Gallium Isotope Effect of Ga-Si Complex Solutions in Water: Theoretical Study Based on Density Functional Theory
Abstract
:1. Introduction
2. Methods
3. Results
3.1. Structural Characteristics of Ga-Si Complexes after Optimization
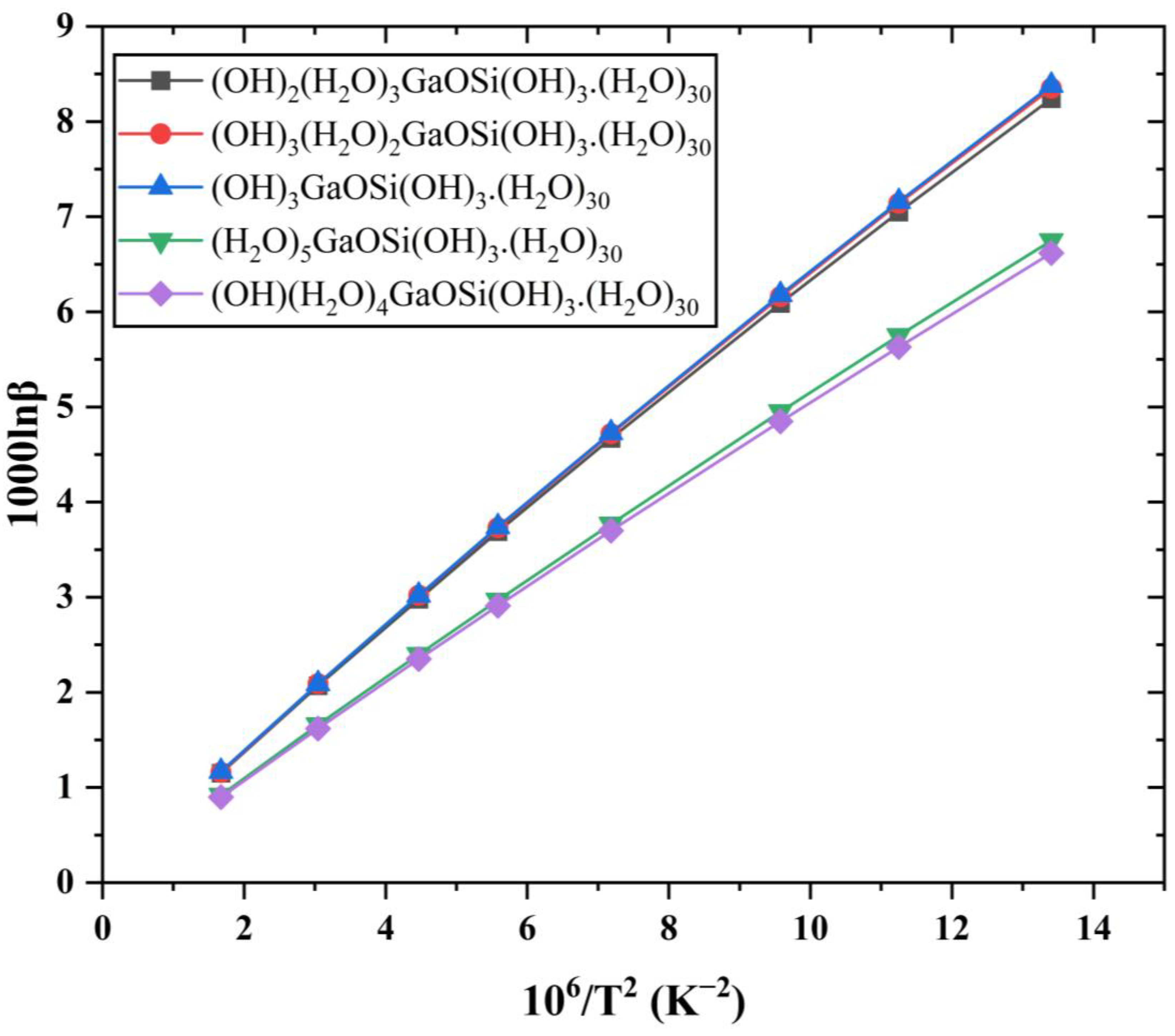
3.2. Ga Isotope Fractionation Parameters (1000lnβ) of Different Ga-Si Complex Aqueous Solutions
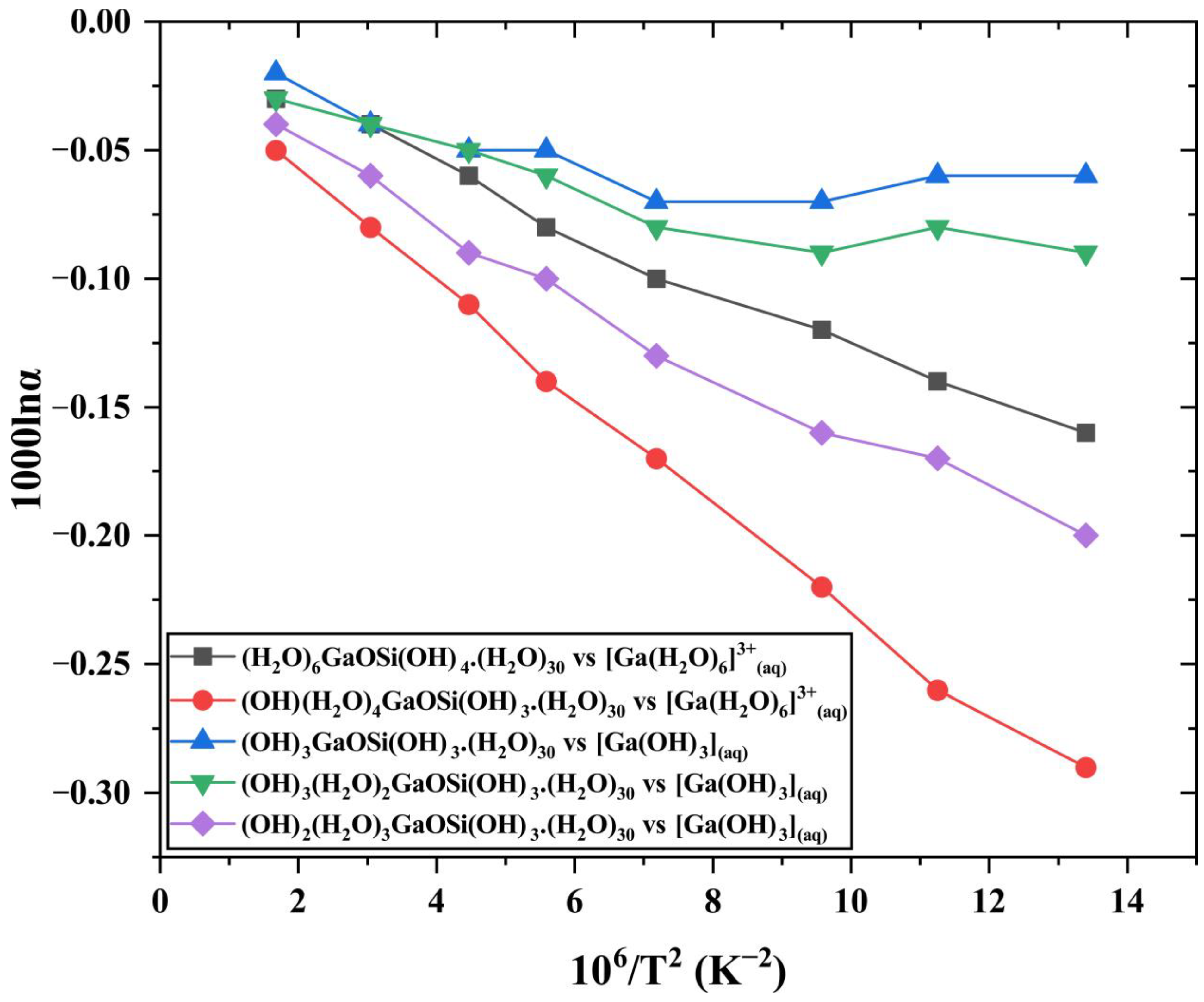
3.3. Ga Isotope Fractionation Factors (1000lnα) between Different Ga-Si Complex Aqueous Solutions and Ga3+-Bearing Aqua-Complex/Hydroxide Solutions
4. Discussion
4.1. Influence of Ga-Si Complex Structures on the Ga Isotope Fractionation Effect
4.2. The 1000lnβs of Different Ga-Si Complex Aqueous Solutions
4.3. Equilibrium Ga Stable Isotope Fractionation Factors of Ga-Si Complexes in Natural Waters (1000lnα, ‰)
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yuan, W.; Chen, J.; Teng, H.; Chetelat, B.; Cai, H.; Liu, J.; Wang, Z.; Bouchez, J.; Moynier, F.; Gaillardet, J.; et al. A Review on the Elemental and Isotopic Geochemistry of Gallium. Glob. Biogeochem. Cycles 2021, 35, e2021GB007033. [Google Scholar] [CrossRef]
- de Laeter, J.R.; Rosman, K.J.R. The atomic weight of gallium. Int. J. Mass Spectrom. Ion Phys. 1976, 21, 403–409. [Google Scholar] [CrossRef]
- Marinenko, G. On the atomic weight of gallium. J. Res. Natl. Bur. Stand. Sect. A Phys. Chem. 1977, 81, 409–420. [Google Scholar] [CrossRef]
- De Laeter, J.R. The isotopic composition and elemental abundance of gallium in meteorites and in terrestrial samples. Geochim. Cosmochim. Acta 1972, 36, 735–743. [Google Scholar] [CrossRef]
- Machlan, L.A.; Gramlich, J.W.; Powell, L.J.; Lambert, G.M. Absolute Isotopic Abundance Ratio and Atomic Weight Of a Reference Sample of Gallium. J. Res. Natl. Bur. Stand. 1986, 91, 323–331. [Google Scholar] [CrossRef]
- Inghram, M.G.; Hess, D.C.; Brown, H.S.; Goldberg, E. On the Isotopic Composition of Meteoritic and Terrestrial Gallium. Phys. Rev. 1948, 74, 343–344. [Google Scholar] [CrossRef]
- Gramlich, J.W.; Machlan, L.A. Isotopic variations in commercial high-purity gallium. Anal. Chem. 1985, 57, 1788–1790. [Google Scholar] [CrossRef]
- Machlan, L.A.; Gramlich, J.W. Isotopic fractionation of gallium on an ion-exchange column. Anal. Chem. 1988, 60, 37–39. [Google Scholar] [CrossRef]
- Kato, C.; Moynier, F.; Foriel, J.; Teng, F.-Z.; Puchtel, I.S. The gallium isotopic composition of the bulk silicate Earth. Chem. Geol. 2017, 448, 164–172. [Google Scholar] [CrossRef]
- Kato, C.; Moynier, F. Gallium isotopic evidence for extensive volatile loss from the Moon during its formation. Sci. Adv. 2017, 3, e1700571. [Google Scholar] [CrossRef]
- Kato, C.; Moynier, F. Gallium isotopic evidence for the fate of moderately volatile elements in planetary bodies and refractory inclusions. Earth Planet. Sci. Lett. 2017, 479, 330–339. [Google Scholar] [CrossRef]
- Wen, J.; Zhang, Y.; Wen, H.; Ling, K.; Zhu, C.; Fan, H.; Shen, N. Gallium isotope fractionation in the Xiaoshanba bauxite deposit, central Guizhou Province, southwestern China. Ore Geol. Rev. 2021, 137, 104299. [Google Scholar] [CrossRef]
- Zhang, Y.; Liao, S.; Tao, C.; Wen, H.; Fan, H.; Wen, J.; Yang, W.; Li, W. Ga isotopic fractionation in sulfides from the Yuhuang and Duanqiao hydrothermal fields on the Southwest Indian Ridge. Geosci. Front. 2021, 12, 101137. [Google Scholar] [CrossRef]
- Yuan, W.; Saldi, G.D.; Chen, J.; Vetuschi Zuccolini, M.; Birck, J.-L.; Liu, Y.; Schott, J. Gallium isotope fractionation during Ga adsorption on calcite and goethite. Geochim. Cosmochim. Acta 2018, 223, 350–363. [Google Scholar] [CrossRef]
- Yuan, W.; Gong, Y.; Chen, J.; Wang, Z.; Huang, F.; Yang, X.; Chetelat, B.; Teng, H.; Schott, J. Gallium isotope constraints on the intense weathering of basalt. Geochim. Cosmochim. Acta 2022, 333, 22–38. [Google Scholar] [CrossRef]
- Yuan, W.; Wang, Z.; Saldi, G.D.; Cai, H.; Zheng, W.; Schott, J.; Chen, J. Gallium isotope fractionation during precipitation of α-GaOOH from aqueous solution. Chem. Geol. 2024, 646, 121923. [Google Scholar] [CrossRef]
- Pokrovski, G.S.; Borisova, A.Y.; Bychkov, A.Y. Speciation and Transport of Metals and Metalloids in Geological Vapors. Rev. Mineral. Geochem. 2013, 76, 165–218. [Google Scholar] [CrossRef]
- Breiter, K.; Gardenová, N.; Kanický, V.; Vaculovič, T. Gallium and germanium geochemistry during magmatic fractionation and post-magmatic alteration in different types of granitoids: A case study from the Bohemian Massif (Czech Republic). Geol. Carpathica 2013, 64, 171–180. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, L.; Yang, L.; Wang, Q.; Feng, L.-P.; Liu, Y.-S. High precision measurements of gallium isotopic compositions in geological materials by, M.C.-I.C.P.-M.S. J. Anal. At. Spectrom. 2016, 31, 1673–1679. [Google Scholar] [CrossRef]
- Yuan, W.; Chen, J.B.; Birck, J.L.; Yin, Z.Y.; Yuan, S.L.; Cai, H.M.; Wang, Z.W.; Huang, Q.; Wang, Z.H. Precise Analysis of Gallium Isotopic Composition by MC-ICP-MS. Anal. Chem. 2016, 88, 9606–9613. [Google Scholar] [CrossRef]
- El Korh, A.; Luais, B.; Boiron, M.-C.; Deloule, E.; Cividini, D. Investigation of Ge and Ga exchange behaviour and Ge isotopic fractionation during subduction zone metamorphism. Chem. Geol. 2017, 449, 165–181. [Google Scholar] [CrossRef]
- Feng, L.-P.; Zhou, L.; Liu, J.; Hu, Z.-C.; Liu, Y.-S. Determination of Gallium Isotopic Compositions in Reference Materials. Geostand. Geoanal. Res. 2019, 43, 701–714. [Google Scholar] [CrossRef]
- Wimpenny, J.; Marks, N.; Knight, K.; Borg, L.; Badro, J.; Ryerson, F. Constraining the behavior of gallium isotopes during evaporation at extreme temperatures. Geochim. Cosmochim. Acta 2020, 286, 54–71. [Google Scholar] [CrossRef]
- Whitmore, L.M.; Pasqualini, A.; Newton, R.; Shiller, A.M. Gallium: A New Tracer of Pacific Water in the Arctic Ocean. J. Geophys. Res. Ocean. 2020, 125, e2019JC015842. [Google Scholar] [CrossRef]
- Wimpenny, J.; Borg, L.; Sio, C.K.I. The gallium isotopic composition of the Moon. Earth Planet. Sci. Lett. 2022, 578, 117318. [Google Scholar] [CrossRef]
- Zhang, J. First-principles calculations of equilibrium Ga isotope fractionations between several important Ga-bearing minerals and aqueous solutions. Sci. Rep. 2023, 13, 6230. [Google Scholar] [CrossRef]
- Render, J.; Wimpenny, J.; Borg, L. Gallium isotopic constraints for the origin of the Earth-Moon system. Earth Planet. Sci. Lett. 2023, 611, 118146. [Google Scholar] [CrossRef]
- Poledniok, J. Speciation of scandium and gallium in soil. Chemosphere 2008, 73, 572–579. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Nuamah, D.O.B.; Kwaah Tandoh, K.; Brako, B. Geochemistry of Minor and Trace Elements in Soils of Akuse Area, Southeastern Ghana. Geosciences 2019, 9, 8–17. [Google Scholar] [CrossRef]
- Urey, H.C. The thermodynamic properties of isotopic substances. J. Chem. Soc. 1947, 562–581. [Google Scholar] [CrossRef]
- Bigeleisen, J.; Mayer, M.G. Calculation of Equilibrium Constants for Isotopic Exchange Reactions. J. Chem. Phys. 1947, 15, 261–267. [Google Scholar] [CrossRef]
- Schauble, E.A. Applying Stable Isotope Fractionation Theory to New Systems. Rev. Mineral. Geochem. 2004, 55, 65–111. [Google Scholar] [CrossRef]
- Seo, J.H.; Lee, S.K.; Lee, I. Quantum chemical calculations of equilibrium copper (I) isotope fractionations in ore-forming fluids. Chem. Geol. 2007, 243, 225–237. [Google Scholar] [CrossRef]
- Sherman, D.M. Complexation of Cu+ in Hydrothermal NaCl Brines: Ab initio molecular dynamics and energetics. Geochim. Cosmochim. Acta 2007, 71, 714–722. [Google Scholar] [CrossRef]
- Li, X.; Zhao, H.; Tang, M.; Liu, Y. Theoretical prediction for several important equilibrium Ge isotope fractionation factors and geological implications. Earth Planet. Sci. Lett. 2009, 287, 1–11. [Google Scholar] [CrossRef]
- Liu, Q.; Tossell, J.A.; Liu, Y. On the proper use of the Bigeleisen–Mayer equation and corrections to it in the calculation of isotopic fractionation equilibrium constants. Geochim. Cosmochim. Acta 2010, 74, 6965–6983. [Google Scholar] [CrossRef]
- Gao, C.; Liu, Y. First-principles calculations of equilibrium bromine isotope fractionations. Geochim. Cosmochim. Acta 2021, 297, 65–81. [Google Scholar] [CrossRef]
- Pokrovski, G.S.; Schott, J.; Hazemann, J.-L.; Farges, F.; Pokrovsky, O.S. An X-ray absorption fine structure and nuclear magnetic resonance spectroscopy study of gallium–silica complexes in aqueous solution. Geochim. Cosmochim. Acta 2002, 66, 4203–4222. [Google Scholar] [CrossRef]
- Zhang, J. Equilibrium sulfur isotope fractionations of several important sulfides. Geochem. J. 2021, 55, 135–147. [Google Scholar] [CrossRef]
- Richet, P.; Bottinga, Y.; Javoy, M. A Review of Hydrogen, Carbon, Nitrogen, Oxygen, Sulphur, and Chlorine Stable Isotope Fractionation Among Gaseous Molecules. Annu. Rev. Earth Planet. Sci. 1977, 5, 65–110. [Google Scholar] [CrossRef]
- Sherman, D.M. Equilibrium isotopic fractionation of copper during oxidation/reduction, aqueous complexation and ore-forming processes: Predictions from hybrid density functional theory. Geochim. Cosmochim. Acta 2013, 118, 85–97. [Google Scholar] [CrossRef]
- Fujii, T.; Moynier, F.; Blichert-Toft, J.; Albarède, F. Density functional theory estimation of isotope fractionation of Fe, Ni, Cu, and Zn among species relevant to geochemical and biological environments. Geochim. Cosmochim. Acta 2014, 140, 553–576. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Petersson, G.; Nakatsuji, H. Gaussian 16 Revision B. 01, 2009; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B Condens. Matter 1988, 37, 785–789. [Google Scholar] [CrossRef]
- de Bruin, T.J.M.; Marcelis, A.T.M.; Zuilhof, H.; Sudhölter, E.J.R. Geometry and electronic structure of bis-(glycinato)-CuII·2H2O complexes as studied by density functional B3LYP computations. Phys. Chem. Chem. Phys. 1999, 1, 4157–4163. [Google Scholar] [CrossRef]
- Gao, C.; Cao, X.; Liu, Q.; Yang, Y.; Zhang, S.; He, Y.; Tang, M.; Liu, Y. Theoretical calculation of equilibrium Mg isotope fractionations between minerals and aqueous solutions. Chem. Geol. 2018, 488, 62–75. [Google Scholar] [CrossRef]
- Liu, Y.; Tossell, J.A. Ab initio molecular orbital calculations for boron isotope fractionations on boric acids and borates. Geochim. Cosmochim. Acta 2005, 69, 3995–4006. [Google Scholar] [CrossRef]
- Pokrovsky, O.S.; Pokrovski, G.S.; Schott, J. Gallium(III) adsorption on carbonates and oxides: X-ray absorption fine structure spectroscopy study and surface complexation modeling. J. Colloid. Interface Sci. 2004, 279, 314–325. [Google Scholar] [CrossRef]
- Persson, P.; Zivkovic, K.; Sjoberg, S. Quantitative adsorption and local structures of gallium(III) at the water-alpha-FeOOH interface. Langmuir 2006, 22, 2096–2104. [Google Scholar] [CrossRef]
- Dooryhee, E.; Greaves, G.N.; Steel, A.T.; Townsend, R.P.; Carr, S.W.; Thomas, J.M.; Catlow, C.R.A. Structural studies of high-area zeolitic adsorbents and catalysts by a combination of high-resolution X-ray powder diffraction and X-ray absorption spectroscopy. Faraday Discuss. Chem. Soc. 1990, 89, 119. [Google Scholar] [CrossRef]
- Gibbs, G.V. Molecules as models for bonding in silicates. Am. Mineral. 1982, 67, 421–450. [Google Scholar]
- Benézéth, P.; Diakonov, I.I.; Pokrovski, G.S.; Dandurand, J.-L.; Schott, J.; Khodakovsky, I.L. Gallium speciation in aqueous solution. Experimental study and modelling: Part 2. Solubility of α-GaOOH in acidic solutions from 150 to 250 °C and hydrolysis constants of gallium (III) to 300 °C. Geochim. Cosmochim. Acta 1997, 61, 1345–1357. [Google Scholar] [CrossRef]

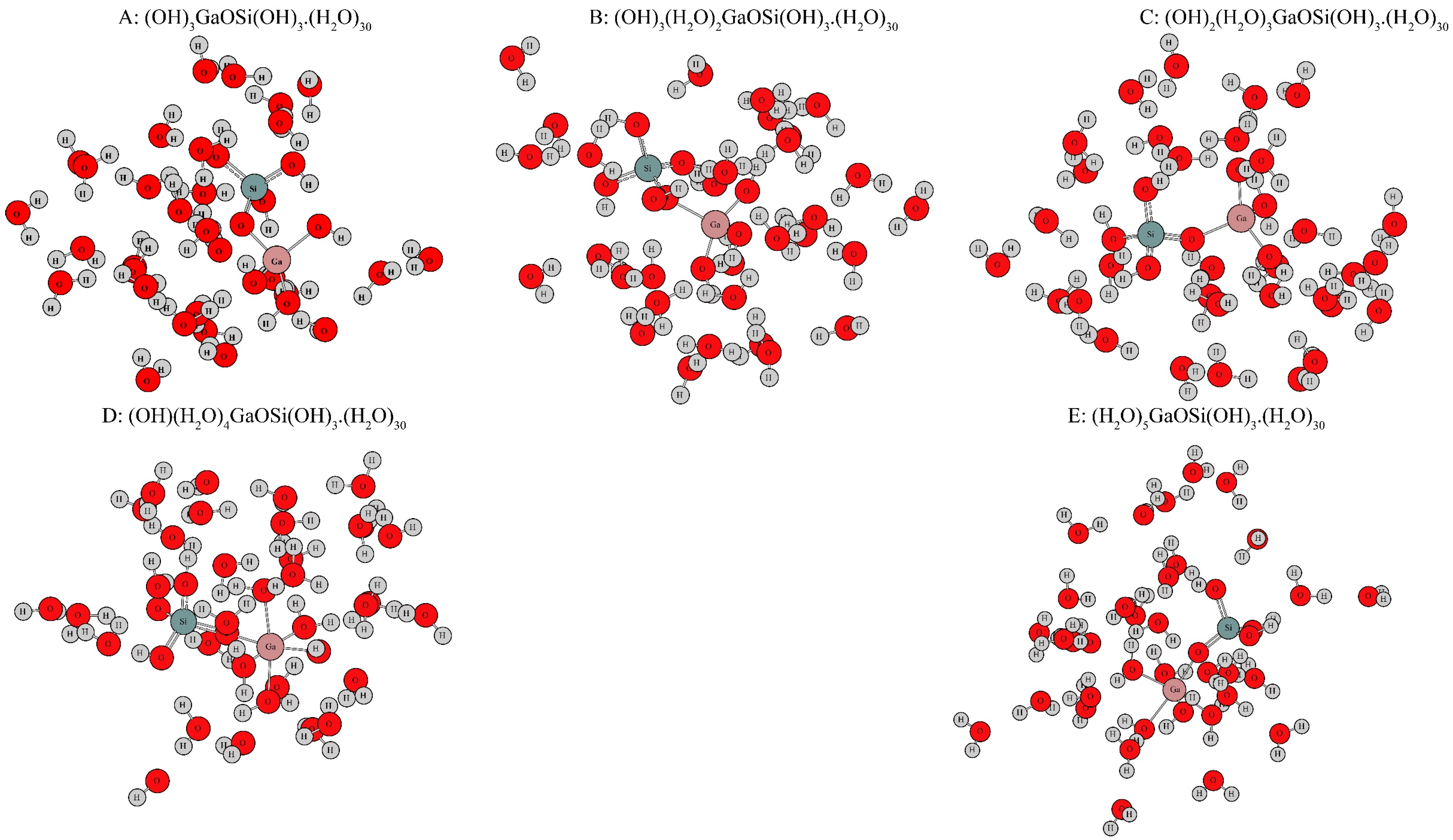

| Parameters | Bond Length (Å) | Bond Angle (°) | |||||
|---|---|---|---|---|---|---|---|
| Species | Ga-Si | Bridge O | Non-Bridge O | ∠Ga-O-Si | |||
| Ga-O | Si-O | Ga-OH | Ga-OH2 | ||||
| (OH)3GaOSi(OH)3 | 3.17 | 1.87 | 1.62 | 1.86 | 130.60 | ||
| (OH)3(H2O)2GaOSi(OH)3 | 3.15 | 1.86 | 1.60 | 1.87 | 130.43 | ||
| (OH)2(H2O)3GaOSi(OH)3 | 3.03 | 1.85 | 1.62 | 1.84 | 2.19 | 121.02 | |
| (OH)(H2O)4GaOSi(OH)3 | 3.17 | 1.85 | 1.61 | 1.81 | 2.13 | 132.49 | |
| (H2O)5GaOSi(OH)3 | 3.09 | 1.82 | 1.63 | 2.05 | 127.46 | ||
| Energy | E(RB3LYP) (Hartree) | |||||||
|---|---|---|---|---|---|---|---|---|
| Species | ||||||||
| n | 0 | 6 | 12 | 18 | 24 | 30 | ||
| (OH)2(H2O)3GaOSi(OH)3.(H2O)n | −2898.64 | A | −3357.50 | −3816.36 | −4275.22 | −4734.09 | −5192.95 | |
| B | −3357.50 | −3816.37 | −4275.23 | −4734.10 | −5192.96 | |||
| C | −3357.51 | −3816.37 | −4275.23 | −4734.08 | −5192.95 | |||
| D | −3357.39 | −3816.35 | −4275.19 | −4734.05 | −5192.95 | |||
| (OH)3(H2O)2GaOSi(OH)3.(H2O)n | −2898.13 | A | −3357.00 | −3815.86 | −4274.72 | −4733.59 | −5192.44 | |
| B | −3357.01 | −3815.87 | −4274.71 | −4733.57 | −5192.44 | |||
| C | −3357.00 | −3815.87 | −4274.73 | −4733.58 | −5192.47 | |||
| D | −3356.99 | −3815.86 | −4274.73 | −4733.60 | −5192.47 | |||
| (OH)3GaOSi(OH)3.(H2O)n | −2745.18 | A | −3204.05 | −3662.91 | −4121.77 | −4580.64 | −5039.49 | |
| B | −3204.04 | −3662.92 | −4121.77 | −4580.62 | −5039.48 | |||
| C | −3204.04 | −3662.90 | −4121.77 | −4580.64 | −5039.47 | |||
| D | −3204.05 | −3662.91 | −4121.77 | −4580.62 | −5039.51 | |||
| (H2O)5GaOSi(OH)3.(H2O)n | −2899.24 | A | −3358.19 | −3817.09 | −4275.96 | −4734.82 | −5193.70 | |
| B | −3358.19 | −3817.08 | −4275.94 | −4734.82 | −5193.67 | |||
| C | −3358.19 | −3817.08 | −4275.96 | −4734.82 | −5193.66 | |||
| D | −3358.18 | −3817.08 | −4275.95 | −4734.81 | −5193.67 | |||
| (OH)(H2O)4GaOSi(OH)3.(H2O)n | −2899.01 | A | −3357.90 | −3816.76 | −4275.63 | −4734.48 | −5193.34 | |
| B | −3357.89 | −3816.76 | −4275.62 | −4734.49 | −5193.35 | |||
| C | −3357.89 | −3816.75 | −4275.62 | −4734.49 | −5193.35 | |||
| D | −3357.90 | −3816.77 | −4275.62 | −4734.49 | −5193.37 | |||
| Temperature (°C) | 0 | 25 | 50 | 100 | 150 | 200 | 300 | 500 | |
|---|---|---|---|---|---|---|---|---|---|
| Species | |||||||||
| (OH)2(H2O)3GaOSi(OH)3 | 7.77 | 6.65 | 5.75 | 4.41 | 3.49 | 2.82 | 1.95 | 1.09 | |
| (OH)2(H2O)3GaOSi(OH)3.(H2O)6 | 7.76 | 6.63 | 5.73 | 4.39 | 3.47 | 2.80 | 1.94 | 1.08 | |
| (OH)2(H2O)3GaOSi(OH)3.(H2O)12 | 8.08 | 6.90 | 5.96 | 4.57 | 3.61 | 2.92 | 2.02 | 1.13 | |
| (OH)2(H2O)3GaOSi(OH)3.(H2O)18 | 8.11 | 6.93 | 5.99 | 4.59 | 3.62 | 2.93 | 2.03 | 1.13 | |
| (OH)2(H2O)3GaOSi(OH)3.(H2O)24 | 8.15 | 6.96 | 6.02 | 4.61 | 3.64 | 2.95 | 2.04 | 1.14 | |
| (OH)2(H2O)3GaOSi(OH)3.(H2O)30 | 8.24 | 7.05 | 6.09 | 4.67 | 3.69 | 2.98 | 2.07 | 1.15 | |
| (OH)3(H2O)2GaOSi(OH)3 | 8.43 | 7.20 | 6.22 | 4.77 | 3.76 | 3.04 | 2.11 | 1.17 | |
| (OH)3(H2O)2GaOSi(OH)3.(H2O)6 | 8.37 | 7.15 | 6.17 | 4.73 | 3.74 | 3.02 | 2.09 | 1.17 | |
| (OH)3(H2O)2GaOSi(OH)3.(H2O)12 | 8.32 | 7.11 | 6.14 | 4.71 | 3.72 | 3.01 | 2.08 | 1.16 | |
| (OH)3(H2O)2GaOSi(OH)3.(H2O)18 | 8.27 | 7.06 | 6.10 | 4.68 | 3.69 | 2.99 | 2.07 | 1.15 | |
| (OH)3(H2O)2GaOSi(OH)3.(H2O)24 | 8.33 | 7.12 | 6.14 | 4.71 | 3.72 | 3.01 | 2.08 | 1.16 | |
| (OH)3(H2O)2GaOSi(OH)3.(H2O)30 | 8.35 | 7.14 | 6.16 | 4.72 | 3.73 | 3.02 | 2.09 | 1.16 | |
| (OH)3GaOSi(OH)3 | 8.41 | 7.19 | 6.21 | 4.76 | 3.76 | 3.04 | 2.10 | 1.17 | |
| (OH)3GaOSi(OH)3.(H2O)6 | 8.39 | 7.17 | 6.20 | 4.75 | 3.75 | 3.03 | 2.10 | 1.17 | |
| (OH)3GaOSi(OH)3.(H2O)12 | 8.36 | 7.15 | 6.17 | 4.73 | 3.73 | 3.02 | 2.09 | 1.17 | |
| (OH)3GaOSi(OH)3.(H2O)18 | 8.35 | 7.13 | 6.16 | 4.72 | 3.73 | 3.01 | 2.08 | 1.16 | |
| (OH)3GaOSi(OH)3.(H2O)24 | 8.40 | 7.17 | 6.19 | 4.75 | 3.75 | 3.03 | 2.10 | 1.17 | |
| (OH)3GaOSi(OH)3.(H2O)30 | 8.38 | 7.16 | 6.18 | 4.73 | 3.74 | 3.02 | 2.09 | 1.17 | |
| (H2O)5GaOSi(OH)3 | 6.73 | 5.73 | 4.94 | 3.77 | 2.97 | 2.40 | 1.66 | 0.92 | |
| (H2O)5GaOSi(OH)3.(H2O)6 | 6.76 | 5.75 | 4.95 | 3.78 | 2.98 | 2.40 | 1.66 | 0.92 | |
| (H2O)5GaOSi(OH)3.(H2O)12 | 6.75 | 5.74 | 4.94 | 3.77 | 2.97 | 2.40 | 1.65 | 0.92 | |
| (H2O)5GaOSi(OH)3.(H2O)18 | 6.77 | 5.76 | 4.96 | 3.79 | 2.98 | 2.41 | 1.66 | 0.92 | |
| (H2O)5GaOSi(OH)3.(H2O)24 | 6.75 | 5.75 | 4.95 | 3.78 | 2.97 | 2.40 | 1.66 | 0.92 | |
| (H2O)5GaOSi(OH)3.(H2O)30 | 6.75 | 5.75 | 4.95 | 3.77 | 2.97 | 2.40 | 1.66 | 0.92 | |
| (OH)(H2O)4GaOSi(OH)3 | 7.01 | 5.99 | 5.17 | 3.97 | 3.13 | 2.54 | 1.75 | 0.98 | |
| (OH)(H2O)4GaOSi(OH)3.(H2O)6 | 6.76 | 5.76 | 4.97 | 3.80 | 3.00 | 2.42 | 1.67 | 0.93 | |
| (OH)(H2O)4GaOSi(OH)3.(H2O)12 | 6.62 | 5.64 | 4.86 | 3.71 | 2.92 | 2.36 | 1.63 | 0.91 | |
| (OH)(H2O)4GaOSi(OH)3.(H2O)18 | 6.55 | 5.58 | 4.81 | 3.67 | 2.89 | 2.33 | 1.61 | 0.90 | |
| (OH)(H2O)4GaOSi(OH)3.(H2O)24 | 6.58 | 5.60 | 4.82 | 3.68 | 2.90 | 2.34 | 1.62 | 0.90 | |
| (OH)(H2O)4GaOSi(OH)3.(H2O)30 | 6.62 | 5.63 | 4.85 | 3.70 | 2.91 | 2.35 | 1.62 | 0.90 | |
| Parameter | a | b | c | d | |
|---|---|---|---|---|---|
| Species | |||||
| (OH)2(H2O)3GaOSi(OH)3.(H2O)30 | 7.0429 × 10−5 | −7.1015 × 10−3 | 6.9692 × 10−1 | 6.2006 × 10−3 | |
| (OH)3(H2O)2GaOSi(OH)3.(H2O)30 | 1.1109 × 10−4 | −7.8732 × 10−3 | 7.0885 × 10−1 | −1.6438 × 10−3 | |
| (OH)3GaOSi(OH)3.(H2O)30 | 6.1993 × 10−5 | −6.5505 × 10−3 | 7.0076 × 10−1 | 1.5884 × 10−2 | |
| (H2O)5GaOSi(OH)3.(H2O)30 | 4.2861 × 10−5 | −4.3055 × 10−3 | 5.5300 × 10−1 | 1.0022 × 10−2 | |
| (OH)(H2O)4GaOSi(OH)3.(H2O)30 | 6.9455 × 10−5 | −4.8448 × 10−3 | 5.4640 × 10−1 | −7.5287 × 10−4 | |
| Temperature (°C) | 0 | 25 | 50 | 100 | 150 | 200 | 300 | 500 | |
|---|---|---|---|---|---|---|---|---|---|
| Species | |||||||||
| (H2O)5GaOSi(OH)3.(H2O)30 vs. [Ga(H2O)6]3+(aq) | −0.16 | −0.14 | −0.12 | −0.1 | −0.08 | −0.06 | −0.04 | −0.03 | |
| (OH)(H2O)4GaOSi(OH)3.(H2O)30 vs. [Ga(H2O)6]3+(aq) | −0.29 | −0.26 | −0.22 | −0.17 | −0.14 | −0.11 | −0.08 | −0.05 | |
| (OH)3GaOSi(OH)3.(H2O)30 vs. [Ga(OH)3](aq) | −0.06 | −0.06 | −0.07 | −0.07 | −0.05 | −0.05 | −0.04 | −0.02 | |
| (OH)3(H2O)2GaOSi(OH)3.(H2O)30 vs. [Ga(OH)3](aq) | −0.09 | −0.08 | −0.09 | −0.08 | −0.06 | −0.05 | −0.04 | −0.03 | |
| (OH)2(H2O)3GaOSi(OH)3.(H2O)30 vs. [Ga(OH)3](aq) | −0.2 | −0.17 | −0.16 | −0.13 | −0.1 | −0.09 | −0.06 | −0.04 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J. Gallium Isotope Effect of Ga-Si Complex Solutions in Water: Theoretical Study Based on Density Functional Theory. Water 2024, 16, 1680. https://doi.org/10.3390/w16121680
Zhang J. Gallium Isotope Effect of Ga-Si Complex Solutions in Water: Theoretical Study Based on Density Functional Theory. Water. 2024; 16(12):1680. https://doi.org/10.3390/w16121680
Chicago/Turabian StyleZhang, Jixi. 2024. "Gallium Isotope Effect of Ga-Si Complex Solutions in Water: Theoretical Study Based on Density Functional Theory" Water 16, no. 12: 1680. https://doi.org/10.3390/w16121680




