Long-Term Performance Evaluation and Fouling Characterization of a Full-Scale Brackish Water Reverse Osmosis Desalination Plant
Abstract
:1. Introduction
2. Materials and Methods
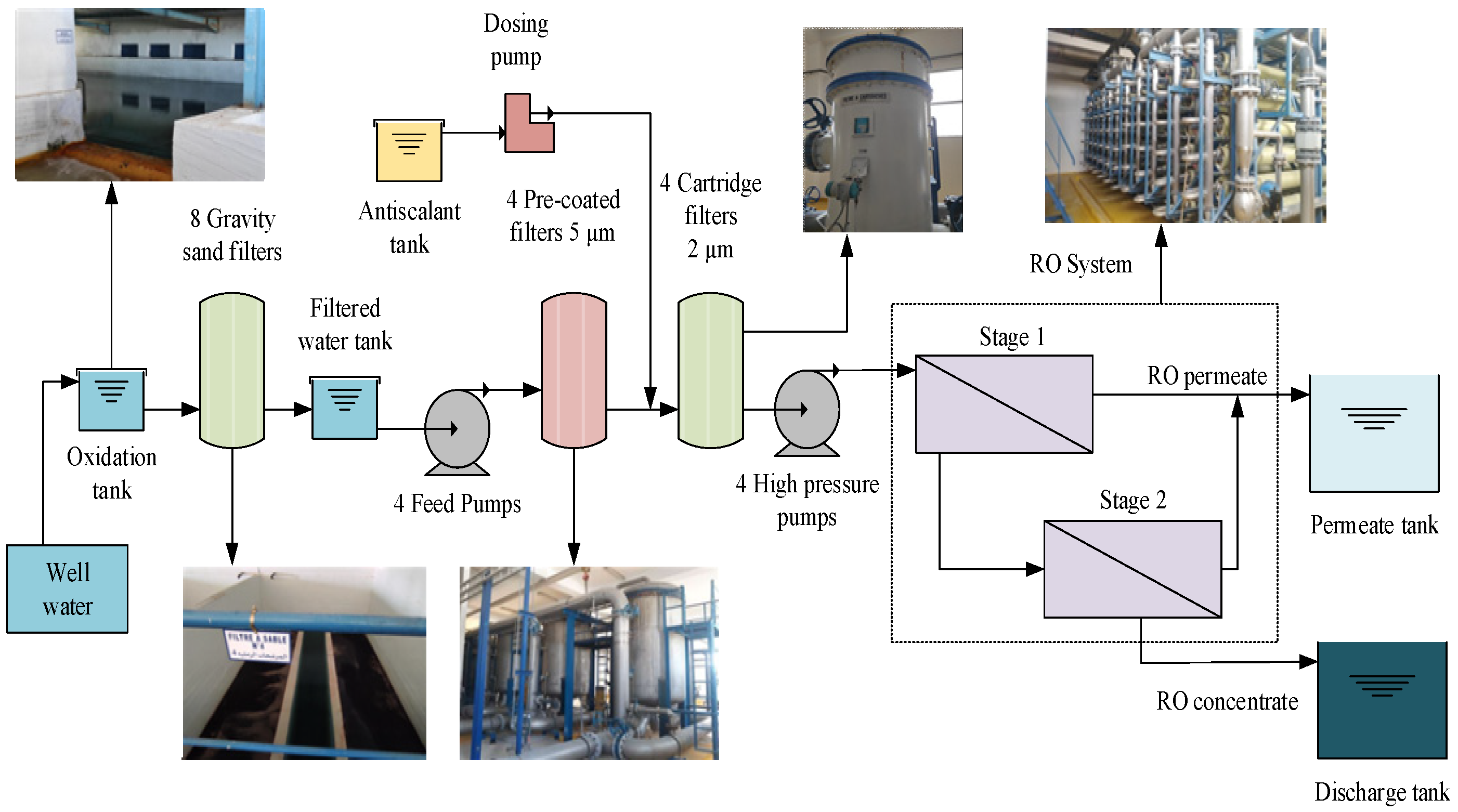
2.1. Plant Description
- (1)
- The pre-treatment phase begins with water oxidation in an aerated tank, followed by filtration through 8 slow sand filters. Each filter consists of two compartments fed by a central column from the oxidation basin, which retains suspended solids and iron oxides. After filtration, water is pumped through 4 pre-coated filters, at a flow rate of 480 m3 h−1, to eliminate impurities larger than 5 µm. Each vessel consists of 330 elements. Several types of antiscalants have been used to prevent scale formation (Polyphosphonate, Polyacroboxic, and Polyacrylate). The antiscalant dose corresponds to the dose at the feed. No additional chemical products have been added to this plant. The water is then subjected to micro-filtration by cartridge filters (1 µm), of which there are four. Each filter contains 102 cartridges, except for the fourth line, which contains 170 elements. Four high-pressure pumps, with an electric power motor with 350 kW for three lines and 200 kW for the fourth line, were installed to boost the filtered water through the BWRO system, with a feed flow of 480 m3 h−1 each and a feed pressure of about 1.5 MPa.
- (2)
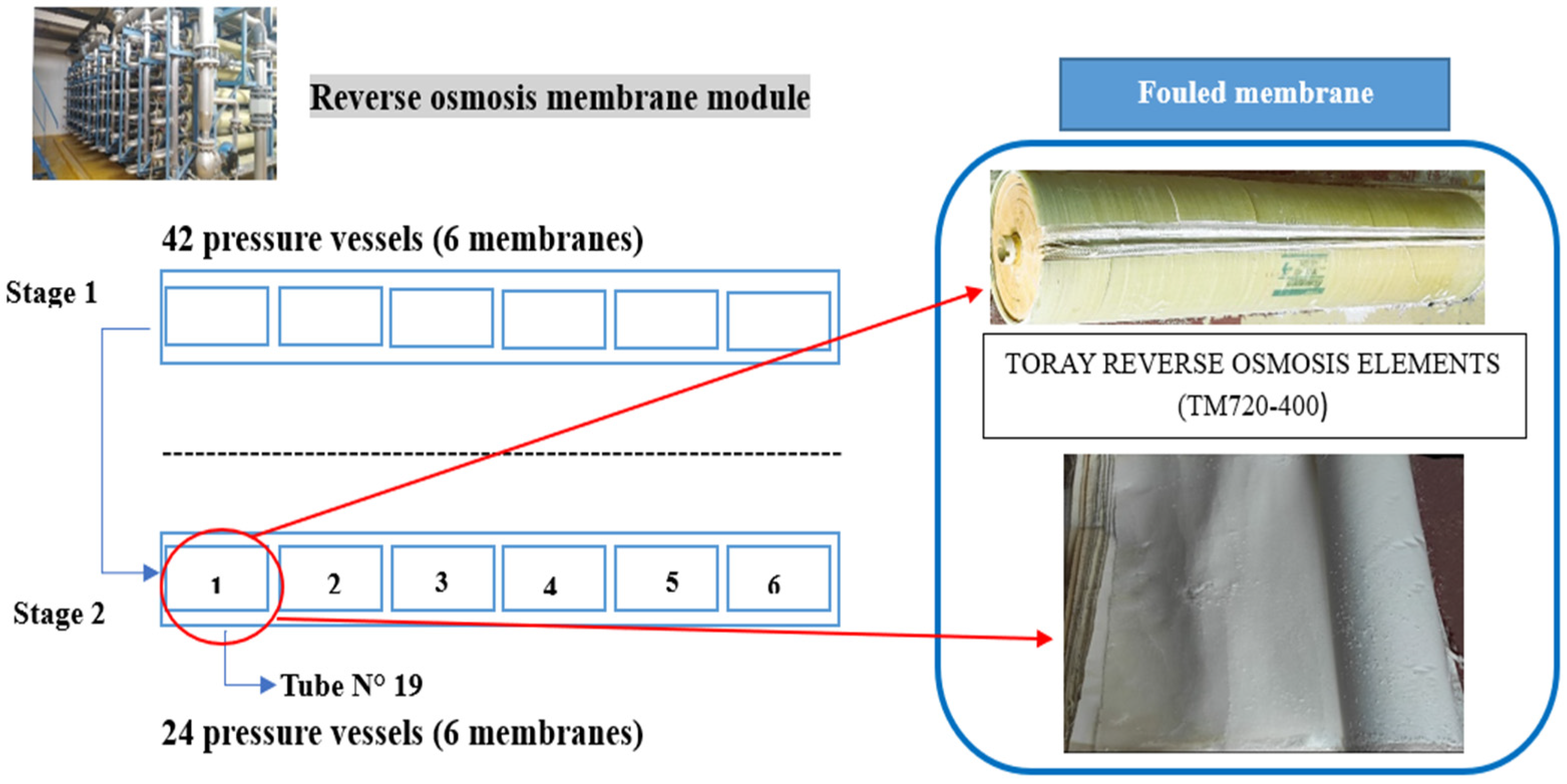
- The BWRO system consists of four production lines with a total capacity of 1250 m3 h−1, the system uses spiral-wound BWRO membranes TORAY TM720-400 (Toray Industries, Inc., Tokyo, Japan) distributed over two stages. The first stage consists of 42 pressure vessels (PVs), and the rejection flow from this stage feeds the second stage, which consists of 24 PVs.
- (3)
- The final stage involves post-treatment, during which the pH of the permeate is adjusted by adding sodium hydroxide (NaOH) and removing CO2.
2.2. Method for Measuring Ion Species
2.3. Standardization of the Operating Data
2.3.1. Standardization of
2.3.2. Standardization of
2.4. Calculation of Permeability Coefficients and
2.5. Fouling Characterization Methods
3. Results and Discussion
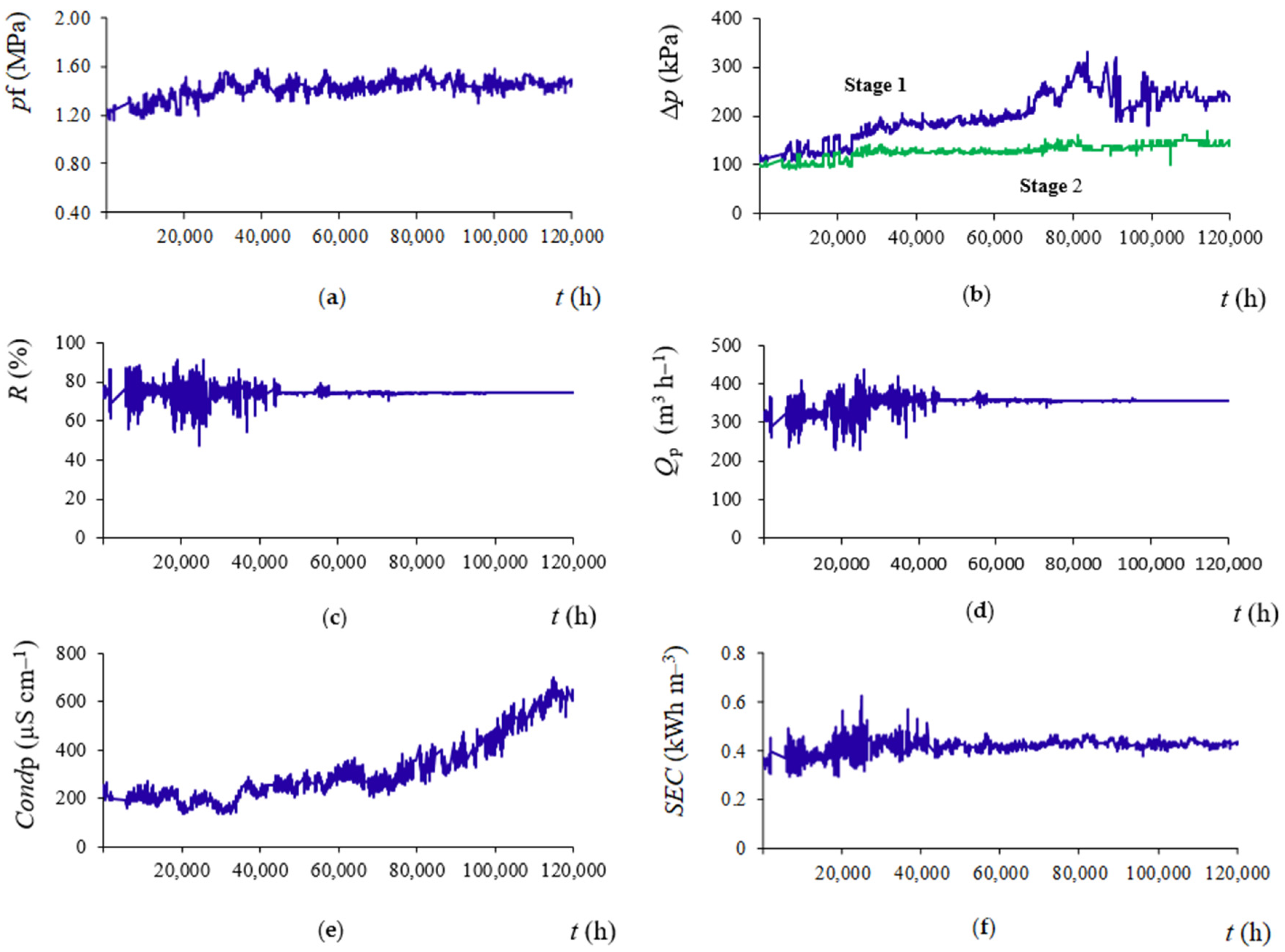
3.1. Operating Data Analysis
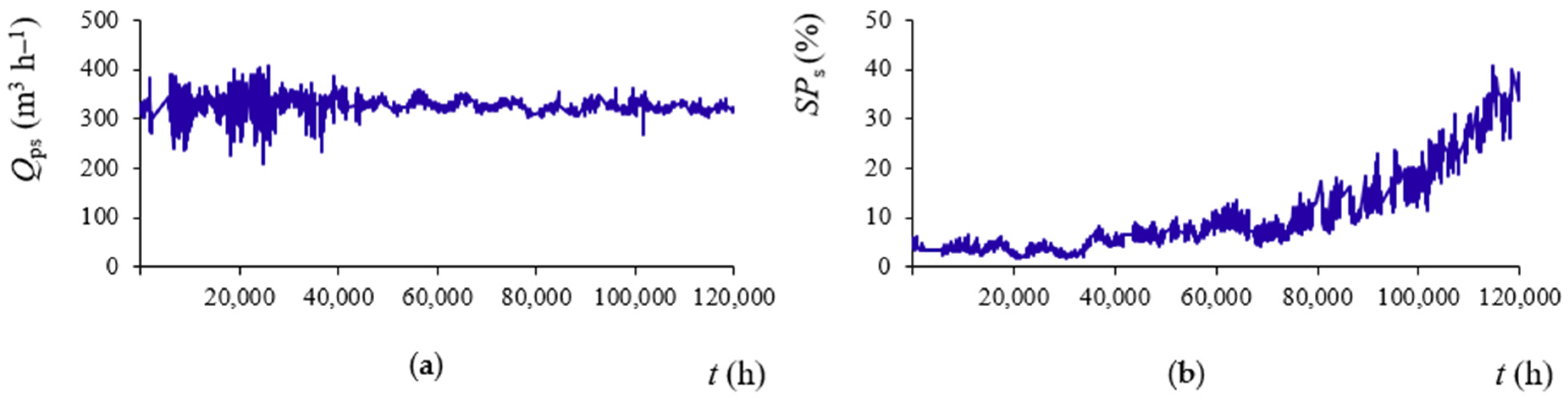
3.2. Standardization of Operating Data
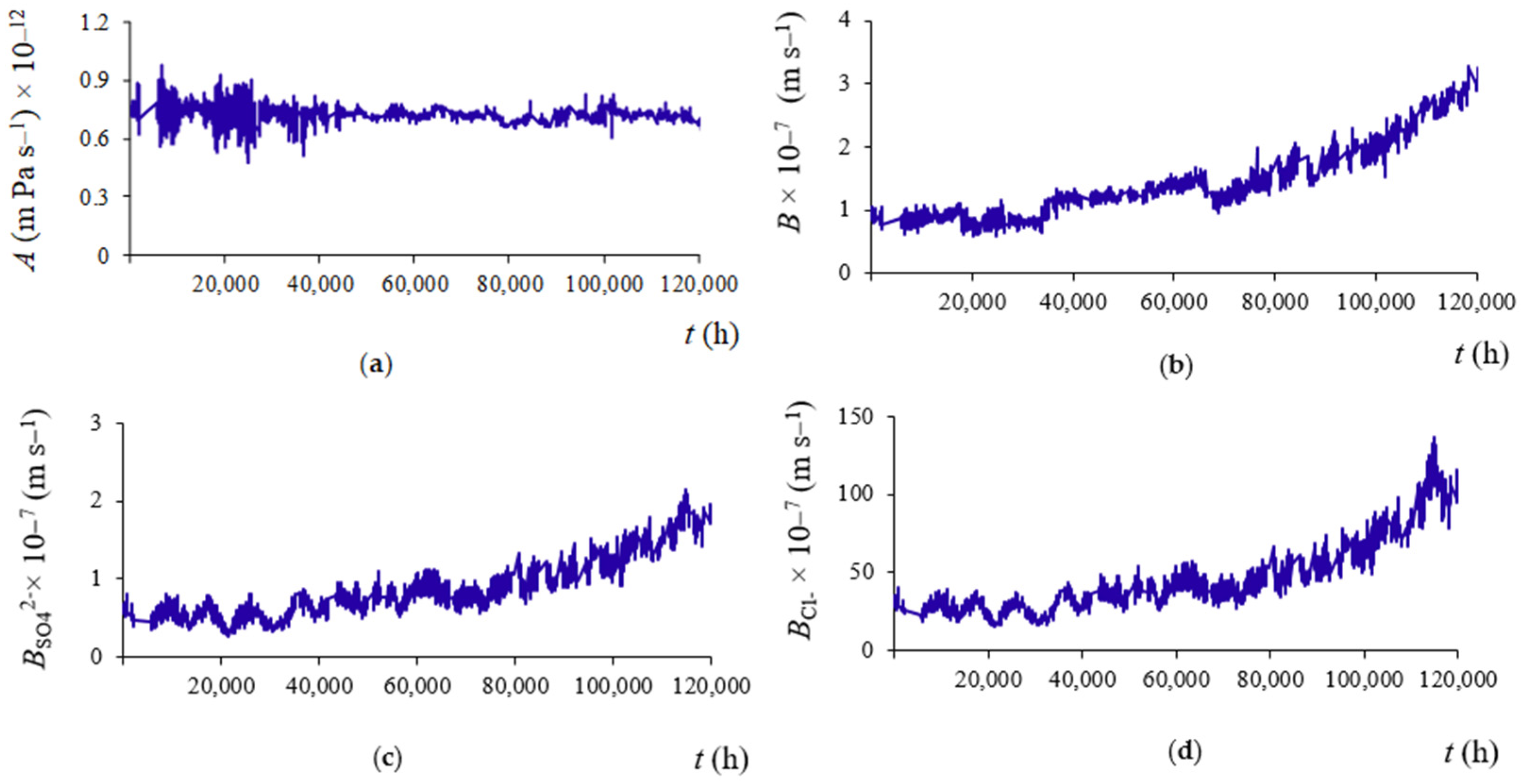
3.3. Permeability Coefficients
3.4. Fouling Characterization
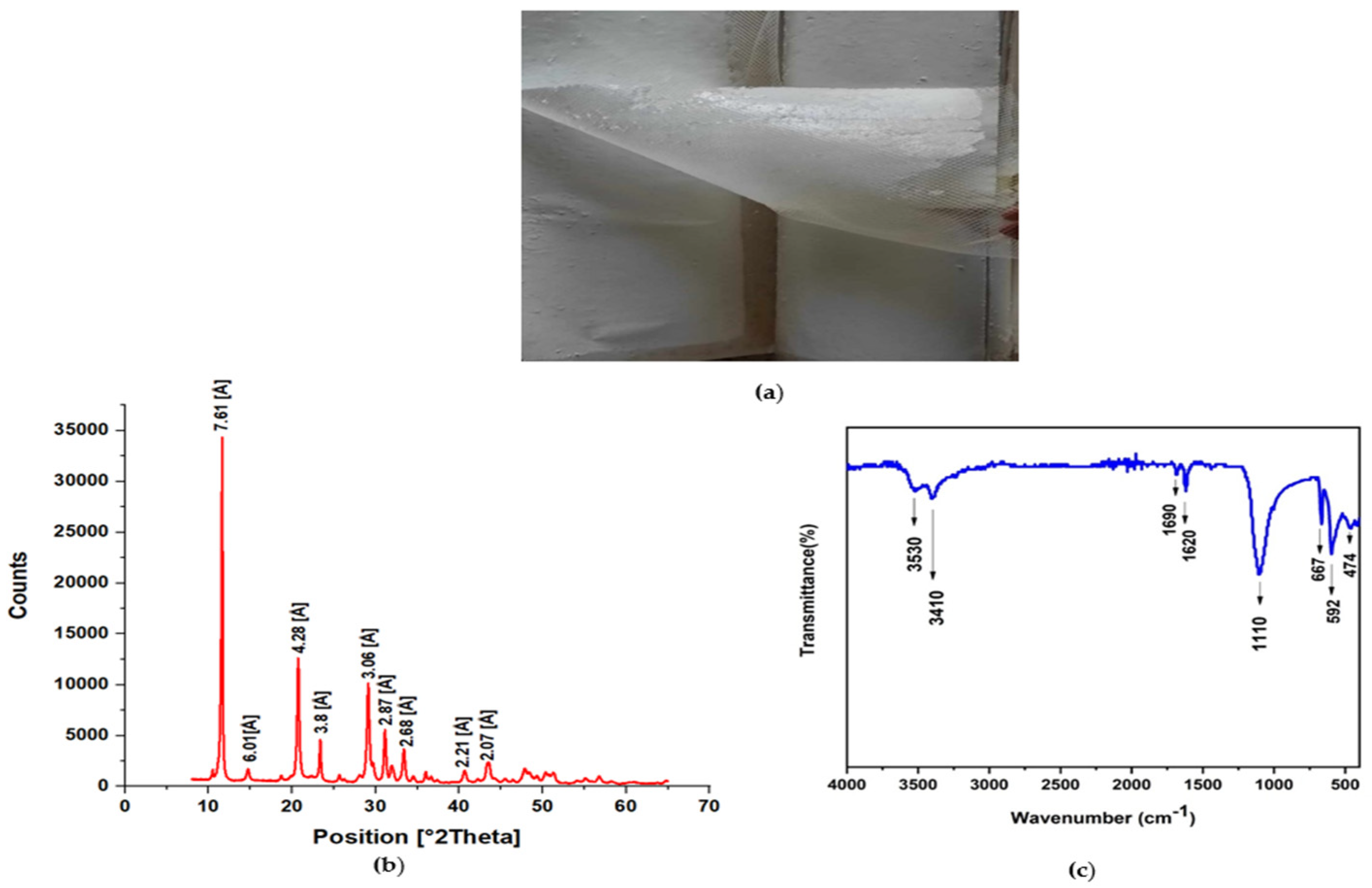
3.4.1. Visual Inspection
3.4.2. X-ray Diffraction (XRD)
3.4.3. FTIR Results
3.4.4. X-ray Fluorescence Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ghazi, Z.M.; Rizvi, S.W.F.; Shahid, W.M.; Abdulhameed, A.M.; Saleem, H.; Zaidi, S.J. An Overview of Water Desalination Systems Integrated with Renewable Energy Sources. Desalination 2022, 542, 116063. [Google Scholar] [CrossRef]
- Ayaz, M.; Namazi, M.A.; ud Din, M.A.; Ershath, M.I.M.; Mansour, A.; Aggoune, E.H.M. Sustainable Seawater Desalination: Current Status, Environmental Implications and Future Expectations. Desalination 2022, 540, 116022. [Google Scholar] [CrossRef]
- Najid, N.; Fellaou, S.; Kouzbour, S.; Gourich, B.; Ruiz-García, A. Energy and Environmental Issues of Seawater Reverse Osmosis Desalination Considering Boron Rejection: A Comprehensive Review and a Case Study of Exergy Analysis. Process Saf. Environ. Prot. 2021, 156, 373–390. [Google Scholar] [CrossRef]
- Belmehdi, F.; Otmani, S.; Taha-Janan, M. Global Trends of Solar Desalination Research: A Bibliometric Analysis during 2010–2021 and Focus on Morocco. Desalination 2023, 555, 116490. [Google Scholar] [CrossRef]
- Shahrim, N.A.; Abounahia, N.M.; El-Sayed, A.M.A.; Saleem, H.; Zaidi, S.J. An Overview on the Progress in Produced Water Desalination by Membrane-Based Technology. J. Water Process Eng. 2023, 51, 103479. [Google Scholar] [CrossRef]
- Voutchkov, N. Energy Use for Membrane Seawater Desalination—Current Status and Trends. Desalination 2018, 431, 2–14. [Google Scholar] [CrossRef]
- Abushawish, A.; Bouaziz, I.; Almanassra, I.W.; AL-Rajabi, M.M.; Jaber, L.; Khalil, A.K.A.; Takriff, M.S.; Laoui, T.; Shanableh, A.; Atieh, M.A.; et al. Desalination Pretreatment Technologies: Current Status and Future Developments. Water 2023, 15, 1572. [Google Scholar] [CrossRef]
- Lou, F.; Nie, S.; Yin, F.; Lu, W.; Ji, H.; Ma, Z.; Kong, X. Numerical and Experimental Research on the Integrated Energy Recovery and Pressure Boost Device for Seawater Reverse Osmosis Desalination System. Desalination 2022, 523, 115408. [Google Scholar] [CrossRef]
- Wang, C.; Meng, P.; Wang, S.; Song, D.; Xiao, Y.; Zhang, Y.; Ma, Q.; Liu, S.; Wang, K.; Zhang, Y. Comparison of Two Types of Energy Recovery Devices: Pressure Exchanger and Turbine in an Island Desalination Project Case. Desalination 2022, 533, 115752. [Google Scholar] [CrossRef]
- Baig, U.; Waheed, A. Fabrication of Polypyrrole-Graphitic Carbon Nitride Nanocomposite Containing Hyper-Cross-Linked Polyamide Photoresponsive Membrane with Self-Cleaning Properties for Water Decontamination and Desalination Applications. J. Water Process Eng. 2022, 47, 102721. [Google Scholar] [CrossRef]
- Thummar, U.G.; Jayalakshmi, J.; Saxena, M.; Suva, Y.; Nandha, N.; Kumar, P.; Aswal, V.K.; Singh, P.S. Highly Water Permeable ‘Reverse Osmosis’ Polyamide Membrane of Folded Nanoscale Film Morphology. J. Water Process Eng. 2023, 55, 104110. [Google Scholar] [CrossRef]
- Tong, Y.; Wei, Y.; Zhang, H.; Wang, L.; Li, L.; Xiao, F.; Gao, C.; Zhu, G. Fabrication of Polyamide Thin Film Nanocomposite Membranes with Enhanced Desalination Performance Modified by Silica Nanoparticles Formed In-Situ Polymerization of Tetramethoxysilane. J. Environ. Chem. Eng. 2023, 11, 109415. [Google Scholar] [CrossRef]
- Ruiz-García, A.; Nuez, I. Simulation-Based Assessment of Safe Operating Windows and Optimization in Full-Scale Seawater Reverse Osmosis Systems. Desalination 2022, 533, 115768. [Google Scholar] [CrossRef]
- Zubair, M.M.; Saleem, H.; Zaidi, S.J. Recent Progress in Reverse Osmosis Modeling: An Overview. Desalination 2023, 564, 116705. [Google Scholar] [CrossRef]
- Naji, O.; Al-juboori, R.A.; Khan, A.; Yadav, S.; Altaee, A.; Alpatova, A.; Soukane, S.; Ghaffour, N. Ultrasound-Assisted Membrane Technologies for Fouling Control and Performance Improvement: A Review. J. Water Process Eng. 2021, 43, 102268. [Google Scholar] [CrossRef]
- Najid, N.; Hakizimana, J.N.; Kouzbour, S.; Gourich, B.; Ruiz-García, A.; Vial, C.; Stiriba, Y.; Semiat, R. Fouling Control and Modeling in Reverse Osmosis for Seawater Desalination: A Review. Comput. Chem. Eng. 2022, 162, 107794. [Google Scholar] [CrossRef]
- Nikkola, J.; Liu, X.; Li, Y.; Raulio, M.; Alakomi, H.L.; Wei, J.; Tang, C.Y. Surface Modification of Thin Film Composite RO Membrane for Enhanced Anti-Biofouling Performance. J. Membr. Sci. 2013, 444, 192–200. [Google Scholar] [CrossRef]
- Goh, P.S.; Zulhairun, A.K.; Ismail, A.F.; Hilal, N. Contemporary Antibiofouling Modifications of Reverse Osmosis Desalination Membrane: A Review. Desalination 2019, 468, 114072. [Google Scholar] [CrossRef]
- Jarma, Y.A.; Thompson, J.; Khan, B.M.; Cohen, Y. Field Evaluation of UF Filtration Pretreatment Impact on RO Membrane Scaling. Water 2023, 15, 847. [Google Scholar] [CrossRef]
- Wilf, M.; Klinko, K. Performance of Commercial Seawater Membranes. Desalination 1994, 96, 465–478. [Google Scholar] [CrossRef]
- Abbas, A.; Al-Bastaki, N. Performance Decline in Brackish Water Film Tec Spiral Wound RO Membranes. Desalination 2001, 136, 281–286. [Google Scholar] [CrossRef]
- Belkacem, M.; Bekhti, S.; Bensadok, K. Groundwater Treatment by Reverse Osmosis. Desalination 2007, 206, 100–106. [Google Scholar] [CrossRef]
- Ruiz-García, A.; Ruiz-Saavedra, E.; Pérez Báez, S.O. Evaluation of the First Seven Years Operating Data of a RO Brackish Water Desalination Plant in Las Palmas, Canary Islands, Spain. Desalin. Water Treat. 2015, 54, 3193–3199. [Google Scholar] [CrossRef]
- Ruiz-García, A.; Ruiz-Saavedra, E. 80,000 h Operational Experience and Performance Analysis of a Brackish Water Reverse Osmosis Desalination Plant. Assessment of Membrane Replacement Cost. Desalination 2015, 375, 81–88. [Google Scholar] [CrossRef]
- Farhat, S.; Bali, M.; Kamel, F. Membrane Autopsy to Provide Solutions to Operational Problems of Jerba Brackish Water Desalination Plant. Desalination 2018, 445, 225–235. [Google Scholar] [CrossRef]
- Adel, M.; Nada, T.; Amin, S.; Anwar, T.; Mohamed, A.A. Characterization of Fouling for a Full-Scale Seawater Reverse Osmosis Plant on the Mediterranean Sea: Membrane Autopsy and Chemical Cleaning Efficiency. Groundw. Sustain. Dev. 2022, 16, 100704. [Google Scholar] [CrossRef]
- Wang, J.; Xing, J.; Li, G.; Yao, Z.; Ni, Z.; Wang, J.; Liang, S.; Zhou, Z.; Zhang, L. How to Extend the Lifetime of RO Membrane? From the Perspective of the End-of-Life RO Membrane Autopsy. Desalination 2023, 561, 116702. [Google Scholar] [CrossRef]
- Tang, F.; Hu, H.Y.; Sun, L.J.; Sun, Y.X.; Shi, N.; Crittenden, J.C. Fouling Characteristics of Reverse Osmosis Membranes at Different Positions of a Full-Scale Plant for Municipal Wastewater Reclamation. Water Res. 2016, 90, 329–336. [Google Scholar] [CrossRef]
- Karime, M.; Bouguecha, S.; Hamrouni, B. RO Membrane Autopsy of Zarzis Brackish Water Desalination Plant. Desalination 2008, 220, 258–266. [Google Scholar] [CrossRef]
- Yang, H.L.; Huang, C.; Pan, J.R. Characteristics of RO Foulants in a Brackish Water Desalination Plant. Desalination 2008, 220, 353–358. [Google Scholar] [CrossRef]
- Kim, S.J.; Oh, B.S.; Yu, H.W.; Kim, L.H.; Kim, C.M.; Yang, E.T.; Shin, M.S.; Jang, A.; Hwang, M.H.; Kim, I.S. Foulant Characterization and Distribution in Spiral Wound Reverse Osmosis Membranes from Different Pressure Vessels. Desalination 2015, 370, 44–52. [Google Scholar] [CrossRef]
- Ruiz-García, A.; Melián-Martel, N.; Mena, V. Fouling Characterization of RO Membranes after 11 Years of Operation in a Brackish Water Desalination Plant. Desalination 2018, 430, 180–185. [Google Scholar] [CrossRef]
- Karmal, I.; Mohareb, S.; El Housse, M.; Hafid, N.; Hadfi, A.; Belattar, M.; Ben-Aazza, S.; Addi, A.A.; Akbour, R.A.; Hamdani, M.; et al. Structural and Morphological Characterization of Scale Deposits on the Reverse Osmosis Membranes: Case of Brackish Water Demineralization Station in Morocco. Groundw. Sustain. Dev. 2020, 11, 100483. [Google Scholar] [CrossRef]
- Tapiero, Y.; Mery, F.; García, A. Understanding of Surface Fouling of Brackish Water Reverse Osmosis Spiral Wound Membrane Using an Integrated Analysis of Seawater Quality and Membrane Autopsy. Chem. Eng. Sci. 2023, 280, 119028. [Google Scholar] [CrossRef]
- Jean, R.; Bernard, L.; Nicole, M. L’Analyse de L’Eau: Eaux Naturelles, Eaux Residuaires, Eau de Mer; R. Jean: Paris, France, 2009; p. 1600. [Google Scholar]
- ISO 8245:1999; Water Quality—Guidelines for the Determination of Total Organic Carbon (TOC) and Dissolved Organic Carbon (DOC). International Organization for Standardization (ISO): Geneva, Switzerland, 1999.
- ASTM D4516-00(2010); Standards Practice for Standardizing Reverse Osmosis Performance Data. ASTM International: West Conshohocken, PA, USA, 2010.
- Gorenflo, A.; Brusilovsky, M.; Faigon, M.; Liberman, B. High PH Operation in Seawater Reverse Osmosis Permeate: First Results from the World’s Largest SWRO Plant in Ashkelon. Desalination 2007, 203, 82–90. [Google Scholar] [CrossRef]
- Zhao, Y.; Taylor, J.S. Assessment of ASTM D 4516 for Evaluation of Reverse Osmosis Membrane Performance. Desalination 2005, 180, 231–244. [Google Scholar] [CrossRef]
- Du, Y.; Xie, L.; Liu, J.; Wang, Y.; Xu, Y.; Wang, S. Multi-Objective Optimization of Reverse Osmosis Networks by Lexicographic Optimization and Augmented Epsilon Constraint Method. Desalination 2014, 333, 66–81. [Google Scholar] [CrossRef]
- Ruiz-García, A.; Nuez, I.; Carrascosa-Chisvert, M.D.; Santana, J.J. Simulations of BWRO Systems under Different Feedwater Characteristics. Analysis of Operation Windows and Optimal Operating Points. Desalination 2020, 491, 114582. [Google Scholar] [CrossRef]
- Schock, G.; Miquel, A. Mass transfer and pressure loss in spiral wound modules. Desalination 1987, 64, 339–352. [Google Scholar] [CrossRef]
- Taniguchi, M.; Kurihara, M.; Kimura, S. Behavior of a Reverse Osmosis Plant Adopting a Brine Conversion Two-Stage Process and Its Computer Simulation. J. Membr. Sci. 2001, 183, 249–257. [Google Scholar] [CrossRef]
- Sharqawy, M.H.; Lienhard, V.J.H.; Zubair, S.M. Thermophysical Properties of Seawater: A Review of Existing Correlations and Data. Desalin. Water Treat. 2010, 16, 354–380. [Google Scholar] [CrossRef]
- Koutsou, C.P.; Yiantsios, S.G.; Karabelas, A.J. A Numerical and Experimental Study of Mass Transfer in Spacer-Filled Channels: Effects of Spacer Geometrical Characteristics and Schmidt Number. J. Membr. Sci. 2009, 326, 234–251. [Google Scholar] [CrossRef]
- Ruiz-García, A.; Nuez, I.; Khayet, M. Performance Assessment and Modeling of an SWRO Pilot Plant with an Energy Recovery Device under Variable Operating Conditions. Desalination 2023, 555, 116523. [Google Scholar] [CrossRef]
- Guissouma, W.; Tarhouni, J. Fluoride in Tunisian Drinking Tap Water. J. Water Ressour. Prot. 2015, 7, 860–870. [Google Scholar] [CrossRef]
- Dudley, L.Y.; Darton, E.G. Pretreatment Procedures to Control Biogrowth and Scale Formation in Membrane Systems. Desalination 1997, 110, 11–20. [Google Scholar] [CrossRef]
- Ruiz-García, A.; Nuez, I. Long-Term Intermittent Operation of a Full-Scale BWRO Desalination Plant. Desalination 2020, 489, 114526. [Google Scholar] [CrossRef]
- Farhat, S.; Kamel, F.; Jedoui, Y.; Kallel, M. The Relation between the RO Fouling Membrane and the Feed Water Quality and the Pretreatment in Djerba Island Plant. Desalination 2012, 286, 412–416. [Google Scholar] [CrossRef]
- Greenlee, L.F.; Lawler, D.F.; Freeman, B.D.; Marrot, B.; Moulin, P. Reverse Osmosis Desalination: Water Sources, Technology, and Today’s Challenges. Water Res. 2009, 43, 2317–2348. [Google Scholar] [CrossRef] [PubMed]
- Matin, A.; Khan, Z.; Zaidi, S.M.J.; Boyce, M.C. Biofouling in Reverse Osmosis Membranes for Seawater Desalination: Phenomena and Prevention. Desalination 2011, 281, 1–16. [Google Scholar] [CrossRef]
- Tay, K.G.; Song, L. A More Effective Method for Fouling Characterization in a Full-Scale Reverse Osmosis Process. Desalination 2005, 177, 95–107. [Google Scholar] [CrossRef]
- Fethi, K.; Habib, C. Performances de La Station de Dessalement de Gabés (22,500 M3/j) Apres Cinq Ans de Fonctionnement. Desalination 2001, 136, 263–272. [Google Scholar] [CrossRef]
- Gryta, M. Influence of Polypropylene Membrane Surface Porosity on the Performance of Membrane Distillation Process. J. Membr. Sci. 2007, 287, 67–78. [Google Scholar] [CrossRef]
- He, F.; Gilron, J.; Lee, H.; Song, L.; Sirkar, K.K. Potential for Scaling by Sparingly Soluble Salts in Crossflow DCMD. J. Membr. Sci. 2008, 311, 68–80. [Google Scholar] [CrossRef]
- Hoek, E.M.V.; Elimelech, M. Cake-Enhanced Concentration Polarization: A New Fouling Mechanism for Salt-Rejecting Membranes. Environ. Sci. Technol. 2003, 37, 5581–5588. [Google Scholar] [CrossRef] [PubMed]
- Park, P.K.; Lee, S.; Cho, J.S.; Kim, J.H. Full-Scale Simulation of Seawater Reverse Osmosis Desalination Processes for Boron Removal: Effect of Membrane Fouling. Water Res. 2012, 46, 3796–3804. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Yun, Z.; Hong, S.; Ahn, K. The Effect of Co-Existing Ions and Surface Characteristics of Nanomembranes of the Removal of Nitrate and Fluoride. Desalination 2001, 133, 53–64. [Google Scholar] [CrossRef]
- Paugam, L.; Diawara, C.K.; Schlumpf, J.P.; Jaouen, P.; Quéméneur, F. Transfer of Monovalent Anions and Nitrates Especially through Nanofiltration Membranes in Brackish Water Conditions. Sep. Purif. Technol. 2004, 40, 237–242. [Google Scholar] [CrossRef]
- Saavedra, E.R.; Gotor, A.G.; Pérez Báez, S.O.; Ramos, M.A.; Ruiz-García, A.; González, A.C. Evaluation of the Five Years Operating Data of a RO Brackish Water Desalination Plant in Las Palmas, Canary Islands, Spain: A Historic Case. Desalin. Water Treat. 2013, 51, 4785–4789. [Google Scholar] [CrossRef]
- Al-Abri, M.; Kyaw, H.H.; Al-Ghafri, B.; Myint, M.T.Z.; Dobretsov, S. Autopsy of Used Reverse Osmosis Membranes from the Largest Seawater Desalination Plant in Oman. Membranes 2022, 12, 671. [Google Scholar] [CrossRef]
- Farrah, H.E.; Lawrance, G.A.; Wanless, E.J. Gypsum-Anhydrite Transformation in Hot Acidic Manganese Sulfate Solution. A Comparative Kinetic Study Employing Several Analytical Methods. Hydrometallurgy 2004, 75, 91–98. [Google Scholar] [CrossRef]
- Ashfaq, M.Y.; Al-Ghouti, M.A.; Da’na, D.A.; Qiblawey, H.; Zouari, N. Effect of Concentration of Calcium and Sulfate Ions on Gypsum Scaling of Reverse Osmosis Membrane, Mechanistic Study. J. Mater. Res. Technol. 2020, 9, 13459–13473. [Google Scholar] [CrossRef]
- Hafid, N.; Belaatar, M.; Ben-Aazza, S.; Hadfi, A.; Ezahri, M.; Driouiche, A. Characterization of Scale Formed in Drinking Water and Hot Water Pipes in the Taliouine Downtown—Morocco. Am. J. Anal. Chem. 2015, 6, 677–686. [Google Scholar] [CrossRef]
- Balcik, C. Understanding the Operational Problems and Fouling Characterization of RO Membrane Used for Brackish Water Treatment via Membrane Autopsy. Water Sci. Technol. 2021, 84, 3653–3662. [Google Scholar] [CrossRef]
- Balan, E.; Fritsch, E.; Radtke, G.; Paulatto, L.; Juillot, F.; Petit, S. First-Principles Modeling of the Infrared Spectrum of Antigorite. Eur. J. Mineral. 2021, 33, 389–400. [Google Scholar] [CrossRef]
- Tang, F.; Hu, H.Y.; Sun, L.J.; Wu, Q.Y.; Jiang, Y.M.; Guan, Y.T.; Huang, J.J. Fouling of Reverse Osmosis Membrane for Municipal Wastewater Reclamation: Autopsy Results from a Full-Scale Plant. Desalination 2014, 349, 73–79. [Google Scholar] [CrossRef]
- Xu, Y.; Liao, Y.; Lin, Z.; Lin, J.; Li, Q.; Lin, J.; Jin, Z. Precipitation of Calcium Sulfate Dihydrate in the Presence of Fulvic Acid and Magnesium Ion. Chem. Eng. J. 2019, 361, 1078–1088. [Google Scholar] [CrossRef]
- Melliti, E.; Touati, K.; Van der Bruggen, B.; Elfil, H. Effect of Fe2+ Ions on Gypsum Precipitation during Bulk Crystallization of Reverse Osmosis Concentrates. Chemosphere 2021, 263, 127866. [Google Scholar] [CrossRef]
- Myka, A.; Łyszczek, R.; Zdunek, A.; Rusek, P. Thermal Analysis of Materials Based on Calcium Sulphate Derived from Various Sources. J. Therm. Anal. Calorim. 2022, 147, 9923–9934. [Google Scholar] [CrossRef]
- El-Zahhar, A.A.; Idris, A.M.; Fawy, K.F.; Arshad, M. SEM, SEM-EDX, µ-ATR-FTIR and XRD for Urban Street Dust Characterisation. Int. J. Environ. Anal. Chem. 2021, 101, 988–1006. [Google Scholar] [CrossRef]
- Xue, W.; Cheng, G.; Wu, Y.; Hou, X.; Wang, W.; Yin, J. Mineral Particles Fouling Analysis and Cleaning in Seawater Reverse Osmosis Desalination. Desalin. Water Treat. 2020, 198, 71–79. [Google Scholar] [CrossRef]
- Sachit, D.E.; Veenstra, J.N. Foulant Analysis of Three RO Membranes Used in Treating Simulated Brackishwater of the Iraqi Marshes. Membranes 2017, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Walha, K.; Amar, R.B.; Firdaous, L.; Quéméneur, F.; Jaouen, P. Brackish Groundwater Treatment by Nanofiltration, Reverse Osmosis and Electrodialysis in Tunisia: Performance and Cost Comparison. Desalination 2007, 207, 95–106. [Google Scholar] [CrossRef]
- Rukapan, W.; Khananthai, B.; Chiemchaisri, C.; Chiemchaisri, W.; Srisukphun, T. Short- and Long-Term Fouling Characteristics of Reverse Osmosis Membrane at Full Scale Leachate Treatment Plant. Water Sci. Technol. 2012, 65, 127–134. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Oddo, J.E.; Tomson, M.B. The Inhibition of Gypsum and Barite Nucleation in NaCl Brines at Temperatures from 25 to 90 °C. Appl. Geochem. 1994, 9, 561–567. [Google Scholar] [CrossRef]
- Pina, C.M.; Putnis, C.V.; Becker, U.; Biswas, S.; Carroll, E.C.; Bosbach, D.; Putnis, A. An Atomic Force Microscopy and Molecular Simulations Study of the Inhibition of Barite Growth by Phosphonates. Surf. Sci. 2004, 553, 61–74. [Google Scholar] [CrossRef]
- Rabizadeh, T.; Peacock, C.L.; Benning, L.G. Investigating the Effectiveness of Phosphonate Additives in Hindering the Calcium Sulfate Dihydrate Scale Formation. Ind. Eng. Chem. Res. 2020, 59, 14970–14980. [Google Scholar] [CrossRef]
- Bosbach, D.; Hochella, M.F. Gypsum Growth in the Presence of Growth Inhibitors: A Scanning Force Microscopy Study. Chem. Geol. 1996, 132, 227–236. [Google Scholar] [CrossRef]
- Akyol, E.; Öner, M.; Barouda, E.; Demadis, K.D. Systematic Structural Determinants of the Effects of Tetraphosphonates on Gypsum Crystallization. Cryst. Growth Des. 2009, 9, 5145–5154. [Google Scholar] [CrossRef]
- Hamouda, S.B.; Akhtar, F.H.; Elfil, H. Diagnosis of Small Capacity Reverse Osmosis Unit for Desalinated Tap Water. Desalin. Water Treat. 2014, 52, 6976–6984. [Google Scholar] [CrossRef]

| Ion | Concentration Range | Limit/Reference [47] |
|---|---|---|
| pH | 7.02–7.84 | >6.5 and <8.5 |
| Ca2+ (mg L−1) | 298–460 | 200 |
| Mg2+ (mg L−1) | 35–112 | 100 |
| Na+ (mg L−1) | 344–500 | 2080 |
| HCO3− (mg L−1) | 77–139 | - |
| SO42− (mg L−1) | 1060–1373 | 500 |
| Cl− (mg L−1) | 638–1080 | 500 |
| SiO2 (mg L−1) | 13–27 | - |
| TDS (mg L−1) | 2960–3350 | >200 and <2000 |
| TOC (mg L−1) | <0.3 | - |
| Turbidity (NTU) | 0.26–1.1 | 3 |
| SDI | 0.5–1.4 | - |
| Ion | Concentration Range (mg L−1) |
|---|---|
| Ca2+ | 16–136 |
| Mg2+ | 3–78 |
| Na+ | 70–102 |
| HCO3− | 9.5–36 |
| SO42− | 45–132 |
| Cl− | 75–180 |
| SiO2 | 1.8–18 |
| TDS | 280–504 |
| Compound | Weight % |
|---|---|
| Calcium oxide (CaO) | 32.498 |
| Loss on ignition (LOI) | 21.56 |
| Sulfur trioxide (SO3) | 45.901 |
| Sodium oxide (Na2O) | 0.047 |
| Magnesium oxide (MgO) | 0.034 |
| Alumina (Al2O3) | 0.021 |
| Silica (SiO2) | 0.130 |
| Phosphorus pentoxide (P2O5) | 0.021 |
| Potassium oxide (K2O) | 0.022 |
| Titanium dioxide (TiO2) | 0.011 |
| Iron oxide (Fe2O3) | 0.007 |
| Strontium oxide (SrO) | 0.230 |
| Manganese oxide (Mn2O3) | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chebil, S.; Ruiz-García, A.; Farhat, S.; Bali, M. Long-Term Performance Evaluation and Fouling Characterization of a Full-Scale Brackish Water Reverse Osmosis Desalination Plant. Water 2024, 16, 1892. https://doi.org/10.3390/w16131892
Chebil S, Ruiz-García A, Farhat S, Bali M. Long-Term Performance Evaluation and Fouling Characterization of a Full-Scale Brackish Water Reverse Osmosis Desalination Plant. Water. 2024; 16(13):1892. https://doi.org/10.3390/w16131892
Chicago/Turabian StyleChebil, Sabrine, A. Ruiz-García, Soumaya Farhat, and Mahmoud Bali. 2024. "Long-Term Performance Evaluation and Fouling Characterization of a Full-Scale Brackish Water Reverse Osmosis Desalination Plant" Water 16, no. 13: 1892. https://doi.org/10.3390/w16131892
APA StyleChebil, S., Ruiz-García, A., Farhat, S., & Bali, M. (2024). Long-Term Performance Evaluation and Fouling Characterization of a Full-Scale Brackish Water Reverse Osmosis Desalination Plant. Water, 16(13), 1892. https://doi.org/10.3390/w16131892










