Hydroxyl Radical-Based Advanced Oxidation Processes of Red Reactive Dyes by Ultrafine Bubbles Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
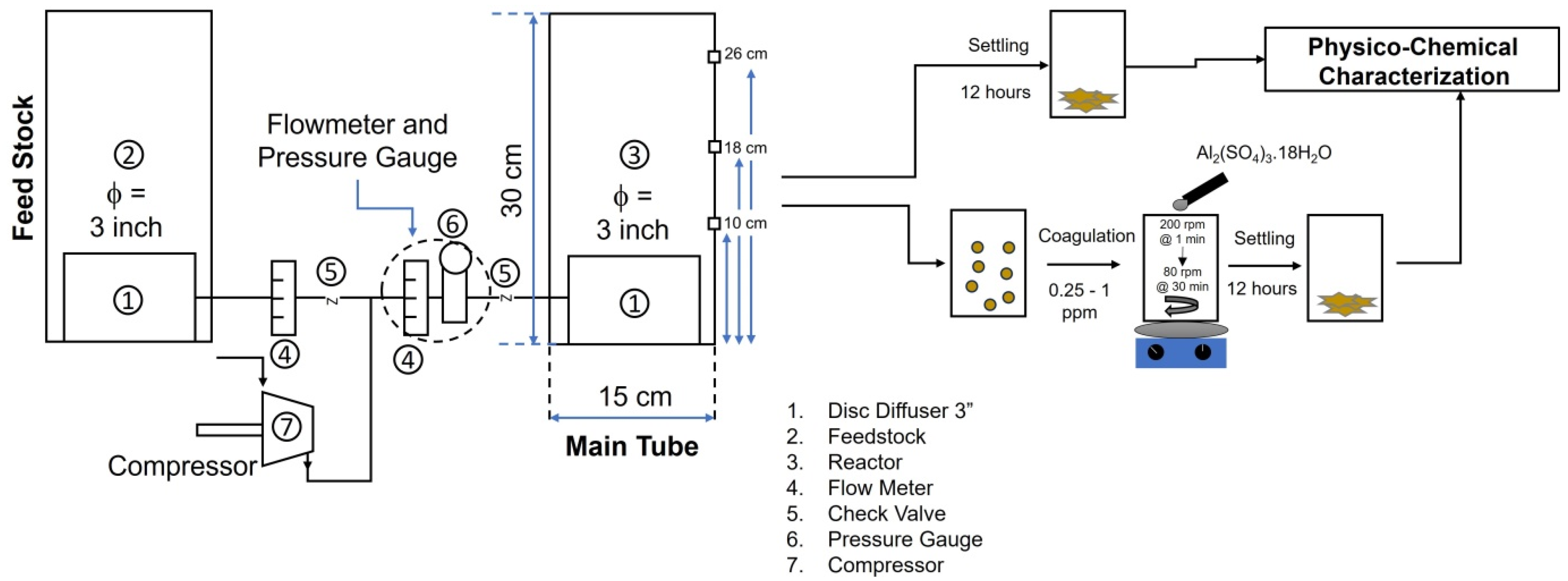
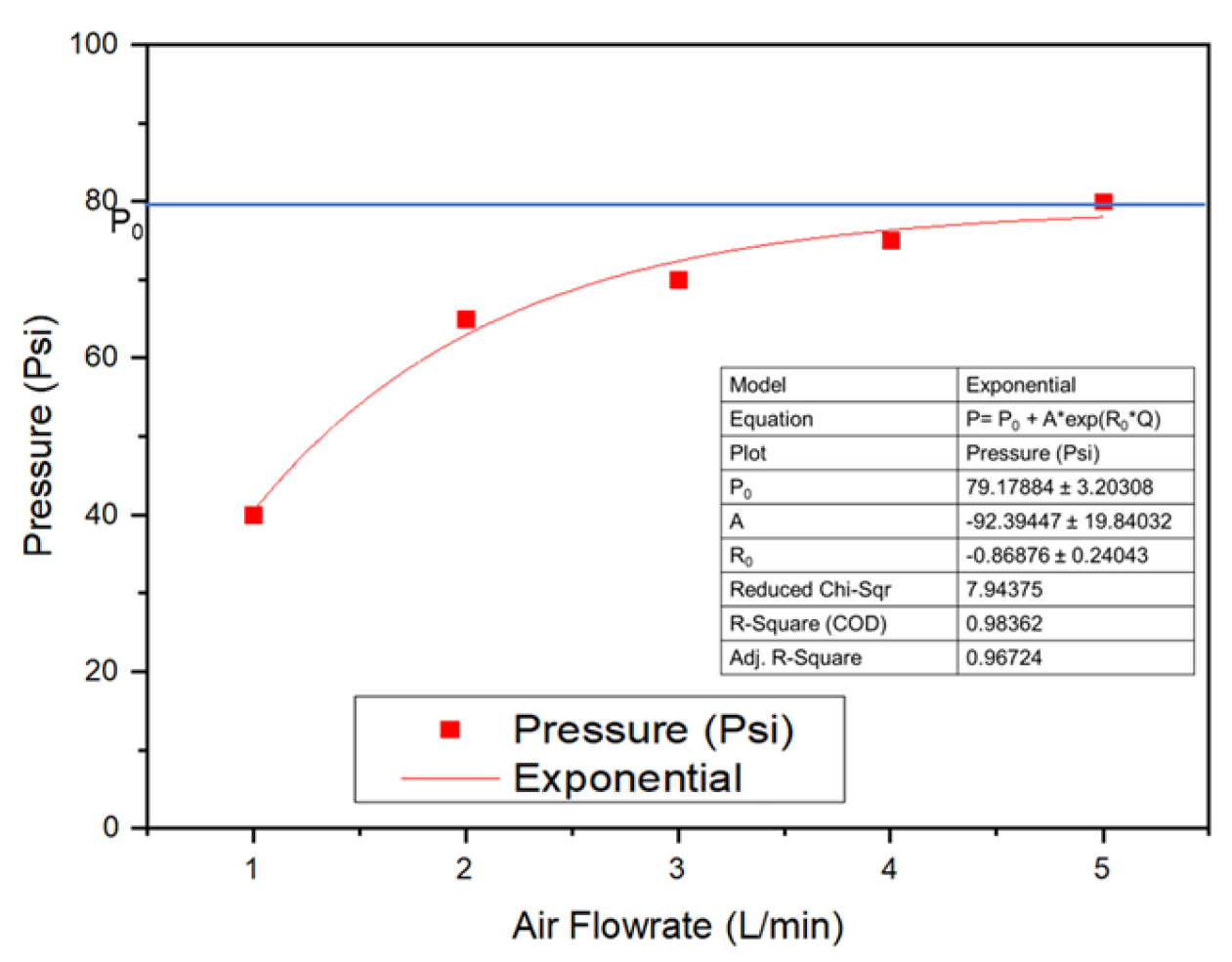
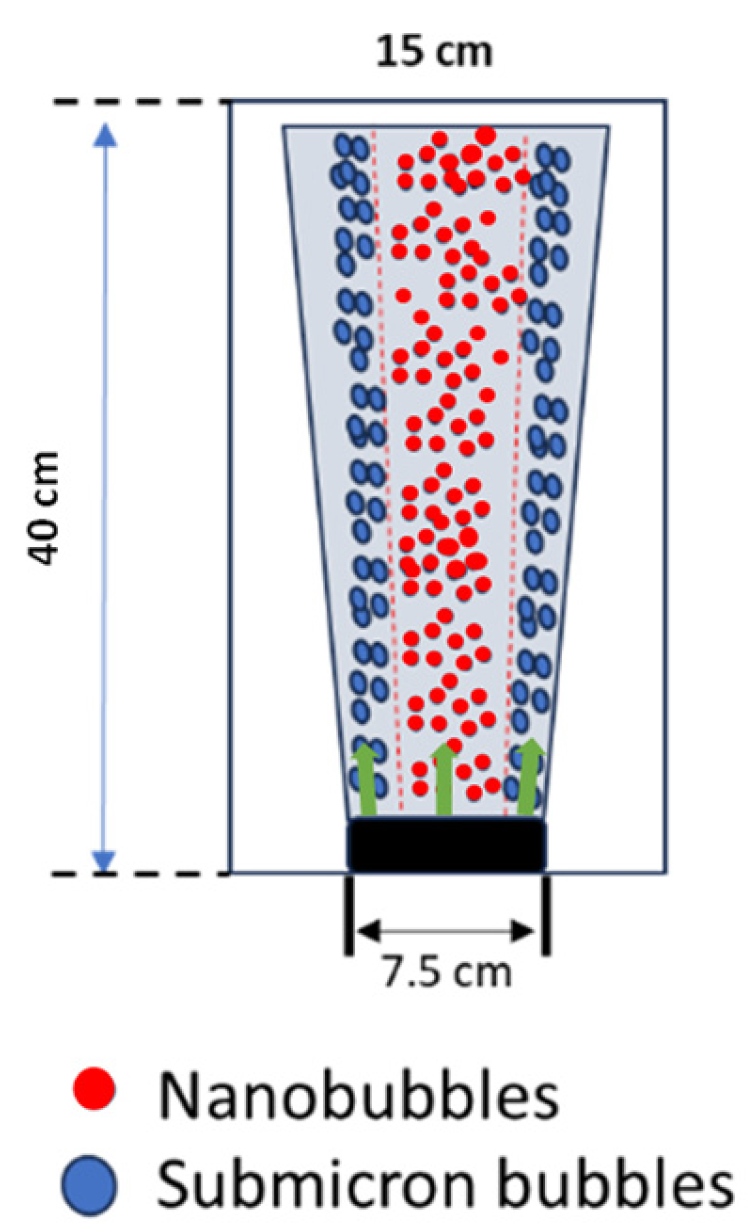
2.2.1. Diffuser Performance Test
2.2.2. Bubble Test with Distilled Water
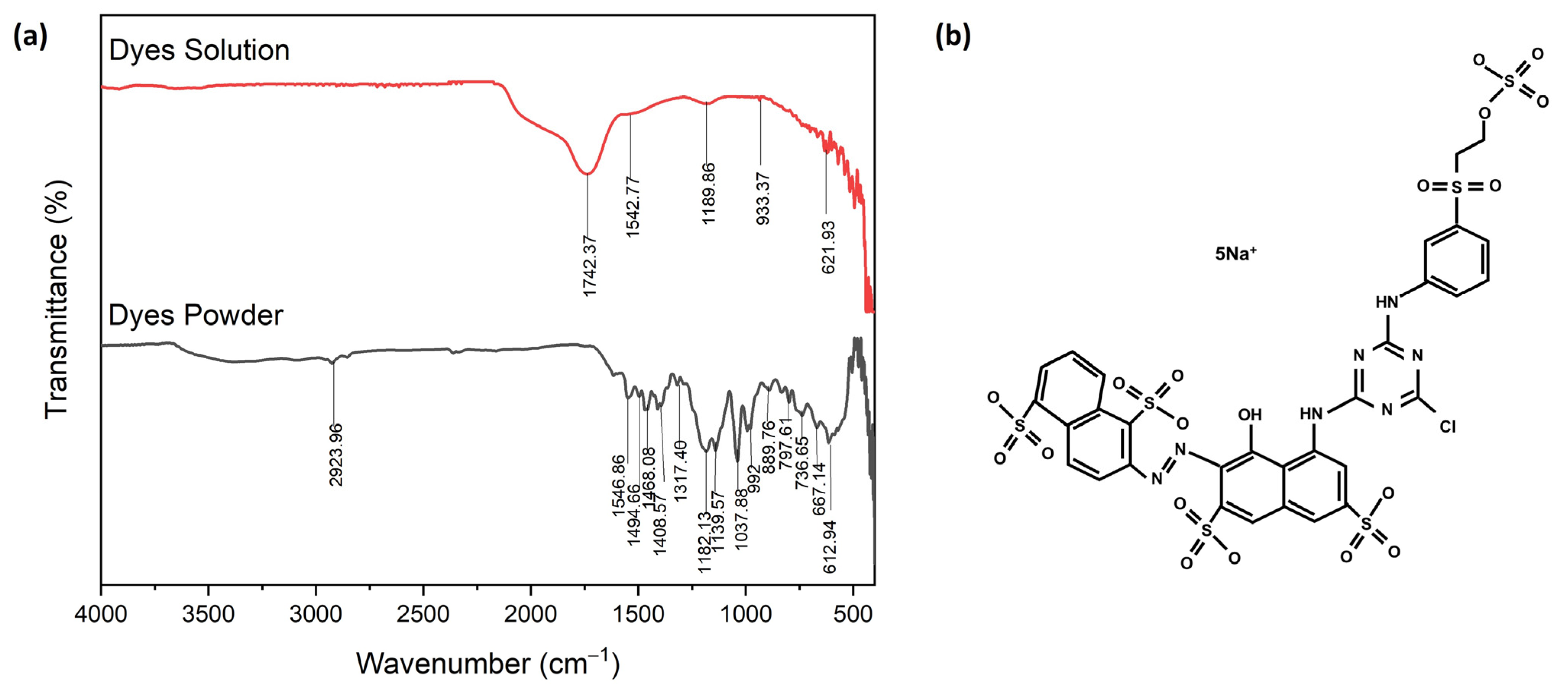
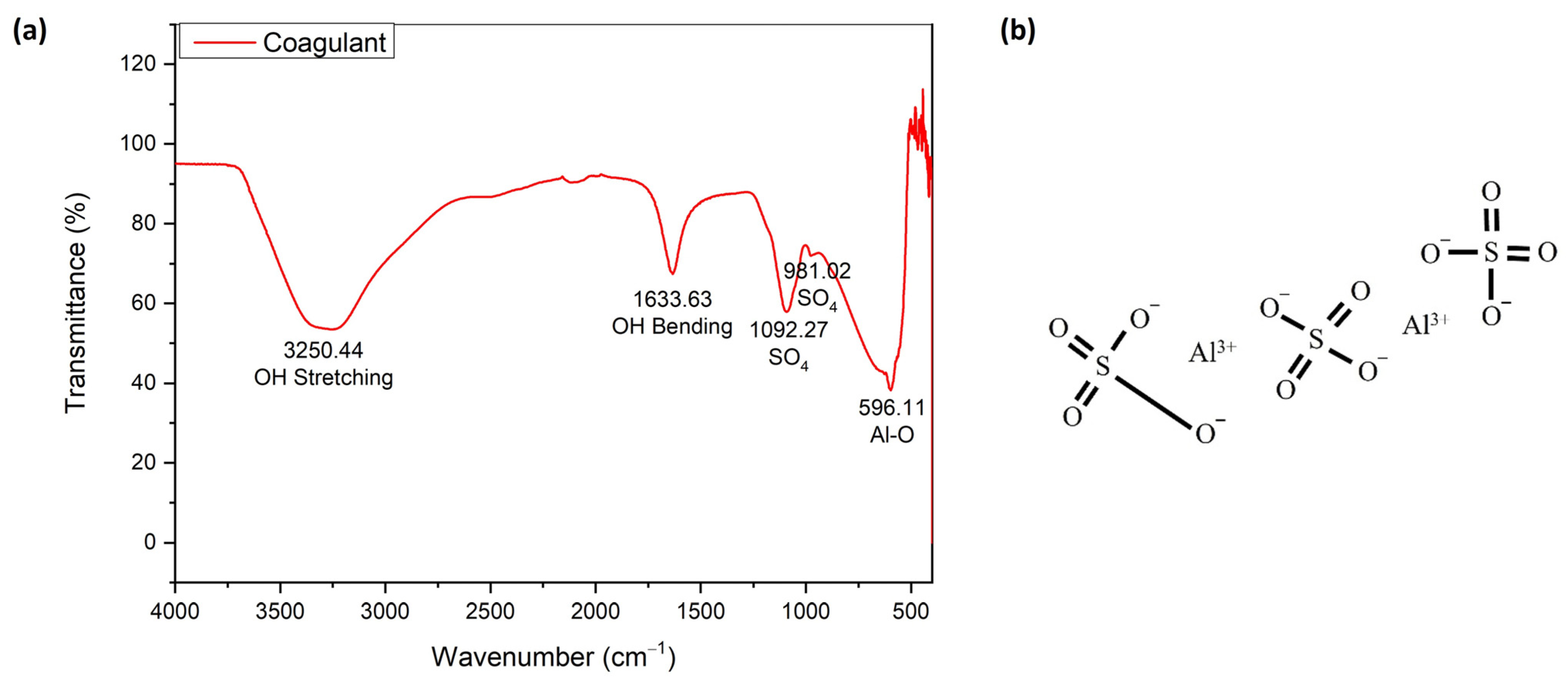
2.2.3. Properties of Dyes and Coagulants
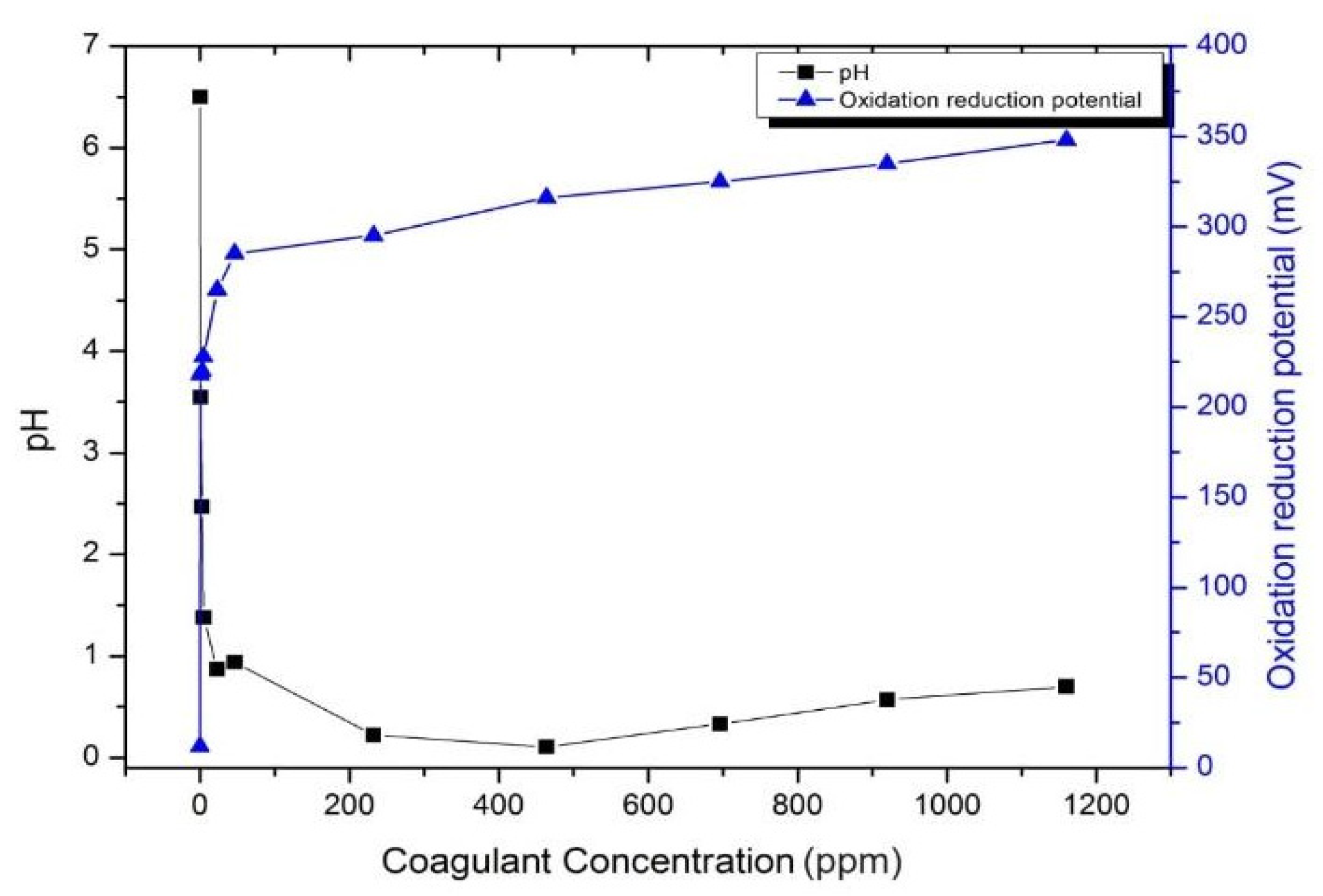
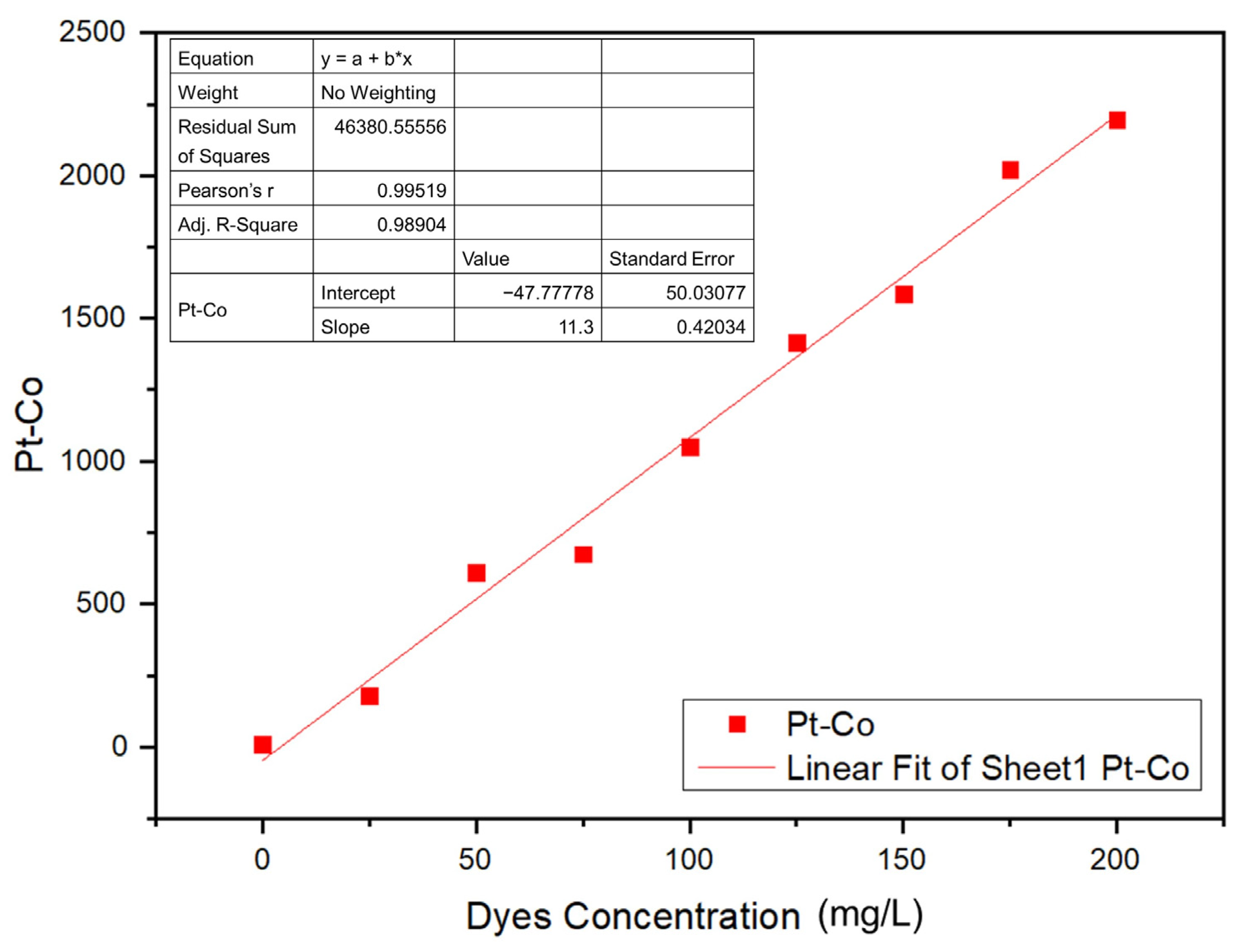
2.2.4. Determination of the Optimal Coagulation Dosage
2.2.5. Treatment of Dyes
3. Results and Discussion
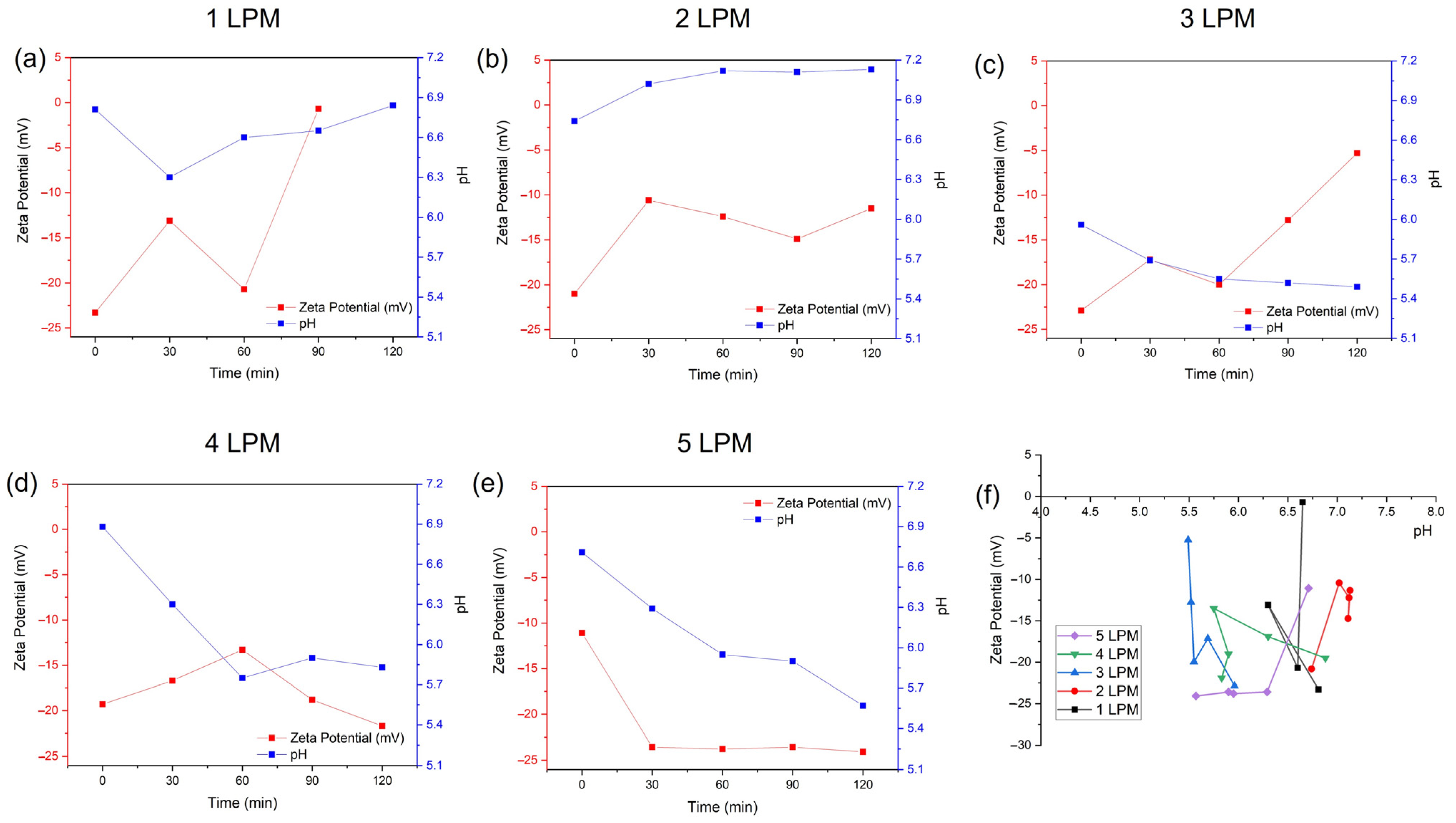
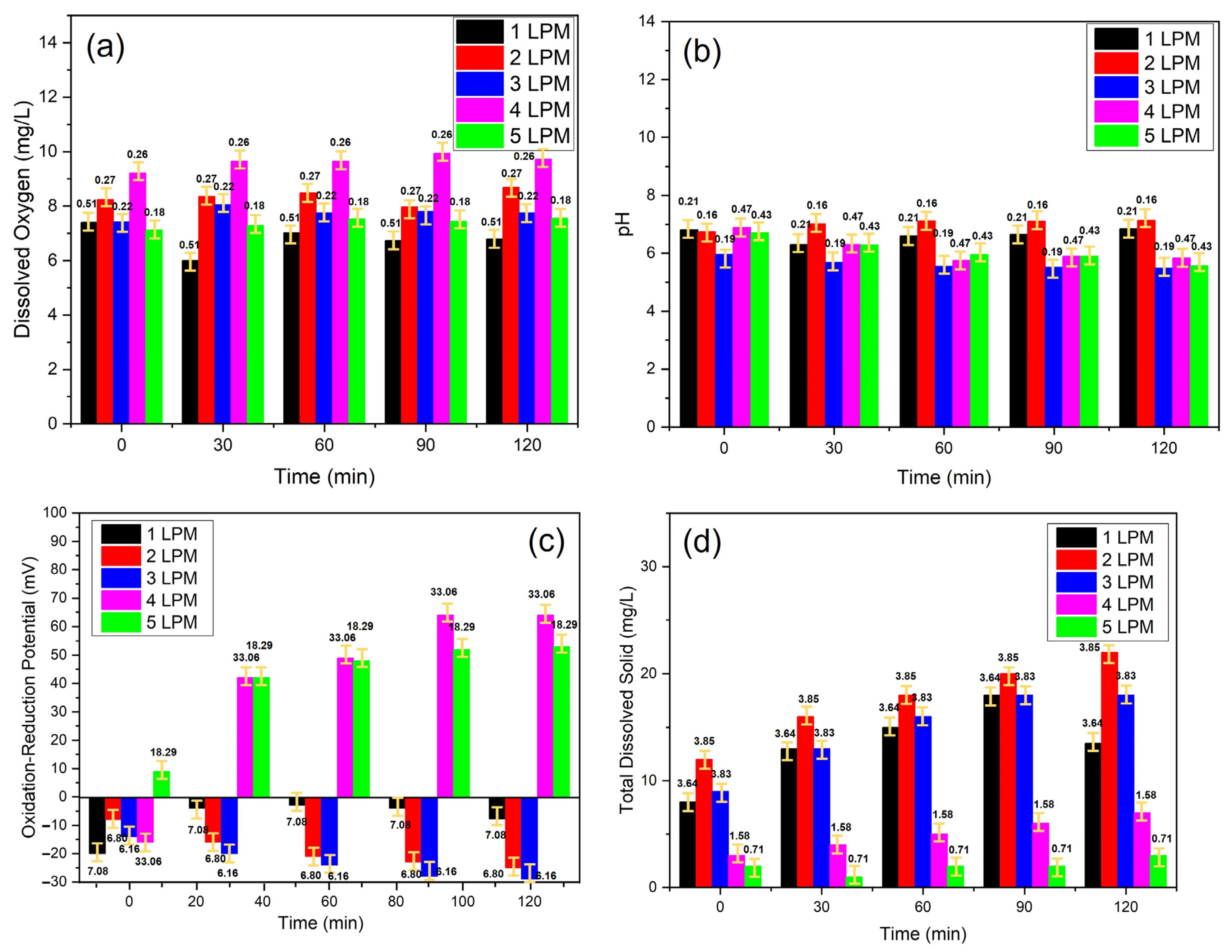
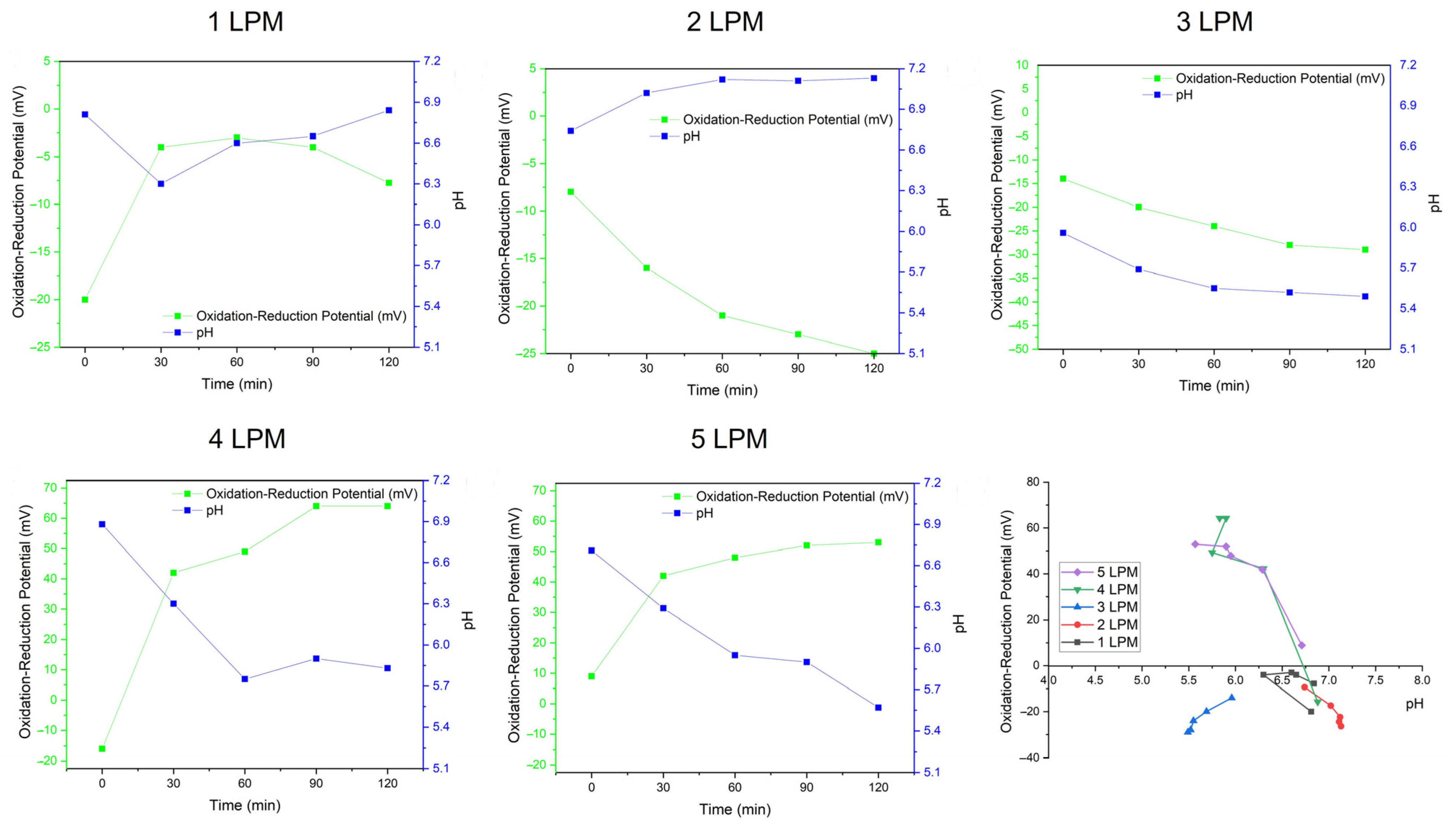
3.1. Characteristics of Disc Diffuser as UFB Generator
3.2. ROS Formation from UFBs Generator
3.3. Characteristics of Red Dye Navacron Ruby S-3B and Coagulants
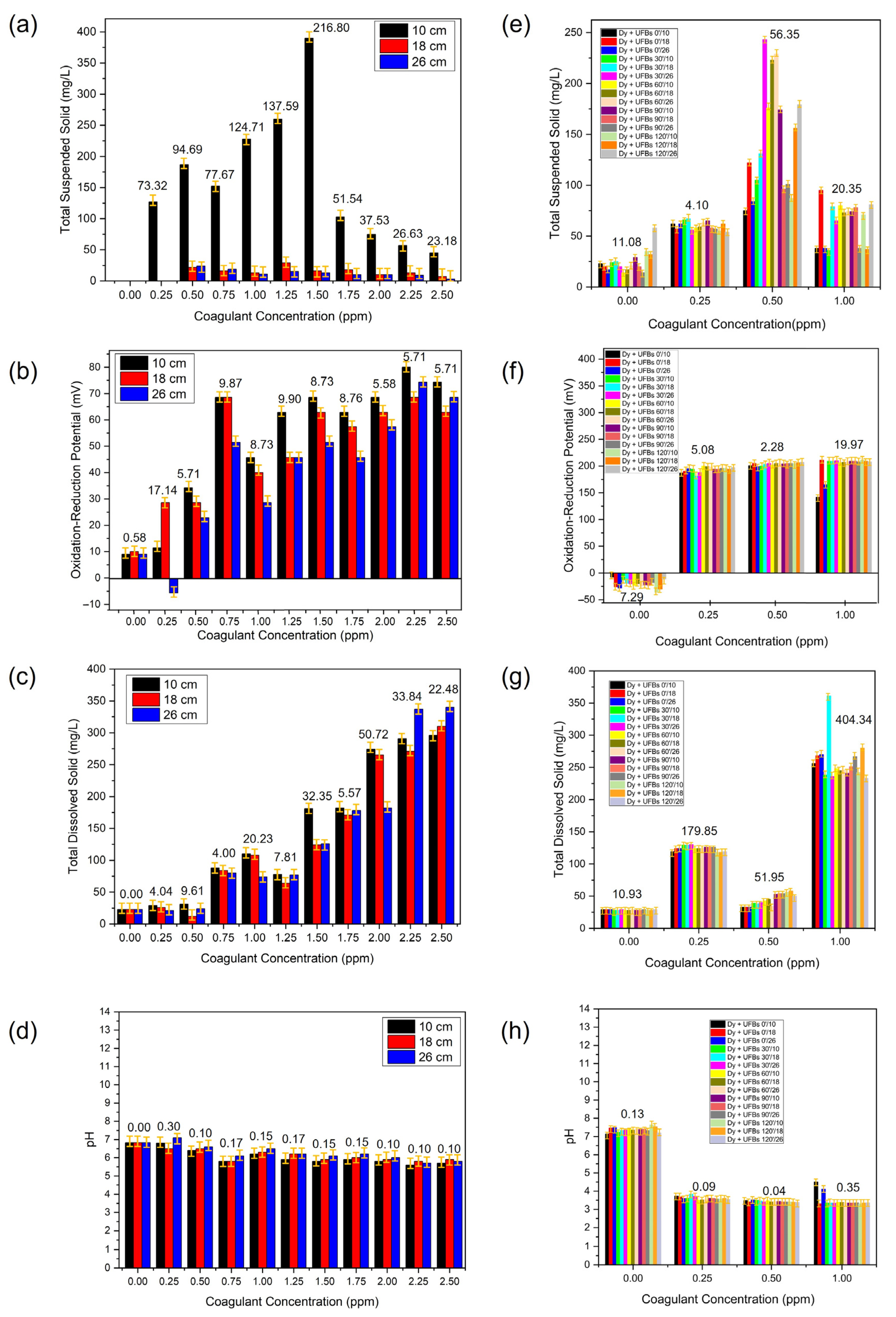
3.4. Determination of the Optimal Coagulation Dosage
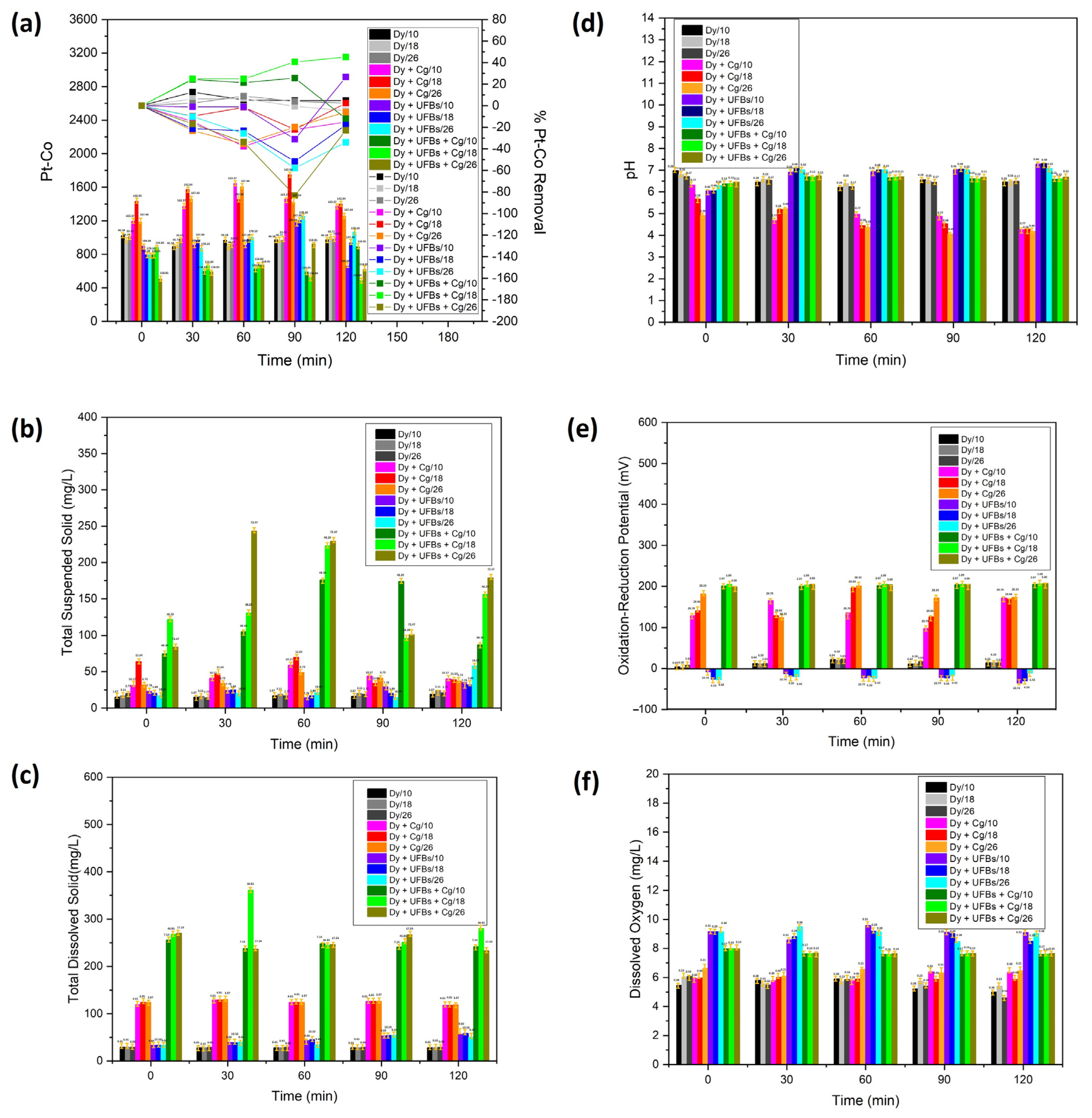
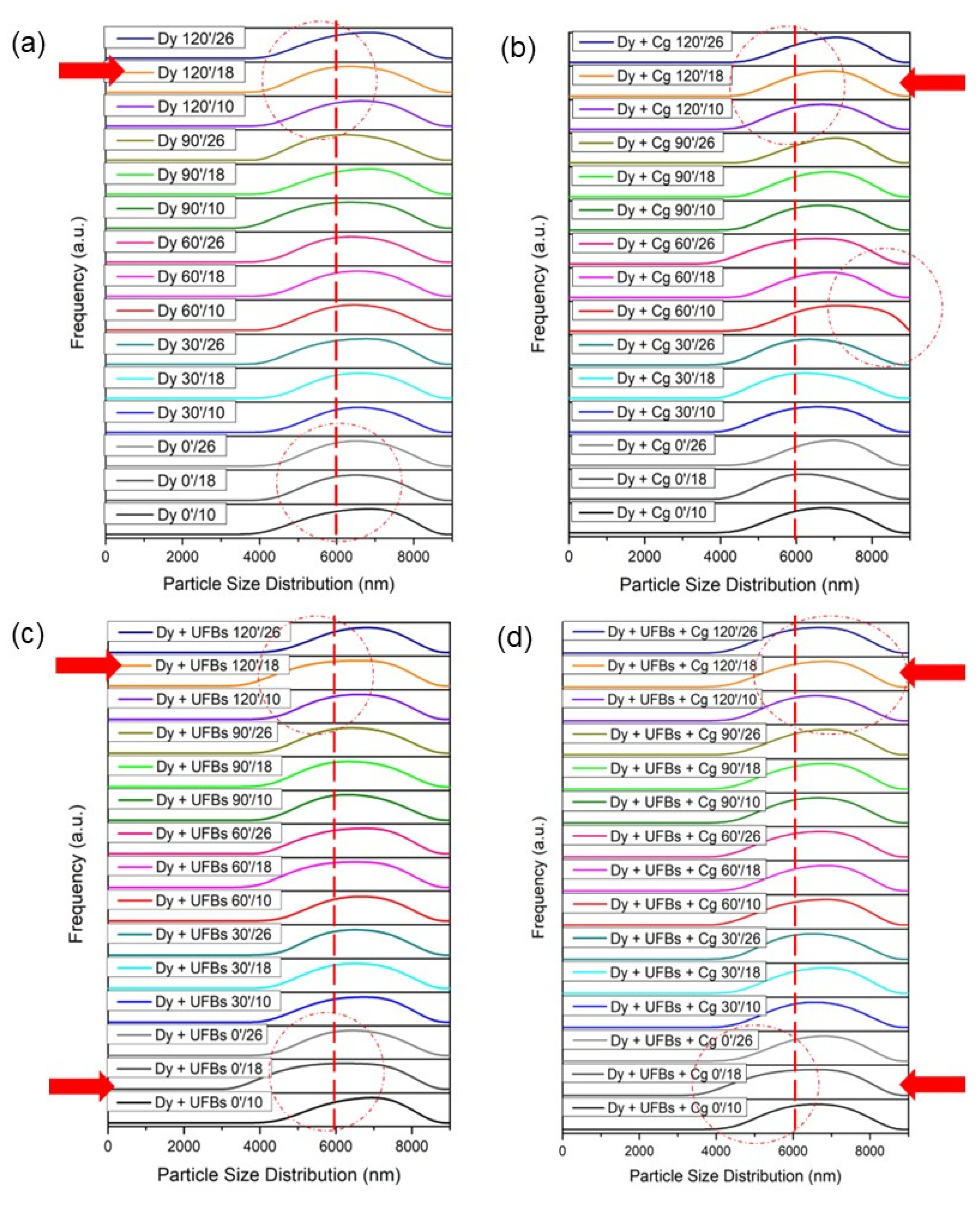
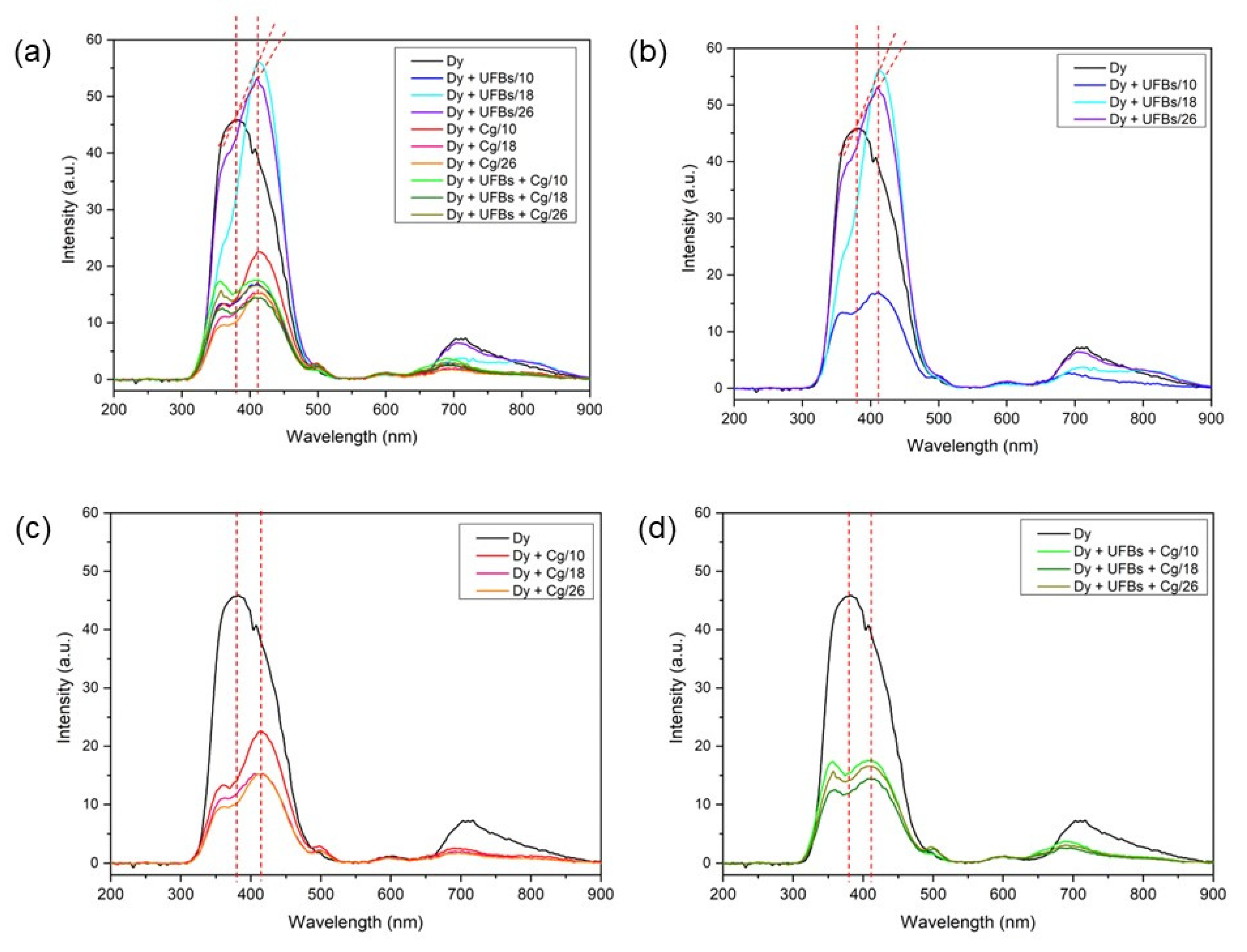
3.5. Test Treatment of Dyes
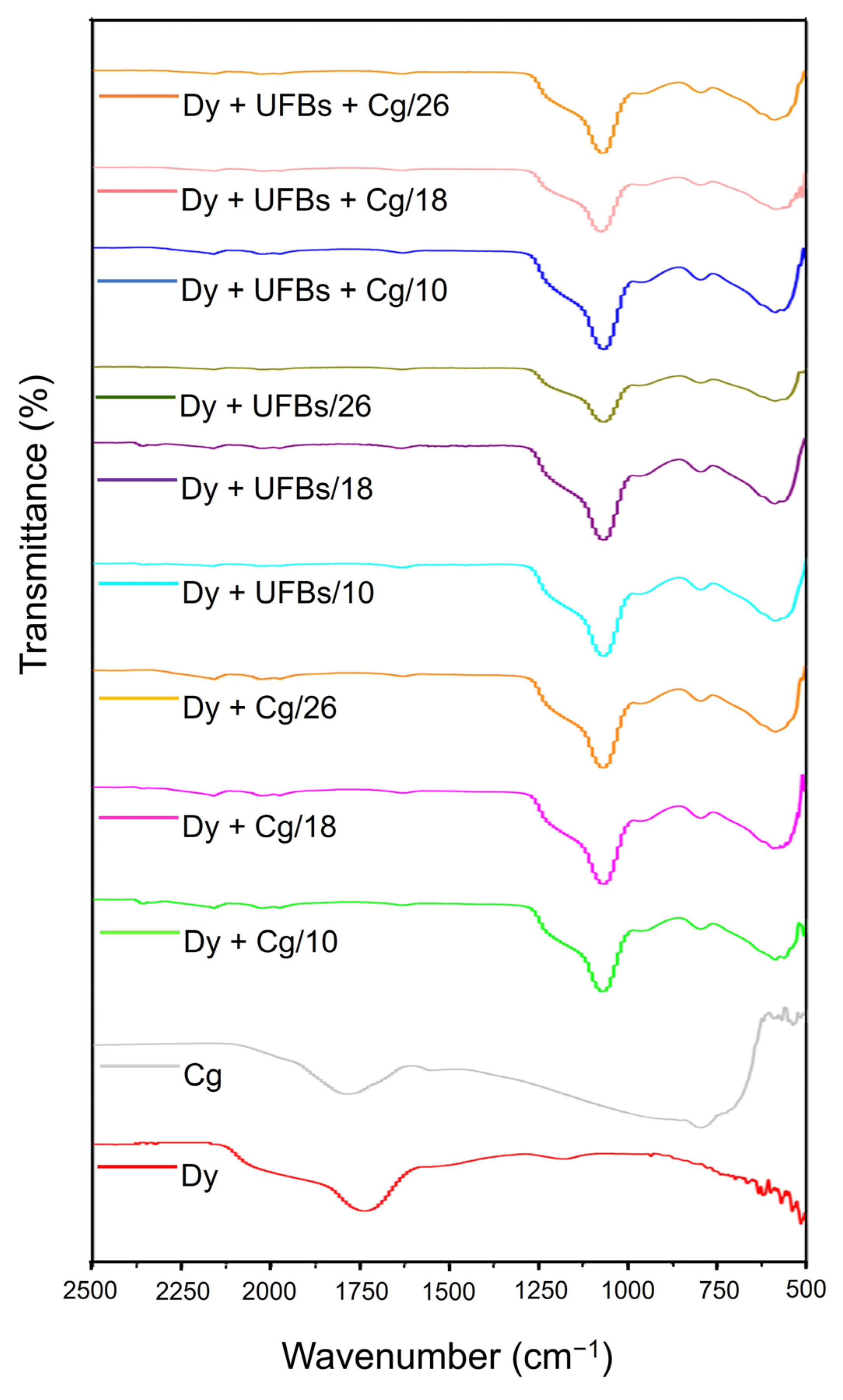
3.6. Proposed Mechanism of Dye Degradation, Mineralization, and Flotation
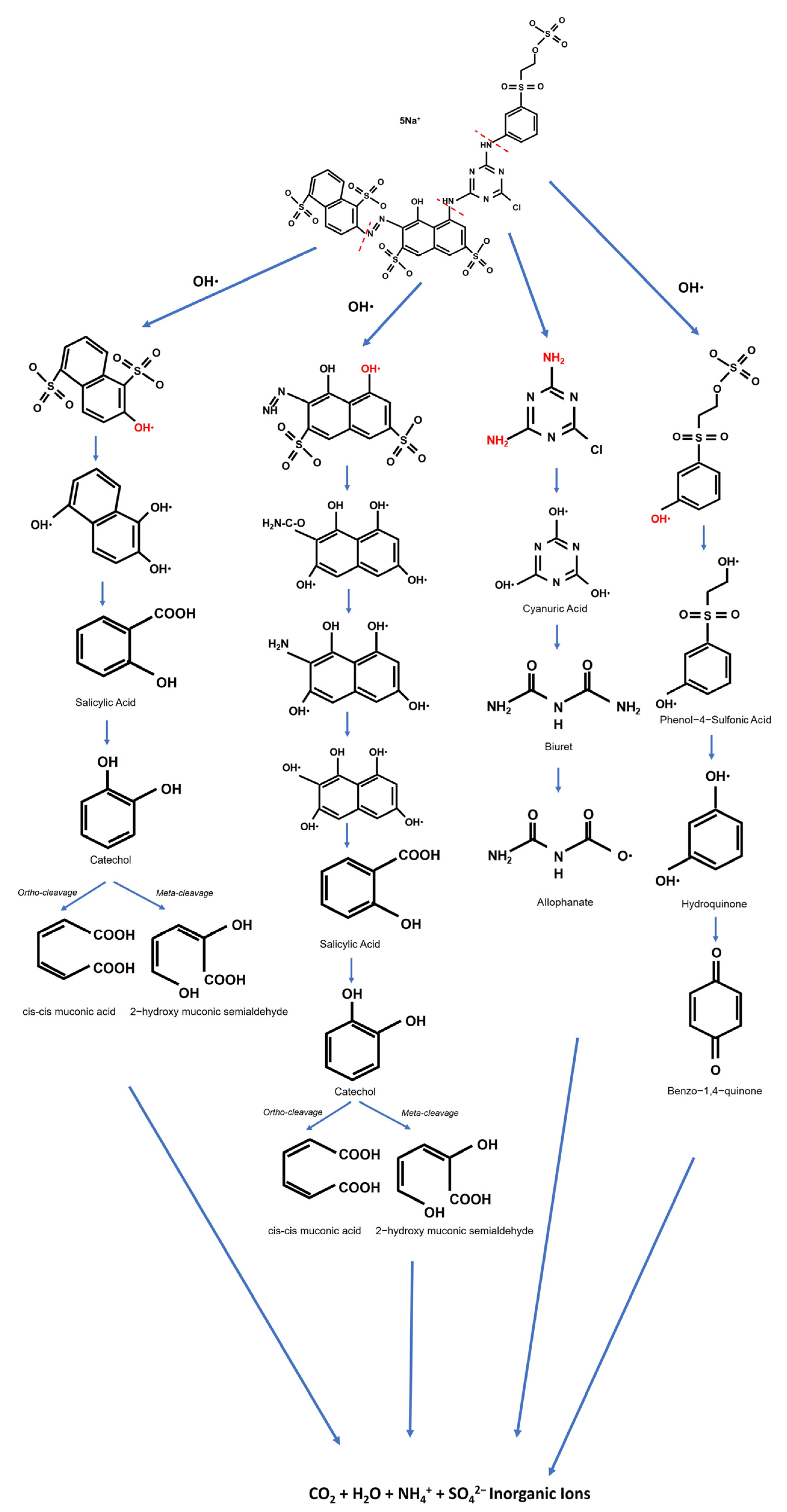
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ismail, G.A.; Sakai, H. Review on effect of different type of dyes on advanced oxidation processes (AOPs) for textile color removal. Chemosphere 2022, 291, 132906. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Kumar, A. Advanced Oxidation Process: A remediation technique for organic and non-biodegradable pollutant. Res. Surf. Interfaces 2023, 11, 100122. [Google Scholar] [CrossRef]
- Pandis, P.K.; Kalogirou, C.; Kanellou, E.; Vaitsis, C.; Savvidou, M.G.; Sourkouni, G.; Zorpas, A.A.; Argirusis, C. Key Points of Advanced Oxidation Processes (AOPs) for Wastewater, Organic Pollutants and Pharmaceutical Waste Treatment: A Mini Review. Chem. Eng. 2022, 6, 8. [Google Scholar] [CrossRef]
- Wang, J.L.; Xu, L.J. Advanced oxidation processes for wastewater treatment: Formation of hydroxyl radical and application. Crit. Rev. Environ. Sci. Technol. 2012, 42, 251–325. [Google Scholar] [CrossRef]
- Guo, W.; Yang, Z.; Zhou, X.; Wu, Q. Degradation and mineralization of dyes with advanced oxidation processes (AOPs): A brief review. In Proceedings of the 2015 International Forum on Energy, Environment Science and Materials (IFEESM 2015), Shenzhen, China, 25–26 September 2015. [Google Scholar] [CrossRef]
- Sivaram, N.M.; Gopal, P.M.; Barik, D. Toxic waste from textile industries. In Woodhead Publishing Series in Energy, Energy from Toxic Organic Waste for Heat and Power Generation, 1st ed.; Barik, D., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; pp. 43–54. [Google Scholar] [CrossRef]
- Azanaw, A.; Birlie, B.; Teshome, B.; Jemberie, M. Textile effluent treatment methods and eco-friendly resolution of textile wastewater. Case Stud. Chem. Environ. Eng. 2022, 6, 100230. [Google Scholar] [CrossRef]
- Mchedlov-Petrossyan, N.O.; Kharchenko, A.Y.; Marfunin, M.O.; Klochaniuk, O.R. Nano-sized bubbles in solution of hydrophobic dyes and the properties of the water/air interface. J. Mol. Liq. 2019, 275, 384–393. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Q.; Ma, H.; Huang, P.; Li, J.; Kikuchi, T. Effect of micro-bubbles on coagulation flotation process of dyeing wastewater. Sep. Purif. Technol. 2010, 71, 337–346. [Google Scholar] [CrossRef]
- Agarwal, A.; Ng, W.J.; Liu, Y. Principle and applications of microbubble and nanobubble technology for water treatment. Chemosphere 2011, 84, 1175–1180. [Google Scholar] [CrossRef]
- Oshita, S.; Boerzhijin, S.; Kameya, H.; Yoshimura, M.; Sotome, I. Promotion Effects of Ultrafine Bubbles/Nanobubbles on Seed Germination. Nanomaterials 2023, 13, 1677. [Google Scholar] [CrossRef]
- Hassaan, M.A.; Nemr, A.E. Advanced Oxidation Processes for Textile Wastewater Treatment. Int. J. Photochem. Photobiol. 2017, 2, 85–93. [Google Scholar]
- Temesgen, T.; Han, M. Ultrafine bubbles as an augmenting agent for ozone-based advanced oxidation. Water Sci. Technol. 2021, 84, 3705–3715. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, A.J.; Apul, O.G.; Schneider, O.; Garcia-Segura, S.; Westerhoff, P. Nanobubble Technologies Offer Opportunities to Improve Water Treatment. Acc. Chem. Res. 2019, 52, 1196–1205. [Google Scholar] [CrossRef] [PubMed]
- Subhan, U.; Muthukannan, V.; Azhary, S.Y.; Mulhadi, M.F.; Rochima, E.; Panatarani, C.; Joni, I.M. Development and performance evaluation of air fine bubbles on water quality of thai catfish rearing. AIP Conf. Proc. 2018, 1927, 30043. [Google Scholar] [CrossRef]
- Han, Z.; Kurokawa, H.; Matsui, H.; He, C.; Wang, K.; Wei, Y.; Dodbiba, G.; Otsuki, A.; Fujita, T. Stability and Free Radical Production for CO2 and H2 in Air Nanobubbles in Ethanol Aqueous Solution. Nanomaterials 2022, 12, 237. [Google Scholar] [CrossRef]
- Takahashi, M.; Chiba, K.; Li, P. Free-Radical Generation from Collapsing Microbubbles in the Absence of a Dynamic Stimulus. J. Phys. Chem. B 2007, 111, 1343–1347. [Google Scholar] [CrossRef]
- Joni, I.M.; Azhary, S.Y.; Ridha, M.; Sulistio, E.; Lukito, H.P.; Wawan, H.; Mia, M.; Panatarani, C. Degradation of methylene blue using bubbles treatment. AIP Conf. Proc. 2020, 2219, 090004. [Google Scholar] [CrossRef]
- Bouaifi, M.; Hebrard, G.; Bastoul, D.; Roustan, M. A comparative study of gas hold-up, bubble size, interfacial area and mass transfer coefficients in stirred gas–liquid reactors and bubble columns. Chem. Eng. Process. Process Intensif. 2011, 40, 97–111. [Google Scholar] [CrossRef]
- Han, Z.; Chen, H.; He, C.; Dodbiba, G.; Otsuki, A.; Wei, Y.; Fujita, T. Nanobubble size distribution measurement by interactive force apparatus under an electric field. Sci. Rep. 2023, 13, 3663. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, E.; Herrmann-Heber, R.; Reinecke, S.F.; Hampel, U. Bubble generation by micro-orifices with application on activated sludge wastewater treatment. Chem. Eng. Process.–Process Intensif. 2019, 143, 107511. [Google Scholar] [CrossRef]
- Fraga, B.; Stoesser, T. Influence of bubble size, diffuser width, and flow rate on the integral behavior of bubble plumes. J. Geophys. Res. Ocean. 2016, 121, 3887–3904. [Google Scholar] [CrossRef]
- Turner, J.S. Turbulent entrainment: The development of the entrainment assumption, and its application to geophysical flows. J. Fluid Mech. 1986, 173, 431–471. [Google Scholar] [CrossRef]
- Eskanlou, A.; Hemmati, M.; Reza, M.; Abdollahy, M. Modeling the bubble loading based on force balance on the particles attached to the bubble. Coll. Surf. A. 2019, 582, 123892. [Google Scholar] [CrossRef]
- Bombardelli, F.A.; Buscaglia, G.C.; Rehmann, C.R.; Rincón, L.E.; García, M.H. Modeling and scaling of aeration bubble plumes: A two-phase flow analysis. J. Hydraul. Res. 2010, 45, 617–630. [Google Scholar] [CrossRef]
- Wüest, A.; Brooks, N.H.; Imboden, D.M. Bubble plume modeling for lake restoration. Water Resour. Res. 1992, 28, 3235–3250. [Google Scholar] [CrossRef]
- Hunter, R.J. Zeta Potential in Colloid Science: Principles and Applications; Academic Press: Cambridge, MA, USA, 1981; ISBN 978-0123619504. [Google Scholar]
- Chen, Y.; Pignatello, J.J. Role of Quinone Intermediates as Electron Shuttles in Hydroxyl Radical Formation during Reductive Transformations of Aromatic Hydroquinones by Iron(III) in Aquatic Solutions. Environ. Sci. Technol. 1997, 31, 2399–2406. [Google Scholar] [CrossRef]
- Ohgaki, S.; Matsumoto, S. Nanobubbles: Generation Using a Multiporous Membrane and Application to Environmental Remediation. J. Environ. Sci. Health Part A 2015, 50, 1–7. [Google Scholar]
- Jameson, G.J. A New Paradigm for Flotation of Sulphide Minerals. J. Phys. Conf. Ser. 2010, 285, 012001. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Y.; Chen, N.; Piao, H.; Sun, D.; Ratnaweera, H.; Maletskyi, Z.; Bi, X. Characterization of Oxidation-Reduction Potential Variations in Biological Wastewater Treatment Processes: A Study from Mechanism to Application. Processes 2022, 10, 2607. [Google Scholar] [CrossRef]
- Copeland, A.; Lytle, D.A. Measuring the oxidation–reduction potential of important oxidants in drinking water. J. AWWA 2014, 106, E10–E20. [Google Scholar] [CrossRef]
- James, C.; Copeland, R.; Lytle, D. Relationships between Oxidation-Reduction Potential, Oxidant, and pH in Drinking Water. In Proceedings of the AWWA Water Quality Technology Conference, San Antonio, TX, USA, 2004; Available online: https://api.semanticscholar.org/CorpusID:51135998 (accessed on 31 May 2024).
- Lyu, T.; Wu, S.; Mortimer, R.J.G.; Pan, G. Nanobubble Technology in Environmental Engineering: Revolutionization Potential and Challenges. Environ. Sci. Technol. 2019, 53, 7175–7176. [Google Scholar] [CrossRef]
- Fujita, T.; Kurokawa, H.; Han, Z.; Zhou, Y.; He, C.; Wei, Y.; Kurokawa, H.; Matsui, H.; Ponou, J.; Dodbiba, G. Free radical degradation in aqueous solution by blowing hydrogen and carbon dioxide nanobubbles. Sci. Rep. 2021, 11, 3068. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Oshita, S.; Kawabata, S.; Makino, Y.; Yoshimoto, T. Identification of ROS Produced by Nanobubbles and Their Positive and Negative Effects on Vegetable Seed Germination. Langmuir 2016, 32, 11295–11302. [Google Scholar] [CrossRef]
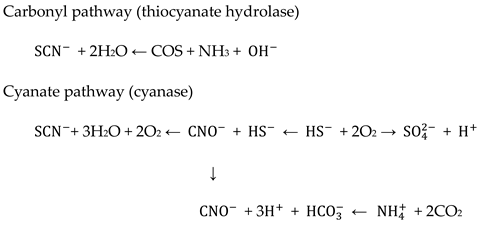
- Harris, R.E.; Bunch, A.W.; Knowles, C.J. Microbial cyanide and nitrile metabolism. Sci. Prog. 1987, 71, 293–304. [Google Scholar]
- Favvas, E.P.; Kyzas, G.Z.; Efthimiadou, E.K.; Mitropoulos, A.C. Bulk nanobubbles, generation methods and potential applications. Curr. Opin. Colloid Interface Sci. 2021, 54, 101455. [Google Scholar] [CrossRef]
- Papapetros, K.; Sygellou, L.; Anastasopoulos, C.; Andrikopoulos, K.S.; Bokias, G.; Voyiatzis, G.A. Spectroscopic Study of the Interaction of Reactive Dyes with Polymeric Cationic Modifiers of Cotton Fabrics. Appl. Sci. 2023, 13, 5530. [Google Scholar] [CrossRef]
- de-Berg, K.C. The Iron(III) Thiocyanate Reaction: Research History and Role in Chemical Analysis. In SpringerBriefs in Molecular Science, 1st ed.; Rasmussen, S.C., Ed.; Springer: Cham, Switzerland, 2019; pp. 19–24, 2191–5415. [Google Scholar] [CrossRef]
- Budaev, S.L.; Batoeva, A.A.; Tsybikova, B.A. Degradation of thiocyanate in aqueous solution by persulfate activated ferric ion. Miner. Eng. 2015, 81, 88–95. [Google Scholar] [CrossRef]
- Galan, J.; Trilleras, J.; Zapata, P.A.; Arana, V.A.; Grande-Tovar, C.D. Optimization of Chitosan Glutaraldehyde-Crosslinked Beads for Reactive Blue 4 Anionic Dye Removal Using a Surface Response Methodology. Life 2021, 11, 85. [Google Scholar] [CrossRef] [PubMed]
- León-Luque, A.J.; Barajas, C.L.; Peña-Guzmán, C.A. Determination of the Optimal Dosage of Aluminum Sulfate in the Coagulation-Flocculation Process Using an Artificial Neural Network. Int. J. Environ. Sci. Dev. 2016, 7, 346–350. [Google Scholar] [CrossRef]
- Cui, H.; Huang, X.; Yu, Z.; Chen, P.; Cao, X. Application progress of enhanced coagulation in water treatment. RSC Adv. 2020, 10, 20231–20244. [Google Scholar] [CrossRef]
- El-taweel, R.M.; Mohamed, N.; Alrefaey, K.A.; Husien, S.; Abdel-Aziz, A.B.; Salim, A.I.; Mostafa, N.G.; Said, L.A.; Fahim, I.S.; Radwan, A.G. A review of coagulation explaining its definition, mechanism, coagulant types, and optimization models; RSM, and ANN. Curr. Res. Green Sustain. Chem. 2023, 6, 100358. [Google Scholar] [CrossRef]
- Sheng, D.P.W.; Bilad, M.R.; Shamsuddin, N. Assessment and Optimization of Coagulation Process in Water Treatment Plant: A Review. ASEAN J. Sci. Eng. 2023, 3, 79–100. [Google Scholar] [CrossRef]
- Koul, B.; Bhat, N.; Abubakar, M.; Mishra, M.; Arukha, A.P.; Yadav, D. Application of Natural Coagulants in Water Treatment: A Sustainable Alternative to Chemicals. Water 2022, 14, 3751. [Google Scholar] [CrossRef]
- Bouznif, S.; Bali, M. Coupling of the coagulation/flocculation and the anodic oxidation processes for the treatment of textile wastewater. AQUA- Water Infrastruct. Ecosyst. Soc. 2021, 70, 587–599. [Google Scholar] [CrossRef]
- Kang, Y.G.; Yoon, H.; Lee, C.S.; Kim, E.J.; Chang, Y.S. Advanced oxidation and adsorptive bubble separation of dyes using MnO2-coated Fe3O4 nanocomposite. Water Res. 2019, 151, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, F.; Vissers, A.J.W.H. Experimental Investigation of Bubble Size in Flotation: Effect of Salt, Coagulant, Temperature, and Organic Compound. SPE Prod. Oper. 2020, 35, 384–392. [Google Scholar] [CrossRef]
- Sharma, M.; Jain, T.; Singh, S.; Pandey, O.P. Photocatalytic degradation of organic dyes under UV-Visible light using capped ZnS nanoparticles. Sol. Energy 2012, 86, 626–633. [Google Scholar] [CrossRef]
- Krumova, K.; Cosa, G. Singlet Oxygen: Applications in Biosciences and Nanosciences; Nonell, S., Flors, C., Nonell, S., Flors, C., Eds.; The Royal Society of Chemistry: London, UK, 2016; Chapter 1; pp. 1–21. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Eugenie, N.; Kamil, K.; Marian, V. Several lines of antioxidant defense against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef] [PubMed]
- Stratford, J.; Dias, A.E.X.O.; Knowles, C.J. The utilization of thiocyanate as a nitrogen source by a heterotrophic bacterium: The degradative pathway involves formation of ammonia and tetrathionate. Microbiology 1994, 140, 2657–2662. [Google Scholar] [CrossRef] [PubMed]
- Ebbs, S. Biological degradation of cyanide compounds. Curr. Opin. Biotechnol. 2004, 15, 231–236. [Google Scholar] [CrossRef]
- Sayan, L.B.; Agnes, A.B.; Belegma, A.T. Effect of Fenton-like reactions on the degradation of thiocyanate in water treatment. J. Environ. Chem. Eng. 2014, 2, 1907–1911. [Google Scholar] [CrossRef]
- Kremer, M.L. Mechanism of the Fenton reaction. Evidence for a new intermediate. Phys. Chem. Chem. Phys. 1999, 1, 3595–3605. [Google Scholar] [CrossRef]
- Kremer, M.L. Initial Steps in the Reaction of H2O2 with Fe2+ and Fe3+ Ions: Inconsistency in the Free Radical Theory. Reactions 2023, 4, 171–175. [Google Scholar] [CrossRef]



| Sample | Mean | ||||||
|---|---|---|---|---|---|---|---|
| pH | Oxidation–Reduction Potential (mV) | Total Dissolved Solid (mg/L) | Initial DO | Saturated Dissolved Oxygen | |||
| mg/L | % | mg/L | % | ||||
| 1 LPM | 6.64 ± 0.21 | −7.75 ± 7.08 | 13.5 ± 3.64 | 6.38 | 85.07 | 6.79 ± 0.51 | 90.53 |
| 2 LPM | 7.02 ± 0.16 | −18.6 ± 6.80 | 17.6 ± 3.85 | 6.48 | 86.40 | 8.35 ± 0.27 | 111.33 |
| 3 LPM | 5.64 ± 0.19 | −23.0 ± 6.16 | 14.8 ± 3.83 | 5.56 | 74.13 | 7.75 ± 0.22 | 103.33 |
| 4 LPM | 6.13 ± 0.47 | 40.6 ± 33.06 | 5.0 ± 1.58 | 5.69 | 75.87 | 9.64 ± 0.26 | 128.53 |
| 5 LPM | 6.08 ± 0.43 | 40.8 ± 18.29 | 2.0 ± 0.71 | 5.17 | 68.93 | 7.39 ± 0.18 | 98.53 |
| Coagulant Concentration (ppm) | Mean | |||
|---|---|---|---|---|
| Total Suspended Solid (mg/L) | Total Dissolved Solid (mg/L) | pH | Oxidation–Reduction Potential (mV) | |
| 0.00 | 0.00 ± 0.00 | 23.00 ± 0.00 | 6.83 ± 0.00 | 9.33 ± 0.58 |
| 0.25 | 42.33 ± 73.32 | 25.33 ± 4.04 | 6.80 ± 0.30 | 11.43 ± 17.14 |
| 0.50 | 77.67 ± 94.69 | 22.33 ± 9.61 | 6.50 ± 0.10 | 28.57 ± 5.71 |
| 0.75 | 62.33 ± 77.67 | 84.00 ± 4.00 | 5.90 ± 0.17 | 62.87 ± 9.87 |
| 1.00 | 84.00 ± 124.71 | 97.33 ± 20.23 | 6.33 ± 0.15 | 38.09 ± 8.73 |
| 1.25 | 101.33 ± 137.59 | 73.00 ± 7.81 | 6.10 ± 0.17 | 51.43 ± 9.90 |
| 1.50 | 139.67 ± 216.80 | 143.67 ± 32.35 | 5.93 ± 0.15 | 60.95 ± 8.73 |
| 1.75 | 43.67 ± 51.54 | 177.00 ± 5.57 | 6.03 ± 0.15 | 55.33 ± 8.76 |
| 2.00 | 31.67 ± 37.53 | 240.33 ± 50.72 | 5.90 ± 0.10 | 62.95 ± 5.58 |
| 2.25 | 26.33 ± 26.63 | 299.67 ± 33.84 | 5.70 ± 0.10 | 74.29 ± 5.71 |
| 2.50 | 18.33 ± 23.18 | 315.33 ± 22.48 | 5.80 ± 0.10 | 68.58 ± 5.71 |
| Coagulant Concentration (ppm) | Mean | |||
|---|---|---|---|---|
| Total Suspended Solid (mg/L) | Total Dissolved Solid (mg/L) | pH | Oxidation–Reduction Potential (mV) | |
| 0.00 | 24.6 ± 11.08 | 23.72 ± 10.93 | 7.37 ± 0.13 | −21.40 ± 7.29 |
| 0.25 | 60.07 ± 4.10 | 45.86 ± 179.85 | 3.61 ± 0.09 | 193.53 ± 5.08 |
| 0.50 | 145.4 ± 56.35 | 21.92 ± 51.95 | 3.43 ± 0.04 | 203.73 ± 2.28 |
| 1.00 | 63.80 ± 20.35 | 83.56 ± 404.34 | 3.47 ± 0.35 | 201.4 ± 19.97 |
| Sample | Mean | |||||
|---|---|---|---|---|---|---|
| Pt-Co | Total Suspended Solid (mg/L) | Total Dissolved Solid (mg/L) | Oxidation–Reduction Potential (mV) | Dissolved Oxygen (mg/L) | pH | |
| Dy/10 | 968 ± 46.58 | 16.4 ± 1.67 | 28.2 ± 0.45 | 12.6 ± 6.46 | 5.48 ± 0.38 | 6.53 ± 0.28 |
| Dy/18 | 950 ± 46.77 | 19.2 ± 3.11 | 28.2 ± 0.45 | 11.6 ± 6.50 | 5.69 ± 0.23 | 6.55 ± 0.16 |
| Dy/26 | 974 ± 33.43 | 18.2 ± 2.77 | 29.0 ± 0.00 | 14.8 ± 5.63 | 5.48 ± 0.56 | 6.48 ± 0.17 |
| Dy + Cg/10 | 1409 ± 163.37 | 43.0 ± 10.17 | 123.2 ± 4.65 | 140.0 ± 29.79 | 6.04 ± 0.30 | 5.02 ± 0.77 |
| Dy + Cg/18 | 1523 ± 142.94 | 50.8 ± 15.64 | 124.2 ± 4.05 | 152.4 ± 29.94 | 5.92 ± 0.06 | 4.82 ± 0.58 |
| Dy + Cg/26 | 1385 ± 167.44 | 38.6 ± 6.73 | 124.6 ± 3.97 | 170.2 ± 28.33 | 6.41 ± 0.21 | 4.55 ± 0.49 |
| Dy + UFBs/10 | 912 ± 184.04 | 25.0 ± 7.78 | 44.8 ± 9.60 | −20.4 ± 10.74 | 9.10 ± 0.35 | 6.84 ± 0.46 |
| Dy + UFBs/18 | 976 ± 147.49 | 22.8 ± 5.89 | 45.6 ± 10.50 | −23.6 ± 4.50 | 8.92 ± 0.29 | 6.90 ± 0.48 |
| Dy + UFBs/26 | 998 ± 178.10 | 26 ± 18.09 | 42.2 ± 9.44 | −20.2 ± 6.42 | 9.05 ± 0.38 | 6.88 ± 0.33 |
| Dy + UFBs + Cg/10 | 700 ± 134.95 | 123.4 ± 48.30 | 245 ± 7.14 | 202.4 ± 2.07 | 7.67 ± 0.17 | 6.58 ± 0.13 |
| Dy + UFBs + Cg/18 | 645 ± 156.84 | 145.6 ± 48.29 | 281 ± 46.81 | 204.8 ± 1.09 | 7.70 ± 0.16 | 6.59 ± 0.12 |
| Dy + UFBs + Cg/26 | 663 ± 158.85 | 167.2 ± 72.47 | 250.4 ± 17.24 | 204.0 ± 3.00 | 7.72 ± 0.14 | 6.65 ± 0.11 |
| Sample | Height (cm) | Compound | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O–H Carboxylic Acid | O=C=O Carbon Dioxide | S-C≡N Thiocyanate | N=C=S Iso Thiocyanate | C=C=C Allene | C=C Alkene | S=O Sulfoxide | C–Cl Halo Compound | N–H Aliphatic Amine | C-O Secondary Alcohol | C-I Halo Compound | ||
| Coagulant | √ | - | - | - | - | √ | - | - | - | √ | √ | |
| Dye Solution | 10 | √ | - | √ | √ | √ | √ | √ | √ | - | - | - |
| 18 | √ | - | √ | √ | √ | √ | √ | √ | - | - | - | |
| 26 | √ | - | √ | √ | √ | √ | √ | √ | - | - | - | |
| Dye + Coagulant | 10 | - | √ | √ | √ | - | √ | √ | √ | - | - | - |
| 18 | - | - | √ | √ | - | √ | √ | √ | - | - | - | |
| 26 | - | - | √ | √ | - | √ | √ | √ | - | - | - | |
| Dye + UFBs | 10 | - | - | √ | - | - | √ | √ | √ | √ | - | - |
| 18 | √ | - | √ | √ | - | √ | √ | √ | - | - | - | |
| 26 | - | - | - | - | - | √ | √ | √ | - | - | - | |
| Dye + UFBs + Coagulant | 10 | √ | - | √ | √ | - | √ | √ | √ | - | - | - |
| 18 | √ | - | √ | √ | - | √ | √ | √ | - | - | - | |
| 26 | - | - | √ | √ | - | √ | √ | - | √ | - | - | |
| Sample | Height (cm) | Elements | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na | Al | Fe | S | Cl | Ba | Si | Zr | Cu | Br | Sn | Rb | Sr | Pt | ||
| Coagulant | ND | 10.5 | 0.923 | 79.8 | ND | 2.47 | ND | ND | 0.103 | 0.008 | 0.076 | 0.0074 | 0.0389 | 0.0093 | |
| Dye Solution | 10 | ND | 2.64 | 0.513 | 0.847 | ND | ND | ND | 93.4 | ND | ND | ND | ND | ND | ND |
| 18 | ND | ND | 4.01 | ND | ND | ND | ND | 94.1 | ND | ND | ND | ND | ND | ND | |
| 26 | ND | ND | 4.66 | ND | ND | ND | ND | 95.2 | ND | ND | ND | ND | ND | ND | |
| Dye + Coagulant | 10 | ND | 89.4 | 0.048 | 4.91 | ND | ND | 5.52 | 0.123 | ND | ND | ND | ND | ND | ND |
| 18 | ND | 26.8 | 1.71 | 15.2 | ND | ND | 17 | 39.1 | ND | ND | ND | ND | ND | ND | |
| 26 | ND | 32.2 | 0.137 | 18.6 | 13.8 | ND | 29.4 | 32.2 | ND | ND | ND | ND | ND | ND | |
| Dye + UFBs | 10 | ND | 15.3 | 0.004 | 2.61 | 0.806 | ND | 7.53 | 0.067 | ND | ND | ND | ND | ND | ND |
| 18 | ND | 24.1 | 2.13 | 5.47 | ND | ND | 16.8 | 47.2 | ND | ND | ND | ND | ND | ND | |
| 26 | ND | 25.8 | 0.507 | 6.66 | 3.62 | ND | 25 | 38.4 | ND | ND | ND | ND | ND | ND | |
| Dye + UFBs + Coagulant | 10 | ND | 6.7 | 79.0 | 13.5 | ND | 0.375 | ND | ND | ND | ND | ND | ND | ND | ND |
| 18 | ND | 7.51 | 79.4 | 13.1 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| 26 | ND | 10.4 | 68.2 | 21.4 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sofia, D.R.; Hanam, E.S.; Sunardi, S.; Sumiarsa, D.; Joni, I.M. Hydroxyl Radical-Based Advanced Oxidation Processes of Red Reactive Dyes by Ultrafine Bubbles Method. Water 2024, 16, 1678. https://doi.org/10.3390/w16121678
Sofia DR, Hanam ES, Sunardi S, Sumiarsa D, Joni IM. Hydroxyl Radical-Based Advanced Oxidation Processes of Red Reactive Dyes by Ultrafine Bubbles Method. Water. 2024; 16(12):1678. https://doi.org/10.3390/w16121678
Chicago/Turabian StyleSofia, Dedeh Rosmaniar, Eko Sulistio Hanam, Sunardi Sunardi, Dadan Sumiarsa, and I Made Joni. 2024. "Hydroxyl Radical-Based Advanced Oxidation Processes of Red Reactive Dyes by Ultrafine Bubbles Method" Water 16, no. 12: 1678. https://doi.org/10.3390/w16121678





