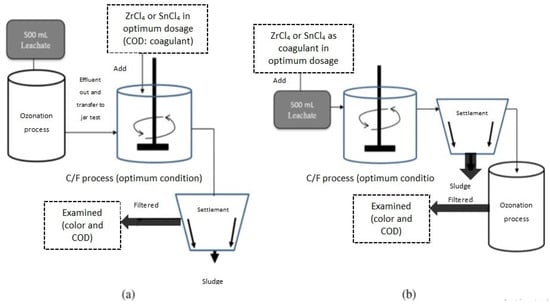
Figure 6.
Schematic diagram of combined treatment: (a) ozonation as pre-treatment followed by coagulation; (b) coagulation followed by ozonation as polishing process [88]. (CCC License Number 5751501056682).
As depicted in Figure 6, four treatment sequences were implemented: SnCl4-O3, ZrCl4-O3, O3-SnCl4, and O3-ZrCl4. The SnCl4-O3 treatment achieved the highest removal efficiencies, with 88% for COD and 97.1% for color. The ZrCl4-O3 treatment had lower removal percentages (70.6% for COD and 90% for color). The SnCl4-O3 treatment significantly improved the leachate’s biodegradability ratio (BOD5/COD), transitioning it from a stabilized to an intermediate state (from 0.03 to 0.28). Overall, the combined treatment process was more effective than C/F alone in treating stabilized LL.
In research by Sun [106], the performance of a combination treatment process that included electrooxidation (EO) and EC (EO + EC) to treat stabilized LL was confirmed. For COD and TOC, the treatment’s efficacy was 80.32% and 79%, respectively. EO could remove HA and FA concurrently and encourage the conversion of FA to hydrophilic (HyI) humus. EC was extremely successful in the removal of a significant amount of humic-like humus from the concentrate but weak in the removal of FA and long-wave humus. When it came to eliminating aromatic organic debris, EC + EO performed better than EO+EC. While EC only effectively removes the HA component with significant sludge generation, EC and EO both have the ability to totally degrade the HA component on their own.
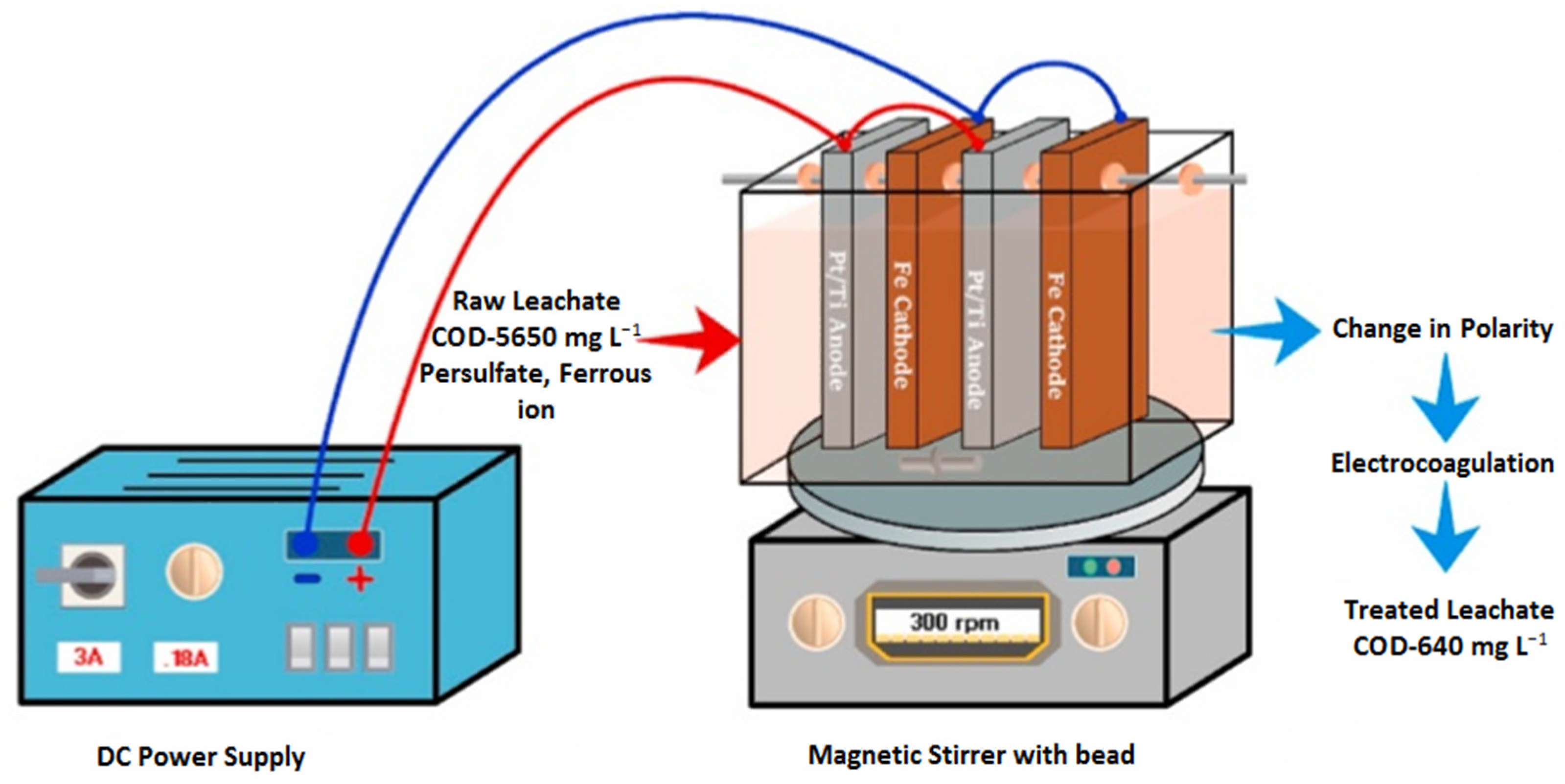
Nidheesh [150] conducted a study to investigate the treatment of stabilized LL utilizing a combination of sulfate-radical-associated electro-chemical AOPs (SR-EAOPs) followed by EC (SR-EAOP + EC). The electrochemical processes were carried out in a single step, as shown in Figure 7.
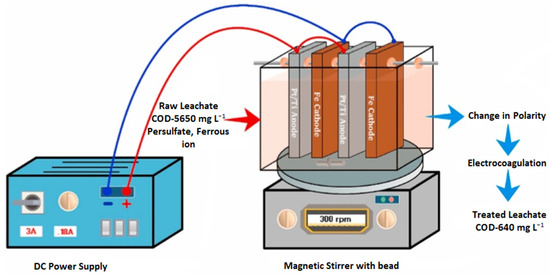
Figure 7.
Electrochemical reactor [150]. (CCC License Number 5751400096307).
As shown in Figure 7, the polarity is switched in the process of EC. In SR-EAOP, Fe was used as the cathode and Pt/Ti was used as the anode. The treatment processes involved the generation of sulfate radicals through the cathodic reduction of persulfate (PS), the anodic oxidation of Pt/Ti, and the activation of these radicals through the addition of externally provided Fe2+ ions in the water medium. The system stimulated the production of active chlorine and hydroxyl radicals through the indirect electrochemical oxidation and anodic oxidation processes, which significantly enhanced the reduction in COD in the leachate.
The study conducted by Lu [105] aimed to enhance the treatment of stabilized LL in China by combining two chemical processes: the electrochemical peroxidation (ECP) process and the EF process. This combined process resulted in a reduction in acute cytotoxicity (ACT) as well as an improvement in the removal of nitrogen and organic contaminants. The ECP process primarily removes the organic compounds in stabilized LL, which are then further mineralized into smaller hydrophobic molecules in the EF process. The combined ECP-EF process effectively reduces the complexity of the organic compounds and aromaticity, leading to decreased ACT. The study demonstrates that the combined ECP-EF process allows for advanced treatment of old LL. Under the optimal parameters outlined in Table 8, this combined process achieved removal efficiencies of 99.2 ± 0.1%, 99.4 ± 0.1%, 85.1 ± 0.8%, and 95.9 ± 0.4% for total nitrogen (TN), NH+4-N, TOC, and COD, respectively. Additionally, the retention of Fe2+ produced by the sacrificial anode in the combined process facilitated the subsequent EF process. However, the study identified a limitation in the proper handling and disposal of resulting by-products, which may be necessary for environmental sustainability. Addressing this issue is crucial to ensure the overall sustainability and environmental impact of the ECP and EF process.
5.2. Chemical + Physical Integrated Processes
The integration of chemical and physical treatment processes to treat LL has gained wide attention due to its ability to remove biodegradable and non-biodegradable organic compounds and toxic metals and nitrogen compounds in a more efficient and effective manner [82]. Table 9 provides an overview of a few chemical + physical combination treatment processes along with an analysis of their effectiveness. Chemical and physical treatment processes are often used as pre-treatment or combined treatments to treat the LL [151]. In the study, chemical treatment processes such as EC, C/F, and Fenton were used as pre-treatments to remove suspended and colloidal solids from LL to make it a viable biological treatment in the young LL, and to decompose some of the heat-resistant organic compounds present in the stabilized LL [84,151]. Although chemical processes have shown effectiveness in reducing COD and toxic metals [95], they alone could not achieve a reduction of more than 69%. Therefore, combining chemical and physical processes can improve treatment efficiency.

Table 9.
A summary of the reviewed chemical + physical integrated treatment processes and their results.
By combining different processes such as adsorption, filtration, C/F, EC, and Fenton, the limitations of each individual process can be overcome, leading to an improvement in overall treatment performance.
As indicated in Table 9, research on the treatment of LL utilizing a combination of pre-treatment processes, including coagulation and adsorption, was undertaken by Gandhimathi [151]. The researchers utilized fly ash as a cost-effective adsorbent for adsorption studies, while alum and ferric chloride were employed to study coagulation. Coagulation experiments were performed on both stabilized and young leachate, whereas adsorption experiments were conducted using alum-pre-treated stabilized leachate. The researchers found that the maximum COD removal for stabilized leachate, which had been pre-treated with alum, was 28% when fly ash was utilized as the adsorbent. By combining coagulation with alum and adsorption with fly ash, an overall COD removal efficiency of 82% was achieved for leachate. During the coagulation experiments, it was observed that the COD removal efficiency using ferric chloride was 59% for stabilized leachate, whereas for alum, it was 75% without prior pH adjustment. Comparatively, for young leachate, the COD removal efficiency using alum was 55%, while it was 35% for ferric chloride without pH correction. The researchers also reported that the removal efficiency of COD using ferric chloride decreased from 53% at pH 2 to alkaline pH levels. Conversely, alum exhibited a COD removal efficiency of 55% at pH 8.
For the purpose of removing contaminants from LL, Pedro-Cedillo [95] compared the Fenton–adsorption and adsorption treatments. In comparison to the adsorption process on raw leachate, the Fenton–adsorption process demonstrated greater removal rates for TOC, TN, COD, and color (over 99%), as well as removal rates for TDS and BOD5 up to 95%. The primary organic substance in the leachate was bisphenol-A, which was only eliminated during the Fenton–adsorption process’s adsorption step. The most abundant organic compound in the leachate was bisphenol-A, which was retained by the carbon in the adsorption process. The combined Fenton–adsorption process was successful for treating LL, but it was expensive because of the adsorption material. Further studies are needed to optimize the use of GAC.
Li [152] conducted a study that examined the use of EC and fiber filtration for treating intermediate LL. The research focused on assessing the effectiveness of EC in removing COD, arsenic, iron, and phosphorus from the LL. By employing a combination of EC and fiber filtration, the study achieved significant reductions in the concentrations of contaminants responsible for suspended solids, color, and odor. Following the EC process using Al or Fe electrodes, the treated LL was passed through two steps of fiber filters. The first-step fiber filter retained the flocs formed during EC, while the second-step biofilter facilitated the removal of organic contaminants through biodegradation.
El Mrabet [103] conducted a study on LL treatment using a combined C/F with FeCl3 as a coagulant, followed by adsorption with a Cupressus sempervirens (CupSem) bioadsorbent. Combining C/F with CupSem adsorption further improved the removal percentages, with 93% color, 96% BOD5, and 86% COD removal. The study found that the pseudo-second-order kinetics and Langmuir isotherm models described the adsorption process well. These findings suggest that the combined C/F and adsorption treatment of LL offers an affordable alternative to existing processes and can be successfully implemented in large-scale sanitary landfills. Similarly, Chaouki [84] used C/F with FeCl3, followed by adsorption onto palm bark powder (PBP), to treat young LL. The Dubinin–Radushkevich isotherm models describe the adsorption process.
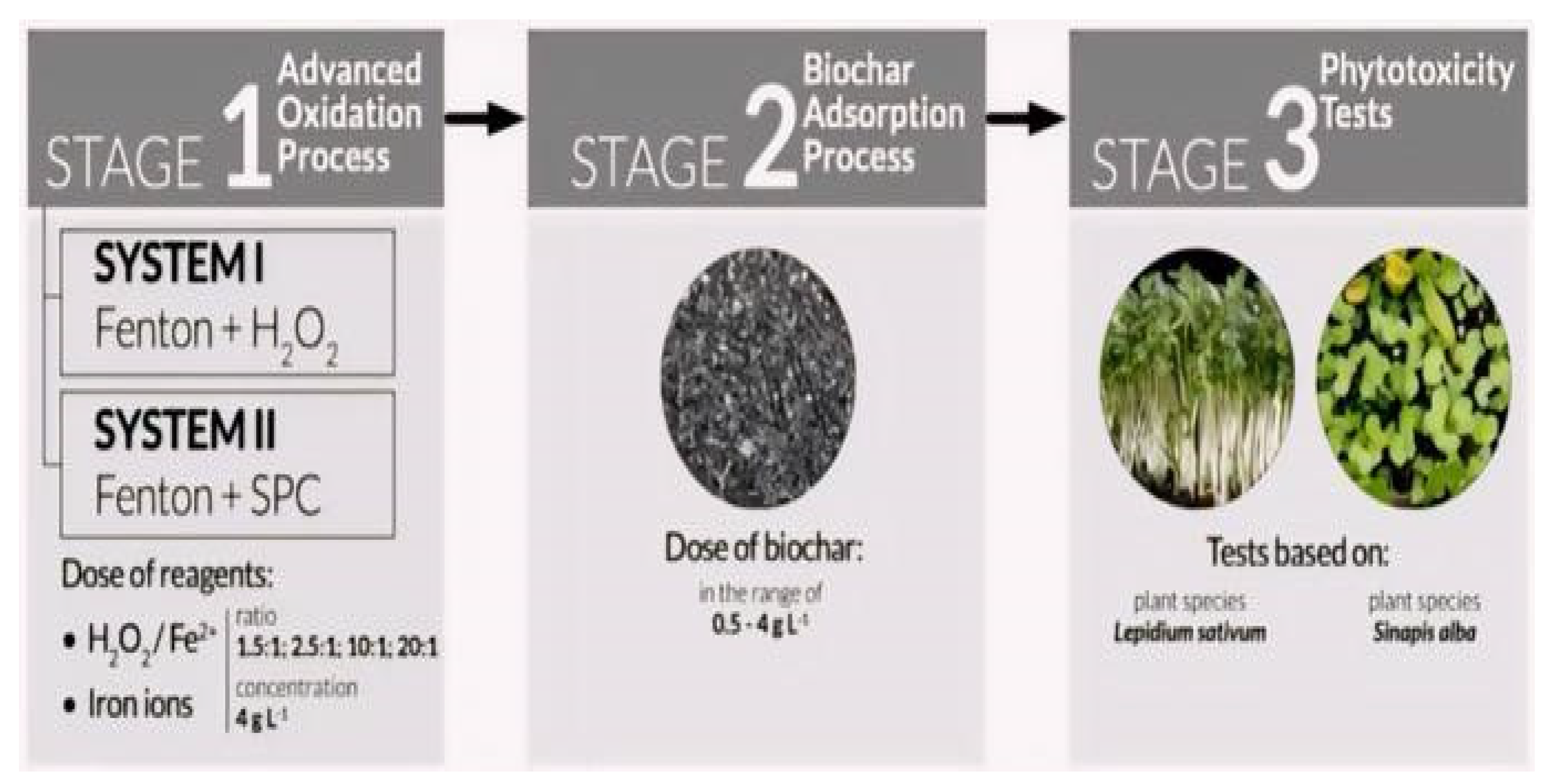
In order to assess the effectiveness of treating LL, Kwarciak-Kozłowska and Fijałkowski [81] combined the AOPs with biochar adsorption (BC), as indicated in Figure 8.
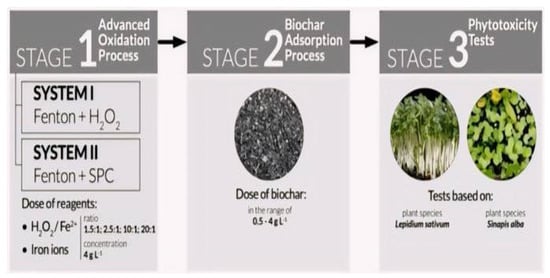
Figure 8.
The schematic of LL treatment by AOP (Systems 1 and 2) associated with the BC adsorption process [81]. (CCC License Number 5751400707357).
Two systems, H2O2+BC and sodium percarbonate (SPC) with BC, were tested, as shown in Figure 8. COD values decreased significantly in both systems, with the H2O2+BC system showing higher elimination efficiencies. The SPC+BC system exhibited better germination and longer plant roots. In comparison to the conventional Fenton process, the suggested system has the benefit of utilizing a safer and more ecologically friendly reagent (SPC), and it is also more affordable to use biochar as an adsorbent than granular activated carbon (GAC).
Yan [102] investigated the combination of a membrane electrochemical reactor (MER) with membrane distillation (MD) for LL treatment. The aim was to control membrane fouling, recover resources, and reduce energy consumption and carbon emissions. The MER-MD system efficiently removed pollutants, with high rejection rates for metal ions, ammonia, total phosphorus, and CODCr. It also recovered humic acid and ammonia nitrogen from LL. The MER-MD system showed greater resistance to membrane fouling and wetting compared to the MD and EO-MD systems. Energy consumption and carbon emissions were reduced by around 20% compared to MD alone and by 8% compared to EO-MD. The MER-MD system’s recovery of ammonia nitrogen can compensate for 8.25 kg of a carbon dioxide equivalent.
5.3. Chemical + Biological Integrated Processes
Biological treatment processes are commonly used to treat LL containing high concentrations of COD and BOD5 due to their simplicity and high cost-effectiveness [29]. Biological treatment processes have proven to be satisfactory. However, the presence of toxic pollutants, recalcitrant organic materials, and non-degradable organic compounds with little carbon and oxygen in LL limits the application of biological treatment alone [97]. In such cases, chemical processes are used to pre-treat LL to make it amenable to biological treatment and to convert non-degradable organic compounds into more stable, non-hazardous materials. It has been found that the use of an integration of chemical and biological treatment processes is the most common and widely used among the combined treatments to treat LL. Table 10 provides an overview of a few chemical + biological combination treatment processes along with an analysis of their effectiveness.

Table 10.
A summary of the reviewed chemical + biological integrated treatment processes and their results.
As indicated in Table 10, in a study conducted by Liu [153], the researchers examined the effectiveness of the combined Fenton–SBR process in removing FA and HA from stabilized LL. The results indicated that the combined Fenton–SBR process was capable of efficiently removing humus from the leachate, with removal rates of 88.2% for FA and 87.3% for HA. Furthermore, the removal rates for DOC and COD of both FA and HA exceeded 80%.
Vilar [107] investigated combining an SPF process with biological oxidation (denitrification and nitrification) for residual DOC and nitrogen removal from LL. They achieved complete removal of nitrites, nitrates, and ammonium through biological nitrification and denitrification after neutralizing Fe sludge. The highest rate of nitrification, 68 mg of NeNH4+/day, was achieved with 33 mmol NaOH/L and 27.5 mmol H2SO4/L of denitrification, respectively.
El-Fadel [60] studied the use of an SBR preceded by a C/F process with phosphorus nutritional balance for LL treatment. The study also identified several factors that influenced the rate of biomass growth resulting from substrate consumption. These factors included a YH (yield of heterotrophic biomass) of 0.18 mg of VSS/mg of COD (volatile suspended solids per milligram of COD), Yobs (observed yield) ranging from 0.12 to 0.17 mg of VSS/mg of COD, qmax (maximum specific substrate utilization rate) ranging from 2.81 to 2.99 day−1, mmax (maximum specific growth rate) ranging from 0.51 to 0.54 day−1, and Kd (endogenous decay coefficient) of 0.10 day−1. COD removal followed a pseudo-first-order model with a constant k of 0.36–1.54 day−1 depending on phosphorus levels.
Djelal [154] investigated the combined use of EC and biological treatment for stabilizing LL in a non-hazardous waste storage facility. The EC treatment reduced COD, with removal rates of 33% and 56% at current densities of 23 and 95 A/m2, respectively. However, ammonium removal was not effective. Subsequent biological treatment using activated sludge (AS) did not significantly enhance the degradation of OM, which mainly consisted of recalcitrant compounds. Both biological treatments, with and without AS, reduced NH4+ with a removal efficiency of 62%. The addition of AS improved nitrification efficiency by 10%. However, the combined treatment achieved only around 80% degradation of NH4+ at a current density of 95 A/m2.
Nivya and Pieus [97] investigated the treatment of highly contaminated LL using the photo-electro-Fenton process (PEF), followed by the MBR. The combined approach showed higher pollutant removal efficiencies compared to PEF alone. Furthermore, the study examined the biodegradability of the synthetic wastewater by measuring the BOD/COD ratio, which increased from 0.19 to 0.34. This indicated improved biodegradability, leading to the adsorption of MBR as a post-treatment. However, when the combination of PEF followed by MBR was applied, the removal percentages increased to 88.2% for chloride, 93.3% for sulfide, 82.7% for sulfate, 100% for phosphate, 88.3% for ammonia nitrogen, 96.2% for COD, 90.2% for BOD, and 95.5% for TSS.
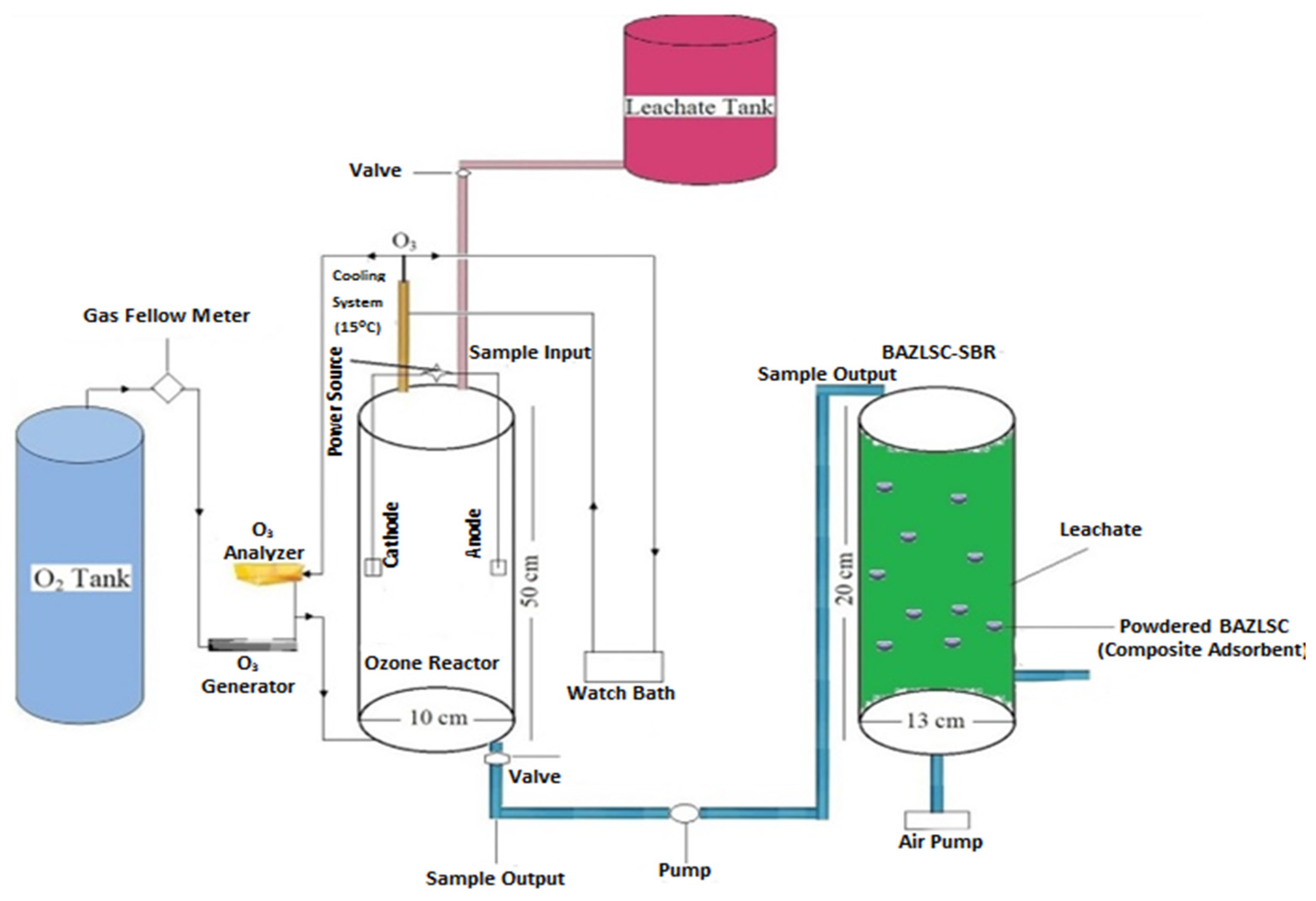
Mojiri [117] conducted a study in which they treated concentrated LL using a combination of electro-ozonation and a composite adsorbent-augmented SBR process, as depicted in Figure 9. The treatment process resulted in removal efficiencies of 52.9% for nickel, 90.4% for color, and 64.8% for COD. The ozone utilization in this process ranged from 0.3 kg to 1.4 kg of COD removed per kg of ozone consumed.
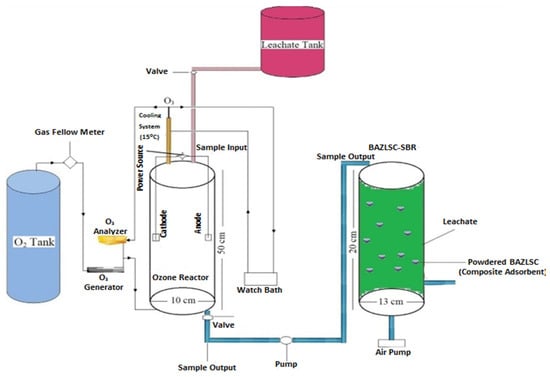
Figure 9.
Schematic diagram of reactor design process [117]. (CCC License Number 5751401403360).
As shown in Figure 9, following the optimal electro-ozonation treatment, the concentrated leachate was then transferred to a powdered composite adsorbent (P-BAZLSC)-augmented SBR reactor (PB-SBR). By employing the PB-SBR, the removal efficiencies for nickel, color, and COD improved to 73.4%, 96.1%, and 88.2%, respectively, compared to the initial values of 52.9%, 90.4%, and 64.8%.
Bhagawan [98] investigated the use of the EC process and biogas generation for treating LL. The study examined various parameters affecting the EC process, including electrode material, electrode reactive surface, in situ peroxi-EC, inter-electrode distance, applied voltage, and initial pH. The metal removal rate increased with higher electrode surface area and applied voltage but decreased with increasing inter-electrode distance. The results showed high removal efficiencies for COD, Fe, Cu, Pb, Zn, and Ni, as shown in Table 10.
Tezcan Un [104] studied the combined treatment of LL using an EC process as pre-treatment followed by anaerobic treatment. The EC process was optimized to achieve a maximum COD removal efficiency of 69%. The effluent was then subjected to anaerobic treatment, and the process was further optimized using a factorial design process. The maximum COD removal through anaerobic treatment was 74%. When the combined EC and anaerobic treatment were applied, it resulted in an overall COD removal efficiency of 92%. The study emphasizes the effectiveness of the combined treatment approach compared to using either process alone for leachate wastewater.
Baiju [155] synthesized iron molybdophosphate (FeMoPO) and used it as a catalyst for the EF treatment of LL. EF achieved 82% COD removal, improving the biodegradability as indicated by an increased BOD5/COD ratio from 0.03 to 0.4. The catalyst could be reused for up to three cycles. When EF was combined with biological treatment, an overall COD removal of 97% was achieved, resulting in a final COD concentration of 192 mg/L. The combination of EF and biological processes proved to be highly efficient for LL treatment.
Colombo [156] investigated the combination of physical–chemical and biological processes for reducing pollutants in LL. They employed a conventional biological treatment followed by a photo-Fenton (PF) process and then combined PF with biological treatment. The PF process was optimized using a central composite rotatable design (CCRD). After a decantation process, the biological treatment was performed over 40 h. When the improved PF process was combined with the biological process, a removal effectiveness of 98% for COD and BOD5 was achieved, meeting the requirements for releasing treated wastewater into water bodies.
5.4. Physical + Physical Integrated Processes
The utilization of physical treatment processes to treat LL has proven effective in terms of high flexibility, high process performance, greater mobility, and low production costs, especially membrane filtration [157]. Membrane filtration processes have been commonly used in recent years, including RO, NF, MF, and UF [135]. Among these, NF and RO have been found to be efficient in the removal of suspended and colloidal solids and organic pollutants from LL [131,135]. Forward osmosis membranes’ excellent treatment efficiency has made them appropriate for a variety of uses [157]. Table 11 provides an overview of a few physical + physical combination treatment processes along with an analysis of their effectiveness.

Table 11.
A summary of the reviewed physical + physical integrated treatment processes and their results.
According to Table 11, forward osmosis (FO) combined with MD processes for treating high-salinity hazardous waste LL gave excellent performance in TN, TOC, and NH4+-N, as well as the toxic element Hg, in a study conducted by Zhou [157]. They used RSM to optimize the operating parameters of the FO process using the Box–Behnken design (BBD), aiming to maximize pollutant removal efficiency, water flux, and salt reverse flux. The independent variables considered were the flow rate of the draw solution (DS) and the feed solution (FS), as well as the concentration of the DS. The FO-MD combined system demonstrated high salt rejection rates (>96%) and excellent removal rates for TN, TOC, and NH4+-N, as well as the toxic elements Hg, As, and Sb (>98% removal). The results demonstrated that the FO-MD hybrid system was effective in treating high-salinity hazardous LL and outperformed individual FO or MD processes in contaminant rejection.
Kulikowska [140] conducted a study to investigate the removal of organics from stabilized LL using a combination of adsorption with Norit SX2 powdered activated carbon (PAC) and fine UF. Fine UF alone showed low efficiency in organics removal. To improve the removal efficiency, two doses of PAC were used before fine UF: 0.2 g/L and 1 g/L. However, Norit SX2 particles caused membrane blockage and reduced the permeate flux, even at the lower dose. The combination of adsorption and fine UF was more effective in removing organics but led to a lower permeate flux compared to fine UF alone. Using a lower dose of Norit SX2 with fine UF provided similar treatment efficiency with less reduction in permeate flux.
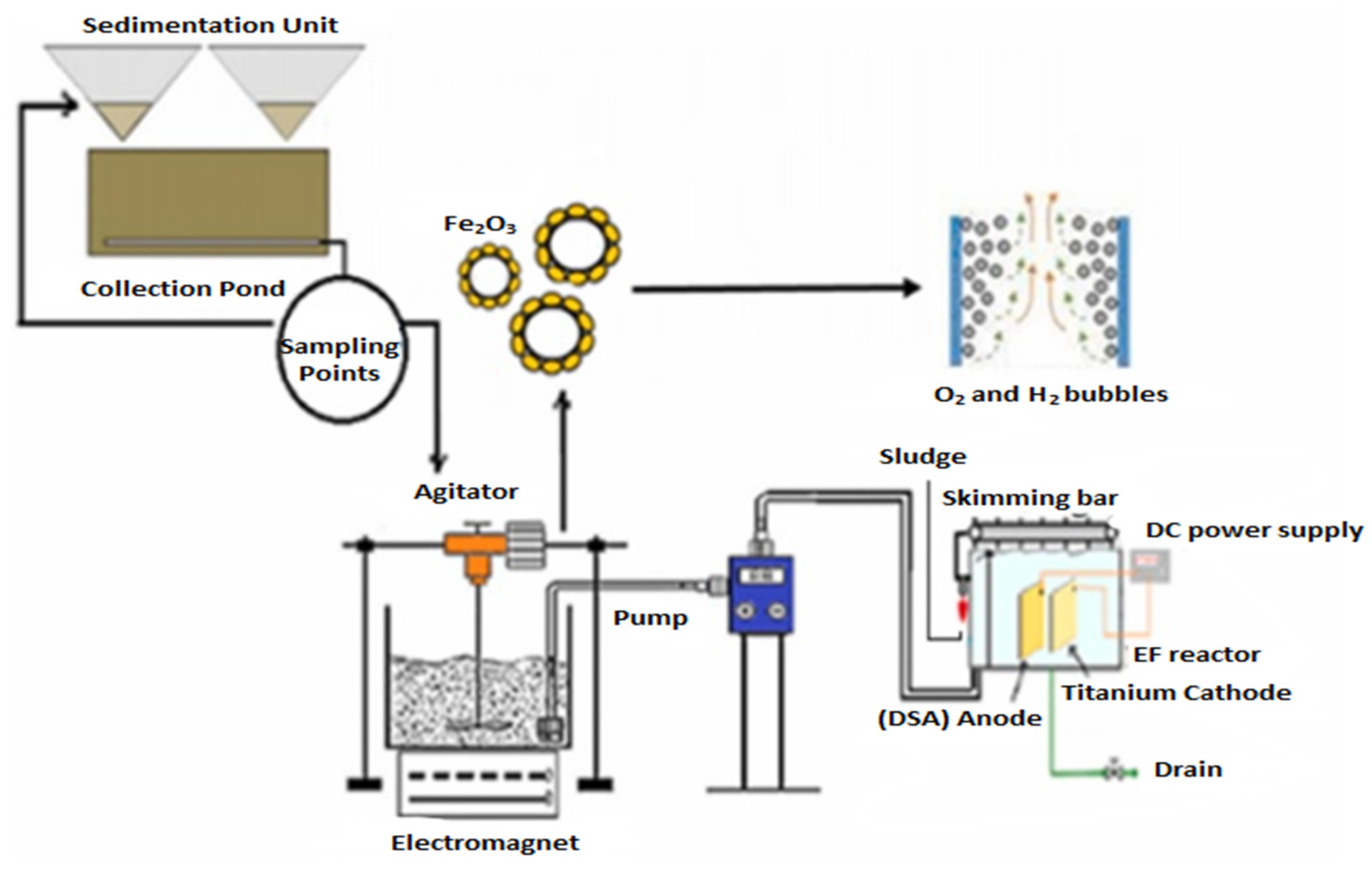
Shadi [85] conducted a study to investigate the treatment of stabilized LL utilizing a combination of iron oxide nanoparticles (Fe2O3 NPs) as adsorbents and an electro-flotation process, as shown in Figure 10. The aim was to remove organic and inorganic pollutants, increase DO levels, and improve water quality.
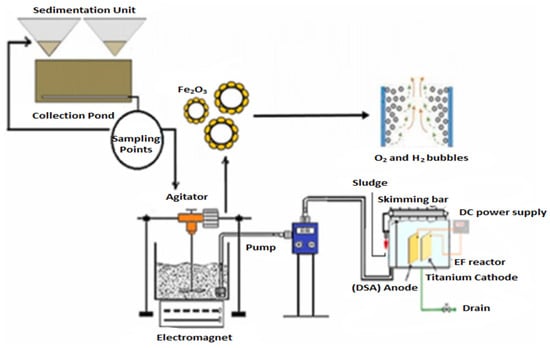
Figure 10.
Schematic diagram of combined Fe3O3 NPs/electro-flotation process [85]. (CCC License Number 5751410653438).
As shown in Figure 10, combining both processes (Fe2O3/electro-flotation) significantly improved removal efficiencies. After 120 min, almost all pollutants were removed (>99% removal) for COD, color, TDS, NH3-N, and turbidity. The combined treatment process was highly effective, achieving over 99% pollutant removal with an estimated operational cost of 3.18 USD/m3. The study demonstrates an efficient treatment process for removing inorganic and organic contaminants from stabilized LL.
5.5. Physical + Chemical Integrated Processes
The efficacy of treating LL by combining chemical and physical treatment modalities has also been reported in the literature. Adsorption and UF by themselves are not sufficient to decrease contaminants to levels that are tolerable. Based on the previous literature, the UF treatment process can remove only 50% of COD from LL [118]. Therefore, the most effective way to satisfy the allowable limits of pollutant discharge is to combine it with chemical treatments such as Fenton and C/F [29,159]. Table 12 provides an overview of a few physical + chemical combination treatment processes along with an analysis of their effectiveness.

Table 12.
A summary of the reviewed physical + chemical integrated treatment processes and their results.
According to Table 12, in order to mineralize and decolorize LL, Silveira [99] undertook research to assess the viability of using electrolysis and FeTiO3, increased by UV-LED, for the activation of persulfate (PS), followed by Fenton oxidation. Increasing current density converted PS to SO4− and generated hypochlorite, enhancing light penetration and promoting Fe(III) photoreduction on the FeTiO3 surface for improved decolorization. The combined use of electrolysis, FeTiO3, and UV-LED demonstrated synergistic effects, achieving a mineralization rate of 53%. Subsequent Fenton oxidation at pH 3 resulted in over 90% overall mineralization. This combined treatment process proved effective for refractory and highly colored effluents, with the remaining TOC consisting mainly of short-chain organic acids and a small amount of chlorinated compounds. However, more improvement is needed. While PS has several advantageous properties, such as high stability, solubility, and versatile applications, there is room to enhance its efficiency. Future research could focus on optimizing the PS dosage and reaction conditions to achieve even higher mineralization rates. Additionally, exploring alternative catalysts or modifications to the Fenton process may help improve the overall performance of the combined approach.
Poblete and Pérez [100] studied the depuration of LL using sawdust (SD) as an activated carbon material for adsorption processes as a pre-treatment for SPF and SPF+O3. Treatment with SD resulted in significant reductions in pollutant concentrations, including copper, iron, ammonium, color, COD, and HA, with reductions of 61.1%, 70.2%, 87%, 19.5%, 33.7%, and 18.3%, respectively. Additionally, the combined process of SPF+O3 resulted in the removal of organic matter, with HA, ammonium, nitrate, color, and COD showing reductions of 73.3%, 12.8%, 50%, 74.9%, and 76.4%, respectively. The overall treatment achieved removal efficiencies of 97.9%, 94.5%, 95%, and 95.1% for pollutants such as HA, ammonium, color, and COD, respectively. The improved LL quality led to reduced toxicity, as indicated by a germination index (GI) of 20% for Lactuca sativa using a 50% diluted LL. The incorporation of SD as a pre-treatment in LL treatment proves to be beneficial. However, more improvement is needed. Future studies could investigate the selection of suitable absorbents and explore different operational parameters to enhance the absorption process. Additionally, optimizing the PF reaction conditions, such as catalyst concentration, pH, and irradiation intensity, could lead to improved degradation efficiency and reduced treatment time.
In the study conducted by Nazia [159], the researchers explored the effectiveness of different coagulants in combination with alum Al(SO4)3 and lime Ca(OH)2 for the pre-treatment of LL. They also developed a polyethersulfone (PES) polymer-based UF membrane for the treatment process. The aim was to reduce impurities such as COD, TDS, turbidity, and color on the membrane. They found that FeCl3 alone was the most effective and cost-efficient coagulant for impurity reduction in LL. The synthesized PES-based UF membrane exhibited good stability. The optimized combination of FeCl3 and a UF membrane effectively removed turbidity, TDS, electrical conductivity, pH, and color, producing clear permeate water for reuse. The integrated process demonstrated the reclamation of at least 70% of LL water for various applications, offering advantages such as a small equipment footprint, cost-effectiveness, environmental safety, low energy consumption, and scalability. While the PES membrane used in the UF process exhibited good stability, further research could focus on enhancing the membrane’s fouling resistance and long-term performance. Additionally, investigating alternative coagulants and flocculants, as well as optimizing the dosage and reaction conditions, may further improve the removal efficiency of the C/F process. This combined approach has the potential to reclaim a significant amount of water from liquid waste for industrial or domestic applications, and further optimization efforts can enhance its overall effectiveness.
5.6. Physical + Biological Integrated Processes
Biological treatment processes are commonly utilized to treat LL containing high concentrations of COD and BOD5 due to their simplicity [29]. Biological treatment processes have proven to be satisfactory. However, the presence of recalcitrant pollutants in LL limits the application of biological treatment alone as they are often difficult to degrade [116,124]. Combined physical and biological treatment processes have been proven to be effective for the treatment of LL. Table 13 provides an overview of a few physical + biological combination treatment processes along with an analysis of their effectiveness.

Table 13.
A summary of the reviewed physical + biological integrated treatment processes and their results.
According to Table 13, Smaoui [142] investigated the efficiency of a combined process involving air stripping, anaerobic digestion (AD), and aerobic AS treatment for LL with a high organic matter content. To address the nitrogen load that hampers biological development, air stripping pre-treatment was conducted. The research aimed to evaluate a combined process of aerobic activated sludge, AD, and air stripping treatment. Air stripping achieved an impressive ammonia removal rate of over 80%, improving biodegradability and the carbon-to-nitrogen (C/N) ratio. The anaerobic treatment effectively removed COD and produced biogas. This indicates the potential for energy recovery from the leachate through biogas production. The raw leachate exhibited a 78% COD removal rate and generated 4 L/d of biogas with a 70% methane content. This approach removed 77% of COD and over 97% of the initial organic compounds in the leachate. Finally, the application of an activated sludge reactor as a post-treatment process efficiently eliminated resistant organic matter. However, additional information on the specific experimental setup, system performance, and any limitations encountered would provide a more comprehensive scientific evaluation of the study.
5.7. Biological + Biological Integrated Processes
Biological treatments are used for biodegradability of organic compounds, particularly in LL, that contain high concentrations of COD and BOD, due to their ease and affordability [29]. By using microorganisms’ degrading activity, these strategies facilitate the transition of the compounds found in LL to the following: under aerobic conditions, biomass in sludge form and carbonic gas; and biogas, when exposed to anaerobic conditions, preventing the contaminants’ phase transition [118,160,161,162,163,164,165,166,167,168,169]. Table 14 provides an overview of a few biological + biological combination treatment processes along with an analysis of their effectiveness.

Table 14.
A summary of the reviewed biological + biological integrated treatment processes and their results.
According to Table 14, Aluko and Sridhar [145] conducted a study to examine the effectiveness of bench-scale treatment processes, specifically a trickling filter (TF) and SBR, in treating LL. The results showed that the TF treatment process resulted in significant reductions in NH3 (59.50%), BOD5 (76.69%), turbidity (71.96%), and SS (73.17%) in the effluents. On the other hand, the SBR process achieved reductions in NH3 (64.83%), BOD5 (84.06%), and SS (62.28%). Although there was a marginal reduction in NH3 levels, significant nitrification occurred within permissible limits. However, the effluents produced by both treatment processes did not meet the national regulatory standards for discharge into surface water bodies, as the residual concentrations of BOD5, iron, dissolved solids, SS, and color exceeded the limits. Therefore, it is recommended to combine the SBR process with other treatment processes in a sequential manner to achieve higher-quality effluents.
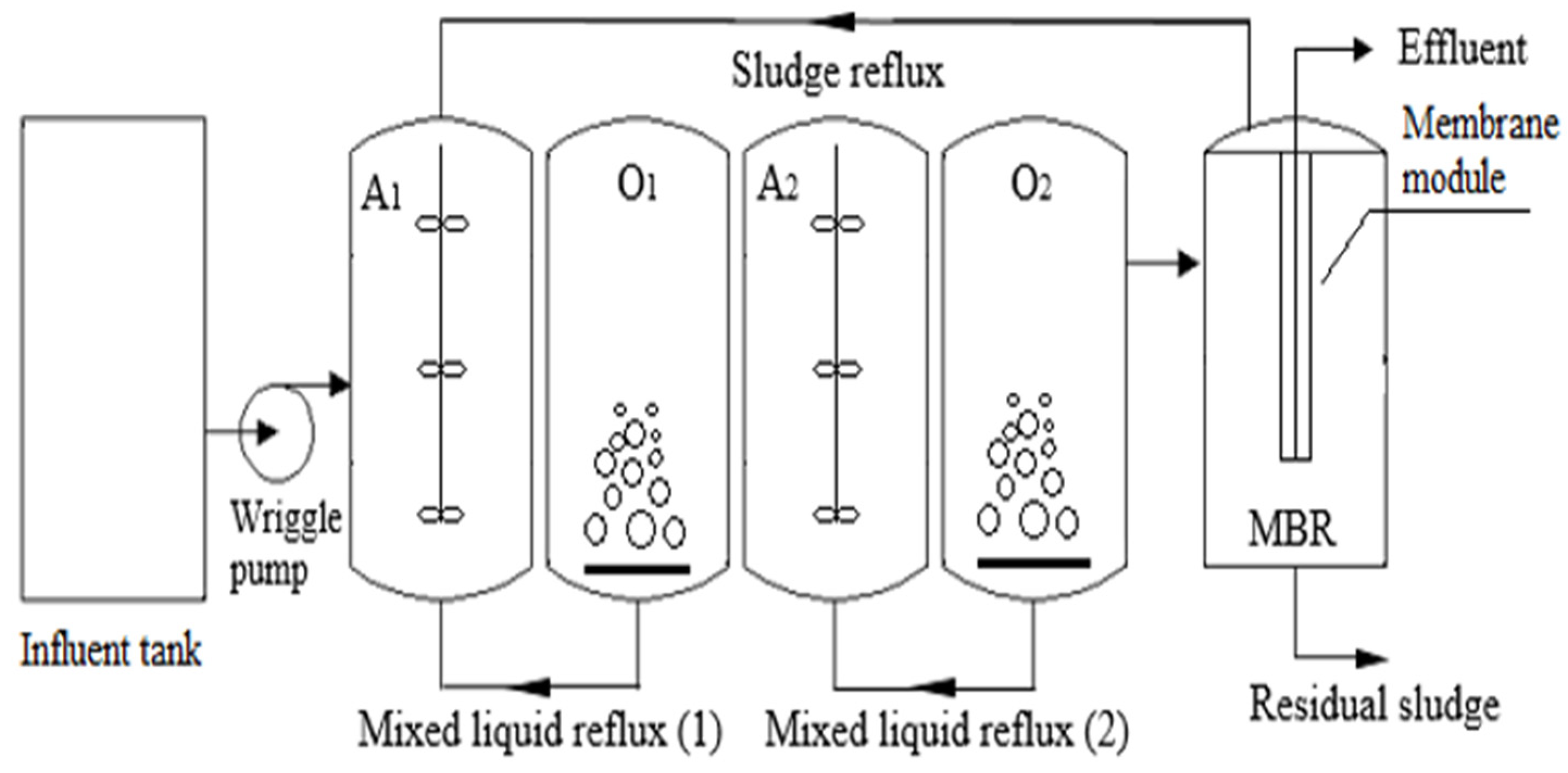
Liu [146] conducted an experiment using a laboratory-scale two-step anoxic/oxic (A/O) combined MBR for 113 days to treat LL with a high NH4+-N concentration and low C/N ratio, as depicted in Figure 11. The average removal rates achieved were 74.87% for TN, 99.04% for NH4+-N, and 80.60% for COD. A mass balance assessment indicated that the second-step A/O process played a crucial role in contaminant removal, with total removal capacities of 22.40 g/d for TN, 24.35 g/d for NH4+-N, and 125.60 g/d for COD. The high-throughput sequencing analysis revealed that Chloroflexi (3.13–4.80%), Firmicutes (3.31–4.53%), Planctomycetes (6.94–8.47%), Bacteroidetes (22.09–27.25%), and Proteobacteria (44.57–50.36%) were the dominant phyla in the bacterial community throughout the operation period. Two-step A/O and MBR showed a pretty good performance for LL treatment.
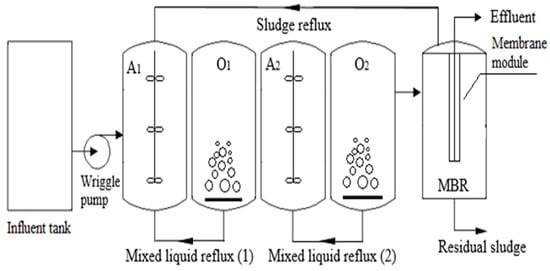
Figure 11.
Schematic diagram of combination of A/O with MBR [146]. (CCC License Number 5751980004456).
The TF process showed substantial reductions in SS, turbidity, BOD5, and NH3. However, the effluents produced by both treatment processes did not meet the national regulatory standards for discharge into surface water bodies. The concentrations of color, SS, dissolved solids, BOD5, and Fe exceeded the limits set by regulatory standards. Additional treatment processes may be necessary to achieve higher-quality effluents that comply with regulatory standards. The performance of this combined process can be enhanced by modifying the filter design or enhancing the filter media. For the combined A/O process followed by the MBR process, the results demonstrated effectiveness in removing TN, NH4+-N, and COD.
According to the previous literature, two studies have investigated combined biological and biological treatment processes for treating LL.
Both studies achieved significant removal of pollutants. The combined TF and SBR processes in Aluko and Sridhar’s work [145] showed reductions in various parameters, but additional treatment processes were recommended for meeting discharge standards. On the other hand, the two-stage A/O-MBR in Liu’s work [146] demonstrated high removal rates for TN, NH4+-N, and COD, indicating its effectiveness for LL treatment.
5.8. Biological + Chemical Integrated Processes
Biological treatment processes are widely used for LL treatment due to their many advantages [29]. Because single biological treatment processes for LL treatment are often unable to break down refractory organic matter, discharge standards are not met. Biological treatments are frequently combined with chemical and physical processes in order to get over this restriction [170,171,172,173,174]. Table 15 provides an overview of a few biological + chemical combination treatment processes along with an analysis of their effectiveness.

Table 15.
A summary of the reviewed biological + chemical integrated treatment processes and their results.
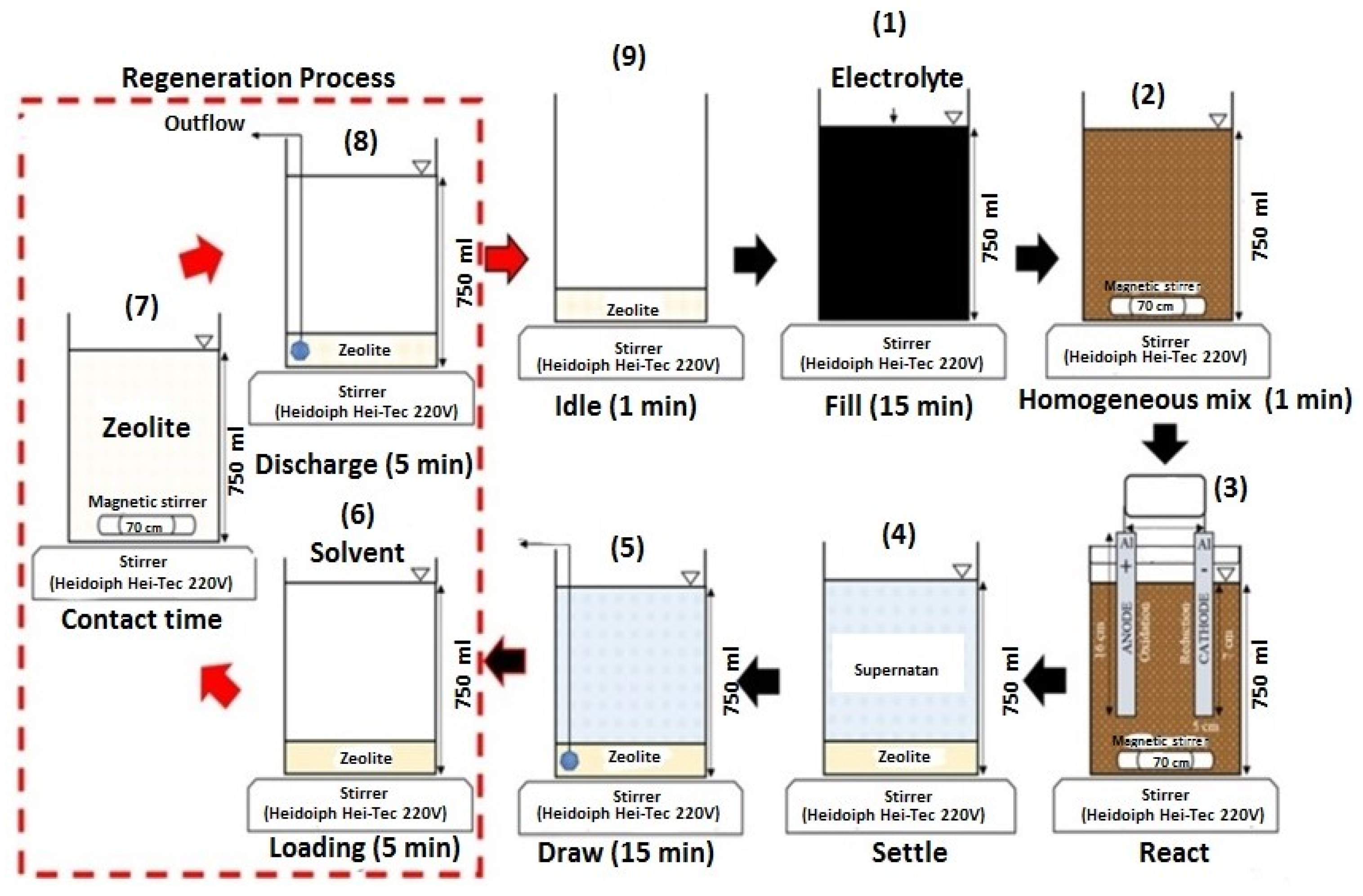
As shown in Figure 12, the proposed system operated continuously. The adsorption kinetics of color and NH3-N were well described by the pseudo-second-order kinetic model (R2 ≈ 1). This research successfully established an advanced technological process for treating leachate using a readily available natural adsorbent, clinoptilolite. The system demonstrated efficient treatment of concentrated leachate at a relatively low overall operating cost of 8.71 USD/m3 per cycle. Therefore, combining biological, physical, and chemical processes into a single process could be a viable option for effectively removing concentrated color and NH3-N from LL.
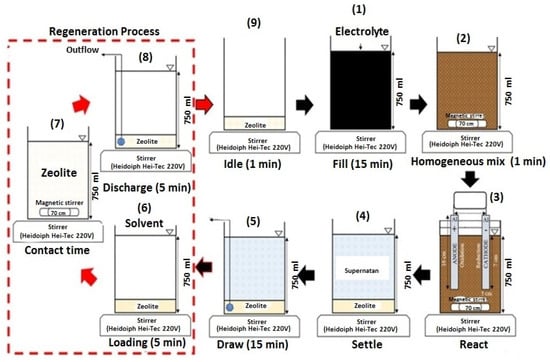
Figure 12.
Schematic diagram of continuous combined treatment process (clinoptilolite + EC with SBR) [164]. (CCC License Number 5751411402364).
5.9. Biological + Physical Integrated Processes
The study by Lim [165] proposed the use of an aerobic SBR (ASBR) followed by zeolite adsorption for treating locally obtained real LL. The ASBR demonstrated the ability to remove 30% of COD and 65% of ammoniacal nitrogen over a seven-day treatment period. Subsequently, zeolite, an effective adsorbent, was employed as a post-treatment step to further polish the LL by adsorbing COD and ammoniacal nitrogen. The combined treatment process exhibited significant removal of COD and heavy metals such as magnesium, aluminum, and lead. In conclusion, this combined treatment approach offers a feasible option for effectively removing contaminants from LL. Table 16 provides an overview of a few biological + physical contamination treatment processes along with an analysis of their effectiveness.

Table 16.
A summary of the reviewed biological + physical integrated treatment processes and their results.
According to Table 16, Chen [80] studied the treatment of LL using a combination of MBR and RO processes. The MBR process alone had limited efficiency in removing organic contaminants from the LL, but when combined with RO, it completely removed macromolecular compounds and produced a treated effluent meeting discharge standards. The LL contained various types of dissolved organic matter (DOM) with a high molecular weight. MBR effectively removed DOM with low saturation and high bioavailability, while RO significantly reduced residual DOM, particularly heteroatom DOM. The combined MBR+RO process produced an effluent mainly consisting of phenolic and aliphatic compounds. The effluent was highly biodegradable, reducing environmental risk compared to untreated LL.
In summary, both studies offer innovative approaches for treating LL, but they have limitations that need to be addressed. The first study [165] lacks information on the long-term performance and stability of the treatment system, while the second study [80] raises concerns about the composition of the treated effluent. Further research and evaluation are needed to fully assess the effectiveness and potential impacts of these treatment approaches on the environment and human health.
6. Conclusions
LL contains complex and refractory pollutants and toxic compounds that can vary depending on landfill maturity, age, and biochemical reactions, making its treatment challenging. Due to its unique characteristics and occurrence in remote locations, LL requires separate treatment from wastewater. Conventional treatment processes, such as biological, physical, and chemical treatment processes, when used alone, are unable to totally remove these contaminants. In order to treat LL, many combinations of treatment techniques have been proposed between 2011 and 2023. Based on the findings of the reviewed studies, the following key conclusions can be drawn:
- It was found that combining successive chemical processes was the most popular and widely used treatment.
- The most successful treatment processes were found to be a sequential combination of chemical treatments, followed by biological–physical treatment processes.
- In comparison to single treatment processes, the use of various combined treatments demonstrated an improvement in efficiency in terms of not only removing COD, TN, TDS, NH3-N, SS, color, turbidity, and heavy metals such as Ni, Cd, Mn, and Fe, but also in terms of process simplicity and sludge reduction.
- Compatible processes that showed higher efficiency in COD removal included MBR with NF (with removal rates reaching 99 ± 1%), Fenton with adsorption (with removal rates up to 99%), PF with biological treatment (with removal rates up to 98%), EF with biological treatment (with removal rates up to 97%), PEF with MBR (with removal rates up to 96.2%), and electrochemical with EF (with removal rates reaching 95.9 ± 0.4%).
- The compatible combination of the Fenton process with adsorption and the EC process with SPF showed improvements in biodegradability, with the BOD5/COD ratio increasing from 0.084 to 0.82 and from 0.35 to 0.75, respectively.
- The co-treatment of LL with wastewater using combined treatment processes has proven to be highly beneficial in terms of pollutant removal and treatment efficiency. Among the commonly employed co-treatment processes, the combination of the SBR with EC demonstrated exceptional performance. This integrated approach achieved remarkable removal efficiencies, with COD, TSS, ammonia, nitrate, and phosphate reaching levels as high as 98% and 99%, respectively. These findings underscore the effectiveness of co-treatment processes in addressing the challenges associated with landfill leachate and wastewater, leading to enhanced removal of pollutants and improved treatment outcomes.
- Countries with heavy rainfall, such as those with tropical Savanna, continental, and humid subtropical climates, tend to generate more waste compared to regions with humid continental, Mediterranean, and semi-arid climates.
- Croatia exhibits the highest waste generation levels, ranging from 1000 to 21,000 mg/L, attributed to its continental climate conditions.
- Significant concentrations of organic LL, measured as COD, were found in Morocco (COD = 22,000 mg/L), China (COD = 6880 ± 180 mg/L), and Algeria (COD = 3847.7 mg/L) for young, intermediate, and stabilized LL.
7. Recommendation
For many years, conventional chemical, physical, and biological treatment processes have been considered the most suitable technologies for managing and manipulating high-strength effluents such as LL. When treating stabilized LL that contains less biodegradable substances, physical and chemical treatment processes have been found to be effective as a refining step after a biological treatment process to remove organic refractory substances. However, when dealing with young LL, biological treatment processes can achieve reasonable treatment performance in terms of NH3-N, COD, and heavy metals. In recent years, due to increasingly stringent discharge standards in most countries and the aging of landfill sites resulting in more stabilized leachates, conventional treatments are no longer sufficient to achieve the required level of purification necessary to fully mitigate the negative environmental impact of LL. This indicates the need for the proposal of new alternative treatment processes.
The combination of chemical, physical, and biological treatment processes, regardless of the order in which they are implemented, addresses the limitations of individual processes and enhances the overall treatment efficacy [29]. The concept of combining treatments has proven to be a more efficient and successful approach. After conducting a thorough literature review on the assessment of combined treatment processes for treating LL, it is recommended that
- The utilization of environmentally friendly technologies, such as solar energy, on an industrial scale, offers a cost-effective solution. By incorporating solar energy into industrial processes, businesses can reduce their environmental footprint while also benefiting from the economic advantages associated with renewable energy sources. This approach aligns with the principles of sustainability and promotes a cleaner and more sustainable industrial sector.
- The recovery and utilization of valuable substances from LL presents a significant opportunity for resource optimization. LL is rich in metal elements, making it a potential source for secondary resources. To fully harness this potential, it is crucial to focus on the advancement of scientifically grounded processes that enable efficient separation and enrichment of valuable substances present in LL. By doing so, we can capitalize on the extensive resources found within LL, consequently reducing the reliance on costly synthetic substances.
- The utilization of kinetic and statistical models to comprehensively describe the various combined processes is currently constrained. However, if these models accurately depict the experimental outcomes, they hold the potential for application in the upscaling of such systems [29,175,176,177,178].
Author Contributions
Conceptualization, T.M.A.-Z. and A.J.; Methodology, T.M.A.-Z.; Formal analysis, T.M.A.-Z. and A.J.; Investigation, Z.A.-Q. and T.M.A.-Z.; Resources, A.J. and T.M.A.-Z.; Data curation, T.M.A.-Z.; Writing—original draft, T.M.A.-Z.; Writing—review and editing, A.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Deanship of Scientific Research at the University of Jordan.
Data Availability Statement
No additional data are available.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Abuabdou, S.M.; Bashir, M.J.; Choon Aun, N.; Sethupathi, S. Applicability of anaerobic membrane bioreactors for landfill leachate treatment: Review and opportunity. IOP Conf. Ser. Earth Environ. Sci. 2018, 140, 012033. [Google Scholar] [CrossRef]
- Bilgili, M.; Demir, A.; Özkaya, B. Influence of leachate recirculation on aerobic and anaerobic decomposition of solid wastes. J. Hazard. Mater. 2007, 143, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhou, Q.; Hua, T. Removal of organic matter from landfill leachate by advanced oxidation processes: A review. Int. J. Chem. Eng. 2010, 2010, 270532. [Google Scholar] [CrossRef]
- Khan, M.A.; Khan, R.; Al-Zghoul, T.M.; Khan, A.; Hussain, A.; Baarimah, A.O.; Arshad, M.A. Optimizing municipal solid waste management in urban Peshawar: A linear mathematical modeling and GIS approach for efficiency and sustainability. Case Stud. Chem. Environ. Eng. 2024, 9, 100704. [Google Scholar] [CrossRef]
- Fan, H.J.; Shu, H.Y.; Yang, H.S.; Chen, W.C. Characteristics of landfill leachates in central Taiwan. Sci. Total Environ. 2006, 361, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Wijekoon, P.; Koliyabandara, P.A.; Cooray, A.T.; Lam, S.S.; Athapattu, B.C.; Vithanage, M. Progress and prospects in mitigation of landfill leachate pollution: Risk, pollution potential, treatment and challenges. J. Hazard. Mater. 2022, 421, 126627. [Google Scholar] [CrossRef] [PubMed]
- Genethliou, C.; Tatoulis, T.; Charalampous, N.; Dailianis, S.; Tekerlekopoulou, A.G.; Vayenas, D.V. Treatment of raw sanitary landfill leachate using a hybrid pilot-scale system comprising adsorption, electrocoagulation and biological process. J. Environ. Manag. 2023, 330, 117129. [Google Scholar] [CrossRef]
- Swar, S.S.; Boonnorat, J.; Ghimire, A. Algae-based treatment of a landfill leachate pretreated by coagulation-flocculation. J. Environ. Manag. 2023, 342, 118223. [Google Scholar] [CrossRef] [PubMed]
- Jotin, R.; Ibrahim, S.; Halimoon, N. Electro coagulation for removal of chemical oxygen demand in sanitary landfill leachate. Int. J. Environ. Sci. 2012, 3, 921. [Google Scholar]
- Elleuch, L.; Messaoud, M.; Djebali, K.; Attafi, M.; Cherni, Y.; Kasmi, M.; Elaoud, A.; Trabelsi, I.; Chatti, A. A new insight into highly contaminated landfill leachate treatment using Kefir grains pre-treatment combined with Ag-doped TiO2 photocatalytic process. J. Hazard. Mater. 2020, 382, 121119. [Google Scholar] [CrossRef]
- Bouchareb, R.; Isik, Z.; Ozay, Y.; Karagunduz, A.; Keskinler, B.; Dizge, N. A hybrid process for leachate wastewater treatment: Evaporation and reverse osmosis/sequencing batch reactor. Water Environ. Res. 2022, 94, e10717. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.N.; Lan, C.Q. Treatment of landfill leachate using membrane bioreactors: A review. Desalination 2012, 287, 41–54. [Google Scholar] [CrossRef]
- Abdelaal, F.B.; Rowe, R.K.; Islam, M.Z. Effect of leachate composition on the long-term performance of a HDPE geomembrane. Geotext. Geomembr. 2014, 42, 348–362. [Google Scholar] [CrossRef]
- Keyikoglu, R.; Karatas, O.; Rezania, H.; Kobya, M.; Vatanpour, V.; Khataee, A. A review on treatment of membrane concentrates generated from landfill leachate treatment processes. Sep. Purif. Technol. 2021, 259, 118182. [Google Scholar] [CrossRef]
- Maiti, S.K.; De, S.; Hazra, T.; Debsarkar, A.; Dutta, A. Characterization of leachate and its impact on surface and groundwater quality of a closed dumpsite–a case study at Dhapa, Kolkata, India. Procedia Environ. Sci. 2016, 35, 391–399. [Google Scholar] [CrossRef]
- Deng, Y.; Zhu, X.; Chen, N.; Feng, C.; Wang, H.; Kuang, P.; Hu, W. Review on electrochemical system for landfill leachate treatment: Performance, mechanism, application, shortcoming, and improvement scheme. Sci. Total Environ. 2020, 745, 140768. [Google Scholar] [CrossRef] [PubMed]
- Aziz, H.A.; Aziz; Bashir, M.J.K.; Mojiri, A. Assessment of various tropical municipal landfill leachate characteristics and treatment opportunities. Glob. Nest J. 2015, 17, 1–13. [Google Scholar]
- Bashir, M.J.; Aziz, H.A.; Amr, S.S.A.; Sethupathi, S.A.P.; Ng, C.A.; Lim, J.W. The competency of various applied strategies in treating tropical municipal landfill leachate. Desalin. Water Treat. 2015, 54, 2382–2395. [Google Scholar] [CrossRef]
- Mojiri, A.; Zhou, J.L.; Ratnaweera, H.; Ohashi, A.; Ozaki, N.; Kindaichi, T.; Asakura, H. Treatment of landfill leachate with different techniques: An overview. Water Reuse 2021, 11, 66–96. [Google Scholar] [CrossRef]
- Hasar, H.; Unsal, S.A.; Ipek, U.; Karatas, S.; Cınar, O.; Yaman, C.; Kınacı, C. Stripping/flocculation/membrane bioreactor/reverse osmosis treatment of municipal landfill leachate. J. Hazard. Mater. 2009, 171, 309–317. [Google Scholar] [CrossRef]
- Assou, M.; El Fels, L.; El Asli, A.; Fakidi, H.; Souabi, S.; Hafidi, M. Landfill leachate treatment by a coagulation–flocculation process: Effect of the introduction order of the reagents. Desalin. Water Treat. 2016, 57, 21817–21826. [Google Scholar] [CrossRef]
- Fernandes, A.; Pacheco, M.J.; Ciríaco, L.; Lopes, A. Review on the electrochemical processes for the treatment of sanitary landfill leachates: Present and future. Appl. Catal. B Environ. 2015, 176, 183–200. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, X.; Qiu, D.; Mao, J.; Zhang, H. Pilot-scale in situ treatment of landfill leachate using combined coagulation–flocculation, hydrolysis acidification, SBR and electro-Fenton oxidation. Environ. Technol. 2019, 40, 2191–2200. [Google Scholar] [CrossRef] [PubMed]
- Singa, P.K.; Isa, M.H.; Sivaprakash, B.; Ho, Y.C.; Lim, J.W.; Rajamohan, N. PAHs remediation from hazardous waste landfill leachate using fenton, photo–fenton and electro-fenton oxidation processes–performance evaluation under optimized conditions using RSM and ANN. Environ. Res. 2023, 231, 116191. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.A.; Galvão, R.B.; Júnior, V.A. Post-treatment of landfill leachate from Cianorte-PR by upward filtration in gravel followed by adsorption on granular activated carbon. Environ. Forum High. Paul. 2014, 10, 220–233. [Google Scholar]
- Contrera, R.C.; da Cruz Silva, K.C.; Morita, D.M.; Rodrigues, J.A.D.; Zaiat, M.; Schalch, V. First-order kinetics of landfill leachate treatment in a pilot-scale anaerobic sequence batch biofilm reactor. J. Environ. Manag. 2014, 145, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Pasalari, H.; Farzadkia, M.; Gholami, M.; Emamjomeh, M.M. Management of landfill leachate in Iran: Valorization, characteristics, and environmental approaches. Environ. Chem. Lett. 2019, 17, 335–348. [Google Scholar] [CrossRef]
- Wijerathna, W.S.M.S.K.; Lindamulla, L.M.L.K.B.; Nanayakkara, K.G.N.; Rathnayake, R.M.L.D.; Jegatheesan, V.; Jinadasa, K.B.S.N. Post-treatment of matured landfill leachate: Synthesis and evaluation of chitosan biomaterial based derivatives as adsorbents. Environ. Res. 2023, 218, 115018. [Google Scholar] [CrossRef]
- Jamrah, A.; Al-Zghoul, T.M.; Darwish, M.M. A comprehensive review of combined processes for olive mill wastewater treatments. Case Stud. Chem. Environ. Eng. 2023, 8, 100493. [Google Scholar] [CrossRef]
- Li, Q.; Cui, H.; Li, Y.; Song, X.; Liu, W.; Wang, Y.; Hou, H.; Zhang, H.; Li, Y.; Wang, F.; et al. Challenges and engineering application of landfill leachate concentrate treatment. Environ. Res. 2023, 231 Pt 1, 116028. [Google Scholar] [CrossRef]
- Mostafa, H.; Iqdiam, B.M.; Abuagela, M.; Marshall, M.R.; Pullammanappallil, P.; Goodrich-Schneider, R. Treatment of olive mill wastewater using High Power Ultrasound (HPU) and Electro-Fenton (EF) method. Chem. Eng. Process.-Process Intensif. 2018, 131, 131–136. [Google Scholar] [CrossRef]
- Igwegbe, C.A.; Ighalo, J.O.; Iwuozor, K.O.; Onukwuli, O.D.; Okoye, P.U.; Al-Rawajfeh, A.E. Prediction and optimisation of coagulation-flocculation process for turbidity removal from aquaculture effluent using Garcinia kola extract: Response surface and artificial neural network methods. Clean. Chem. Eng. 2022, 4, 100076. [Google Scholar] [CrossRef]
- Jegadeesan, C.; Somanathan, A.; Jeyakumar, R.B.; Godvin Sharmila, V. Combination of electrocoagulation with solar photo Fenton process for treatment of landfill leachate. Environ. Technol. 2022, 44, 4441–4459. [Google Scholar] [CrossRef] [PubMed]
- Tałałaj, I.A.; Bartkowska, I.; Biedka, P. Treatment of young and stabilized landfill leachate by integrated sequencing batch reactor (SBR) and reverse osmosis (RO) process. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100502. [Google Scholar]
- Al-Zghoul, T.M.; Al-Qodah, Z.; Al-Jamrah, A. Performance, Modeling, and Cost Analysis of Chemical Coagulation-Assisted Solar Powered Electrocoagulation Treatment System for Pharmaceutical Wastewater. Water 2023, 15, 980. [Google Scholar] [CrossRef]
- Al-Rawajfeh, A.E.; Jawabreh, B.; Ahmad, M. The Prophetic Speeches (Hadith) on Sciences and Scientists: Application of the “Text from Text and D+” Theory. J. Coll. Educ. Women 2023, 34, 1–19. [Google Scholar] [CrossRef]
- Grosser, A.; Neczaj, E.; Madela, M.; Celary, P. Ultrasound-assisted treatment of landfill leachate in a sequencing batch reactor. Water 2019, 11, 516. [Google Scholar] [CrossRef]
- Salem, Z.; Hamouri, K.; Djemaa, R.; Allia, K. Evaluation of landfill leachate pollution and treatment. Desalination 2008, 220, 108–114. [Google Scholar] [CrossRef]
- Schiopu, A.M.; Gavrilescu, M. Options for the treatment and management of municipal landfill leachate: Common and specific issues. CLEAN–Soil Air Water 2010, 38, 1101–1110. [Google Scholar] [CrossRef]
- Reinhart, D.R.; Townsend, T.G. Landfill Bioreactor Design & Operation; CRC: Boca Raton, FL, USA, 1997. [Google Scholar]
- Abbas, A.A.; Jingsong, G.; Ping, L.Z.; Ya, P.Y.; Al-Rekabi, W.S. Review on LandWll leachate treatments. J. Appl. Sci. Res. 2009, 5, 534–545. [Google Scholar]
- Miao, L.; Yang, G.; Tao, T.; Peng, Y. Recent advances in nitrogen removal from landfill leachate using biological treatments—A review. J. Environ. Manag. 2019, 235, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Badri Narayan, R.; Zargham, B.I.; Ngambia, A.; Riyanto, A.R. Economic and environmental impact analysis of ammoniacal nitrogen removal from landfill leachate using sequencing batch reactor: A case study from Czech Republic. J. Water Supply Res. Technol.—AQUA 2019, 68, 816–828. [Google Scholar] [CrossRef]
- Lema, J.M.; Mendez, R.; Blazquez, R. Characteristics of landfill leachates and alternatives for their treatment: A review. Water Air Soil Pollut. 1988, 40, 223–250. [Google Scholar] [CrossRef]
- Bove, D.; Merello, S.; Frumento, D.; Arni, S.A.; Aliakbarian, B.; Converti, A. A critical review of biological processes and technologies for landfill leachate treatment. Chem. Eng. Technol. 2015, 38, 2115–2126. [Google Scholar] [CrossRef]
- Cossu, R.; Raga, R. Test methods for assessing the biological stability of biodegradable waste. Waste Manag. 2008, 28, 381–388. [Google Scholar] [CrossRef]
- Collivignarelli, C. II Trattamento del Percolato da Discarica RSU Situazioni e Prospettive; CIPA: Brescia, Italy, 1995. [Google Scholar]
- Shooshtari, A.A.; Amin, M.M.; Nabizadeh, R.; Jaafarzadeh, N. Treating municipal solid waste leachate in a pilot scale upflow anaerobic sludge blanket reactor under tropical temperature. Int. J. Environ. Health Eng. 2012, 1, 36–40. [Google Scholar]
- Torretta, V.; Ferronato, N.; Katsoyiannis, I.A.; Tolkou, A.K.; Airoldi, M. Novel and conventional technologies for landfill leachates treatment: A review. Sustainability 2016, 9, 9. [Google Scholar] [CrossRef]
- Christensen, T. (Ed.) Solid Waste Technology and Management; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Song, N.; Zhang, Q.; Wang, Y.; Gao, L.; Liu, S.; Yao, H.; Liu, R.; Xu, H. Investigation on molecular characteristics of organic compounds during a full-scale landfill leachate treatment process based on non-targeted analysis. Environ. Res. 2023, 238 Pt 2, 117258. [Google Scholar] [CrossRef] [PubMed]
- Orescanin, V.; Ruk, D.; Kollar, R.; Mikelic, I.L.; Nad, K.; Mikulic, N. A combined treatment of landfill leachate using calcium oxide, ferric chloride and clinoptilolite. J. Environ. Sci. Health 2011, 46, 323–328. [Google Scholar] [CrossRef]
- Top, S.; Sekman, E.; Hoşver, S.; Bilgili, M.S. Characterization and electrocaogulative treatment of nanofiltration concentrate of a full-scale landfill leachate treatment plant. Desalination 2011, 268, 158–162. [Google Scholar] [CrossRef]
- Aziz, S.Q.; Aziz, H.A.; Yusoff, M.S.; Bashir, M.J. Landfill leachate treatment using powdered activated carbon augmented sequencing batch reactor (SBR) process: Optimization by response surface methodology. J. Hazard. Mater. 2011, 189, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhou, S.; Ye, X.; Chen, D.; Zheng, K.; Qin, F. Transformation of pollutants in landfill leachate treated by a combined sequence batch reactor, coagulation, Fenton oxidation and biological aerated filter technology. Process Saf. Environ. Prot. 2011, 89, 112–120. [Google Scholar] [CrossRef]
- Orkun, M.O.; Kuleyin, A. Treatment performance evaluation of chemical oxygen demand from landfill leachate by electro-coagulation and electro-fenton technique. Environ. Prog. Sustain. Energy 2012, 31, 59–67. [Google Scholar] [CrossRef]
- Zhang, H.; Xiang, L.; Zhang, D.; Qing, H. Treatment of landfill leachate by internal microelectrolysis and sequent Fenton process. Desalin. Water Treat. 2012, 47, 243–248. [Google Scholar] [CrossRef]
- Amr, S.S.A.; Aziz, H.A.; Adlan, M.N.; Bashir, M.J. Pretreatment of stabilized leachate using ozone/persulfate oxidation process. Chem. Eng. J. 2013, 221, 492–499. [Google Scholar] [CrossRef]
- Moravia, W.G.; Amaral, M.C.; Lange, L.C. Evaluation of landfill leachate treatment by advanced oxidative process by Fenton’s reagent combined with membrane separation system. Waste Manag. 2013, 33, 89–101. [Google Scholar] [CrossRef] [PubMed]
- El-Fadel, M.; Matar, F.; Hashisho, J. Combined coagulation–flocculation and sequencing batch reactor with phosphorus adjustment for the treatment of high-strength landfill leachate: Experimental kinetics and chemical oxygen demand fractionation. J. Air Waste Manag. Assoc. 2013, 63, 591–604. [Google Scholar] [CrossRef] [PubMed]
- Campagna, M.; Çakmakcı, M.; Yaman, F.B.; Özkaya, B. Molecular weight distribution of a full-scale landfill leachate treatment by membrane bioreactor and nanofiltration membrane. Waste Manag. 2013, 33, 866–870. [Google Scholar] [CrossRef]
- Abood, A.R.; Bao, J.; Du, J.; Zheng, D.; Luo, Y. Non-biodegradable landfill leachate treatment by combined process of agitation, coagulation, SBR and filtration. Waste Manag. 2014, 34, 439–447. [Google Scholar] [CrossRef]
- Güneş, E. Seasonal Characterization of Landfill Leachate and Effect of Seasonal Variations on Treatment Processes of Coagulation/Flocculation and Adsorption. Pol. J. Environ. Stud. 2014, 23, 1155–1163. [Google Scholar]
- Moradi, M.; Ghanbari, F. Application of response surface method for coagulation process in leachate treatment as pretreatment for Fenton process: Biodegradability improvement. J. Water Process Eng. 2014, 4, 67–73. [Google Scholar] [CrossRef]
- Amor, C.; De Torres-Socías, E.; Peres, J.A.; Maldonado, M.I.; Oller, I.; Malato, S.; Lucas, M.S. Mature landfill leachate treatment by coagulation/flocculation combined with Fenton and solar photo-Fenton processes. J. Hazard. Mater. 2015, 286, 261–268. [Google Scholar] [CrossRef]
- Košutić, K.; Dolar, D.; Strmecky, T. Treatment of landfill leachate by membrane processes of nanofiltration and reverse osmosis. Desalin. Water Treat. 2015, 55, 2680–2689. [Google Scholar] [CrossRef]
- Bakraouy, H.; Souabi, S.; Digua, K.; Dkhissi, O.; Sabar, M.; Fadil, M. Optimization of the treatment of an anaerobic pretreated landfill leachate by a coagulation–flocculation process using experimental design methodology. Process Saf. Environ. Prot. 2017, 109, 621–630. [Google Scholar] [CrossRef]
- Rasool, M.A.; Tavakoli, B.; Chaibakhsh, N.; Pendashteh, A.R.; Mirroshandel, A.S. Use of a plant-based coagulant in coagulation–ozonation combined treatment of leachate from a waste dumping site. Ecol. Eng. 2016, 90, 431–437. [Google Scholar] [CrossRef]
- Zolfaghari, M.; Jardak, K.; Drogui, P.; Brar, S.K.; Buelna, G.; Dubé, R. Landfill leachate treatment by sequential membrane bioreactor and electro-oxidation processes. J. Environ. Manag. 2016, 184, 318–326. [Google Scholar] [CrossRef]
- Azari, M.; Walter, U.; Rekers, V.; Gu, J.D.; Denecke, M. More than a decade of experience of landfill leachate treatment with a full-scale anammox plant combining activated sludge and activated carbon biofilm. Chemosphere 2017, 174, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Klauck, C.R.; Giacobbo, A.; de Oliveira, E.D.L.; da Silva, L.B.; Rodrigues, M.A.S. Evaluation of acute toxicity, cytotoxicity and genotoxicity of landfill leachate treated by biological lagoon and advanced oxidation processes. J. Environ. Chem. Eng. 2017, 5, 6188–6193. [Google Scholar] [CrossRef]
- Klein, K.; Kivi, A.; Dulova, N.; Zekker, I.; Mölder, E.; Tenno, T.; Trapido, M.; Tenno, T. A pilot study of three-stage biological–chemical treatment of landfill leachate applying continuous ferric sludge reuse in Fenton-like process. Clean Technol. Environ. Policy 2017, 19, 541–551. [Google Scholar] [CrossRef]
- Hassan, M.; Pous, N.; Xie, B.; Colprim, J.; Balaguer, M.D.; Puig, S. Influence of iron species on integrated microbial fuel cell and electro-Fenton process treating landfill leachate. Chem. Eng. J. 2017, 328, 57–65. [Google Scholar] [CrossRef]
- Ye, Z.L.; Hong, Y.; Pan, S.; Huang, Z.; Chen, S.; Wang, W. Full-scale treatment of landfill leachate by using the mechanical vapor recompression combined with coagulation pretreatment. Waste Manag. 2017, 66, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Ates, H.; Argun, M.E. Removal of PAHs from leachate using a combination of chemical precipitation and Fenton and ozone oxidation. Water Sci. Technol. 2018, 78, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Donneys-Victoria, D.; Marriaga-Cabrales, N.; Camargo-Amado, R.J.; Machuca-Martinez, F.; Peralta-Hernandez, J.M.; Martinez-Huitle, C.A. Treatment of landfill leachate by a combined process: Iron electrodissolution, iron oxidation by H2O2 and chemical flocculation. Sustain. Environ. Res. 2018, 28, 12–19. [Google Scholar] [CrossRef]
- Zhang, M.H.; Dong, H.; Zhao, L.; Wang, D.X.; Meng, D. A review on Fenton process for organic wastewater treatment based on optimization perspective. Sci. Total Environ. 2019, 670, 110–121. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, R.; Moraes Costa, A.; de Almeida Oroski, F.; Carbonelli Campos, J. Evaluation of coagulation–flocculation and nanofiltration processes in landfill leachate treatment. J. Environ. Sci. Health 2019, 54, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Antony, J.; Niveditha, S.V.; Gandhimathi, R.; Ramesh, S.T.; Nidheesh, P.V. Stabilized landfill leachate treatment by zero valent aluminium-acid system combined with hydrogen peroxide and persulfate based advanced oxidation process. Waste Manag. 2020, 106, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhuo, X.; He, C.; Shi, Q.; Li, Q. Molecular investigation into the transformation of dissolved organic matter in mature landfill leachate during treatment in a combined membrane bioreactor-reverse osmosis process. J. Hazard. Mater. 2020, 397, 122759. [Google Scholar] [CrossRef] [PubMed]
- Kwarciak-Kozłowska, A.; Fijałkowski, K.L. Efficiency assessment of municipal landfill leachate treatment during advanced oxidation process (AOP) with biochar adsorption (BC). J. Environ. Manag. 2021, 287, 112309. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Zhang, C.; Yang, X.; Zhou, T.; Yang, S. Combining chemical coagulation with activated coke adsorption to remove organic matters and retain nitrogen compounds in mature landfill leachate. Environ. Technol. 2021, 42, 3487–3495. [Google Scholar] [CrossRef]
- Viegas, C.; Nobre, C.; Mota, A.; Vilarinho, C.; Gouveia, L.; Gonçalves, M. A circular approach for landfill leachate treatment: Chemical precipitation with biomass ash followed by bioremediation through microalgae. J. Environ. Chem. Eng. 2021, 9, 105187. [Google Scholar] [CrossRef]
- Chaouki, Z.; Hadri, M.; Nawdali, M.; Benzina, M.; Zaitan, H. Treatment of a landfill leachate from Casablanca city by a coagulation-flocculation and adsorption process using a palm bark powder (PBP). Sci. Afr. 2021, 12, e00721. [Google Scholar] [CrossRef]
- Shadi, A.M.H.; Kamaruddin, M.A.; Niza, N.M.; Emmanuel, M.I.; Ismail, N.; Hossain, S. Effective removal of organic and inorganic pollutants from stabilized sanitary landfill leachate using a combined Fe2O3 nanoparticles/electroflotation process. J. Water Process Eng. 2021, 40, 101988. [Google Scholar] [CrossRef]
- Le, T.S.; Dang, N.M.; Tran, D.T. Performance of coupling electrocoagulation and biofiltration processes for the treatment of leachate from the largest landfill in Hanoi, Vietnam: Impact of operating conditions. Sep. Purif. Technol. 2021, 255, 117677. [Google Scholar] [CrossRef]
- Brasil, Y.L.; Silva, A.F.; Gomes, R.F.; Amaral, M.C. Technical and economic evaluation of the integration of membrane bioreactor and air-stripping/absorption processes in the treatment of landfill leachate. Waste Manag. 2021, 134, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, S.N.F.; Abdul Aziz, H.; Mohamad, M. Comparison performance of coagulation flocculation process and combination with ozonation process of stabilized landfill leachate treatment. Water Environ. Res. 2022, 94, e10770. [Google Scholar] [CrossRef] [PubMed]
- Elfilali, N.; Elazhar, F.; Dhiba, D.; Elmidaoui, A.; Taky, M. Performances of various hybrids systems coagulation–ultrafiltration/nanofiltration-reverse osmosis in the treatment of stabilized landfill leachate. Desalin. Water Treat. 2022, 257, 55–63. [Google Scholar] [CrossRef]
- De, S.; Hazra, T.; Dutta, A. Application of integrated sequence of air stripping, coagulation flocculation, electrocoagulation and adsorption for sustainable treatment of municipal landfill leachate. Clean. Waste Syst. 2022, 3, 100033. [Google Scholar] [CrossRef]
- Kumar, R.N.; Sadaf, S.; Verma, M.; Chakraborty, S.; Kumari, S.; Polisetti, V.; Kallem, P.; Iqbal, J.; Banat, F. Old Landfill Leachate and Municipal Wastewater Co-Treatment by Sequencing Batch Reactor Combined with Coagulation–Flocculation Using Novel Flocculant. Sustainability 2023, 15, 8205. [Google Scholar] [CrossRef]
- Ye, Z.; Zhang, H.; Zhang, X.; Zhou, D. Treatment of landfill leachate using electrochemically assisted UV/chlorine process: Effect of operating conditions, molecular weight distribution and fluorescence EEM-PARAFAC analysis. Chem. Eng. J. 2016, 286, 508–516. [Google Scholar] [CrossRef]
- Majlesi, M.; Yazdanbakhsh, A.R.; Jabbari, V.; Sheikhmohammadi, A. Landfill leachate treatment using electro-coagulation-flotation process. Adv. Environ. Biol. 2015, 9, 167–173. [Google Scholar]
- Vedrenne, M.; Vasquez-Medrano, R.; Prato-Garcia, D.; Frontana-Uribe, B.A.; Ibanez, J.G. Characterization and detoxification of a mature landfill leachate using a combined coagulation–flocculation/photo Fenton treatment. J. Hazard. Mater. 2012, 205, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Pedro-Cedillo, S.; Méndez-Novelo, R.I.; Rojas-Valencia, M.N.; Barceló-Quintal, M.; Castillo-Borges, E.R.; Sauri-Riancho, M.R.; Marrufo-Gómez, J.M. Evaluation of adsorption and Fenton-adsorption processes for landfill leachate treatment. Rev. Mex. Ing. Química 2015, 14, 745–755. [Google Scholar]
- Al-Wabel, M.I.; Al Yehya, W.S.; Al-Farraj, A.S.; El-Maghraby, S.E. Characteristics of landfill leachates and bio-solids of municipal solid waste (MSW) in Riyadh City, Saudi Arabia. J. Saudi Soc. Agric. Sci. 2011, 10, 65–70. [Google Scholar] [CrossRef]
- Nivya, T.K.; Pieus, T.M. Comparison of Photo ElectroFenton Process (PEF) and combination of PEF Process and Membrane Bioreactor in the treatment of Landfill Leachate. Procedia Technol. 2016, 24, 224–231. [Google Scholar] [CrossRef]
- Bhagawan, D.; Poodari, S.; Chaitanya, N.; Ravi, S.; Rani, Y.M.; Himabindu, V.; Vidyavathi, S. Industrial solid waste landfill leachate treatment using electrocoagulation and biological methods. Desalin. Water Treat. 2017, 68, 137–142. [Google Scholar] [CrossRef]
- Silveira, J.E.; Zazo, J.A.; Pliego, G.; Casas, J.A. Landfill leachate treatment by sequential combination of activated persulfate and Fenton oxidation. Waste Manag. 2018, 81, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Poblete, R.; Pérez, N. Use of sawdust as pretreatment of photo-Fenton process in the depuration of landfill leachate. J. Environ. Manag. 2020, 253, 109697. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Pang, Y.; Li, X.; Chen, F.; Liao, X.; Lei, M.; Song, Y. Landfill leachate treatment by coagulation/flocculation combined with microelectrolysis-Fenton processes. Environ. Technol. 2019, 40, 1862–1870. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Zhu, Z.; Chang, H.; Fan, G.; Wang, Q.; Fu, X.; Qu, F.; Liang, H. Integrated membrane electrochemical reactor-membrane distillation process for enhanced landfill leachate treatment. Water Res. 2023, 230, 119559. [Google Scholar] [CrossRef]
- El Mrabet, I.; Nawdali, M.; Rafqah, S.; Valdés, H.; Benzina, M.; Zaitan, H. Low-cost biomass for the treatment of landfill leachate from Fez City: Application of a combined coagulation–adsorption process. Euro-Mediterr. J. Environ. Integr. 2020, 5, 63. [Google Scholar] [CrossRef]
- Tezcan Un, U.; Filik Iscen, C.; Oduncu, E.; Akcal Comoglu, B.; Ilhan, S. Treatment of landfill leachate using integrated continuous electrocoagulation and the anaerobic treatment technique. Environ. Prog. Sustain. Energy 2018, 37, 1668–1676. [Google Scholar] [CrossRef]
- Lu, W.; Lei, S.; Chen, N.; Feng, C. Research on two-step advanced treatment of old landfill leachate by sequential electrochemical peroxidation-electro-Fenton process. Chem. Eng. J. 2023, 451, 138746. [Google Scholar] [CrossRef]
- Sun, X.; Wang, X.; Liu, Y.; Lian, Y.; Meng, L.; Su, Z. Removing refractory organic matter from nanofiltration concentrated landfill leachate by electrooxidation combined with electrocoagulation: Characteristics and implication for leachate management. J. Water Process Eng. 2022, 47, 102747. [Google Scholar] [CrossRef]
- Vilar, V.J.; Rocha, E.M.; Mota, F.S.; Fonseca, A.; Saraiva, I.; Boaventura, R.A. Treatment of a sanitary landfill leachate using combined solar photo-Fenton and biological immobilized biomass reactor at a pilot scale. Water Res. 2011, 45, 2647–2658. [Google Scholar] [CrossRef] [PubMed]
- Cheibub, A.F.; Campos, J.C.; Da Fonseca, F.V. Removal of COD from a stabilized landfill leachate by physicochemical and advanced oxidative process. J. Environ. Sci. Health 2014, 49, 1718–1726. [Google Scholar] [CrossRef] [PubMed]
- Van Tuyen, T. Treatment of organic compounds of landfill leachate in Vietnam by combining coagulation and ozonation process. Am. J. Environ. Sci. 2013, 9, 518. [Google Scholar]
- Boumechhour, F.; Rabah, K.; Lamine, C.; Said, B.M. Treatment of landfill leachate using F enton process and coagulation/flocculation. Water Environ. J. 2013, 27, 114–119. [Google Scholar] [CrossRef]
- Wang, N.; Zhou, Q. Electrochemical treatment of solid waste Leachate using combined electrocoagulation and electrochemical oxidation treatment. Int. J. Electrochem. Sci. 2022, 17, 220820. [Google Scholar] [CrossRef]
- Mojiri, A.; Aziz, H.A.; Aziz, S.Q. Trends in physical-chemical methods for landfill leachate treatment. Int. J. Sci. Res. Environ. 2013, 1, 16–25. [Google Scholar] [CrossRef]
- Diamadopoulos, E.; Samaras, P.; Dabou, X.; Sakellaropoulos, G.P. Combined treatment of landfill leachate and domestic sewage in a sequencing batch reactor. Water Sci. Technol. 1997, 36, 61–68. [Google Scholar] [CrossRef]
- Abdulhussain, A.A.; Guo, J.; Liu, Z.P.; Pan, Y.Y.; Wisaam, S. Review on landfill leachate treatments. Am. J. Appl. Sci. 2009, 6, 672–684. [Google Scholar]
- Gao, J.; Oloibiri, V.; Chys, M.; Audenaert, W.; Decostere, B.; He, Y.; Van Langenhove, H.; Demeestere, K.; Van Hulle, S.W. The present status of landfill leachate treatment and its development trend from a technological point of view. Rev. Environ. Sci. Bio/Technol. 2015, 14, 93–122. [Google Scholar] [CrossRef]
- Mojiri, A.; Aziz, H.A.; Zaman, N.Q.; Aziz, S.Q.; Zahed, M.A. Metals removal from municipal landfill leachate and wastewater using adsorbents combined with biological method. Desalin. Water Treat. 2016, 57, 2819–2833. [Google Scholar] [CrossRef]
- Mojiri, A.; Ziyang, L.; Hui, W.; Ahmad, Z.; Tajuddin, R.M.; Amr, S.S.A.; Kindaichi, T.; Aziz, H.A.; Farraji, H. Concentrated landfill leachate treatment with a combined system including electro-ozonation and composite adsorbent augmented sequencing batch reactor process. Process Saf. Environ. Prot. 2017, 111, 253–262. [Google Scholar] [CrossRef]
- Lippi, M.; Ley, M.B.R.G.; Mendez, G.P.; Junior, R.A.F.C. State of art of landfill leachate treatment: Literature review and critical evaluation. Ciência Nat. 2018, 40, e78. [Google Scholar] [CrossRef]
- Chakraborty, S.; Mohanty, P.K.; Iqbal, J.; Kumar, R.N. Can electrocoagulation be an effective post-treatment option for SBR treated landfill leachate and municipal wastewater mixture? J. Water Sanit. Hyg. Dev. 2020, 10, 86–95. [Google Scholar] [CrossRef]
- Jagaba, A.H.; Kutty, S.R.M.; Lawal, I.M.; Abubakar, S.; Hassan, I.; Zubairu, I.; Umaru, I.; Abdurrasheed, A.S.; Adam, A.A.; Ghaleb, A.A.S.; et al. Sequencing batch reactor technology for landfill leachate treatment: A state-of-the-art review. J. Environ. Manag. 2021, 282, 111946. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Shafy, H.I.; Ibrahim, A.M.; Al-Sulaiman, A.M.; Okasha, R.A. Landfill leachate: Sources, nature, organic composition, and treatment: An environmental overview. Ain Shams Eng. J. 2023, 15, 102293. [Google Scholar] [CrossRef]
- Verma, M.; Chakraborty, S.; Kumari, S.; Gupta, A.; Kumar, D.; Iqbal, J.; Banu, J.R.; Pugazhendi, A.; Kumar, R.N. Co-treatment of stabilized landfill leachate and municipal wastewater in a granular activated carbon-sequencing batch reactor (GAC-SBR). Process Saf. Environ. Prot. 2023, 174, 424–432. [Google Scholar] [CrossRef]
- Baun, D.L.; Christensen, T.H. Speciation of heavy metals in landfill leachate: A review. Waste Manag. Res. 2004, 22, 3–23. [Google Scholar] [CrossRef]
- Mojiri, A.; Ziyang, L.; Tajuddin, R.M.; Farraji, H.; Alifar, N. Co-treatment of landfill leachate and municipal wastewater using the ZELIAC/zeolite constructed wetland system. J. Environ. Manag. 2016, 166, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Carvajal-Flórez, E.; Cardona-Gallo, S.A. Technologies applicable to the removal of heavy metals from landfill leachate. Environ. Sci. Pollut. Res. 2019, 26, 15725–15753. [Google Scholar] [CrossRef]
- Warith, M.; Li, X.; Jin, H. Bioreactor landfills: State-of-the-art review. Emir. J. Eng. Res. 2005, 10, 1–14. [Google Scholar]
- Zhang, G.; Liu, K.; Lv, L.; Gao, W.; Li, W.; Ren, Z.; Yan, W.; Wang, P.; Liu, X.; Sun, L. Enhanced landfill process based on leachate recirculation and micro-aeration: A comprehensive technical, environmental, and economic assessment. Sci. Total Environ. 2023, 857, 159535. [Google Scholar] [CrossRef] [PubMed]
- Al-Rawajfeh, A.E. A Conjecture from Collatz Conjecture: Elevation by Folding. Gen. Lett. Math. (GLM) 2023, 13, 1–4. [Google Scholar] [CrossRef]
- Feng, S.; Fu, W.; Zhou, A.; Lyu, F. A coupled hydro-mechanical-biodegradation model for municipal solid waste in leachate recirculation. Waste Manag. 2019, 98, 81–91. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, J.; Pérez, M.; Romero, L.I. Comparison of mesophilic and thermophilic dry anaerobic digestion of OFMSW: Kinetic analysis. Chem. Eng. J. 2013, 232, 59–64. [Google Scholar] [CrossRef]
- Renou, S.; Givaudan, J.G.; Poulain, S.; Dirassouyan, F.; Moulin, P. Landfill leachate treatment: Review and opportunity. J. Hazard. Mater. 2008, 150, 468–493. [Google Scholar] [CrossRef]
- Lee, H.; Coulon, F.; Beriro, D.J.; Wagland, S.T. Recovering metal (loids) and rare earth elements from closed landfill sites without excavation: Leachate recirculation opportunities and challenges. Chemosphere 2022, 292, 133418. [Google Scholar] [CrossRef]
- Huang, T.; Tang, Y.; Sun, Y.; Zhang, C.; Ma, X. Life cycle environmental and economic comparison of thermal utilization of refuse derived fuel manufactured from landfilled waste or fresh waste. J. Environ. Manag. 2022, 304, 114156. [Google Scholar] [CrossRef]
- Sohoo, I.; Ritzkowski, M.; Kuchta, K. Influence of moisture content and leachate recirculation on oxygen consumption and waste stabilization in post aeration phase of landfill operation. Sci. Total Environ. 2021, 773, 145584. [Google Scholar] [CrossRef] [PubMed]
- Foo, K.Y.; Hameed, B.H. An overview of landfill leachate treatment via activated carbon adsorption process. J. Hazard. Mater. 2009, 171, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, T.; Wang, H.; Yang, K.; Fang, C.; Lv, B.; Yang, X. Study on treating old landfill leachate by Ultrasound–Fenton oxidation combined with MAP chemical precipitation. Chem. Speciat. Bioavailab. 2015, 27, 175–182. [Google Scholar] [CrossRef]
- Tejera, J.; Hermosilla, D.; Gascó, A.; Miranda, R.; Alonso, V.; Negro, C.; Blanco, Á. Treatment of mature landfill leachate by electrocoagulation followed by Fenton or UVA-LED photo-Fenton processes. J. Taiwan Inst. Chem. Eng. 2021, 119, 33–44. [Google Scholar] [CrossRef]
- Chen, L.; Li, F.; He, F.; Mao, Y.; Chen, Z.; Wang, Y.; Cai, Z. Membrane distillation combined with electrocoagulation and electrooxidation for the treatment of landfill leachate concentrate. Sep. Purif. Technol. 2022, 291, 120936. [Google Scholar] [CrossRef]
- de Almeida, R.; Campos, J.; de Oroski, F. Techno-economic evaluation of landfill leachate treatment by hybrid lime application and nanofiltration process. Multidiscip. J. Waste Resour. Residues 2020, 10, 170–181. [Google Scholar]
- Kulikowska, D.; Zielińska, M.; Konopka, K. Treatment of stabilized landfill leachate in an integrated adsorption–fine-ultrafiltration system. Int. J. Environ. Sci. Technol. 2019, 16, 423–430. [Google Scholar] [CrossRef]
- Amaral, M.C.S.; Pereira, H.V.; Nani, E.; Lange, L.C. Treatment of landfill leachate by hybrid precipitation/microfiltration/nanofiltration process. Water Sci. Technol. 2015, 72, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Smaoui, Y.; Bouzid, J.; Sayadi, S. Combination of air stripping and biological processes for landfill leachate treatment. Environ. Eng. Res. 2020, 25, 80–87. [Google Scholar] [CrossRef]
- Yu, T.; Huang, T.; Pan, Y.X.; Yang, L.H. The Experimental Study on Landfill Leachate Treatment by Coagulation-Sedimentation+ Electro-Oxidation Joint Reactor. Appl. Mech. Mater. 2013, 295, 1472–1477. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Singh, D.; Xue, W.; Avtar, R.; Othman, M.H.D.; Hwang, G.H.; Setiadi, T.; Albadarin, A.B.; Shirazian, S. Resource recovery toward sustainability through nutrient removal from landfill leachate. J. Environ. Manag. 2021, 287, 112265. [Google Scholar] [CrossRef] [PubMed]
- Aluko, O.O.; Sridhar, M.K. Evaluation of leachate treatment by trickling filter and sequencing batch reactor processes in Ibadan, Nigeria. Waste Manag. Res. 2013, 31, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, H.; Zhang, P.; Wu, Y.; Gou, X.; Song, Y.; Tian, Z.; Zeng, G. Two-stage anoxic/oxic combined membrane bioreactor system for landfill leachate treatment: Pollutant removal performances and microbial community. Bioresour. Technol. 2017, 243, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Cortez, S.; Teixeira, P.; Oliveira, R.; Mota, M. Evaluation of Fenton and ozone-based advanced oxidation processes as mature landfill leachate pre-treatments. J. Environ. Manag. 2011, 92, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Ishak, A.R.; Hamid, F.S.; Mohamad, S.; Tay, K.S. Stabilized landfill leachate treatment by coagulation-flocculation coupled with UV-based sulfate radical oxidation process. Waste Manag. 2018, 76, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Rebolledo, L.P.; Arana, V.A.; Trilleras, J.; Barros, G.E.; González-Solano, A.J.; Maury-Ardila, H. Efficiency of combined processes coagulation/solar photo Fenton in the treatment of landfill leachate. Water 2019, 11, 1351. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Murshid, A.; Chanikya, P. Combination of electrochemically activated persulfate process and electro-coagulation for the treatment of municipal landfill leachate with low biodegradability. Chemosphere 2023, 338, 139449. [Google Scholar] [CrossRef] [PubMed]
- Gandhimathi, R.; Durai, N.J.; Nidheesh, P.V.; Ramesh, S.T.; Kanmani, S. Use of combined coagulation-adsorption process as pretreatment of landfill leachate. Iran. J. Environ. Health Sci. Eng. 2013, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, B.; Owete, O.; Dertien, J.; Lin, C.; Ahmad, H.; Chen, G. Landfill Leachate Treatment by Electrocoagulation and Fiber Filtration. Water Environ. Res. 2017, 89, 2015–2020. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, J.; Fang, F. Transformation and fluorescence spectroscopy of dissolved organic matter (dom) in landfill leachate treated by combined process. Environ. Eng. Manag. J. (EEMJ) 2011, 10, 913–918. [Google Scholar] [CrossRef]
- Djelal, H.; Lelievre, Y.; Ricordel, C. Combination of Electro-Coagulation and biological treatment by bioaugmentation for landfill leachate. Desalin. Water Treat. 2015, 54, 2986–2993. [Google Scholar] [CrossRef]
- Baiju, A.; Gandhimathi, R.; Ramesh, S.T.; Nidheesh, P.V. Combined heterogeneous Electro-Fenton and biological process for the treatment of stabilized landfill leachate. J. Environ. Manag. 2018, 210, 328–337. [Google Scholar] [CrossRef]
- Colombo, A.; Módenes, A.N.; Trigueros, D.E.G.; da Costa, S.I.G.; Borba, F.H.; Espinoza-Quiñones, F.R. Treatment of sanitary landfill leachate by the combination of photo-Fenton and biological processes. J. Clean. Prod. 2019, 214, 145–153. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, M.; Deng, Q.; Cai, T. Combination and performance of forward osmosis and membrane distillation (FO-MD) for treatment of high salinity landfill leachate. Desalination 2017, 420, 99–105. [Google Scholar] [CrossRef]
- Zielińska, M.; Kulikowska, D.; Stańczak, M. Adsorption–Membrane process for treatment of stabilized municipal landfill leachate. Waste Manag. 2020, 114, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Nazia, S.; Sahu, N.; Jegatheesan, V.; Bhargava, S.K.; Sridhar, S. Integration of ultrafiltration membrane process with chemical coagulation for proficient treatment of old industrial landfill leachate. Chem. Eng. J. 2021, 412, 128598. [Google Scholar] [CrossRef]
- Zhang, D.B.; Wu, X.G.; Wang, Y.S.; Zhang, H. Landfill leachate treatment using the sequencing batch biofilm reactor method integrated with the electro-Fenton process. Chem. Pap. 2014, 68, 782–787. [Google Scholar] [CrossRef]
- Oumar, D.; Patrick, D.; Gerardo, B.; Rino, D.; Ihsen, B.S. Coupling biofiltration process and electrocoagulation using magnesium-based anode for the treatment of landfill leachate. J. Environ. Manag. 2016, 181, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Yong, Z.J.; Bashir, M.J.; Ng, C.A.; Sethupathi, S.; Lim, J.W. A sequential treatment of intermediate tropical landfill leachate using a sequencing batch reactor (SBR) and coagulation. J. Environ. Manag. 2018, 205, 244–252. [Google Scholar] [CrossRef]
- Ai, J.; Wu, X.; Wang, Y.; Zhang, D.; Zhang, H. Treatment of landfill leachate with combined biological and chemical processes: Changes in the dissolved organic matter and functional groups. Environ. Technol. 2019, 40, 2225–2231. [Google Scholar] [CrossRef]
- Abd Hamid, M.A.; Aziz, H.A.; Yusoff, M.S.; Rezan, S.A. A continuous clinoptilolite augmented SBR-electrocoagulation process to remove concentrated ammonia and colour in landfill leachate. Environ. Technol. Innov. 2021, 23, 101575. [Google Scholar] [CrossRef]
- Lim, C.K.; Seow, T.W.; Neoh, C.H.; Md Nor, M.H.; Ibrahim, Z.; Ware, I.; Mat Sarip, S.H. Treatment of landfill leachate using ASBR combined with zeolite adsorption technology. 3 Biotech 2016, 6, 195. [Google Scholar] [CrossRef]
- Silva, N.C.M.; Moravia, W.G.; Amaral, M.C.S.; Figueiredo, K.C.S. Evaluation of fouling mechanisms in nanofiltration as a polishing step of yeast MBR-treated landfill leachate. Environ. Technol. 2018, 40, 3611–3621. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Englehardt, J.D. Treatment of landfill leachate by the Fenton process. Water Res. 2006, 40, 3683–3694. [Google Scholar] [CrossRef]
- Nabi, M.; Gao, D.; Liang, H.; Cheng, L.; Yang, W.; Li, Y. Landfill leachate treatment by graphite engineered anaerobic membrane bioreactor: Performance enhancement and membrane fouling mitigation. Environ. Res. 2022, 214, 114010. [Google Scholar] [CrossRef]
- Jamrah, A.; Al-Zghoul, T.; Baarimah, A.O.; Al-Karablieh, E. A Bibliometric Analysis of Olive Mill Wastewater Treatment Methods from 1988 to 2023. Case Stud. Chem. Environ. Eng. 2024, 9, 100736. [Google Scholar] [CrossRef]
- Teng, C.; Zhou, K.; Peng, C.; Chen, W. Characterization and treatment of landfill leachate: A review. Water Res. 2021, 203, 117525. [Google Scholar] [CrossRef] [PubMed]
- Al-Qodah, Z.; Al-Bsoul, A.; Assirey, E.; Al-Shannag, M. Combined ultrasonic irradiation and aerobic biodegradation treatment for olive mills wastewaters. Environ. Eng. Manag. J. (EEMJ) 2014, 13, 2109–2118. [Google Scholar]
- Faraj, H.; Jamrah, A.; Al-Omari, S.; Al-Zghoul, T.M. Optimization of an electrocoagulation-assisted adsorption treatment system for dairy wastewater. Case Stud. Chem. Environ. Eng. 2024, 9, 100574. [Google Scholar] [CrossRef]
- Rana, R.; Ganguly, R.; Gupta, A.K. Indexing method for assessment of pollution potential of leachate from non-engineered landfill sites and its effect on ground water quality. Environ. Monit. Assess. 2018, 190, 46. [Google Scholar] [CrossRef]
- Sharma, A.; Ganguly, R.; Kumar Gupta, A. Impact assessment of leachate pollution potential on groundwater: An indexing method. J. Environ. Eng. 2020, 146, 05019007. [Google Scholar] [CrossRef]
- Al-Qodah, Z.; Al-Zghoul, T.M.; Jamrah, A. The performance of pharmaceutical wastewater treatment system of electrocoagulation assisted adsorption using perforated electrodes to reduce passivation. Environ. Sci. Pollut. Res. 2024, 31, 20434–20448. [Google Scholar] [CrossRef] [PubMed]
- Bin Mokaizh, A.A.; Shariffuddin, J.H.; Baarimah, A.O.; Al-Fakih, A.; Mohamed, A.; Baarimah, S.O.; Al-Mekhlafi, A.B.A.; Alenezi, H.; Olalere, O.A.; Saeed, A.A.H. Elucidating the Effects of Reaction Time on the Physicochemical Characterization of Valorized Synthesized Alumina. Materials 2022, 15, 3046. [Google Scholar] [CrossRef] [PubMed]
- Al-Qodah, Z.; Dweiri, R.; Khader, M.; Al-Sabbagh, S.; Al-Shannag, M.; Qasrawi, S.; Al-Halawani, M. Processing and characterization of magnetic composites of activated carbon, fly ash, and beach sand as adsorbents for Cr (VI) removal. Case Stud. Chem. Environ. Eng. 2023, 7, 100333. [Google Scholar] [CrossRef]
- Al-Qodah, Z.; Al-Shannag, M. Separation of yeast cells from aqueous solutions using magnetically stabilized fluidized beds. Lett. Appl. Microbiol. 2006, 43, 652–658. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).