CTAB Surfactant Promotes Rapid, Efficient, and Simultaneous Removal of Cationic and Anionic Dyes through Adsorption on Glycerol/Citrate Polyester
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
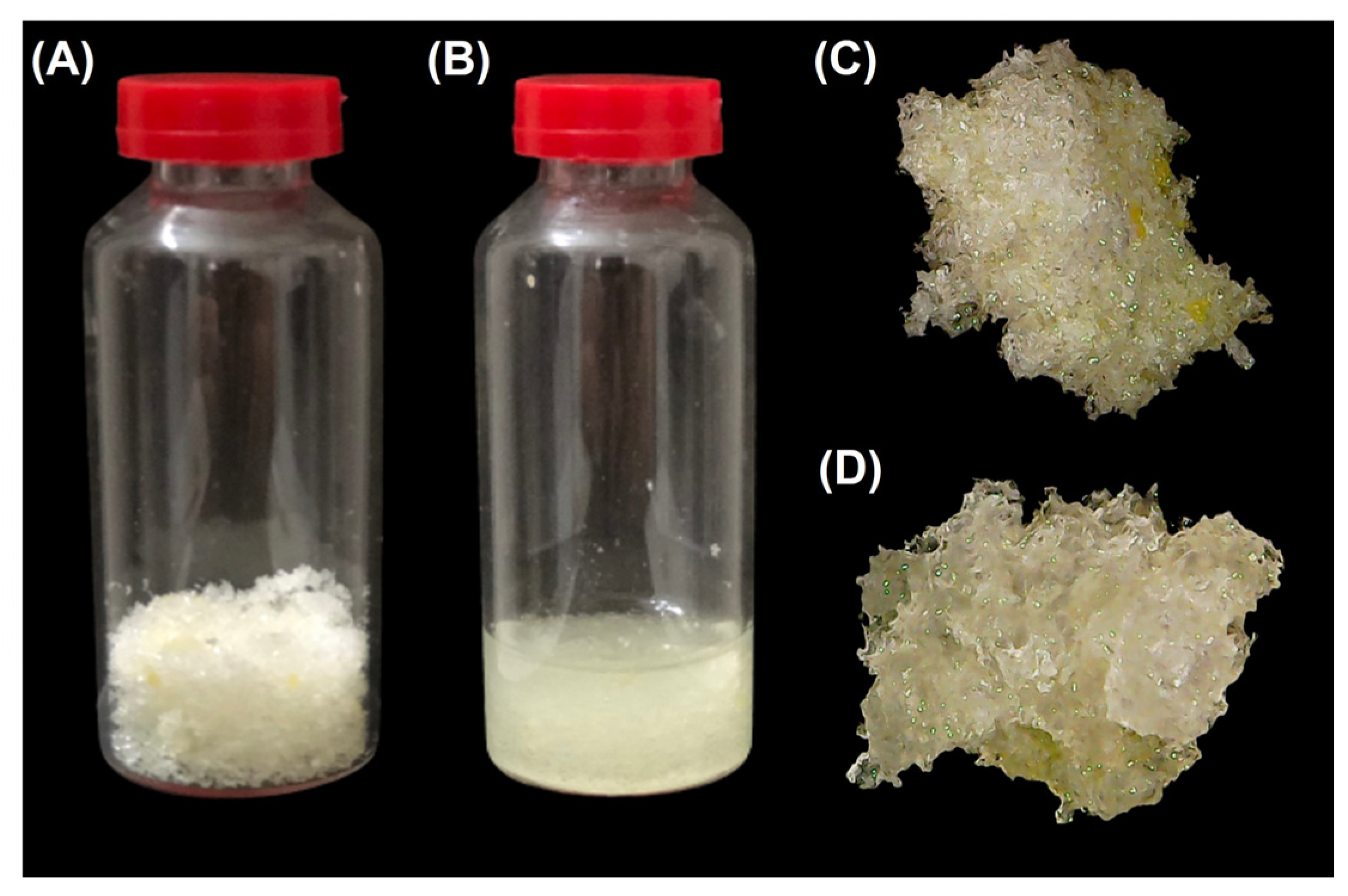
2.2. Preparation of Poly(Glycerol Citrate) Material
2.3. Physiochemical Characterization of Poly(Glycerol Citrate) Material
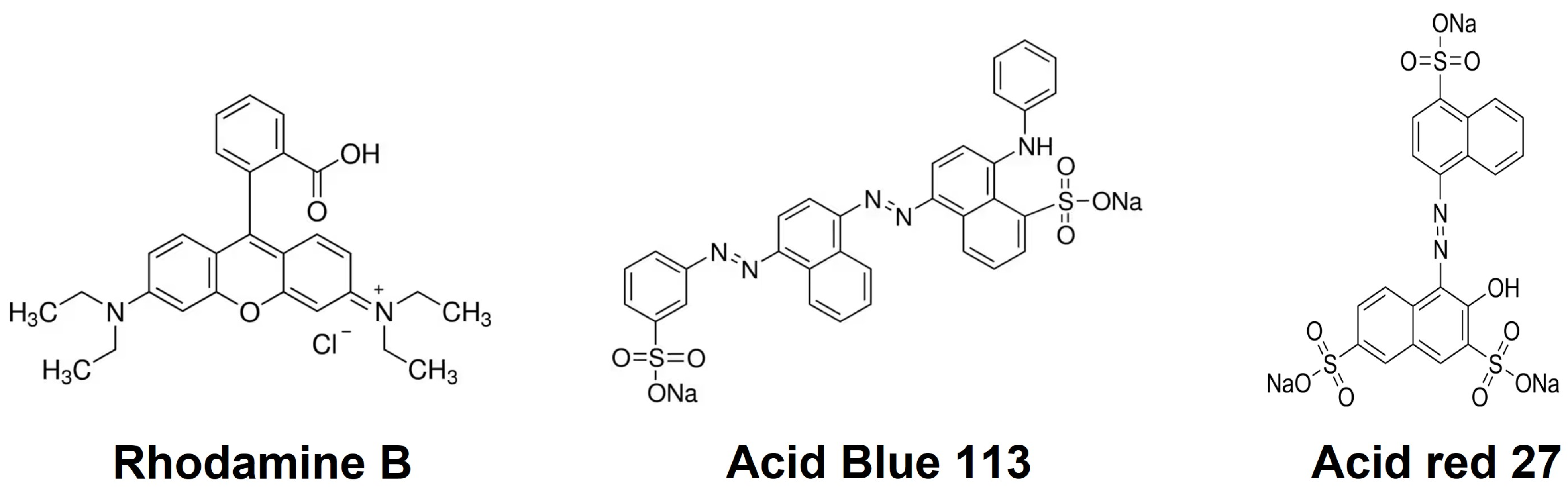
2.4. Dye Adsorption Studies
2.4.1. Dye Removal Efficiency
2.4.2. Adsorption Capacity
2.4.3. Adsorption kinetics
2.4.4. Selectivity and Remotion Mix of Dyes
3. Results and Discussion
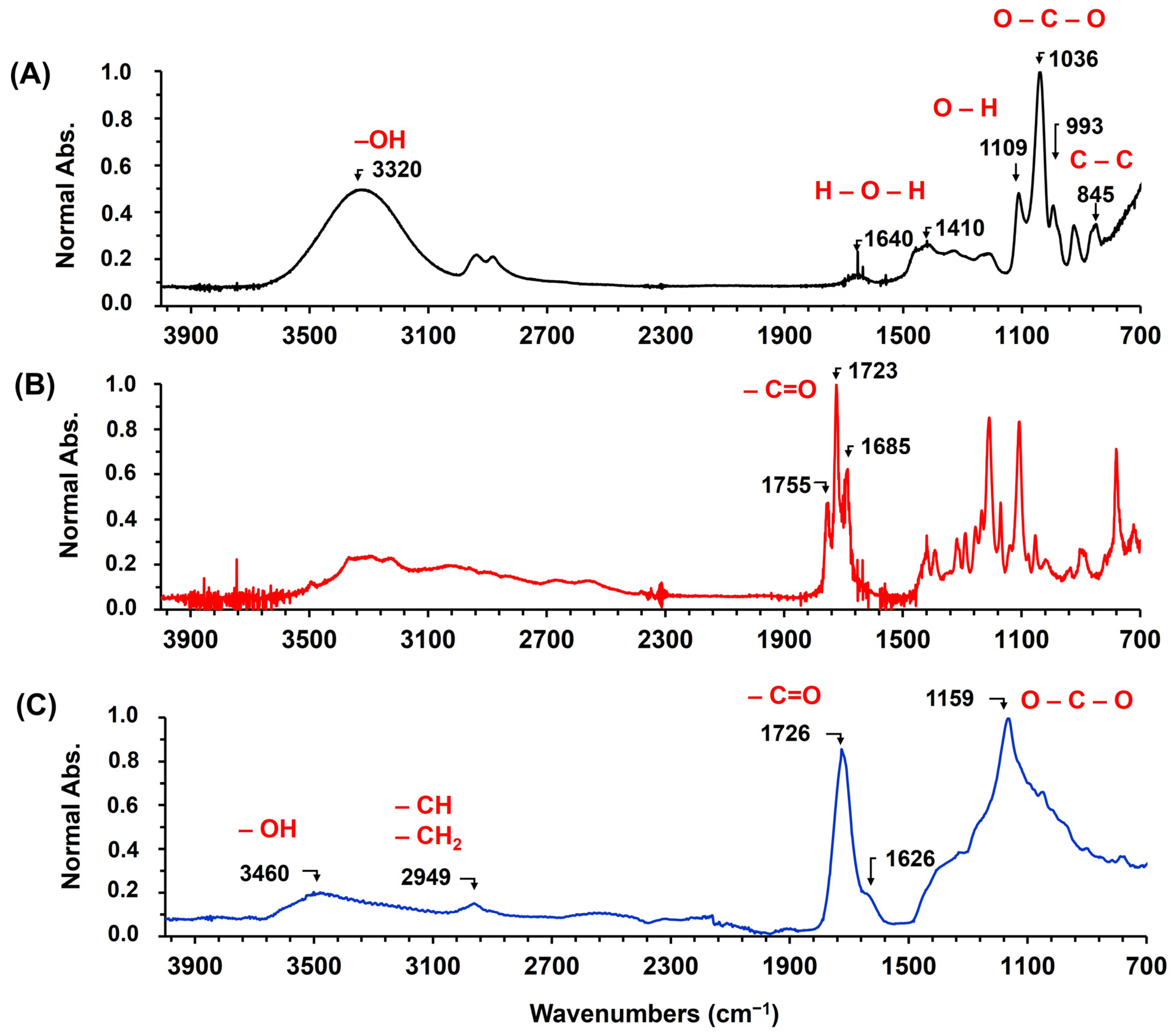
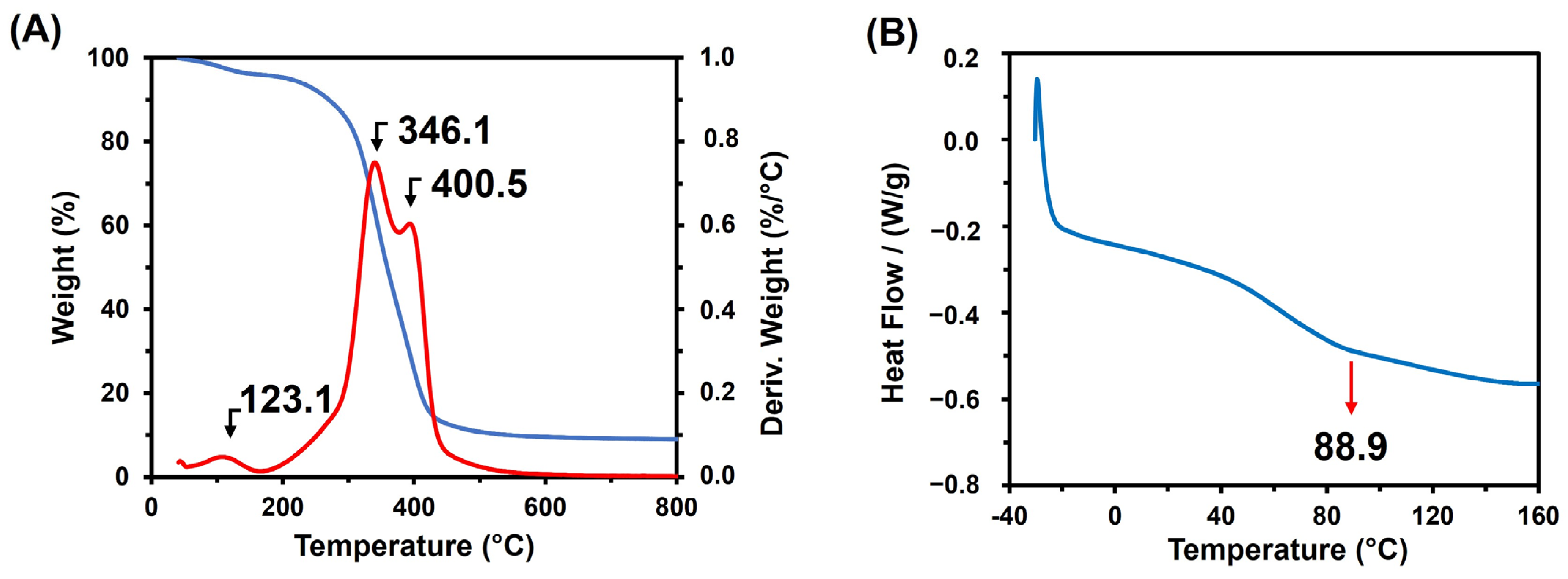
3.1. Synthesis and Characterization of Poly(Glycerol Citrate)
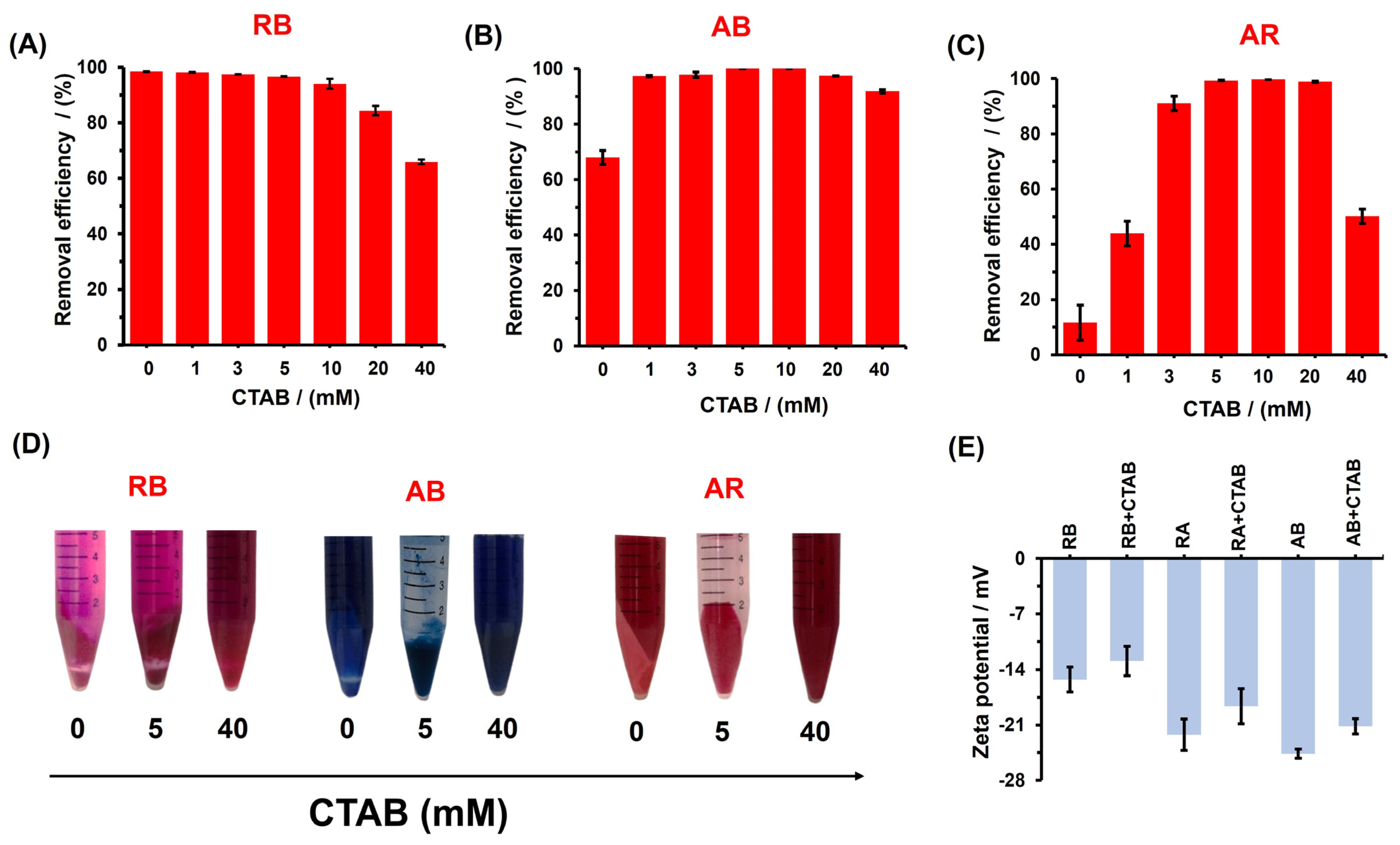
3.2. Adsorption Studies
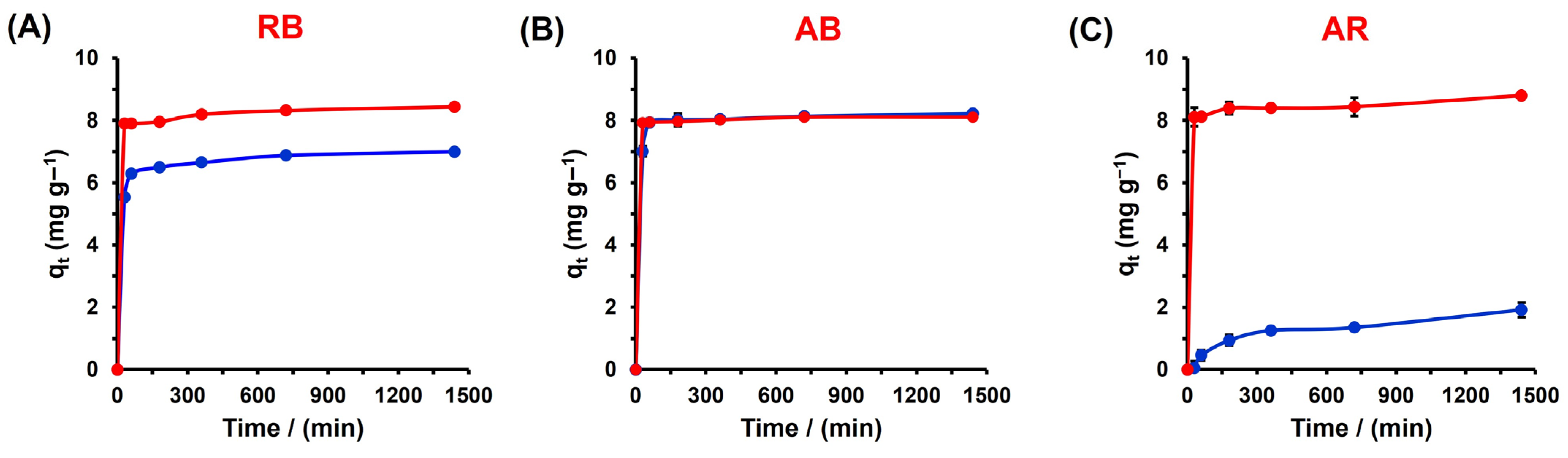
3.3. Adsorption Kinetics
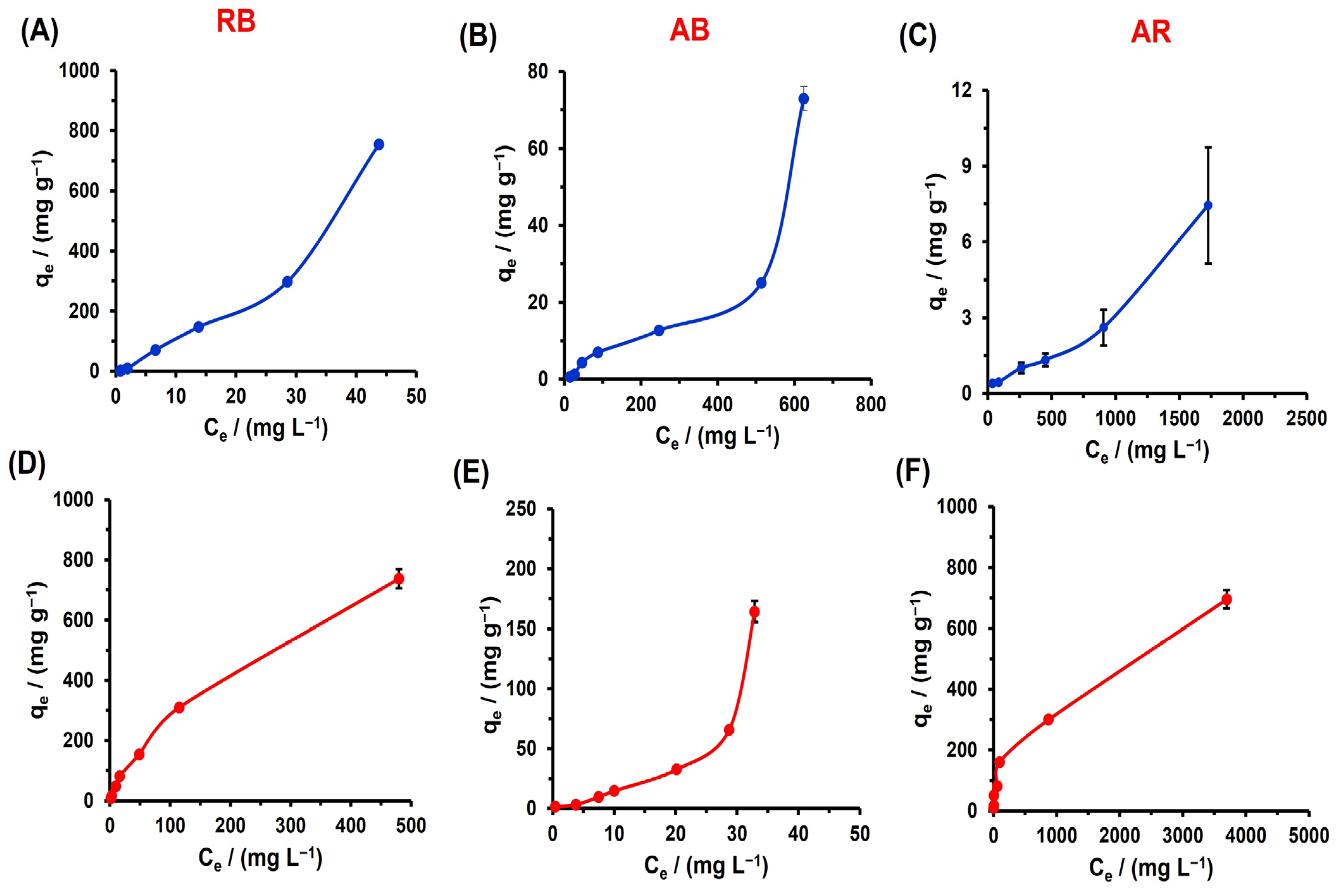
3.4. Adsorption Isotherm Study
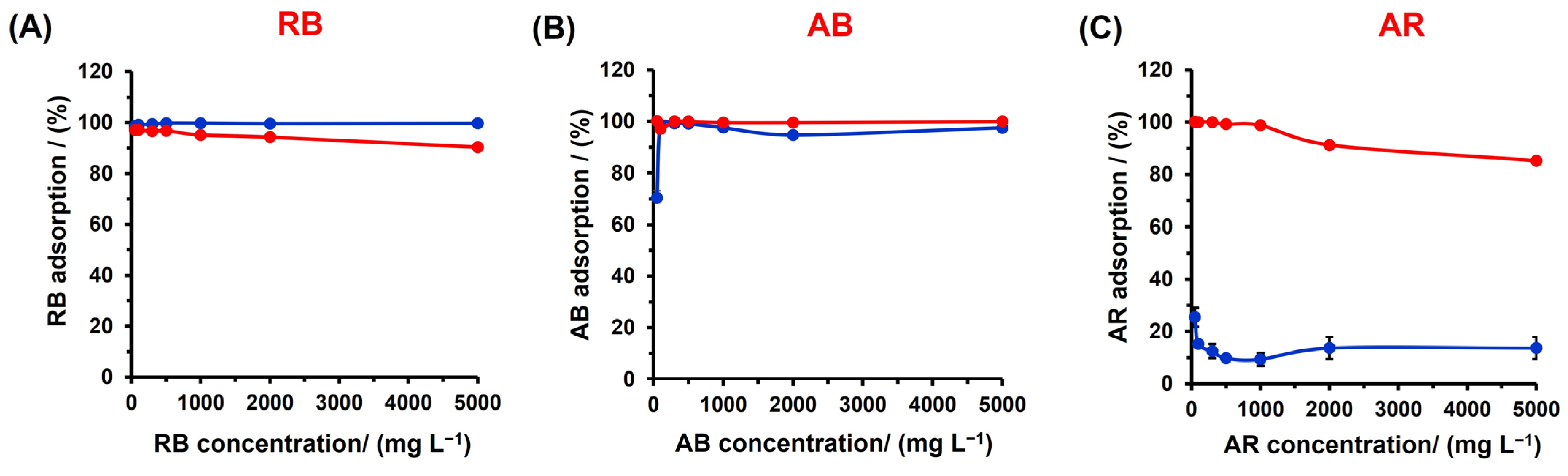
3.5. Selectivity, Separation, and Dye Remotion Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Benkhaya, S.; M’rabet, S.; El Harfi, A. Classifications, properties, recent synthesis, and applications of azo dyes. Heliyon 2020, 6, e03271. [Google Scholar] [CrossRef] [PubMed]
- Rápó, E.; Tonk, S. Factors affecting synthetic dye adsorption—Desorption studies: A review of results from the last five years (2017–2021). Molecules 2021, 26, 5419. [Google Scholar] [CrossRef] [PubMed]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and Adsorption Capacities of Low-Cost Sorbents for Wastewater Treatment: A Review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef]
- Bouabidi, Z.B.; El-Naas, M.H.; Cortes, D.; McKay, G. Steel-Making Dust as a Potential Adsorbent for the Removal of Lead (II) from an Aqueous Solution. Chem. Eng. J. 2018, 334, 837–844. [Google Scholar] [CrossRef]
- Pei, Y.C.; Wang, J.J.; Xuan, X.P.; Fan, J.; Fan, M. Factors affecting ionic liquids-based removal of anionic dyes from water. Environ. Sci. Technol. 2007, 41, 5090–5509. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Yang, Y.; Zhang, X.; Niu, J.; Yang, H.; Tian, S.; Lu, M. Highly efficient, rapid, and simultaneous removal of cationic dyes from aqueous solution using monodispersed mesoporous silica nanoparticles as the adsorbent. Nanomaterials 2017, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xiong, Z.; Zhang, J.; Wu, C. The Strengthening Role of the Amino Group in Metal-Organic Framework MIL-53 (Al) for Methylene Blue and Malachite Green Dye Adsorption. J. Chem. Eng. Data 2015, 60, 3414–3422. [Google Scholar] [CrossRef]
- Farhadi, S.; Mahmoudi, F.; Amini, M.M.; Dusek, M.; Jarosova, M. Synthesis and characterization of a series of novel perov-skite-type LaMnO3/Keggin-type polyoxometalate hybrid nanomaterials for fast and selective removal of cationic dyes from aqueous solutions. Dalton. Trans. 2017, 46, 3252–3264. [Google Scholar] [CrossRef] [PubMed]
- Ardila-Leal, L.D.; Poutou-Piñales, R.A.; Pedroza-Rodríguez, A.M.; Quevedo-Hidalgo, B.E. A Brief History of Colour, the Environmental Impact of Synthetic Dyes and Removal by Using Laccases. Molecules 2021, 26, 3813. [Google Scholar] [CrossRef]
- Chahar, D.; Kumar, D.; Thakur, P.; Thakur, A. Visible light induced photocatalytic degradation of methylene blue dye by using Mg doped Co-Zn nanoferrites. Mater. Res. Bull. 2023, 162, 112205. [Google Scholar] [CrossRef]
- Belal, R.M.; Zayed, M.A.; El-Sherif, R.M.; Ghany, N.A. Electrochemical Degradation and Degree of Mineralization of the BY28 Dye in a Supporting Electrolyte Mixture Using an Expanded Dimensionally Stable Anode. Electrocatalysis 2022, 13, 26–36. [Google Scholar] [CrossRef]
- Jain, D.; Navariya, J.K.; Bhojiya, A.A.; Singh, A.; Mohanty, S.R.; Upadhyay, S.K. Bioprospecting of novel ligninolytic bacteria for effective bioremediation of agricultural by-product and synthetic pollutant dyes. Microbiol. Res. 2023, 270, 127330. [Google Scholar] [CrossRef] [PubMed]
- Shamrock, W.; Abdelhamid, H.N. Fenton-like Cerium Metal–Organic Frameworks (Ce-MOFs) for Catalytic Oxidation of Olefins, Alcohol, and Dyes Degradation. J. Clust. Sci. 2023, 34, 2509–2519. [Google Scholar]
- Karma, V.; Gande, V.V.; Pushpavanam, S. Simultaneous extraction and enrichment of sunset yellow dye in an aqueous two-phase system. Dyes. Pigm. 2023, 212, 111100. [Google Scholar] [CrossRef]
- Mu, B.; Wang, A. Adsorption of dyes onto palygorskite and its composites: A review. J. Environ. Chem. Eng. 2016, 4, 1274–1294. [Google Scholar] [CrossRef]
- Tony, M.A. Low-Cost Adsorbents for Environmental Pollution Control: A Concise Systematic Review from the Prospective of Principles, Mechanism and Their Applications. J. Dispers. Sci. Technol. 2022, 43, 1612–1633. [Google Scholar] [CrossRef]
- Salunkhe, B.; Schuman, T.P. Super-Adsorbent Hydrogels for Removal of Methylene Blue from Aqueous Solution: Dye Ad-sorption Isotherms, Kinetics, and Thermodynamic Properties. Macromoleclues 2021, 1, 256–275. [Google Scholar] [CrossRef]
- Azari, A.; Nabizadeh, R.; Mahvi, A.H.; Nasseri, S. Magnetic multi-walled carbon nanotubes-loaded alginate for treatment of industrial dye manufacturing effluent: Adsorption modelling and process optimisation by central composite face-central design. Int. J. Environ. Anal. Chem. 2023, 103, 1509–1529. [Google Scholar] [CrossRef]
- Lourens, A.; Falch, A.; Malgas-Enus, R. Magnetite immobilized metal nanoparticles in the treatment and removal of pollutants from wastewater: A review. J. Mater. Sci. 2023, 58, 2951–2970. [Google Scholar] [CrossRef]
- Liang, C.; Shi, Q.; Feng, J.; Yao, J.; Huang, H.; Xie, X. Adsorption Behaviors of Cationic Methylene Blue and Anionic Reactive Blue 19 Dyes onto Nano-Carbon Adsorbent Carbonized from Small Precursors. Nanomaterials 2022, 12, 1814. [Google Scholar] [CrossRef]
- Tisserat, B.; O’kuru, R.H.; Hwang, H.; Mohamed, A.A.; Holser, R. Glycerol citrate polyesters produced through heating without catalysis. J. Appl. Polym. Sci. 2012, 125, 3429–3437. [Google Scholar] [CrossRef]
- Lee, S.H.; Md Tahir, P.; Lum, W.C.; Tan, L.P.; Bawon, P.; Park, B.-D.; Osman Al Edrus, S.S.; Abdullah, U.H. A Review on Citric Acid as Green Modifying Agent and Binder for Wood. Polymers 2020, 12, 1692. [Google Scholar] [CrossRef] [PubMed]
- Berube, M.A.; Schorr, D.; Ball, R.J.; Landry, V.; Blanchet, P. Determination of in situ esterification parameters of citric acid-glycerol based polymers for wood impregnation. J. Polym. Environ. 2018, 26, 970–979. [Google Scholar] [CrossRef]
- Franklin, D.S.; Guhanathan, S. Investigation of citric acid–glycerol-based pH-sensitive biopolymeric hydrogels for dye removal applications: A green approach. Ecotoxicol. Environ. Saf. 2015, 121, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Pan, D.; Ding, L.; Tang, F.; Zhang, Q.; Liu, Q.; Yao, S. Mixed hemimicelles SPE based on CTAB-coated Fe3O4/SiO2 NPs for the determination of herbal bioactive constituents from biological samples. Talanta 2010, 80, 1873–1880. [Google Scholar] [CrossRef] [PubMed]
- Bejaoui, B.; Nefzi, K.; Bouchmila, I.; Koumba, S.; Joly, N.; M’Hamdi, N.; Martin, P. Solid phase extraction of polycyclic aromatic hydrocarbons from water samples using CTAB-TIO2 modified nanotubes. Microchemistry J. 2023, 193, 109027. [Google Scholar] [CrossRef]
- Zolgharnein, J.; Rajabalipour, F.; Dermanaki Farahani, S. Indigo Carmine Dye Adsorptive Removal by Polyethylene Glycol-Modified Hydroxyapatite Nanoparticles as an Efficient Adsorbent. Water. Air. Soil. Pollut. 2023, 234, 210. [Google Scholar] [CrossRef]
- Dhaif-Allah, M.A.; Taqui, S.N.; Syed, U.T.; Syed, A.A. Kinetic and isotherm modeling for acid blue 113 dye adsorption onto low-cost nutraceutical industrial fenugreek seed spent. Appl. Water Sci. 2020, 10, 1–16. [Google Scholar] [CrossRef]
- Xiao, W.; Garba, Z.N.; Sun, S.; Lawan, I.; Wang, L.; Lin, M.; Yuan, Z. Preparation and evaluation of an effective activated carbon from white sugar for the adsorption of rhodamine B dye. J. Clean. Prod. 2020, 253, 119989. [Google Scholar] [CrossRef]
- de Santana, J.E.; de Andrade, F.G.S.; Ferreira, A.F.; Ghislandi, M.G.; da Motta Sobrinho, M.A. Isotherms, kinetics and thermodynamics of industrial dye acid red 27 adsorption on Sugarcane Bagasse Ash. Environ. Sci. Pollut. Res. Int. 2024, 1–15. [Google Scholar] [CrossRef]
- Alharbi, R.A.; Alminderej, F.M.; Al-Harby, N.F.; Elmehbad, N.Y.; Mohamed, N.A. Preparation and Characterization of a New Bis-Uracil Chitosan-Based Hydrogel as Efficient Adsorbent for Removal of Anionic Congo Red Dye. Polymers 2023, 15, 1529. [Google Scholar] [CrossRef] [PubMed]
- Razali, N.A.; Conte, M.; McGregor, J. The role of impurities in the La2O3 catalysed carboxylation of crude glycerol. Catal. Lett. 2019, 149, 1403–1414. [Google Scholar] [CrossRef]
- Zahlan, H.; Saeed, W.S.; Alrasheed, R.; Alandes, N.M.; Aouak, T. Synthesis of poly (citric acid-co-glycerol) and its application as an inhibitor of CaCO3 deposition. Materials 2019, 12, 3800. [Google Scholar] [CrossRef]
- Gahrooee, T.R.; Moud, A.A.; Danesh, M.; Hatzikiriakos, S.G. Rheological characterization of CNC-CTAB network below and above critical micelle concentration (CMC). Carbohydr. Polym. 2021, 257, 117552. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Chang, Q.; Zhang, T.; Song, G.; Sun, Y.; Ding, G. A review on selective dye adsorption by different mechanisms. J. Environ. Chem. Eng. 2022, 10, 108639. [Google Scholar] [CrossRef]
- Tan, K.B.; Vakili, M.; Horri, B.A.; Poh, P.E.; Abdullah, A.Z.; Salamatinia, B. Adsorption of dyes by nanomaterials: Recent developments and adsorption mechanisms. Sep. Purif. Technol. 2015, 150, 229–242. [Google Scholar] [CrossRef]
- Zhao, D.; Shen, X. Preparation of Chitosan-Diatomite/Calcium Alginate Composite Hydrogel Beads for the Adsorption of Congo Red Dye. Water 2023, 15, 2254. [Google Scholar] [CrossRef]
- Purnomo, A.S.; Prasetyoko, D.; Bahruji, H. Single-step synthesis and modification of CTAB-hectorite for efficient adsorption of methyl orange dye. Mater. Chem. Phys. 2022, 291, 126749. [Google Scholar]
- Yao, G.; Li, S.; Xu, J.; Liu, H. Dual-responsive graphene oxide/poly (NIPAM-co-AA) hydrogel as an adsorbent for rhodamine B and imidacloprid. J. Chem. Amp. Eng. Data 2019, 64, 4054–4065. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, X.; Lou, T. Simultaneous adsorption for cationic and anionic dyes using chitosan/electrospun sodium alginate nanofiber composite sponges. Carbohydr. Polym. 2022, 276, 118728. [Google Scholar] [CrossRef]
- Khan, T.A.; Dahiya, S.; Ali, I. Use of kaolinite as adsorbent: Equilibrium, dynamics and thermodynamic studies on the adsorption of Rhodamine B from aqueous solution. Appl. Clay Sci. 2012, 69, 58–66. [Google Scholar] [CrossRef]
- Nascimento, R.J.; Pereira, K.R.A.; Avelino, F. Parametric and modeling studies of Rhodamine-B adsorption using coconut coir-based materials as eco-friendly adsorbents. J. Environ. Chem. Eng. 2021, 9, 105943. [Google Scholar] [CrossRef]
- Gupta, V.K.; Gupta, B.; Rastogi, A.; Agarwal, S.; Nayak, A. A comparative investigation on adsorption performances of mesoporous activated carbon prepared from waste rubber tire and activated carbon for a hazardous azo dye—Acid Blue 113. J. Hazard. Mater. 2011, 186, 891–901. [Google Scholar] [CrossRef]
- Al-Musawi, T.J.; Mengelizadeh, N.; Al Rawi, O.; Balarak, D. Capacity and modeling of acid blue 113 dye adsorption onto chitosan magnetized by Fe2O3 nanoparticles. J. Polym. Environ. 2022, 30, 344–359. [Google Scholar] [CrossRef]
- Sodhani, H.; Hedaoo, S.; Murugesan, G.; Pai, S.; Vinayagam, R.; Varadavenkatesan, T.; Selvaraj, R. Adsorptive removal of Acid Blue 113 using hydroxyapatite nanoadsorbents synthesized using Peltophorum pterocarpum pod extract. Chemosphere 2022, 299, 134752. [Google Scholar] [CrossRef]
- Khan, M.; Lo, I.M. Removal of ionizable aromatic pollutants from contaminated water using nano γ-Fe2O3 based magnetic cationic hydrogel: Sorptive performance, magnetic separation, and reusability. J. Hazard. Mater. 2017, 322, 195–204. [Google Scholar] [CrossRef]
- Yusof, N.H.; Foo, K.Y.; Wilson, L.D.; Hameed, B.H.; Hussin, M.H.; Sabar, S. Microwave-assisted synthesis of polyeth-yleneimine grafted chitosan beads for the adsorption of acid red 27. J. Polym. Environ. 2020, 28, 542–552. [Google Scholar] [CrossRef]
- Ebrahimpoor, S.; Kiarostami, V.; Khosravi, M.; Davallo, M.; Ghaedi, A. Bees metaheuristic algorithm with the aid of artificial neural networks for optimization of acid red 27 dye adsorption onto novel polypyrrole/SrFe12O19/graphene oxide nano-composite. Polym. Bull. 2019, 76, 6529–6553. [Google Scholar] [CrossRef]
- Tseng, R.L.; Wu, F.C. Inferring the favorable adsorption level and the concurrent multi-stage process with the Freundlich constant. J. Hazard. Mater. 2008, 155, 277–287. [Google Scholar] [CrossRef]
- Debnath, S.; Das, R. Strong adsorption of CV dye by Ni ferrite nanoparticles for wastewater purification: Fits well the pseudo second order kinetic and Freundlich isotherm model. Ceram. Int. 2023, 49, 16199–16215. [Google Scholar] [CrossRef]
- Ferreira, D.C.M.; Dos Santos, T.C.; dos Reis Coimbra, J.S.; de Oliveira, E.B. Chitosan/carboxymethylcellulose polyelectrolyte complexes (PECs) are an effective material for dye and heavy metal adsorption from water. Carbohydr. Polym. 2023, 315, 120977. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.X.; Lee, L.; Wang, H.H.; Wang, Z. Removal of an anionic dye by adsorption/precipitation processes using alkaline white mud. J. Hazard. Mater. 2007, 149, 735–741. [Google Scholar] [CrossRef] [PubMed]


| Model | Parameter | Units | RB | RB-CTAB | AB | AB-CTAB | AR | AR-CTAB |
|---|---|---|---|---|---|---|---|---|
| Pseudo-first-order | qe | mg/g | 1.82 | 0.20 | 1.09 | 0.62 | 2.13 | 0.62 |
| K1 | ×10−3 (/min) | 27.60 | 2.07 | 3.22 | 2.30 | 0.92 | 0.92 | |
| R2 | 0.6570 | 0.9891 | 0.9245 | 0.9756 | 0.6443 | 0.6750 | ||
| Pseudo-second-order | qe | mg/g | 8.24 | 8.12 | 7.04 | 8.47 | 3.01 | 8.80 |
| K2 | ×10−3 (/min) | 2.09 | 5.48 | 1.09 | 1.52 | 0.07 | 1.23 | |
| R2 | 1.0000 | 1.0000 | 0.9999 | 0.9999 | 0.7746 | 0.9996 | ||
| Boyd liquid-film diffusion model | KFd | ×10−3 (/min) | 2.70 | 6.40 | 3.10 | 2.30 | 0.90 | 0.90 |
| R2 | 0.6570 | 0.8933 | 0.9245 | 0.9756 | 0.6443 | 0.6750 |
| Adsorbent | RB (mg/g) | AB (mg/g) | AR (mg/g) | Ref. |
|---|---|---|---|---|
| Poly(glycerol citrate) | 73.7 ± 3.2 | 82.1 ± 4.4 | 21.2 ± 0.6 | This work |
| Kaolinite | 46.1 | - | - | [41] |
| Coconut fibers | 22.0 | - | - | [42] |
| Graphene oxide/Poly(NIPAM-co-AA) Hydrogel | 193.1 | - | - | [39] |
| Chitosan/electrospun sodium alginate nanofiber | 695.4 ± 17.0 | 926.2 ± 25.7 | - | [40] |
| Mesoporous activated carbon | - | 9.20 | - | [43] |
| Chitosan magnetized by Fe2O3 nanoparticles | - | 124.2 | - | [44] |
| Hydroxyapatite | - | 153.8 | - | [45] |
| Nano-Fe2O3-based magnetic cationic hydrogel | - | - | 833 | [46] |
| Polyethyleneimine grafted chitosan beads | - | - | 48.3 | [47] |
| Polypyrrole/SrFe12O19/graphene oxide nanocomposite | - | - | 294.1 | [48] |
| Model | Parameter | Units | RB | RB-CTAB | AB | AB-CTAB | AR | AR-CTAB |
|---|---|---|---|---|---|---|---|---|
| Langmuir isotherm | qm | mg/g | 98.0 | 104.2 | 119.0 | 38.8 | 20.2 | 21.5 |
| KL (×10−3) | L/mg | 13.56 | 4.58 | 0.45 | 16.50 | 0.09 | 20.34 | |
| R2 | 0.9359 | 0.9476 | 0.1124 | 0.2455 | 0.1124 | 0.9981 | ||
| Freundlich isotherm | n | 1.36 | 1.29 | 0.90 | 1.00 | 1.07 | 4.10 | |
| 1/n | 0.74 | 0.78 | 1.11 | 1.00 | 0.94 | 0.24 | ||
| KF (×103) | L/mg | 173.2 | 73.03 | 3.73 | 99.39 | 0.36 | 28.87 | |
| R2 | 0.9548 | 0.9904 | 0.9494 | 0.8530 | 0.9003 | 0.7951 | ||
| Temkin isotherm | BT | 11.30 | 11.40 | 13.64 | 12.40 | 2.84 | 1.71 | |
| KT | L/mg | 0.72 | 0.26 | 0.04 | 0.66 | 0.008 | 27.51 | |
| R2 | 0.8241 | 0.7909 | 0.5969 | 0.4065 | 0.4969 | 0.8686 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chamorro, A.F.; Lerma, T.A.; Palencia, M. CTAB Surfactant Promotes Rapid, Efficient, and Simultaneous Removal of Cationic and Anionic Dyes through Adsorption on Glycerol/Citrate Polyester. Water 2024, 16, 1860. https://doi.org/10.3390/w16131860
Chamorro AF, Lerma TA, Palencia M. CTAB Surfactant Promotes Rapid, Efficient, and Simultaneous Removal of Cationic and Anionic Dyes through Adsorption on Glycerol/Citrate Polyester. Water. 2024; 16(13):1860. https://doi.org/10.3390/w16131860
Chicago/Turabian StyleChamorro, Andrés F., Tulio A. Lerma, and Manuel Palencia. 2024. "CTAB Surfactant Promotes Rapid, Efficient, and Simultaneous Removal of Cationic and Anionic Dyes through Adsorption on Glycerol/Citrate Polyester" Water 16, no. 13: 1860. https://doi.org/10.3390/w16131860
APA StyleChamorro, A. F., Lerma, T. A., & Palencia, M. (2024). CTAB Surfactant Promotes Rapid, Efficient, and Simultaneous Removal of Cationic and Anionic Dyes through Adsorption on Glycerol/Citrate Polyester. Water, 16(13), 1860. https://doi.org/10.3390/w16131860








