Carbon Reduction Associated with Sediment Reworking through Burrows of the Thalassinid Mud Shrimp Laomedia sp. (Crustacea: Laomediidae) from Korean Intertidal Sediments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Quantification of Reworked Sediments
2.3. Organic Carbon Concentration Analysis
2.4. Grain Size Distribution Analysis
2.5. Burrow Wall Characteristics Analysis
2.6. Statistical Analysis
3. Results
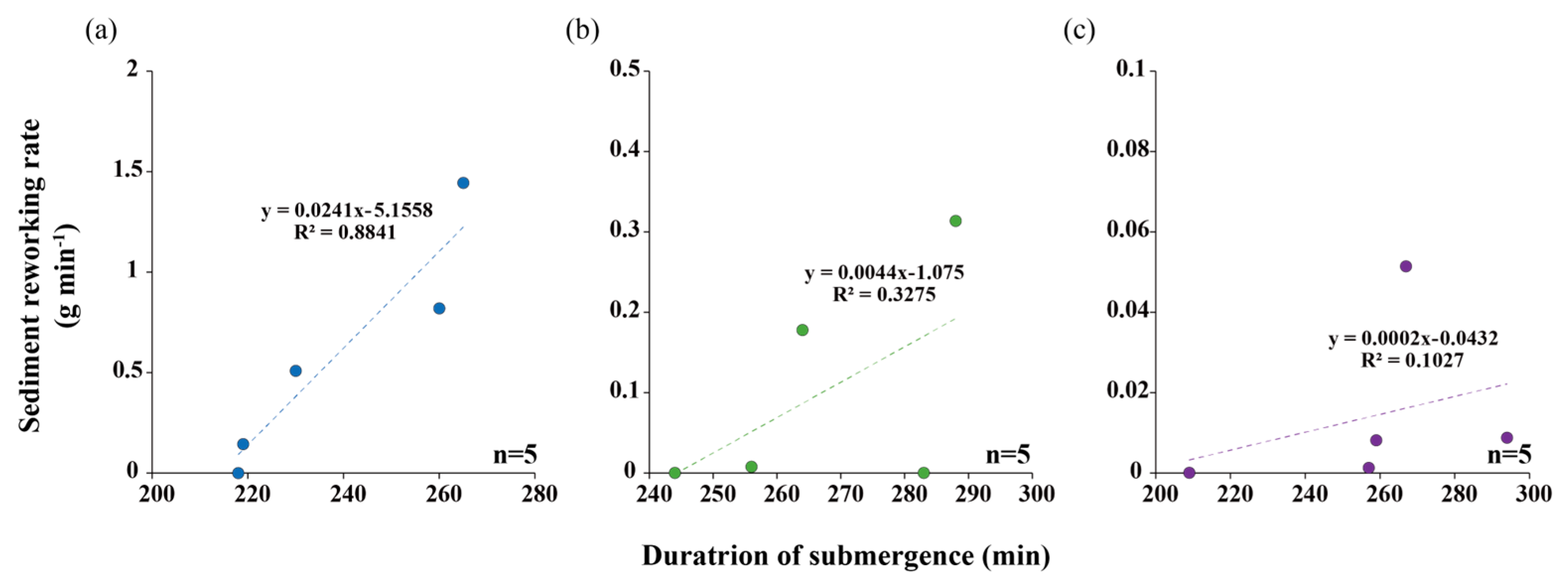
3.1. Biotic and Abiotic Factors
3.2. SRR
3.3. Grain Size Distribution
3.4. Organic Carbon Reduction
3.5. Burrow Wall Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ciutat, A.; Anschutz, P.; Gerino, M.; Boudou, A. Effects of bioturbation on cadmium transfer and distribution into freshwater sediments. Environ. Toxicol. Chem. 2005, 24, 1048–1058. [Google Scholar] [CrossRef]
- Posey, M.H.; Dumbauld, B.R.; Armstrong, D.A. Effects of a burrowing mud shrimp, Upogebia pugettensis (Dana), on abundances of macro-Infauna. J. Exp. Mar. Biol. Ecol. 1991, 148, 283–294. [Google Scholar] [CrossRef]
- Roberts, H.; Wiseman, H.W.J.; Suchanek, T.H. Lagoon sediment transport: The significant effect of Callianassa bioturbation. In Proceedings of the Fourth International Coral Reef Symposium, Manila, Philippines, 18–22 May 1981; pp. 459–465. [Google Scholar]
- Tudhope, A.; Scoffin, T.P. The effects of Callianassa bioturbation on the preservation of carbonate grains in Davies Reef Lagoon, Great Barrier Reef, Australia. J. Sed. Petr. 1984, 54, 1091–1096. [Google Scholar]
- de Vaugelas, J.; Buscail, R. Organic matter distribution in burrows of the thalassinid crustacean Callichirus laurae, Gulf of Aqaba (Red Sea). Hydrobiologia 1990, 207, 269–277. [Google Scholar] [CrossRef]
- Aller, J.Y.; Aller, R.C. Evidence for localized enhancement of biological activity associated with tube and burrow structures in deep-sea sediments at the HEBBLE site, western North Atlantic. Deep-Sea Res. 1986, 33, 755–790. [Google Scholar] [CrossRef]
- Kristensen, E. Benthic fauna and biogeochemical processes in marine sediments: Microbial activities and fluxes. In Nitrogen Cycling in Coastal Marine Environments; Blackburn, T.H., Sorensen, J., Eds.; John Wiley & Son: Chichester, UK, 1988; pp. 275–299. [Google Scholar]
- Furukawa, Y. Biogeochemical consequences of macrofauna burrow ventilation. Geochem. Trans. 2001, 2, 83–91. [Google Scholar] [CrossRef]
- Andreetta, A.; Fusi, M.; Cameldi, I.; Cimo, F.; Carnicelli, S.; Cannicci, S. Mangrove carbon sink. Do burrowing crabs contribute to sediment carbon storage? Evidence from a Kenyan mangrove system. J. Sea Res. 2014, 85, 524–533. [Google Scholar] [CrossRef]
- Atwood, T.B.; Connolly, R.M.; Ritchie, E.G.; Lovelock, C.E.; Heithaus, M.R.; Hays, G.C.; Fourqurean, J.W.; Macreadie, P.I. Predators help protect carbon stocks in blue carbon ecosystems. Nat. Clim. Change 2015, 5, 1038–1045. [Google Scholar] [CrossRef]
- Mehring, A.S.; Cook, P.L.M.; Evrard, V.; Grant, S.B.; Levin, L.A. Pollution-tolerant invertebrates enhance greenhouse gas flux in urban estuaries. Ecol. Appl. 2017, 27, 1852–1861. [Google Scholar] [CrossRef]
- Thomson, A.C.G.; Trevathan-Tackett, S.M.; Maher, D.T.; Ralph, P.J.; Macreadie, P.I. Bioturbator-stimulated loss of seagrass sediment carbon stocks. Limnol. Oceanogr. 2019, 64, 342–356. [Google Scholar] [CrossRef]
- Lee, S.Y. Ecological role of grapsid crabs in mangrove ecosystems: A review. Mar. Freshw. Res. 1998, 49, 335–343. [Google Scholar] [CrossRef]
- Kristensen, E.; Bouillon, S.; Dittmar, T.; Marchand, C. Organic carbon dynamics in mangrove ecosystems: A review. Aquat. Bot. 2008, 89, 201–219. [Google Scholar] [CrossRef]
- Kristensen, E.; Penha-Lopes, G.; Delefosse, M.; Valdemarsen, T.; Quintana, C.O.; Banta, G.T. What is bioturbation? The need for a precise definition for fauna in aquatic sciences. Mar. Ecol. Prog. Ser. 2012, 446, 285–302. [Google Scholar] [CrossRef]
- Kristensen, E. Impact of polychaetes (Nereis spp. and Arenicola marina) on carbon biogeochemistry in coastal marine sediments. Geochem. Trans. 2001, 2, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Papaspyrou, S.; Thessalou-Legaki, M.; Kristensen, E. Impact of Pestarella tyrrhenaon benthic metabolism in sediment microcosms enriched with seagrass and macroalgal detritus. Mar. Ecol. Prog. Ser. 2004, 281, 165–179. [Google Scholar] [CrossRef]
- Papaspyrou, S.; Gregersen, T.; Cox, R.P.; Thessalou-Legaki, M.; Kristensen, E. Sediment properties and bacterial community in burrows of the ghost shrimp Pestarella tyrrhena (Decapoda: Thalassinidea). Aquat. Microb. Ecol. 2005, 38, 181–190. [Google Scholar] [CrossRef]
- Papaspyrou, S. The influence of infaunal (Nereis diversicolor) abundance on degradation of organic matter in sandy sediments. J. Exp. Mar. Biol. Ecol. 2010, 393, 148–157. [Google Scholar] [CrossRef]
- Maher, D.; Eyre, B.D. Insights into estuarine benthic dissolved organic carbon (DOC) dynamics using δ13 C-DOC values, phospholipid fatty acids and dissolved organic nutrient fluxes. Geochim. Cosmochim. Acta 2011, 75, 1889–1902. [Google Scholar] [CrossRef]
- Aller, R.C. The importance of the diffusive permeability of animal burrow linings in determining marine sediment chemistry. J. Mar. Res. 1983, 41, 299–322. [Google Scholar] [CrossRef]
- Kristensen, E.; Jensen, M.H.; Andersen, T.K. The impact of polychaete (Nereis virens Sars) burrows on nitrification and nitrate reduction in estuarine sediments. J. Exp. Mar. Biol. Ecol. 1985, 85, 75–91. [Google Scholar] [CrossRef]
- Koo, B.J. Effects of Macrofaunal Bioturbation on Dynamics of Oxygen and Nutrients in Tidal Sediments of the West Coast of Korea. Ph.D. Thesis, Seoul National University, Seoul, Republic of Korea, 2009. [Google Scholar]
- Koo, B.J. Burrows of Macroinvertebrates in the Korean Tidal Flats; KIOST: Ansan, Republic of Korea, 2017; ISBN 978-89-444-9060-6. [Google Scholar]
- Seo, J.; Koo, B.K. The Sediment Reworking of the Mud Shrimp Laomedia sp. (Crustacea: Laomediidae) with Tidal Conditions in the Intertidal Sediments of Gomso Bay, Korea. J. Mar. Sci. Eng. 2021, 9, 1251. [Google Scholar] [CrossRef]
- Mermillod-Blondin, F.; François-Caracaillet, F.; Rosenberg, R. Biodiversity of benthic invertebrates and organic matter processing in shallow marine sediments: An experimental study. J. Exp. Mar. Biol. Ecol. 2005, 315, 187–209. [Google Scholar] [CrossRef]
- Maire, O.; Duchêne, J.C.; Grémare, A.; Malyuga, V.S.; Meysman, F.J.R. A comparison of sediment reworking rates by the surface depositfeeding bivalve Abra ovate during summertime and wintertime, with a comparison between two models of sediment reworking. J. Exp. Mar. Biol. Ecol. 2007, 343, 21–36. [Google Scholar] [CrossRef]
- Seo, J.; Koo, B.J. Spring-neap variation on sediment reworking with organic matter contents by a polychaete, Perinereis aibuhitensis, in the intertidal sediments of the Gomso Bay, Korea. Mar. Biol. 2019, 166, 124. [Google Scholar] [CrossRef]
- Folk, R.L. Petrology of Sedimentary Rocks; University of Texas: Hemphills, TX, USA, 1968. [Google Scholar]
- Folk, R.L.; Ward, W. Brazos River bar: A study in the significance of grain size parameters. J. Sediment. Pet. 1957, 27, 3–26. [Google Scholar] [CrossRef]
- Suchanek, T.H.; Colin, P.L.; McMurty, G.M.; Suchanek, C.S. Bioturbation and redistribution of sediment radionuclides in Enewetak Atoll lagoon by cailianassid shrimp: Biological aspects. Bull. Mar. Sci. 1986, 38, 144–152. [Google Scholar]
- Nickell, L.A.; Hughes, D.J.; Atkinson, R.J.A. Megafaunal bioturbation in organically enriched Scottish sea lochs. In Biology and Ecology of Shallow Coastal Waters: Proceedings of the 28th European Marine Biology Symposium, Institute of Marine Biology of Crete, Hersonissos, Greece, 23–28 September 1993; Olsen and Olsen: Fredensborg, Denmark, 1997; pp. 315–322. [Google Scholar]
- Berkenbusch, K.; Rowden, A. Factors influencing sediment turnover by the burrowing ghost shrimp Callianassa filholi (Decapoda: Thalassinidea). J. Exp. Mar. Biol. Ecol. 1999, 238, 283–292. [Google Scholar] [CrossRef]
- Rowden, A.; Jones, M.; Morris, A. The role of Callianassa subterranea (Montagu) (THALASSINIDEA) in sediment resuspension in the North Sea. Cont. Shelf Res. 1998, 18, 1365–1380. [Google Scholar] [CrossRef]
- Koo, B.J.; Seo, J.; Jang, M.S. The Relationship between Burrow Opening Dimensions and Biomass of Intertidal Macroinvertebrates by Feeding Mode (Surface Deposit Feeders vs. Suspension Feeders). Animals 2022, 12, 2878. [Google Scholar] [CrossRef]
- Kinoshita, K.; Wada, M.; Kogure, K.; Furota, T. Mud shrimp burrows as dynamic traps and processors of tidal-flat materials. Mar. Ecol. Prog. Ser. 2003, 247, 159–164. [Google Scholar] [CrossRef]
- Dobbs, F.C.; Guckert, J.B. Callianassa trilobata (Crustacea: Thalassinidea) influences abundance of meiofauna and biomass, composition, and physiologic state of microbial communities within its burrow. Mar. Ecol. Prog. Ser. 1988, 45, 69–79. [Google Scholar] [CrossRef]
- Over, D.J. Trace metals in burrow walls and sediments, Georgia Bight, USA. Ichnos 1990, 1, 31–41. [Google Scholar] [CrossRef]
- Stamhuis, E.; Schreurs, C.; Videler, J. Burrow architecture and turbative activity of the thalassinid shrimp Callianassa subterranean from the central North Sea. Mar. Ecol. Prog. Ser. 1997, 151, 155–163. [Google Scholar] [CrossRef]
- Nickell, L.A.; Atkinson, R.J.A.; Pinn, E.H. Morphology of thalassinidean (Crustacea: Decapoda) mouthparts and pereiopods in relation to feeding, ecology and grooming. J. Nat. Hist. 1998, 32, 733–761. [Google Scholar] [CrossRef]
- Bird, F.L.; Boon, P.I.; Nichols, P.D. Physicochemical and microbial properties of burrows of the deposit feeding thalassinidean ghost shrimp Biffarius arenosus (Decapoda:Callianassidae). Estuar. Coast. Shelf Sci. 2000, 51, 279–291. [Google Scholar] [CrossRef]
- Contessa, L.; Bird, F.L. The impact of bait-pumping on populations of the ghost shrimp Trypaea australiensis Dana (Decapoda: Callianassidae) and the sediment environment. J. Exp. Mar. Biol. Ecol. 2004, 304, 75–97. [Google Scholar] [CrossRef]
- Webb, A.P.; Eyre, B.D. Effect of natural populations of burrowing thalassinidean shrimp on sediment irrigation, benthic metabolism, nutrient fluxes and denitrification. Mar. Ecol. Prog. Ser. 2004, 268, 205–220. [Google Scholar] [CrossRef]
- Kinoshita, K. Burrow structure of the mud shrimp Upogebia major (Decapoda: Thalassinidea: Upogebiidae). J. Crust. Biol. 2002, 22, 474–480. [Google Scholar] [CrossRef]
- Kinoshita, K.; Wada, M.; Kogure, K.; Furota, T. Microbial activity and accumulation of organic matter in the burrow of the mud shrimp, Upogebia major (Crustacea: Thalassinidea). Mar. Biol. 2008, 153, 277–283. [Google Scholar] [CrossRef]
- Duarte, C.M.; Middelburg, J.J.; Caraco, N. Major role of marine vegetation on the oceanic carbon cycle. Biogeosciences 2005, 2, 1–8. [Google Scholar] [CrossRef]
- Alongi, D.M. Carbon cycling and storage in mangrove forests. Annu. Rev. Mar. Sci. 2014, 6, 195–219. [Google Scholar] [CrossRef]
- Fourqurean, J.W.; Duarte, C.M.; Kennedy, H.; Marbà, N.; Holmer, M.; Mateo, M.A.; Apostolaki, E.T.; Kendrick, G.A.; Krause-Jensen, D.; McGlathery, K.J.; et al. Seagrass ecosystems as a globally significant carbon stock. Nat. Geosci. 2012, 5, 505–509. [Google Scholar] [CrossRef]
- Lee, J.; Kim, B.; Noh, J.; Lee, C.; Kwon, I.; Kwon, B.; Ryu, J.; Park, J.; Hong, S.; Lee, S.; et al. The first national scale evaluation of organic carbon stocks and sequestration rates of coastal sediments along the West Sea, South Sea, and East Sea of South Korea. Sci. Total Environ. 2021, 793, 148568. [Google Scholar] [CrossRef]
- Fanjul, E.; Grela, M.A.; Iribarne, O. Effects of the dominant SW Atlantic intertidal burrowing crab Chasmagnathus granulatus on sediment chemistry and nutrient distribution. Mar. Ecol. Prog. Ser. 2007, 341, 177–190. [Google Scholar] [CrossRef]
- Fanjul, E.; Grela, M.A.; Canepuccia, A.; Iribarne, O. The Southwest Atlantic intertidal burrowing crab Neohelice granulata modifies nutrient loads of phreatic waters entering coastal area. Estuar. Coast. Shelf Sci. 2008, 79, 300–306. [Google Scholar] [CrossRef]
- Wang, J.Q.; Zhang, X.D.; Jiang, L.F.; Bertness, M.D.; Fang, C.M.; Chen, J.K.; Hara, T.; Li, B. Bioturbation of burrowing crabs promotes sediment turnover and carbon and nitrogen movements in an estuarine salt march. Ecosystems 2010, 13, 586–599. [Google Scholar] [CrossRef]
- Gutierrez, J.L.; Jones, C.G.; Groffman, P.M.; Findlay, S.E.G.; Iribarne, O.O.; Ribeiro, P.D.; Bruschetti, C.M. The contribution of crab burrow excavation to carbon availability in surficial saltmarsh sediments. Ecosystems 2006, 9, 647–658. [Google Scholar] [CrossRef]






| Mean Mound Diameter (mm) | Mean Mound Height (mm) | Elevation from MSL (m) | Mean Duration of Submergence (min) | Mean Duration of Emergence (min) | |
|---|---|---|---|---|---|
| E1 | 7.6 ± 2.1 a | 34.0 ± 10.7 a | 1.83 | 238 ± 23 a | 532 ± 23 a |
| E2 | 7.3 ± 1.2 a | 29.2 ± 3.6 a | 1.49 | 267 ± 18 ab | 503 ± 18 ab |
| E3 | 8.5 ± 1.9 a | 34.9 ± 8.3 a | 1.27 | 257 ± 31 b | 513 ± 31 b |
| Reworked Sediments over the Entire Sampling Period (g) | Overall Mean Reworked Sediments (g) | Overall Mean SRR (g min−1) | |||||
|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | 5th | |||
| E1 | <0.1 | 213.0 | 116.9 | 110.4 | 31.6 | 94.4 ± 83.2 a | 0.58 ± 0.58 a |
| E2 | <0.1 | <0.1 | <0.1 | 90.3 | 4.3 | 18.9 ± 40.1 ab | 0.10 ± 0.14 ab |
| E3 | 0.34 | 2.6 | 1.5 | 0.0001 | 3.3 | 1.5 ± 1.4 b | 0.01 ± 0.02 b |
| Composition (%) | Mean Grain Size (∅) | |||
|---|---|---|---|---|
| Sand | Silt | Clay | ||
| SP | 4.0 ± 1.6 | 67.9 ± 6.1 | 28.1 ± 6.6 | 7.0 ± 0.5 |
| RS | 32.9 ± 16.9 | 57.6 ± 14.8 | 9.4 ± 2.6 | 4.9 ± 0.6 |
| p value | <0.05 | >0.05 | <0.05 | <0.05 |
| Organic Carbon Concentration over the Entire Sampling Period (mg g−1) | Mean Organic Carbon Concentration (mg g−1) | |||||
|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | 5th | ||
| SP | 9.94 | 6.29 | 5.32 | 6.17 | 7.16 | 6.98 ± 1.78 |
| RS | 2.42 | 2.09 | 1.95 | 2.41 | 2.20 | 2.22 ± 0.20 |
| p value | <0.05 | |||||
| Composition (%) | Mean Grain Size (∅) | Mean Organic Carbon Concentration (mg g−1) | |||
|---|---|---|---|---|---|
| Sand | Silt | Clay | |||
| BS | 20.1 ± 4.4 | 72.7 ± 2.6 | 7.2 ± 2.5 | 4.6 ± 0.2 | 4.15 ± 0.93 |
| AS | 25.5 ± 0.7 | 69.2 ± 0.5 | 5.3 ± 0.8 | 4.4 ± 0.1 | 3.02 ± 0.91 |
| p value | <0.05 | <0.05 | >0.05 | <0.05 | >0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, J.; Koo, B.J. Carbon Reduction Associated with Sediment Reworking through Burrows of the Thalassinid Mud Shrimp Laomedia sp. (Crustacea: Laomediidae) from Korean Intertidal Sediments. Water 2024, 16, 1806. https://doi.org/10.3390/w16131806
Seo J, Koo BJ. Carbon Reduction Associated with Sediment Reworking through Burrows of the Thalassinid Mud Shrimp Laomedia sp. (Crustacea: Laomediidae) from Korean Intertidal Sediments. Water. 2024; 16(13):1806. https://doi.org/10.3390/w16131806
Chicago/Turabian StyleSeo, Jaehwan, and Bon Joo Koo. 2024. "Carbon Reduction Associated with Sediment Reworking through Burrows of the Thalassinid Mud Shrimp Laomedia sp. (Crustacea: Laomediidae) from Korean Intertidal Sediments" Water 16, no. 13: 1806. https://doi.org/10.3390/w16131806






