A Comparative Study of Carbon Emissions from Industrial and Domestic Wastewater Treatment Plants under the Background of Carbon Neutralization
Abstract
:1. Introduction
2. Methods and Materials
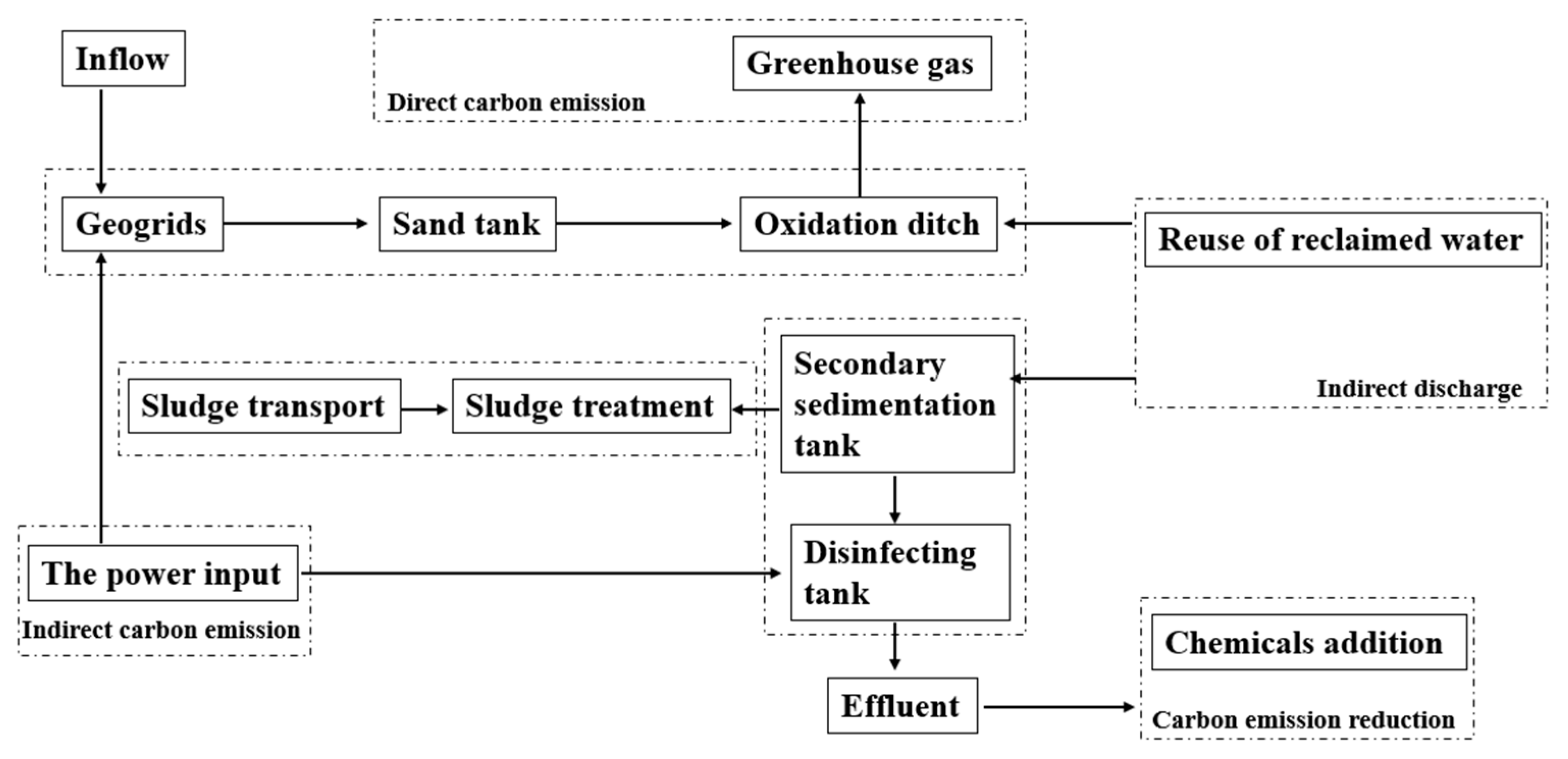
2.1. An Overview of the IWWTP
2.2. An Overview of the DWWTP
2.3. Analytic Methods
2.3.1. Direct Carbon Discharge Calculation
2.3.2. Indirect Carbon Discharge Calculation
2.3.3. Carbon Emission Reduction Calculation
3. Results
3.1. Direct Carbon Emission Accounting
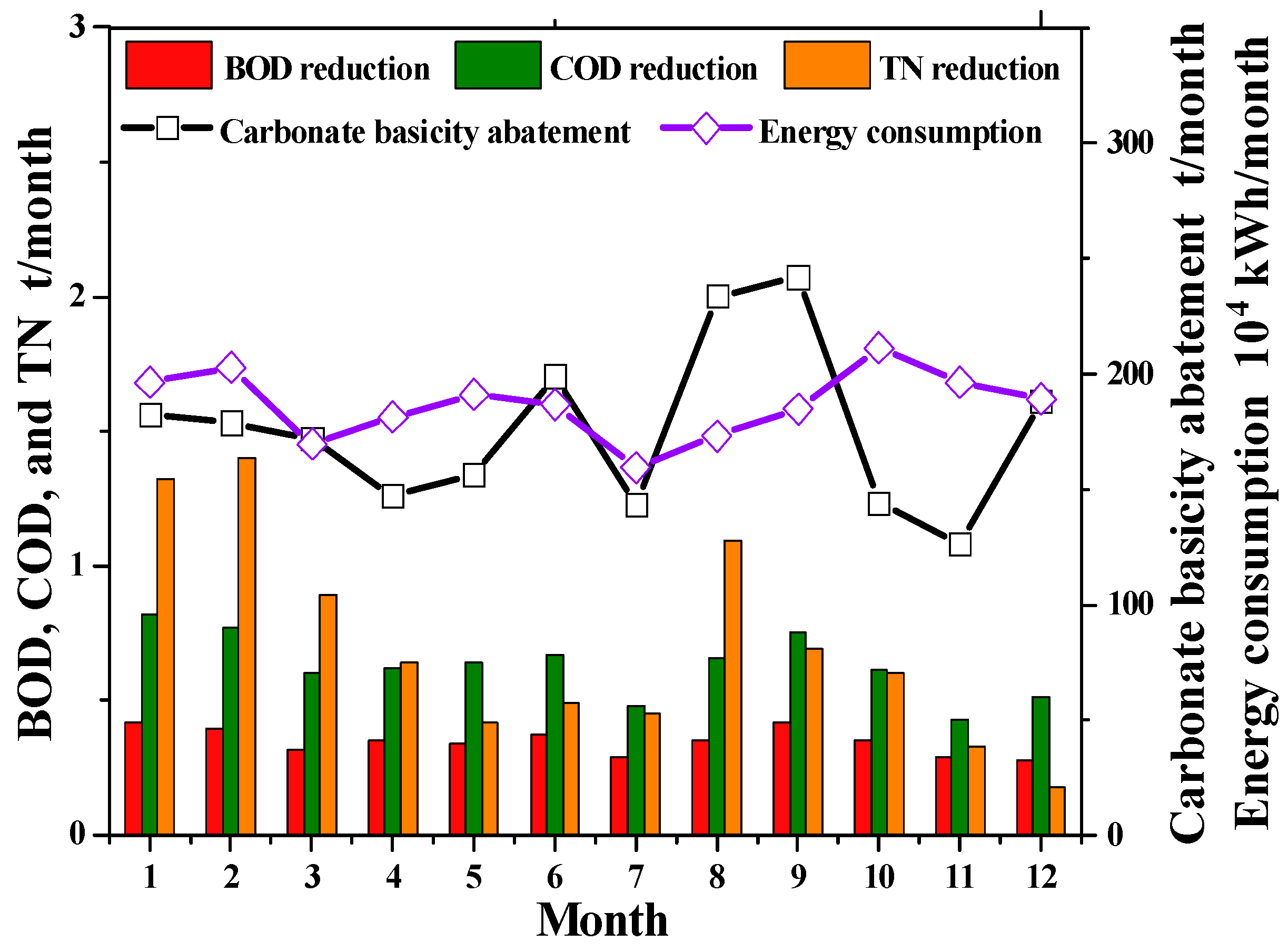
3.1.1. Analysis of Direct Carbon Emission Characteristics of IWWTP
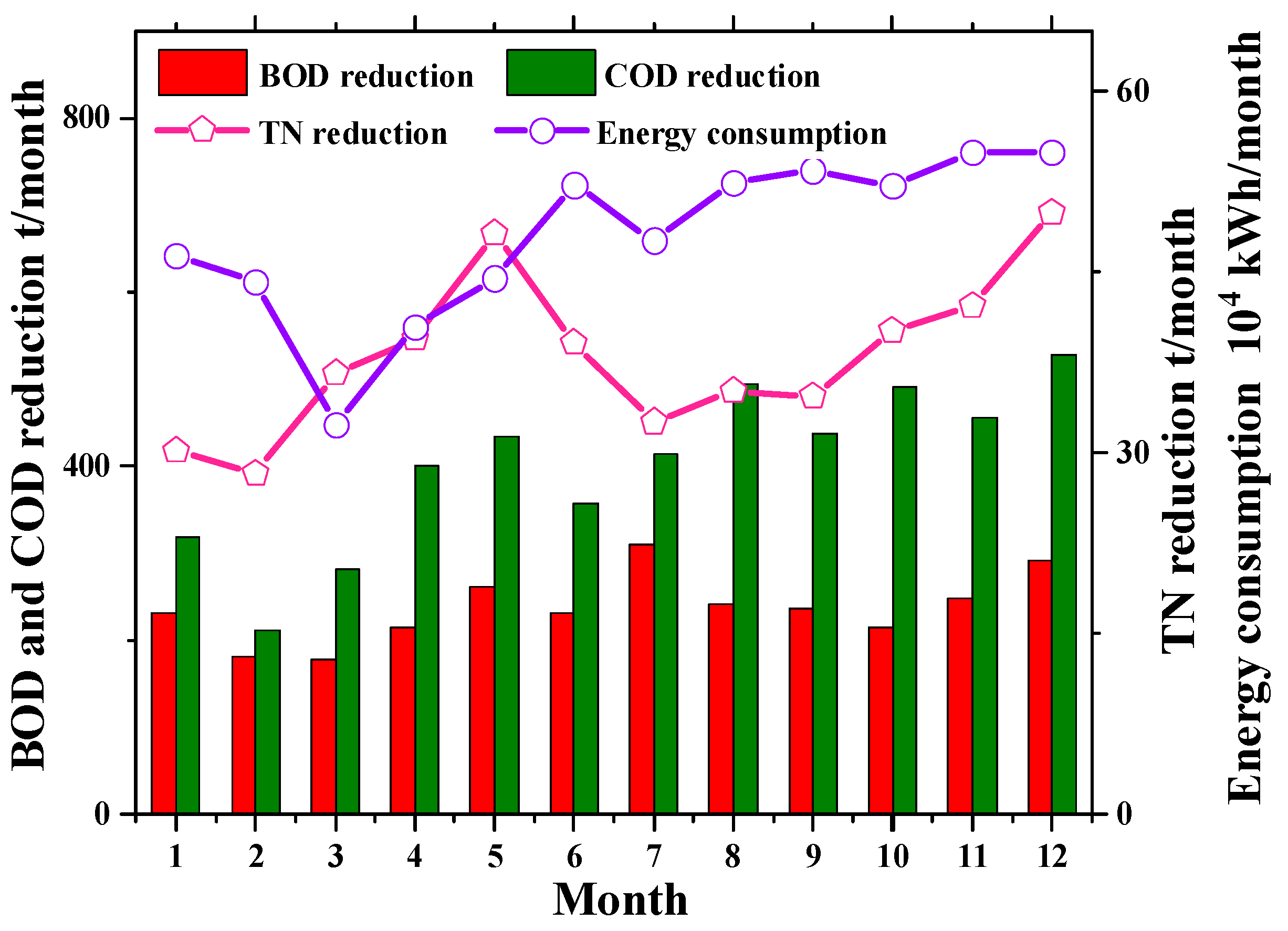
3.1.2. Analysis of Direct Carbon Emission Characteristics of DWWTP
3.2. Indirect Carbon Emission Accounting
3.2.1. Analysis of Indirect Carbon Emission Characteristics of IWWTP
3.2.2. Analysis of Indirect Carbon Emission Characteristics of DWWTP
3.3. Carbon Emission Calculation
3.3.1. Analysis of Total Carbon Emission Characteristics of IWWTP
3.3.2. Analysis of Total Carbon Emission Characteristics of DWWTP
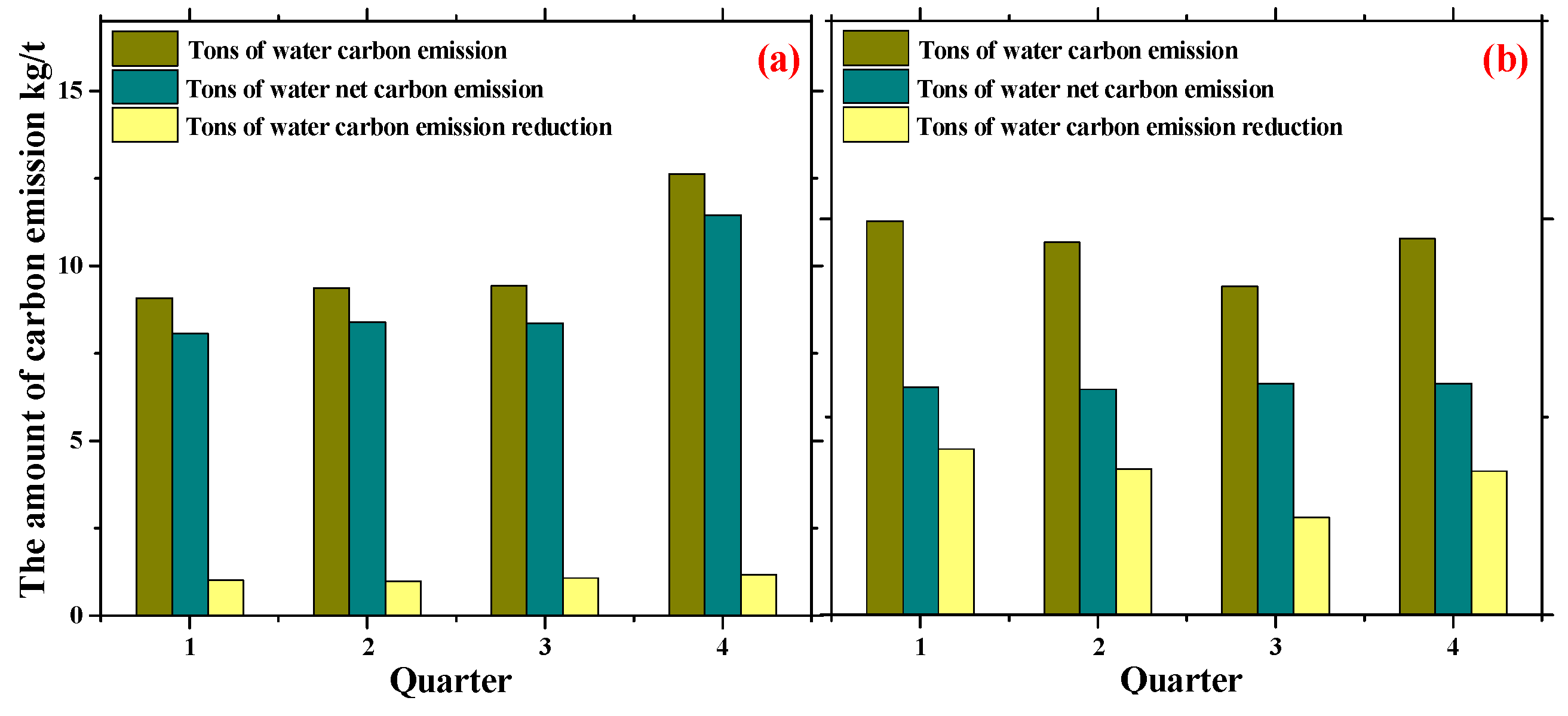
3.4. The Analysis of the Carbon Emission Reduction Effect
4. Discussion
4.1. Analysis of Carbon Emission Characteristics of Industrial and Domestic Wastewater Treatment
4.2. Benefit Analysis of Carbon Emission Reduction in Industrial and Domestic Wastewater Treatment
4.3. Strategy Analysis of Carbon Neutralization in Industrial and Domestic Wastewater Treatment
5. Conclusions
- (1)
- In the present work, the carbon emissions of the IWWTP and DWWTP were 10.13 kg/t and 1.84 kg/t in 2020, respectively, leading to a corresponding total carbon emission of 67,080.46 and 17,533.57 tCO2e. The huge difference is caused by their varied influent characteristics.
- (2)
- Indirect carbon emissions (chemical and energy consumption) play a dominant role in the total carbon emission of the IWWTP, which account for about 97.6% of the total amount. Meanwhile, direct carbon emissions account for about 62% of the total carbon emissions in the DWWTP, followed by indirect carbon emissions generated due to electricity consumption (31.06%) and chemical consumption (6.94%).
- (3)
- Wastewater reclamation and reuse from industrial and domestic WWTPs could reduce the carbon emission per ton of water by 1.06 kg/t and 1.16 kg/t, accounting for about 10.46% and 62.45% of the current emission, respectively. The carbon emission reduction efficiency of the DWWTP is significant. Recycling industrial waste salt and organic chemical energy can further reduce carbon emissions and achieve the goal of the carbon-neutral operation of WWTPs.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vymazal, J.; Zhao, Y.; Mander, Ü. Recent research challenges in constructed wetlands for wastewater treatment: A review. Ecol. Eng. 2021, 169, 106318. [Google Scholar] [CrossRef]
- Senatore, V.; Zarra, T.; Pahunang, R.; Oliva, G.; Belgiorno, V.; Ballesteros, F., Jr.; Naddeo, V. Sustainable Odour and Greenhouse Gas Emissions Control in Wastewater Treatment Plant by Advanced Biotechnology-based System. Chem. Eng. Trans. 2021, 85, 25–30. [Google Scholar]
- Li, W.-W.; Yu, H.-Q.; Rittmann, B.E. Chemistry: Reuse water pollutants. Nature 2015, 528, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; van den Akker, B.; Thwaites, B.J.; Peng, L.; Herman, C.; Pan, Y.; Ni, B.-J.; Watt, S.; Yuan, Z.; Ye, L. Mitigating nitrous oxide emissions at a full-scale wastewater treatment plant. Water Res. 2020, 185, 116196. [Google Scholar] [CrossRef] [PubMed]
- Malila, R.; Lehtoranta, S.; Viskari, E.L. The role of source separation in nutrient recovery—Comparison of alternative wastewater treatment systems. J. Clean. Prod. 2019, 219, 350–358. [Google Scholar] [CrossRef]
- Pahunang, R.R.; Buonerba, A.; Senatore, V.; Oliva, G.; Ouda, M.; Zarra, T.; Muñoz, R.; Puig, S.; Ballesteros, F.C.; Li, C.-W.; et al. Advances in technological control of greenhouse gas emissions from wastewater in the context of circular economy. Sci. Total Environ. 2021, 792, 148479. [Google Scholar] [CrossRef] [PubMed]
- Velazquez Abad, A.; Dodds, P.E. Green hydrogen characterization initiatives: Definitions, standards, guarantees of origin, and challenges. Energy Policy 2020, 138, 111300. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change; Kanagawa, H. 2006 IPCC Guidelines for National Greenhouse Gas Inventories; IPCC: Geneva, Switzerland, 2006. [Google Scholar]
- Xi, J.; Gong, H.; Zhang, Y.; Dai, X.; Chen, L. The evaluation of GHG emissions from Shanghai municipal wastewater treatment plants based on IPCC and operational data integrated methods (ODIM). Sci. Total Environ. 2021, 797, 148967. [Google Scholar] [CrossRef]
- Yu, J.; Zhao, R.; Xiao, L.; Zhang, L.; Wang, S.; Chuai, X.; Han, Y.; Jiao, S. Carbon emissions of urban wastewater treatment system based on the “water- energy-carbon” nexus. Resour. Sci. 2020, 42, 1052–1062. [Google Scholar] [CrossRef]
- Cheng, Z. The Study on Quantitative Evaluation of Carbon Emission in Sewage Treatment System. Master’s Thesis, Xi’an University of Technology, Xi’an, China, 2017. [Google Scholar]
- Nguyen, T.K.L.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Nghiem, L.D.; Liu, Y.; Ni, B.; Hai, F.I. Insight into greenhouse gases emissions from the two popular treatment technologies in municipal wastewater treatment processes. Sci. Total Environ. 2019, 671, 1302–1313. [Google Scholar] [CrossRef]
- Dai, X.H. Current status and development trend of sludge treatment and disposal in China. Science 2020, 72, 30–34. [Google Scholar]
- Chen, G.; Zhang, R.; Guo, X.; Wu, W.; Guo, Q.; Zhang, Y.; Yan, B. Comparative evaluation on municipal sewage sludge utilization processes for sustainable management in Tibet. Sci. Total Environ. 2021, 765, 142676. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Zhu, F.; Li, Q.; Xue, C.; Xia, X.; Yu, H.; Zhao, Q.; Jiang, J.; Bai, S. Development, current state and future trends of sludge management in China: Based on exploratory data and CO2-equivaient emissions analysis. Environ. Int. 2020, 144, 106093. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Li, J.; Van Loosdrecht, M.C.M.; Jiang, H.; Liu, R. Energy recovery from wastewater: Heat over organics. Water Res. 2019, 161, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Masłoń, A.; Czarnota, J.; Szczyrba, P.; Szaja, A.; Szulżyk-Cieplak, J.; Łagód, G. Assessment of Energy Self-Sufficiency of Wastewater Treatment Plants—A Case Study from Poland. Energies 2024, 17, 1164. [Google Scholar] [CrossRef]
- Hu, Y.; Liang, M.; Zhang, Y.; Wang, K. Study on operation efficiency and carbon emission characteristics of A2/O + MBR process. China Environ. Sci. 2021, 41, 4439–4446. [Google Scholar]
- Guo, Y.; Tian, J.; Chen, L. Managing energy infrastructure to decarbonize industrial parks in China. Nat. Commun. 2020, 11, 981. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Y.X.; Cao, X.X.; Liu, X.H.; Zhang, S.Y. Research Progress of Carbon-neutral Operation Technology in Sewage Treatment. J. Beijing Univ. Technol. 2022, 48, 292–305. [Google Scholar]
- Demir, Z.; Yapcolu, P. Investigation of GHG emission sources and reducing GHG emissions in a municipal wastewater treatment plan. Greenh. Gases Sci. Technol. 2019, 9, 948–964. [Google Scholar] [CrossRef]
- Kang, Z.; Ye, Z.; Hsu, S.-C. Developing an hourly-resolution well-to-wheel carbon dioxide emission inventory of electric vehicles. Resour. Conserv. Recycl. 2023, 190, 106819. [Google Scholar] [CrossRef]
- Zhai, G.H.; Zuo, X.F. Spatial differences and influencing factors of carbon emissions from urban wastewater treatment in China. J. Shijiazhuang Tiedao Univ. 2020, 14, 1–10+24. [Google Scholar]
- Song, B.M.; Qin, H.P.; Ma, G.Q. Analysis for dynamic changes of wastewater treatment plant carbon emissions in operation phase: With a wastewater treatment plant in Shenzhen as an example. Environ. Sci. Technol. 2015, 38, 204–209. [Google Scholar]
- Chen, K.; Wang, H.; Valverde-Pérez, B.; Zhai, S.; Vezzaro, L.; Wang, A. Optimal control towards sustainable wastewater treatment plants based on multi-agent reinforcement learning. Chemosphere 2021, 279, 130498. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y. Engineering design and carbon emission analysis of municipal WWTP under stricter discharge criteria—Case of a WWTP in Ningbo. Water Purif. Technol. 2023, 42, 158–163. [Google Scholar]
- Wang, F.; Harindintwali, J.D.; Yuan, Z.; Wang, M.; Wang, F.; Li, S.; Yin, Z.; Huang, L.; Fu, Y.; Li, L.; et al. Technologies and perspectives for achieving carbon neutrality. Innovation 2021, 2, 100180. [Google Scholar] [CrossRef] [PubMed]
- Yapcolu, P. Minimization of greenhouse gas emissions from extended aeration activated sludge process. Water Pract. Technol. 2021, 16, 96–107. [Google Scholar]
- Gruber, W.; Niederdorfer, R.; Ringwald, J.; Morgenroth, E.; Bürgmann, H.; Joss, A. Linking seasonal N2O emissions and nitrification failures to microbial dynamics in a SBR wastewater treatment plant. Water Res. X 2021, 11, 100098. [Google Scholar] [CrossRef] [PubMed]
- Jing, B.Y.; Shi, Y.T.; Chen, Z. Application of Membrane Separation Technology in Advanced Treatment of High Salt Mine Water. Coal Eng. 2019, 51, 47–51. [Google Scholar]
- Suo, Y.; Ren, Y.J. Research on the mechanism of nanofiltration membrane fouling in zero discharge process of high salty wastewater from coal chemical industry. Chem. Eng. Sci. 2021, 245, 116810. [Google Scholar] [CrossRef]
- Guo, L.; Xie, Y.; Sun, W.; Xu, Y.; Sun, Y. Research Progress of High-Salinity Wastewater Treatment Technology. Water 2023, 15, 684. [Google Scholar] [CrossRef]
- Singh, J.; Dhar, D.W. Overview of Carbon Capture Technology: Microalgal Biorefinery Concept and State-of-the-Art. Front. Matine Sci. 2019, 6, 29. [Google Scholar] [CrossRef]
- Shtull-Trauring, E.; Cohen, A.; Ben-Hur, M.; Tanny, J.; Bernstein, N. Reducing salinity of treated waste water with large scale desalination. Water Res. 2020, 186, 116322. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.L.; Kang, D.X.; Rong, Z.Y.; Zhang, R.M.; Sui, B.J. Separation of Lithium Sulfate and Recovery of Acid and Base by Three-compartment Membrane Electrodialysis. Technol. Water Treat. 2022, 10, 72–76+81. [Google Scholar]
- Sarpong, G.; Gude, V.G.; Magbanua, B.S.; Truax, D.D. Evaluation of energy recovery potential in wastewater treatment based on codigestion and combined heat and power schemes. Energy Convers. Manag. 2020, 222, 113147. [Google Scholar] [CrossRef]
- Rueda, E.; García-Galán, M.J.; Díez-Montero, R.; Vila, J.; Grifoll, M.; García, J. Polyhydroxybutyrate and glycogen production in photobioreactors inoculated with wastewater borne cyanobacteria monocultures. Bioresour. Technol. 2020, 295, 122233. [Google Scholar] [CrossRef] [PubMed]
- Almomani, F.; Al Ketife, A.; Judd, S.; Shurair, M.; Bhosale, R.R.; Znad, H.; Tawalbeh, M. Impact of CO2 concentration and ambient conditions on microalgal growth and nutrient removal from wastewater by a photobioreactor. Sci. Total Environ. 2019, 662, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Barboríková, J.; Šutovská, M.; Kazimierová, I.; Jošková, M.; Fraňová, S.; Kopecký, J.; Capek, P. Extracellular polysaccharide produced by Chlorella vulgaris—Chemical characterization and anti-asthmatic profile. Int. J. Biol. Macromol. 2019, 135, 1–11. [Google Scholar] [CrossRef]
- Molazadeh, M.; Ahmadzadeh, H.; Pourianfar, H.R.; Lyon, S.; Rampelotto, P.H. The Use of Microalgae for Coupling Wastewater Treatment with CO2 Biofixation. Front. Bioeng. Biotechnol. 2019, 7, 42. [Google Scholar] [CrossRef]
- Yadav, G.; Dash, S.K.; Sen, R. A biorefinery for valorization of industrial waste-water and flue gas by microalgae for waste mitigation, carbon-dioxide sequestration and algal biomass production. Sci. Total Environ. 2019, 688, 129–135. [Google Scholar] [CrossRef]


| Month | IWWTP (×104 m3) | DWWTP (×104 m3) |
|---|---|---|
| 1 | 66.7139 | 68.485 |
| 2 | 58.3853 | 50.443 |
| 3 | 54.2453 | 64.163 |
| 4 | 58.8956 | 73.127 |
| 5 | 50.8384 | 81.678 |
| 6 | 54.8501 | 83.109 |
| 7 | 46.5732 | 91.719 |
| 8 | 62.419 | 92.397 |
| 9 | 67.9071 | 88.735 |
| 10 | 54.1464 | 86.85 |
| 11 | 46.666 | 84.894 |
| 12 | 46.913 | 85.535 |
| COD | HCO3− | BOD5 | TN | ||||
|---|---|---|---|---|---|---|---|
| Influent | Effluent | Influent | Effluent | Influent | Effluent | Influent | Effluent |
| 30.43 ± 5.5 | 2.22 ±1.6 | 328.29 ± 60.1 | 12.26 ± 4.0 | 6.05 ± 2.4 | 5.49 ± 2.2 | 438.50 ± 83.6 | 72.00 ± 13.7 |
| Quarter | CH4 | N2O | CO2 | Direct Carbon Emission |
|---|---|---|---|---|
| 1 | 2.42 | 37.66 | 384.34 | 424.42 |
| 2 | 2.28 | 16.25 | 362.27 | 380.80 |
| 3 | 2.28 | 23.19 | 446.51 | 471.98 |
| 4 | 1.97 | 11.57 | 330.78 | 344.31 |
| Sum | 8.95 | 88.67 | 1523.90 | 1621.51 |
| COD | BOD5 | TN | |||
|---|---|---|---|---|---|
| Influent | Effluent | Influent | Effluent | Influent | Effluent |
| 523.80 ± 103 | 21.13 ± 3.9 | 271.44 ± 56 | 3.13 ± 1.9 | 61.93 ± 24 | 13.22 ± 2.7 |
| Quarter | CH4 | N2O | Direct Carbon Emission |
|---|---|---|---|
| 1 | 1268.62 | 992.15 | 2260.77 |
| 2 | 1521.12 | 1323.73 | 2844.85 |
| 3 | 1693.36 | 1068.54 | 2761.91 |
| 4 | 1624.54 | 1379.88 | 3004.42 |
| Sum | 6107.64 | 4764.30 | 10,871.94 |
| Quarter | Electricity | Chemical Addition | Indirect Carbon Emission | |||
|---|---|---|---|---|---|---|
| NaOH | Coagulation | HCl | Sum | |||
| 1 | 5378.59 | 7029.93 | 59.38 | 3396.01 | 10,485.31 | 15,863.90 |
| 2 | 5286.07 | 6830.96 | 65.63 | 2858.47 | 9755.06 | 15,041.14 |
| 3 | 4893.97 | 7729.56 | 71.88 | 3523.66 | 11,325.09 | 16,219.06 |
| 4 | 5643.34 | 8630.78 | 61.25 | 3999.48 | 12,691.51 | 18,334.84 |
| Sum | 21,201.97 | 30,221.24 | 258.13 | 13,777.62 | 44,256.98 | 65,458.95 |
| Quarter | Electricity | Chemical Addition | Indirect Carbon Emission | ||
|---|---|---|---|---|---|
| PAM | Disinfectant | Sum | |||
| 1 | 1162.62 | 101.88 | 110.61 | 212.49 | 1375.11 |
| 2 | 1297.45 | 157.25 | 175.59 | 332.84 | 1630.29 |
| 3 | 1451.76 | 125.00 | 184.44 | 309.44 | 1761.20 |
| 4 | 1532.62 | 184.50 | 177.91 | 362.41 | 1895.03 |
| sum | 5444.45 | 568.63 | 648.55 | 1217.18 | 6661.63 |
| Quarter | Direct Carbon Emission/t | Indirect Carbon Emission/t | Treated Volume/10,000 m3 | Carbon Emission per Ton Water kg/t | ||||
|---|---|---|---|---|---|---|---|---|
| IWWTP | DWWTP | IWWTP | DWWTP | IWWTP | DWWTP | IWWTP | DWWTP | |
| 1 | 424.42 | 2260.77 | 15,863.90 | 1375.11 | 179.34 | 183.09 | 9.08 | 1.99 |
| 2 | 380.80 | 2844.85 | 15,041.14 | 1630.29 | 164.58 | 237.91 | 9.37 | 1.88 |
| 3 | 471.98 | 2761.91 | 16,219.06 | 1761.20 | 176.90 | 272.85 | 9.44 | 1.66 |
| 4 | 344.31 | 3004.42 | 18,334.84 | 1895.03 | 147.73 | 257.28 | 12.64 | 1.90 |
| Sum | 1621.51 | 10,871.94 | 65,458.95 | 6661.63 | 668.55 | 951.14 | 10.13 | 1.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Li, Z.; Li, P.; Xu, W.; Liu, T.; Cheng, S.; Li, J.; Miao, Z.; Zhang, L.; Zheng, X. A Comparative Study of Carbon Emissions from Industrial and Domestic Wastewater Treatment Plants under the Background of Carbon Neutralization. Water 2024, 16, 1652. https://doi.org/10.3390/w16121652
Liu X, Li Z, Li P, Xu W, Liu T, Cheng S, Li J, Miao Z, Zhang L, Zheng X. A Comparative Study of Carbon Emissions from Industrial and Domestic Wastewater Treatment Plants under the Background of Carbon Neutralization. Water. 2024; 16(12):1652. https://doi.org/10.3390/w16121652
Chicago/Turabian StyleLiu, Xiaoping, Zhanbin Li, Peng Li, Weiqing Xu, Tong Liu, Shengdong Cheng, Jiajia Li, Ziyao Miao, Lin Zhang, and Xing Zheng. 2024. "A Comparative Study of Carbon Emissions from Industrial and Domestic Wastewater Treatment Plants under the Background of Carbon Neutralization" Water 16, no. 12: 1652. https://doi.org/10.3390/w16121652





