Eco-Engineering Improves Water Quality and Mediates Plankton–Nutrient Interactions in a Restored Wetland
Abstract
:1. Introduction
2. Materials and Methods
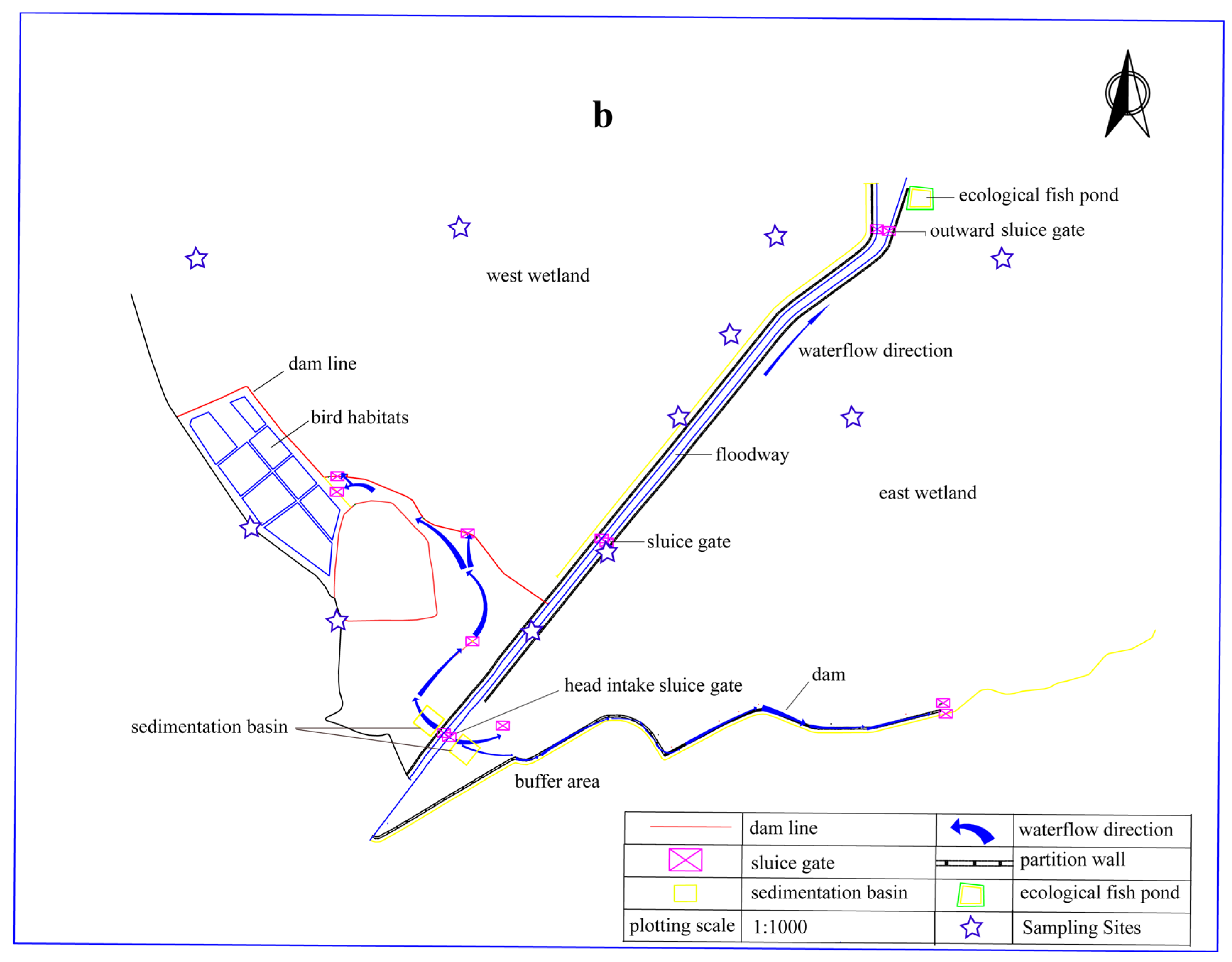
2.1. Study Area and Sampling Sites
2.2. Sample Collection and Analyses
2.2.1. Water Sample Collection and Testing
2.2.2. Trophic Level Index
- TLI(Σ ) means the trophic level index;
- Wj is the relevant weight of the nutritional status index of the jth parameter;
- TLI(j) is the nutritional status index representing the jth parameter.
2.2.3. Phytoplankton and Zooplankton Identification
2.2.4. Plankton Diversity
- ni means the number of the ith plankton;
- N means the total number of individual plankton identified in the sample;
- S means the total number of plankton species in the sample;
- fi means the frequency of the ith plankton;
- Pi means the percentage of the ith plankton individual number in the total individual number.
2.3. Statistical Analysis
3. Results
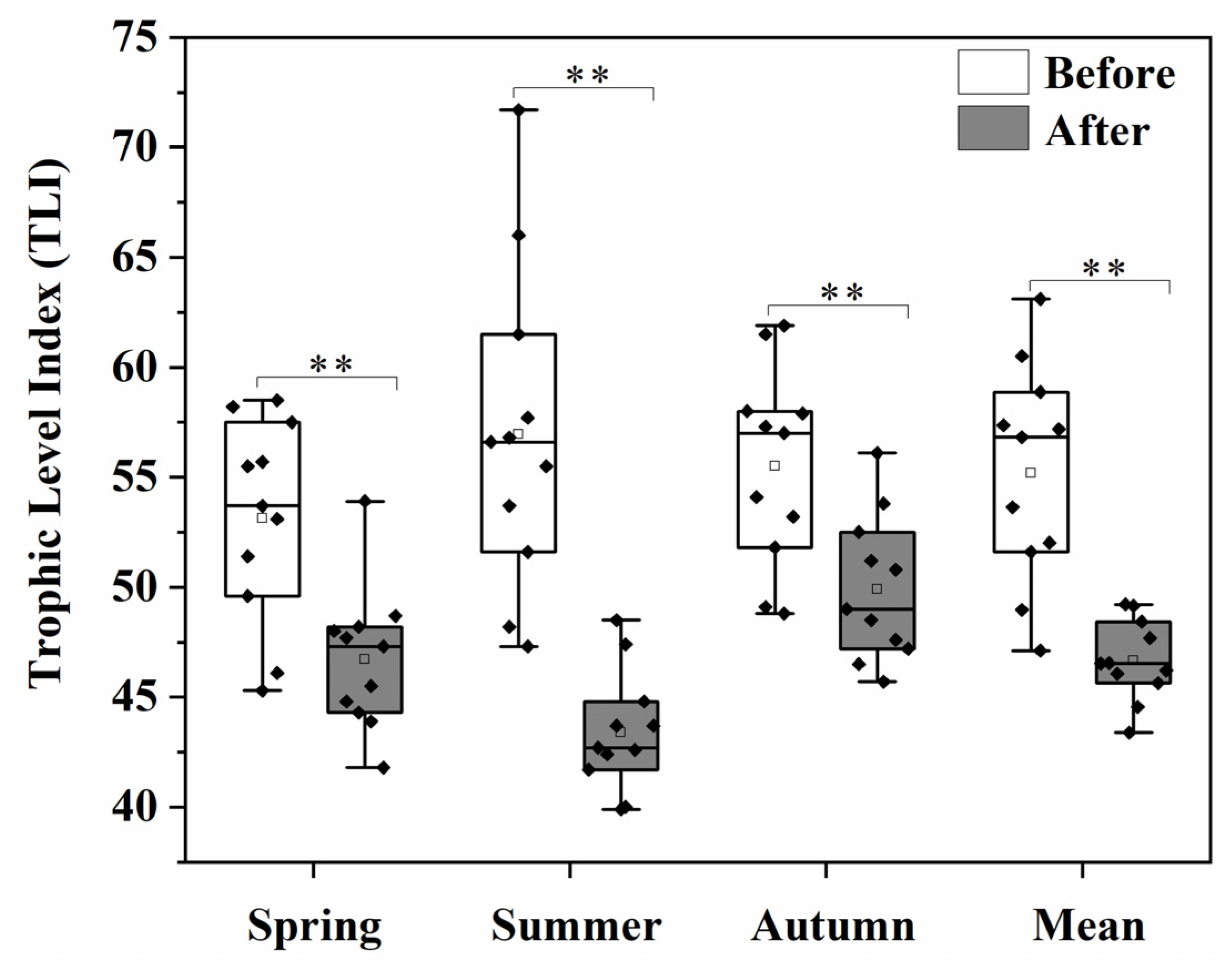
3.1. Environmental Variables and TLI
3.2. Changes in the Plankton Community
3.2.1. Species Composition, Abundance, and Biomass of Plankton
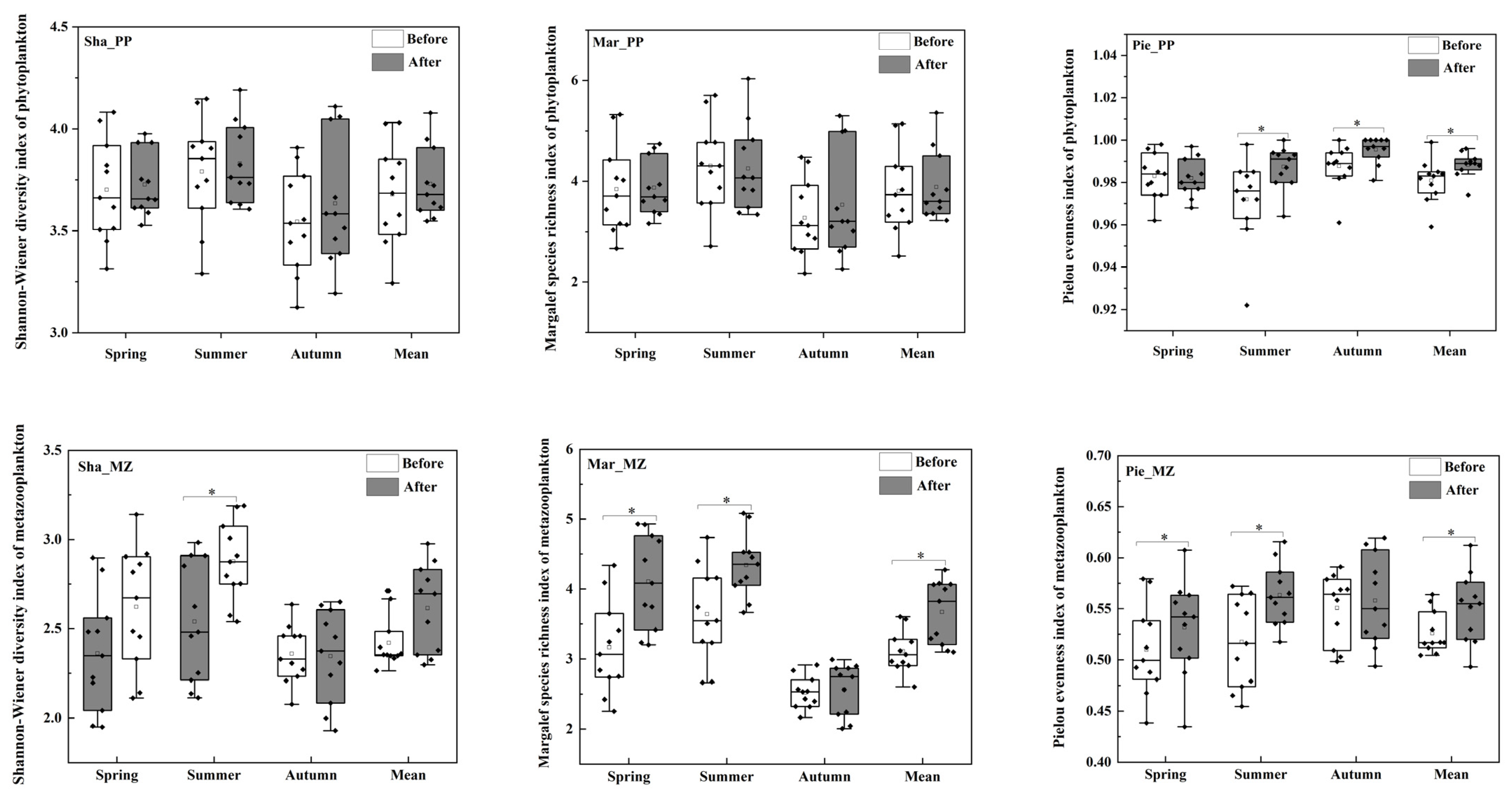
3.2.2. Plankton Diversity
3.2.3. Dominant Species
3.3. Relationships between Plankton Community and Environmental Factors
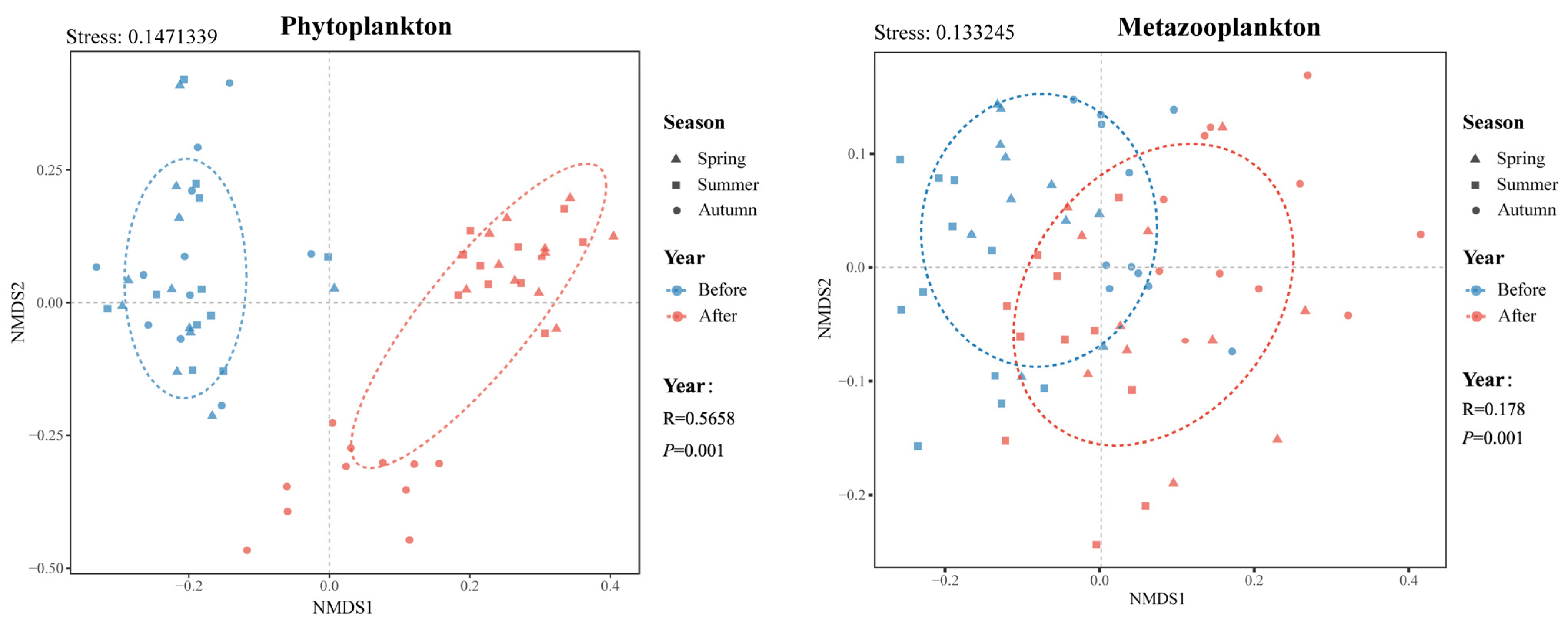
3.3.1. Non-Metric Multidimensional Scale Analysis (NMDS)
3.3.2. Structural Equation Modeling of Planktonic Biomass and Environmental Factors
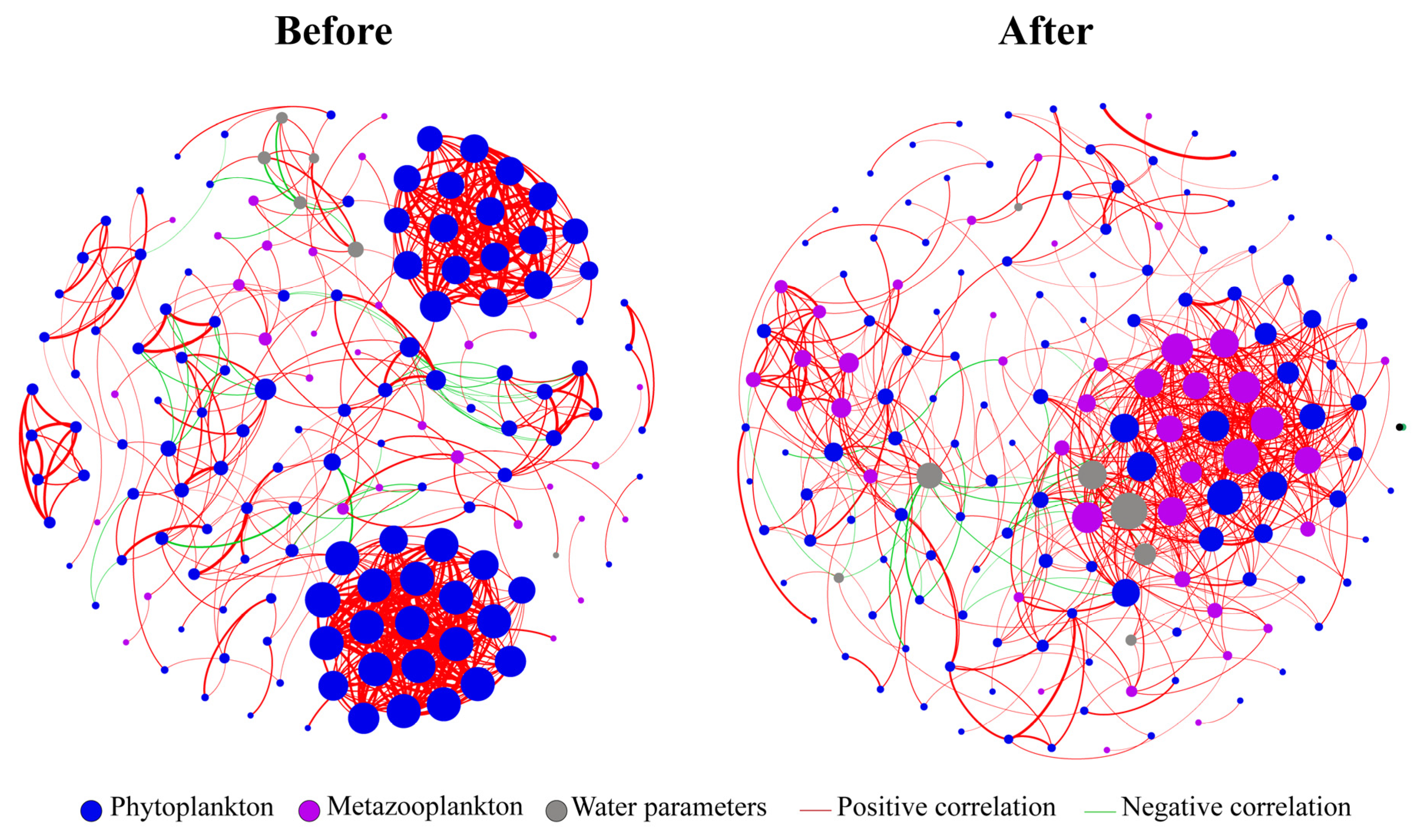
3.3.3. Ecological Network Analysis of the Plankton Community
4. Discussion
4.1. Effect of Eco-Engineering Implementation on Wetland Trophic Status
4.2. Pattern of Plankton Community Structure Variation before and after Eco-Engineering
4.3. Effect of Wetland Eco-Engineering on Planktonic Interactions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schuerch, M.; Spencer, T.; Temmerman, S.; Kirwan, M.L.; Wolff, C.; Lincke, D.; McOwen, C.J.; Pickering, M.D.; Reef, R.; Vafeidis, A.T.; et al. Future response of global coastal wetlands to sea-level rise. Nature 2018, 561, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Gopal, B. Should ‘wetlands’ cover all aquatic ecosystems and do macrophytes make a difference to their ecosystem services? Folia Geobot. 2016, 51, 209–226. [Google Scholar] [CrossRef]
- Fluet-Chouinard, E.; Stocker, B.D.; Zhang, Z.; Malhotra, A.; Melton, J.R.; Poulter, B.; Kaplan, J.O.; Goldewijk, K.K.; Siebert, S.; Minayeva, T.; et al. Extensive global wetland loss over the past three centuries. Nature 2023, 614, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xiong, Z.; Ouyang, L.; He, G.; Liu, W.; Cai, M. Macrohabitat and microhabitat mediate the relationships between wetland multifaceted biodiversity and multifunctionality. Catena 2024, 241, 108023. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Lu, X.G. Wetland function and research direction of wetland science. Wetl. Sci. 2003, 1, 7–11. (In Chinese) [Google Scholar] [CrossRef]
- Jiang, Y.-J.; He, W.; Liu, W.-X.; Qin, N.; Ouyang, H.-L.; Wang, Q.-M.; Kong, X.-Z.; He, Q.-S.; Yang, C.; Yang, B.; et al. The seasonal and spatial variations of phytoplankton community and their correlation with environmental factors in a large eutrophic Chinese lake (Lake Chaohu). Ecol. Indic. 2014, 40, 58–67. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, K.; Cheng, Y.; Li, J.; Lu, W. Investigation of Dominant Populations of Late-summer Phytoplankton and Comprehensive Nutritional Evaluation of Water Quality in Bailang Lake. Agric. Sci. Technol. 2013, 14, 453–457. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, Y.; Huang, Z.; Zheng, B.; Wen, Y.; Liu, G. Investigation of phytoplankton community structure and formation mechanism: A case study of Lake Longhu in Jinjiang. Front. Microbiol. 2023, 14, 1267299. [Google Scholar] [CrossRef]
- Xu, R.; Cai, Y.; Wang, X.; Li, C.; Liu, Q.; Yang, Z. Agricultural nitrogen flow in a reservoir watershed and its implications for water pollution mitigation. J. Clean. Prod. 2020, 267, 122034. [Google Scholar] [CrossRef]
- Beaver, J.R.; Miller-Lemke, A.M.; Acton, J.K. Midsummer zooplankton assemblages in four types of wetlands in the Upper Midwest, USA. Hydrobiologia 1998, 380, 209–220. [Google Scholar] [CrossRef]
- Nielsen, D.L.; Smith, D.; Petrie, R. Resting egg banks can facilitate recovery of zooplankton communities after extended exposure to saline conditions. Freshw. Biol. 2012, 57, 1306–1314. [Google Scholar] [CrossRef]
- Gerhard, M.; Koussoroplis, A.M.; Hillebrand, H.; Striebel, M. Phytoplankton community responses to temperature fluctuations under different nutrient concentrations and stoichiometry. Ecology 2019, 100, e02834. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, H.; Zhang, Y.; Li, Y.; Wei, J.; Pei, H. Variation of phytoplankton communities and their driving factors along a disturbed temperate river-to-sea ecosystem. Ecol. Indic. 2020, 118, 106776. [Google Scholar] [CrossRef]
- Tian, W.; Zhang, H.; Zhao, L.; Zhang, F.; Huang, H. Phytoplankton Diversity Effects on Community Biomass and Stability along Nutrient Gradients in a Eutrophic Lake. Int. J. Environ. Res. Public Health 2017, 14, 95. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Gao, H.; Zhang, J. Mycoloop: Modeling phytoplankton–chytrid–zooplankton interactions in aquatic food webs. Math. Biosci. 2024, 368, 109134. [Google Scholar] [CrossRef] [PubMed]
- Severiano, J.d.S.; Almeida-Melo, V.L.d.S.; Bittencourt-Oliveira, M.D.C.; Chia, M.A.; Moura, A.D.N. Effects of increased zooplankton biomass on phytoplankton and cyanotoxins: A tropical mesocosm study. Harmful Algae 2018, 71, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Gusha, M.N.; Dalu, T.; Wasserman, R.J.; McQuaid, C.D. Zooplankton grazing pressure is insufficient for primary producer control under elevated warming and nutrient levels. Sci. Total. Environ. 2019, 651, 410–418. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, Y.; Chen, Y.; Li, S.; Zhan, A. Interactome-based abiotic and biotic impacts on biodiversity of plankton communities in disturbed wetlands. Divers. Distrib. 2019, 25, 1416–1428. [Google Scholar] [CrossRef]
- Song, J.; Hou, C.; Liu, Q.; Wu, X.; Wang, Y.; Yi, Y. Spatial and temporal variations in the plankton community because of water and sediment regulation in the lower reaches of Yellow River. J. Clean. Prod. 2020, 261, 120972. [Google Scholar] [CrossRef]
- Li, C.; Feng, W.; Chen, H.; Li, X.; Song, F.; Guo, W.; Giesy, J.P.; Sun, F. Temporal variation in zooplankton and phytoplankton community species composition and the affecting factors in Lake Taihu—A large freshwater lake in China. Environ. Pollut. 2019, 245, 1050–1057. [Google Scholar] [CrossRef]
- GB3838-2002; Surface water environmental quality Standard. China Environmental Science Press: Beijing, China, 2002.
- Carlson, R.E. A trophic state index for lakes. Limnol. Oceanogr. 1977, 22, 361–369. [Google Scholar] [CrossRef]
- Aizaki, M. Application of Modified Carlson’s Trophic State Index to Japanese Lakes and Its Relationships to Other Parameters Related to Trophic State; National Institute of Environmental Study: Tsukuba, Japan, 1981; pp. 13–31. [Google Scholar]
- Wang, J.; Fu, Z.; Qiao, H.; Liu, F. Assessment of eutrophication and water quality in the estuarine area of Lake Wuli, Lake Taihu, China. Sci. Total. Environ. 2019, 650, 1392–1402. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.C.; Liu, X.Q.; Zhang, J.H. Evaluate method and classification standard on lake eutrophicatio. Environ. Monit. China 2002, 18, 47–49. [Google Scholar] [CrossRef]
- Shen, Y.F.; Gu, M.R.; Feng, W.S. Micro-biological monitoring of water pollution. Life Sci. 1997, 1997, 81–85. (In Chinese) [Google Scholar]
- Wang, J.J. Freshwater Rotifers of China; Science Press: Beijing, China, 1961; pp. 1–343. [Google Scholar]
- Jiang, X.Z.; Du, N.S. Zoography of China, Arthropods, Crustaceans, Freshwater Cladoceras; Science Press: Beijing, China, 1979. [Google Scholar]
- Zhou, F.X.; Chen, J.H. Freshwater Microbiomes and Benthos; Chemical Industry Press: Beijing, China, 2011. [Google Scholar]
- Shannon, E.; Weaver, W. The Mathematical Theory of Communication; University Illinois Press: London, UK, 1949; pp. 296–297. [Google Scholar]
- Margalef, R. Information theory in ecology. Gensyst 1958, 3, 36–71. [Google Scholar]
- Pielou, C. An Introduction to Mathematical Ecology; Wiley Interscience: New York, NY, USA, 1969. [Google Scholar]
- Lampitt, R.S.; Wishner, K.F.; Turley, C.M.; Angel, M.V. Marine snow studies in the Northeast Atlantic Ocean: Distribution, composition and role as a food source for migrating plankton. Mar. Biol. 1993, 116, 689–702. [Google Scholar] [CrossRef]
- Jiao, S.; Liu, Z.; Lin, Y.; Yang, J.; Chen, W.; Wei, G. Bacterial communities in oil contaminated soils: Biogeography and co-occurrence patterns. Soil Biol. Biochem. 2016, 98, 64–73. [Google Scholar] [CrossRef]
- Bastian, M.; Heymann, S.; Jacomy, M. Gephi: An open source software for exploring and manipulating networks. Icwsm 2009, 8, 361–362. [Google Scholar] [CrossRef]
- Albarico, F.P.J.B.; Lim, Y.C.; Chen, C.-W.; Chen, C.-F.; Wang, M.-H.; Dong, C.-D. Linking seasonal plankton succession and cellular trace metal dynamics in marine assemblages. Sci. Total. Environ. 2024, 907, 167805. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.; Wang, L.; Li, Y.; Yan, Z.; Liu, H.; Yu, D.; Liu, C. Response of sediment and water microbial communities to submerged vegetations restoration in a shallow eutrophic lake. Sci. Total. Environ. 2021, 801, 149701. [Google Scholar] [CrossRef]
- Chen, C.-C.; Meng, P.-J.; Hsieh, C.-H.; Jan, S. Plankton Community Respiration and Particulate Organic Carbon in the Kuroshio East of Taiwan. Plants 2022, 11, 2909. [Google Scholar] [CrossRef]
- Peng, Z.; Hu, W.; Zhang, Y.; Liu, G.; Zhang, H.; Gao, R. Modelling the effects of joint operations of water transfer project and lake sluice on circulation and water quality of a large shallow lake. J. Hydrol. 2020, 593, 125881. [Google Scholar] [CrossRef]
- Lucas, C.; Chalar, G.; Ibarguren, E.; Baeza, S.; De Giacomi, S.; Alvareda, E.; Brum, E.; Paradiso, M.; Mejía, P.; Crossa, M. Nutrient levels, trophic status and land-use influences on streams, rivers and lakes in a protected floodplain of Uruguay. Limnologica 2022, 94, 125966. [Google Scholar] [CrossRef]
- Li, N.; Tian, X.; Li, Y.; Fu, H.; Jia, X.; Jin, G.; Jiang, M. Seasonal and Spatial Variability of Water Quality and Nutrient Removal Efficiency of Restored Wetland: A Case Study in Fujin National Wetland Park, China. Chin. Geogr. Sci. 2018, 28, 1027–1037. [Google Scholar] [CrossRef]
- Hu, L.; Hu, W.; Zhai, S.; Wu, H. Effects on water quality following water transfer in Lake Taihu, China. Ecol. Eng. 2010, 36, 471–481. [Google Scholar] [CrossRef]
- Mai, S.; He, Y.; Li, W.; Zhao, T. Effects of environmental factors on vertical distribution of the eukaryotic plankton community in early summer in Danjiangkou Reservoir, China. Front. Ecol. Evol. 2023, 11, 1324932. [Google Scholar] [CrossRef]
- Lowery, C.M.; Bown, P.R.; Fraass, A.J.; Hull, P.M. Ecological Response of Plankton to Environmental Change: Thresholds for Extinction. Annu. Rev. Earth Planet. Sci. 2020, 48, 403–429. [Google Scholar] [CrossRef]
- Yang, C.; Nan, J.; Yu, H.; Li, J. Embedded reservoir and constructed wetland for drinking water source protection: Effects on nutrient removal and phytoplankton succession. J. Environ. Sci. 2020, 87, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Qin, B.; Paerl, H.W.; Brookes, J.D.; Hall, N.S.; Shi, K.; Zhou, Y.; Guo, J.; Li, Z.; Xu, H.; et al. The persistence of cyanobacterial (M icrocystis spp.) blooms throughout winter in Lake Taihu, China. Limnol. Oceanogr. 2016, 61, 711–722. [Google Scholar] [CrossRef]
- Xu, Y.; Li, A.J.; Qin, J.; Li, Q.; Ho, J.G.; Li, H. Seasonal patterns of water quality and phytoplankton dynamics in surface waters in Guangzhou and Foshan, China. Sci. Total. Environ. 2017, 590–591, 361–369. [Google Scholar] [CrossRef]
- Srichandan, S.; Baliarsingh, S.K.; Prakash, S.; Panigrahy, R.C.; Sahu, K.C. Zooplankton Research in Indian Seas: A Review. J. Ocean Univ. China 2018, 17, 1149–1158. [Google Scholar] [CrossRef]
- Ren, L.; Rabalais, N.N.; Turner, R.E. Effects of Mississippi River water on phytoplankton growth and composition in the upper Barataria estuary, Louisiana. Hydrobiologia 2020, 847, 1831–1850. [Google Scholar] [CrossRef]
- Pohnert, G.; Poulin, R.X.; Baumeister, T.U.H. The making of a plankton toxin. Science 2018, 361, 1308–1309. [Google Scholar] [CrossRef]
- Gu, P.; Jia, J.; Qi, D.; Gao, Q.; Zhang, C.; Yang, X.; Nie, M.; Liu, D.; Luo, Y. Response of phytoplankton composition to environmental stressors under humidification in three alpine lakes on the Qinghai-Tibet Plateau, China. Front. Microbiol. 2024, 15, 1370334. [Google Scholar] [CrossRef] [PubMed]
- Ersoy, Z.; Brucet, S.; Bartrons, M.; Mehner, T. Short-term fish predation destroys resilience of zooplankton communities and prevents recovery of phytoplankton control by zooplankton grazing. PLoS ONE 2019, 14, e0212351. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, S.R.; Kitchell, J.F.; Hodgson, J.R. Cascading Trophic Interactions and Lake Productivity. BioScience 1985, 35, 634–639. [Google Scholar] [CrossRef]
- Carvalho, S.A.; Martins, M.L. Community structures in allelopathic interaction networks: An ecoevolutionary approach. Phys. Rev. E 2018, 102, 042305. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, X.; Han, X.; Deng, Y. Higher precipitation strengthens the microbial interactions in semi-arid grassland soils. Glob. Ecol. Biogeogr. 2018, 27, 570–580. [Google Scholar] [CrossRef]
- Steinberger, Y.; Zelles, L.; Bai, Q.Y.; von Lützow, M.; Munch, J.C. Phospholipid fatty acid profiles as indicators for the microbial community structure in soils along a climatic transect in the Judean Desert. Biol. Fertil. Soils 1999, 28, 292–300. [Google Scholar] [CrossRef]
- Moreno-Mateos, D.; Alberdi, A.; Morriën, E.; van der Putten, W.H.; Rodríguez-Uña, A.; Montoya, D. The long-term restoration of ecosystem complexity. Nat. Ecol. Evol. 2020, 4, 676–685. [Google Scholar] [CrossRef]
- Mougi, A.; Kondoh, M. Diversity of Interaction Types and Ecological Community Stability. Science 2012, 337, 349–351. [Google Scholar] [CrossRef] [PubMed]
- Ghoul, M.; Mitri, S. The Ecology and Evolution of Microbial Competition. Trends Microbiol. 2016, 24, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Morriën, E.; Hannula, S.E.; Snoek, L.B.; Helmsing, N.R.; Zweers, H.; de Hollander, M.; Soto, R.L.; Bouffaud, M.-L.; Buée, M.; Dimmers, W.; et al. Soil networks become more connected and take up more carbon as nature restoration progresses. Nat. Commun. 2017, 8, 14349. [Google Scholar] [CrossRef] [PubMed]



| Before | After | |||||||
|---|---|---|---|---|---|---|---|---|
| Phylum | Species | Spring | Summer | Autumn | Spring | Summer | Autumn | |
| PP | Cyanobacteria | M. marssonii | 0.027 | 0.022 | / | / | / | / |
| M. aeruginosa | / | / | 0.021 | / | / | / | ||
| C. minutus | 0.020 | 0.027 | 0.025 | 0.020 | / | / | ||
| P. allorgei | 0.021 | 0.029 | / | / | / | / | ||
| Bacillariophyta | S. amphicephala | / | / | 0.023 | / | / | / | |
| C. meneghiniana | / | / | / | 0.024 | / | 0.024 | ||
| F. brevistriata | 0.022 | / | / | 0.020 | / | / | ||
| N. exigua | / | / | / | 0.029 | / | 0.024 | ||
| C. placentula | 0.027 | / | / | / | 0.031 | / | ||
| M. Granulata var. angustissima | / | 0.029 | / | / | 0.022 | / | ||
| S. acusvar | / | / | / | / | 0.020 | 0.024 | ||
| Chlorophyta | S. platydiscus | 0.025 | / | 0.035 | / | / | / | |
| A. acicularis | / | / | / | 0.02 | / | / | ||
| C. vulgaris | / | / | / | / | / | / | ||
| S. gracile | 0.025 | 0.026 | 0.035 | / | / | 0.020 | ||
| A. angustus | / | / | / | / | 0.029 | 0.020 | ||
| Chrysophyta | D. divergens | 0.025 | 0.026 | 0.035 | / | / | / | |
| C. elegans | / | / | / | / | 0.020 | / | ||
| MZ | Cladoceran | B. longirostris | / | 0.030 | 0.034 | / | 0.020 | 0.059 |
| Copepod | M. leuckarti | / | / | 0.098 | / | / | 0.037 | |
| nauplius.sp | 0.095 | 0.072 | / | 0.075 | 0.059 | 0.13 | ||
| Rotifer | B. calyciflorus | / | / | 0.028 | 0.021 | 0.021 | 0.042 | |
| V. limax | / | / | 0.022 | 0.026 | / | 0.032 | ||
| L. buna | / | / | / | / | / | 0.029 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, X.; Qin, L.; Zou, Y.; Yu, H.; Li, Y.; Yuan, Y.; Jiang, M. Eco-Engineering Improves Water Quality and Mediates Plankton–Nutrient Interactions in a Restored Wetland. Water 2024, 16, 1821. https://doi.org/10.3390/w16131821
Tian X, Qin L, Zou Y, Yu H, Li Y, Yuan Y, Jiang M. Eco-Engineering Improves Water Quality and Mediates Plankton–Nutrient Interactions in a Restored Wetland. Water. 2024; 16(13):1821. https://doi.org/10.3390/w16131821
Chicago/Turabian StyleTian, Xue, Lei Qin, Yuanchun Zou, Han Yu, Yu Li, Yuxiang Yuan, and Ming Jiang. 2024. "Eco-Engineering Improves Water Quality and Mediates Plankton–Nutrient Interactions in a Restored Wetland" Water 16, no. 13: 1821. https://doi.org/10.3390/w16131821
APA StyleTian, X., Qin, L., Zou, Y., Yu, H., Li, Y., Yuan, Y., & Jiang, M. (2024). Eco-Engineering Improves Water Quality and Mediates Plankton–Nutrient Interactions in a Restored Wetland. Water, 16(13), 1821. https://doi.org/10.3390/w16131821







