Subsurface Hydrodynamics of the Southeastern Taoudéni Basin (West Africa) through Hydrogeochemistry and Isotopy
Highlights
- The Taoudéni Basin aquifers are crucial for Sahel's water security and sustainable development.
- Hydrogeochemical and isotopic analyses validate the groundwater flow and reveal multiple eventual aquifer systems.
- Mineralization is primarily driven by silicate hydrolysis and carbonate dissolution.
- Isotopes (δ¹⁸O, δ²H, and ³H) indicate a minimal recent recharge and the presence of ancient, mixed waters.
- The findings highlight the need for sustainable groundwater management due to slow renewal and anthropogenic impacts.
Abstract
:1. Introduction
2. Study Area
2.1. Location
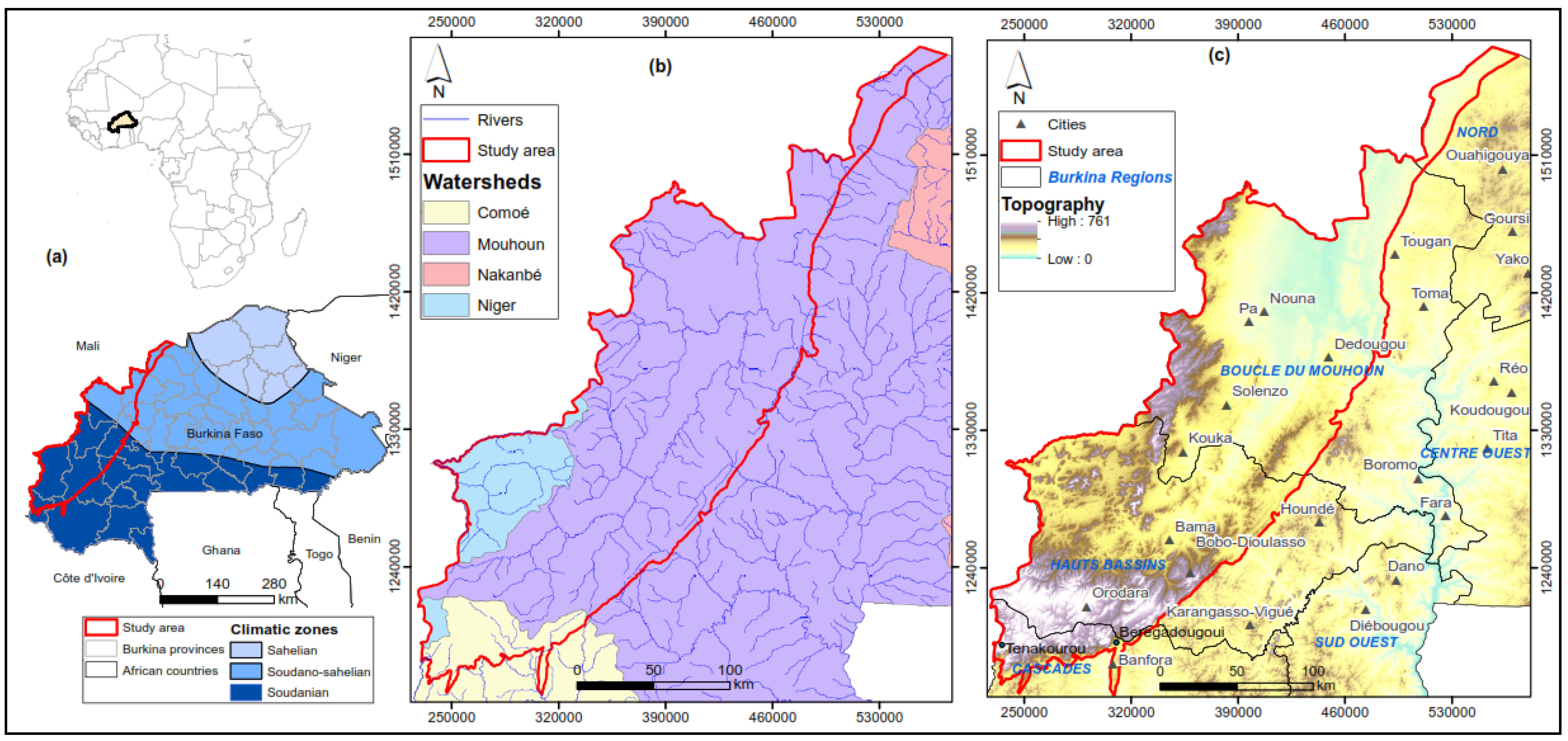
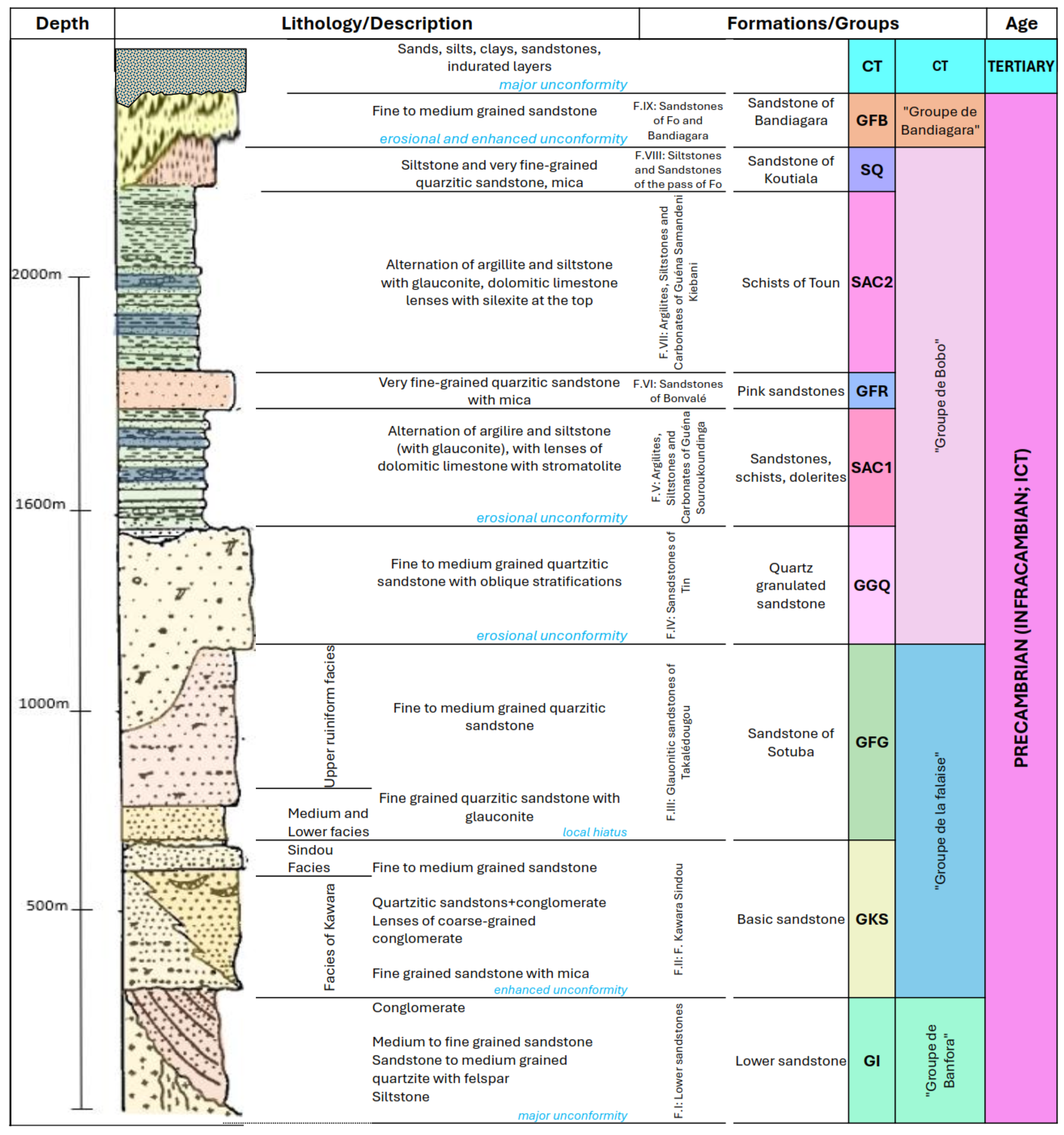
2.2. Geology and Hydrogeology

3. Material and Methods
3.1. Data Collection and Analysis
- Resampling of several points to verify potential geochemical and isotopic changes. Emphasis was placed on points located in the aquifer’s supposed recharge zone (corresponding to high piezometric heights) to verify hypotheses on recharge zones.
- The need to add new points not sampled in previous work, to extend surveys to little-explored areas or aquifer levels. Particular attention was paid to the quartz granulate sandstones (GGQ) level, which appears to be the most productive.
- The need to sample points whose lithological section is available in our borehole database.
- Laboratoire d’Analyse Structurale et Isotopique (LASI) at Centre National de l’Energie des Sciences et des Techniques Nucléaires (CNESTEN), Morocco.
- Laboratoire de Radio-Analyses et Environnement at École Nationale d’Ingénieurs de Sfax, Tunisia.
3.2. Hydrodynamics and Mineralization
- If IS = 0, the water is in equilibrium (saturated) with the mineral.
- If IS < 0, the water is undersaturated with respect to the mineral, meaning that the water will dissolve the mineral.
- If IS > 0, the water is supersaturated, meaning that the water will precipitate the mineral.
3.3. Resource Renewal
4. Results
4.1. Hydrogeochemistry
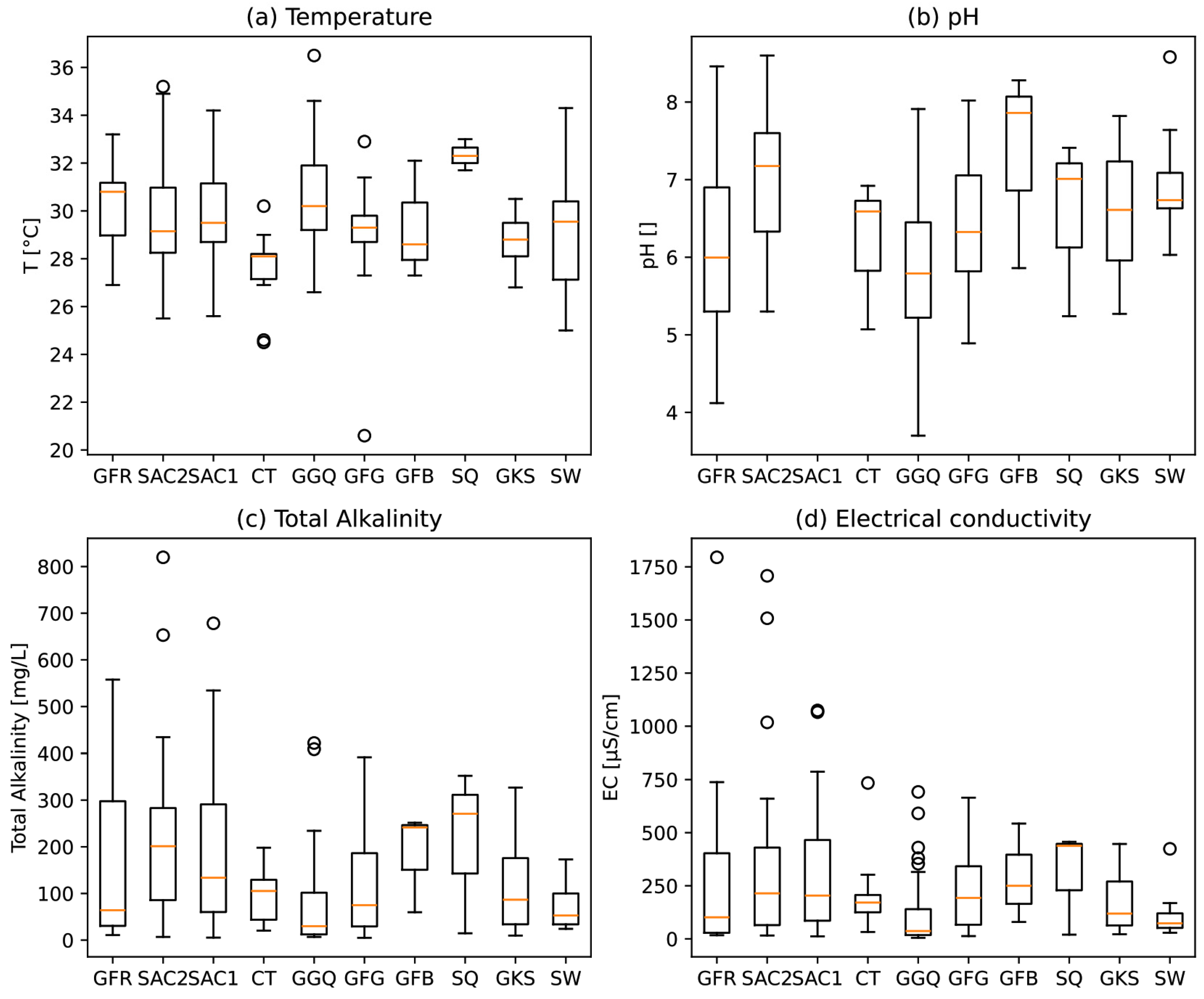
4.1.1. Physico-Chemical Parameters
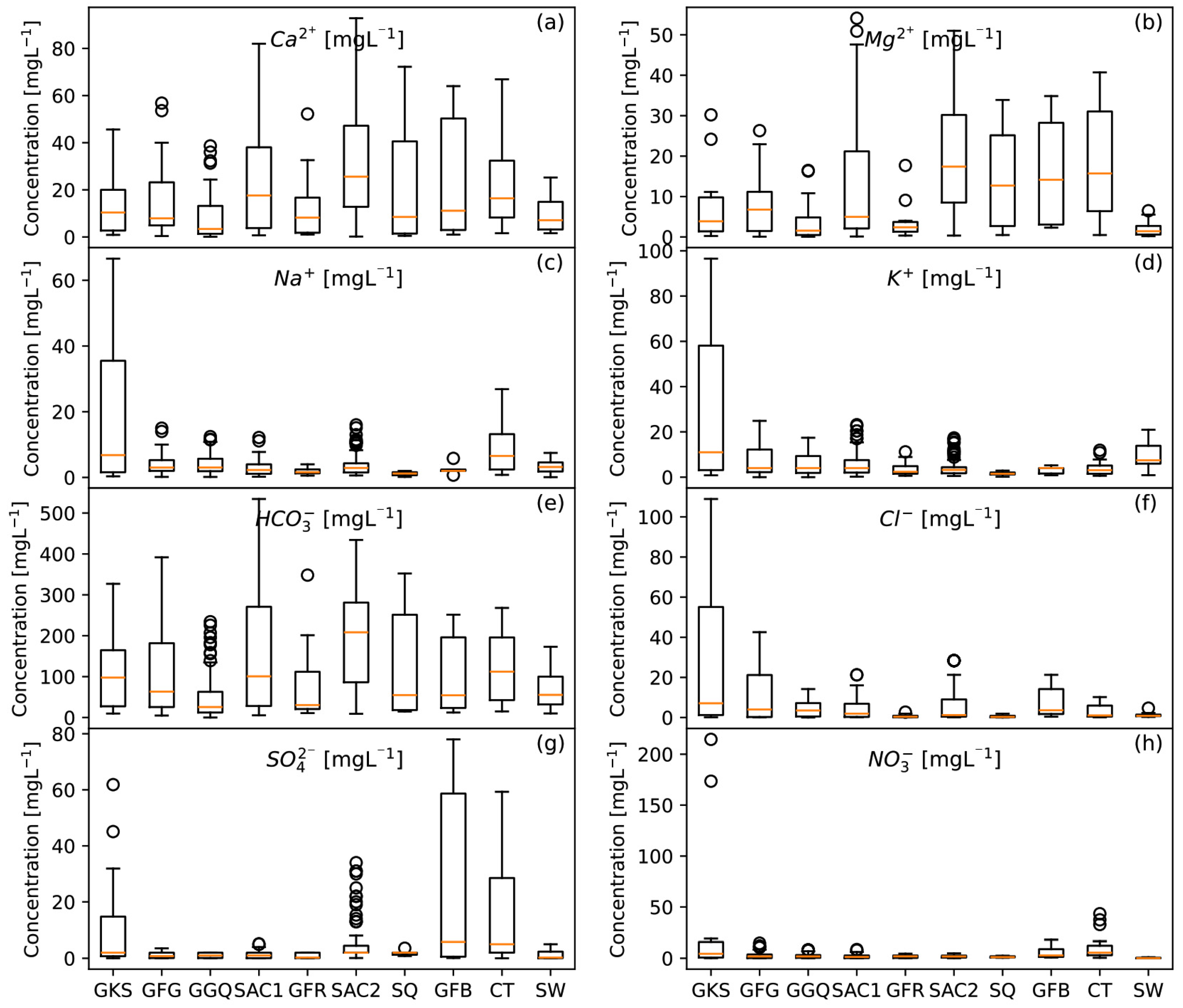
4.1.2. Major Elements
- Bicarbonates: The high concentration of bicarbonates (12 to 850 mg/L) observed in the study area likely originates from several sources. These include carbonate minerals in the soil and underlying formations (SAC1 and SAC2), as well as the contribution of CO2 from decomposing organic matter in the recharge zone, carried by infiltrating rainwater. This is attributed to the presence of carbonate minerals in the soil and geological formations (SAC1 and SAC2) [38].
- Sulfates: Sulfates present concentrations ranging from 0.01 to 167 mg/L, with five samples exceeding potability standards (250 mg/L) [39], mainly from the SAC2 and GFR formations. Potential sources include natural processes such as evaporite dissolution and anthropogenic processes such as sewage infiltration, fertilizer use, and industrial wastewater [35,57]. Although the SAC1 and SAC2 formations originate from marine sedimentation [25,30] favorable to evaporitic dissolution, the low sulfate concentrations suggest a limited contribution to water mineralization.
- Chlorides: Chlorides in groundwater can be of natural origin (precipitation, dissolution of evaporites) or anthropogenic (fertilizers, industrial wastewater). The observed concentrations (0.1 to 108.9 mg/L) show high values, mainly in the SAC1, SAC2, GKS, GFG, and CT formations, indicating a probable marine origin with a likely anthropogenic contribution, notably linked to fertilizer use.
- Nitrates: The presence of nitrates in groundwater (0.01 to 48.8 mg/L) can be attributed to agricultural activities and wastewater discharges. Some 14 samples show nitrate levels over potability standards set at 50 mg/L [39]. The probable reasons also include the low static level of some sampling points and the proximity of faults, facilitating nitrate infiltration.
- Calcium: Calcium is present in all the formations, with an average concentration of 23.96 mg/L, with a high concentration in the SAC1 and SAC2 geological formations. The abundance of calcium can mainly be attributed to the hydrolysis of silicates, the dissolution of carbonates, and, to a small degree, of evaporites.
- Magnesium: The magnesium concentrations, due to silicate hydrolysis and dissolution of carbonate minerals, exceed the limit (≤50 mg/L) in some samples, notably in the SAC1 and SAC2 formations.
- Sodium: Sodium is derived from silicate hydrolysis and evaporite dissolution, and to a lesser extent, from cation exchange [35,40,58]. In terms of the water quality, the water quality of the waterworks in the region meets drinking water standards (≤200 mg/L) [39], suggesting the predominant influence of sandstone formations rich in silicate minerals. In terms of the water quality for irrigation, the sodium levels encountered remain within the required standard [59] except for a single sample, as shown in the Wilcox diagram (Figure 8).
- Potassium: Th potassium concentrations in the groundwater vary between 0.1 and 41 mg/L, generally meeting WHO standards (≤50 mg/L) [39], but 9 out of 347 samples exceed them up to 297.83 mg/L. In addition to silicate hydrolysis (a natural process), the widespread use of potassium fertilizers, notably NPK in agriculture, can also be identified as a likely source of contamination, underlining the need to monitor agricultural practices to ensure groundwater quality in the region.
4.1.3. Hydrochemical Facies
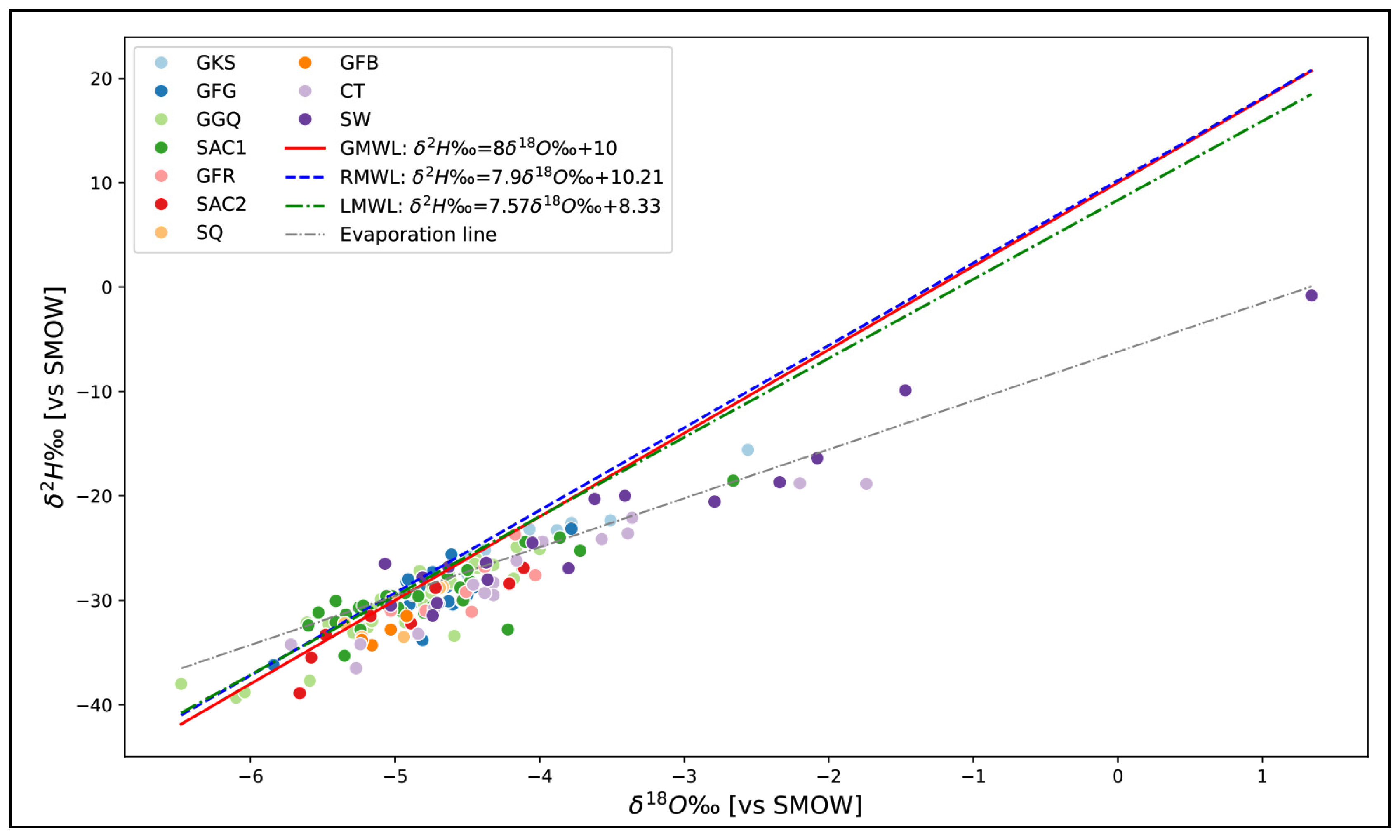
4.2. Isotopic Composition
4.2.1. Isotopic Composition of Precipitation
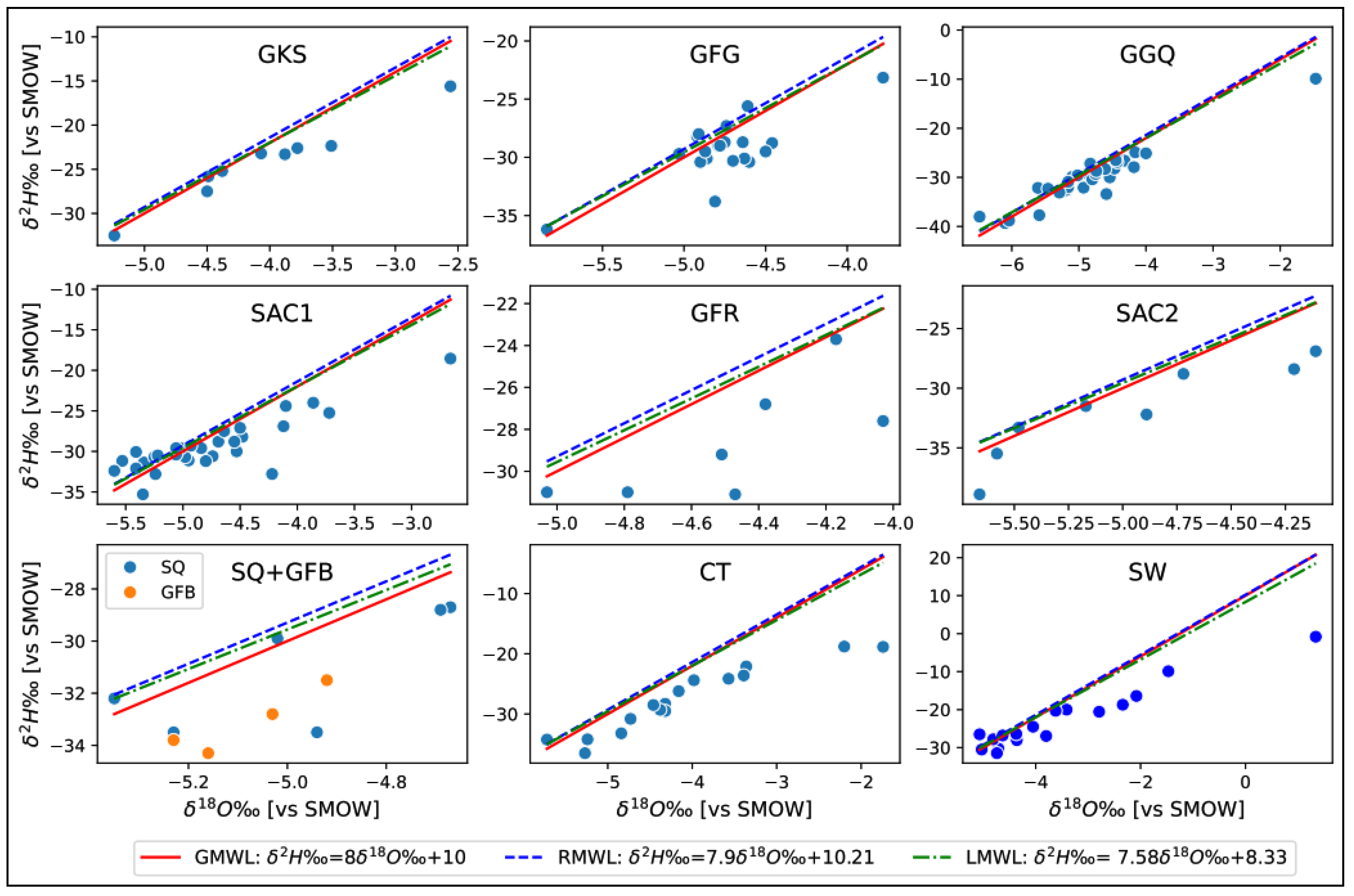
4.2.2. Isotopic Composition of Groundwater
5. Discussion
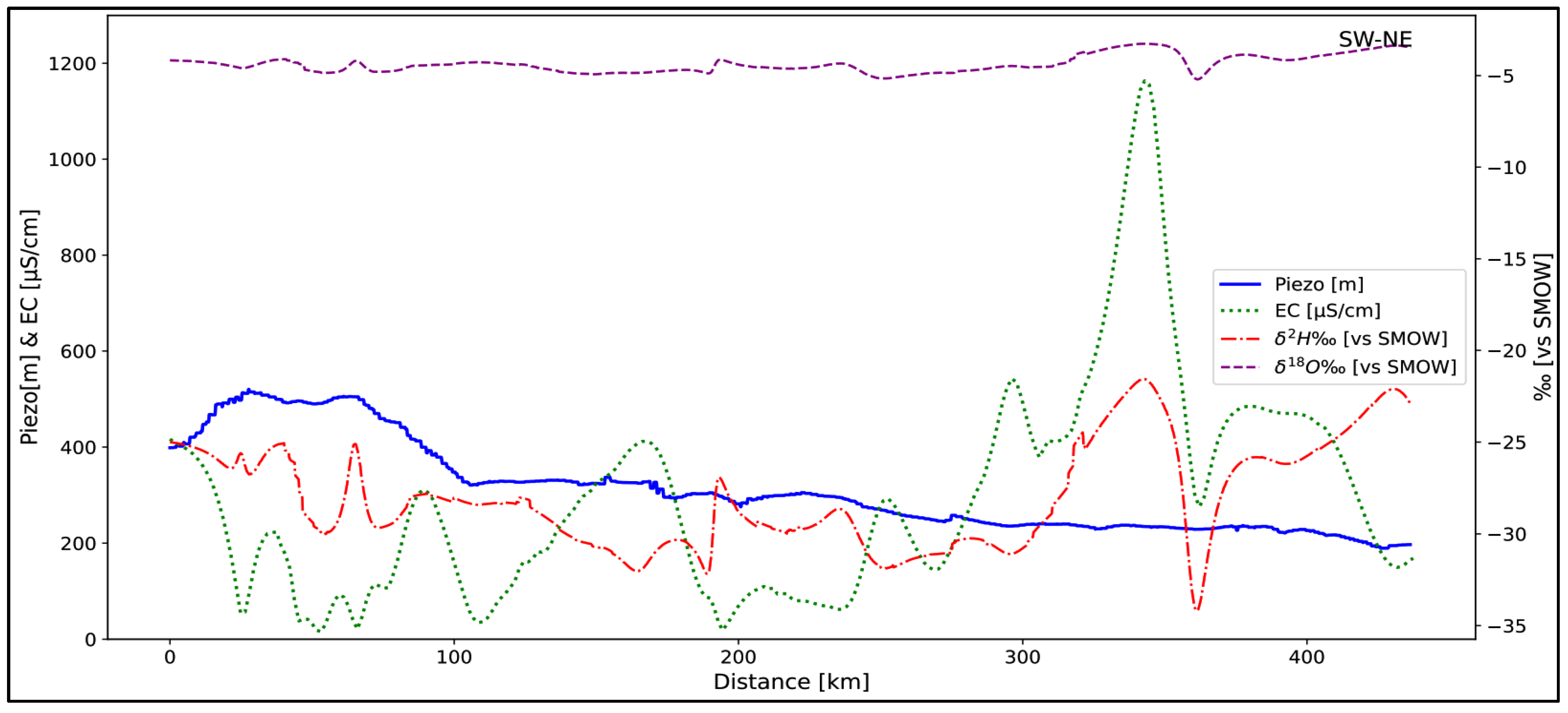
5.1. Hydrodynamics
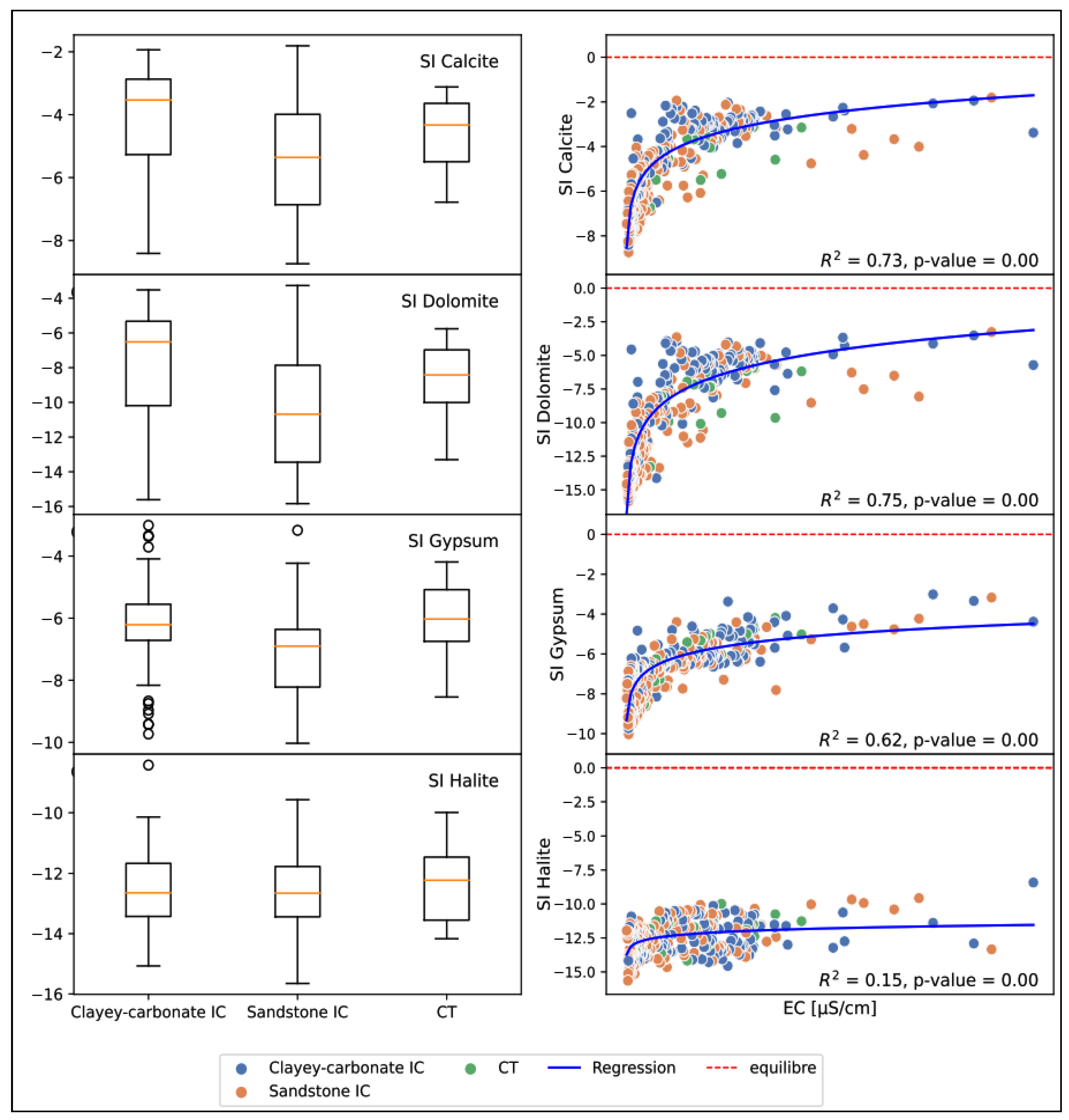
5.2. Groundwater Mineralization
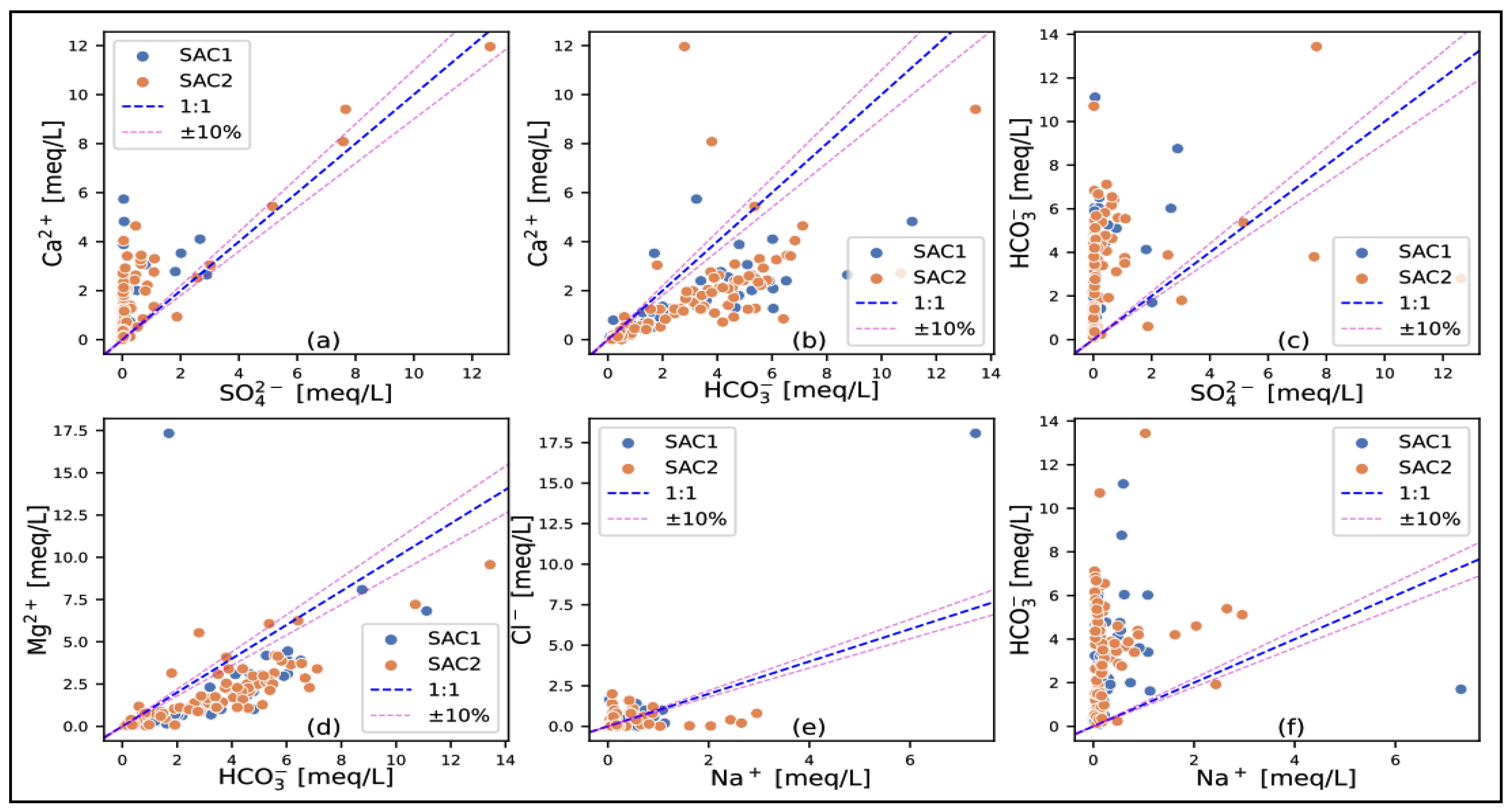
- Evaporitic dissolution: In all three reservoirs, calcium and bicarbonate are generally more abundant than sulfate (Figure 12a,c, Figure 13a,c and Figure 14a,c). However, there are a few samples, mainly from the carbonate and sandstone-carbonate reservoirs of the CT, which are in the equilibrium zone. This indicates that the gypsum (or anhydrite) dissolution process is present but less significant. This may be supported by a general undersaturation to gypsum, with some high values observed in the formations mentioned above due to the presence of gypsum in the SAC1 and SAC2 formations. In terms of halite dissolution, sodium is more abundant than chlorine in the IC sandstone and CT sandstone-carbonate reservoirs, with a few points on the equilibrium line in the IC sandstone formations (Figure 12e and Figure 14e). For the carbonate formations (Figure 13e), we find roughly the same quantities on either side of the equilibrium line, with points also located on the line. The high halite undersaturation observed in all the formations indicates that the presence of halite is not due to the evaporitic dissolution of halite but rather to anthropogenic input, particularly in the shallows where water evaporation contributes to increasing sodium concentration.
- Carbonate dissolution: The diagrams and show the contribution of carbonate dissolution (calcite, dolomite) to water mineralization. The sandstone IC (Figure 12b,d) shows little influence of carbonate dissolution on water mineralization, with calcium and magnesium coming more from other mechanisms (silicate hydrolysis, cation exchange) than from carbonate dissolution. Clay-carbonate IC (Figure 13b,d) and CT (Figure 14b,d) show a significant contribution of carbonate dissolution to water mineralization. These findings are confirmed by the saturation indices for dolomite and calcite; the waters in general show a slight undersaturation of these two indices. It should be noted, however, that the clay-carbonate IC and the CT have higher average contents than the sandstone IC.
- Cation exchange: The diagram shows the same trend for all three reservoir types (Figure 12f, Figure 13f and Figure 14f). We note that the points are more toward the bicarbonate pole and only a few toward the sodium pole, marking here the cation exchange mechanism. This diagram shows that silicate hydrolysis remains the major process responsible for groundwater mineralization.
- In the sandstone aquifer (IC), most water samples plot close to and below the equilibrium line, tending toward the pole side. This suggests silicate hydrolysis as the dominant process governing their chemistry. However, the presence of a few samples above the line indicates that carbonate dissolution can also play a secondary role in influencing the water composition of these specific samples.
- In the case of the clay-carbonate IC, the number of samples on either side of the equilibrium line is roughly the same, indicating that silicate hydrolysis and carbonate dissolution are the two major processes controlling water mineralization.
- For the CT, most water samples are located near and above the equilibrium line on the This indicates that dissolution of carbonate rocks is the main hydrochemical process in the system, followed by the hydrolysis of silicates.
- In the CT formation, water samples primarily cluster near and above the equilibrium line. This distribution suggests that carbonate rock dissolution is the dominant hydrogeochemical process, with silicate hydrolysis playing a secondary role.
5.3. Groundwater Renewal
- Water below the global meteoric line: This is deep water from a paleorecharge. They account for 80% of samples.
- Water located above the meteoric line: This water is mainly located at the level of the piezometric dome and is mostly superficial. This is water from current recharges, representing 20% of samples.
- Contains less than 0.30 TU: These are ancient waters from deep aquifers.
- Contents between 0.30 and 3 TU: These are waters resulting from a mixture of old and young waters.
- Content over 3 TU: This refers to water from current recharge.
- Old water accounts for 12% of the samples collected.
- Mixed water accounts for 74% of the samples collected.
- And recent waters account for 14% of the samples collected.
5.4. Aquifer’s Discrimination
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dakoure, D. Etude Hydrogéologique et Géochimique de La Bordure Sud-Est Du Bassin de Taoudéni (Burkina Faso-Mali)-Essai de Modélisation. Ph.D. Dissertation, Université Paris VI-Pierre et Marie Curie, Paris, France, 2003. [Google Scholar]
- Tirogo, J.; Jost, A.; Biaou, A.; Valdes-Lao, D.; Koussoubé, Y.; Ribstein, P.; Tirogo, J.; Jost, A.; Biaou, A.; Valdes-Lao, D.; et al. Climate Variability and Groundwater Response: A Case Study in Burkina Faso (West Africa). Water 2016, 8, 171. [Google Scholar] [CrossRef]
- Gramont, H.M.D.; Savadogo, A.N.; Dakoure, D. Amélioration de la Connaissance et de la Gestion des Eaux au Burkina Faso. P162723. Annexe 1: Diagnostic sur les Eaux Souterraines; La Banque Mondiale: Washington, DC, USA, 2017. [Google Scholar]
- AIEA. Adding the Groundwater Dimension to the Understanding and Management of Shared Water Resources in the Sahel Region; Vienna International Centre: Vienna, Austria, 2018. [Google Scholar]
- Kouanda, B. Modélisation Intégrée Du Complexe Mouhoun Supérieur-Sourou Dans Le Contexte Des Changements Climatiques. Ph.D. Dissertation, Institut International d’Ingénierie de l’Eau et de l’Environnement (2iE), Ouagadougou, Burkina Faso, 2019. [Google Scholar]
- Tirogo, Y.J. Etude Du Fonctionnement Hydrodynamique de l’aquifère Sédimentaire Du Bassin de Kou Au Sud-Ouest Du Burkina Faso. Ph.D. Dissertation, Institut International d’Ingénierie de l’Eau et de l’Environnement (2iE)-Université Pierre et Marie Curie (UPMC), Paris, France, 2016. [Google Scholar]
- Khan, M.Y.A.; El Kashouty, M.; Gusti, W.; Kumar, A.; Subyani, A.M.; Alshehri, A. Geo-Temporal Signatures of Physicochemical and Heavy Metals Pollution in Groundwater of Khulais Region—Makkah Province, Saudi Arabia. Front. Environ. Sci. 2022, 9, 800517. [Google Scholar] [CrossRef]
- Bhat, M.A.; Zhong, J.; Dar, T.; Kumar, A.; Li, S.-L. Spatial Distribution of Stable Isotopes in Surface Water on the Upper Indus River Basin (UIRB): Implications for Moisture Source and Paleoelevation Reconstruction. Appl. Geochem. 2022, 136, 105137. [Google Scholar] [CrossRef]
- Krishan, G.; Rao, M.S.; Vashisht, R.; Chaudhary, A.; Singh, J.; Kumar, A. Isotopic Assessment of Groundwater Salinity: A Case Study of the Southwest (SW) Region of Punjab, India. Water 2022, 14, 133. [Google Scholar] [CrossRef]
- Sandao, I.; Babaye, M.S.A.; Ousmane, B.; Michelot, J.L. Apports Des Isotopes Naturels de l’eau à La Caractérisation Des Mécanismes de Recharge Des Aquifères Du Bassin de La Korama, Région de Zinder, Niger. Int. J. Biol. Chem. Sci. 2018, 12, 1931–1954. [Google Scholar] [CrossRef]
- Fang, C.; Sun, S.; Jia, S.; Li, Y. Groundwater Level Analysis Using Regional Kendall Test for Trend with Spatial Autocorrelation. Groundwater 2019, 57, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Murhula, E.M.; Kutangila, S.M.; Bihenjira, E.M.; Muyisa, S.K. Hydrogéochimie et susceptibilité à la contamination des eaux souterraines dans le secteur de Panzi, ville de Bukavu, RD Congo (Hydrogeochemistry and susceptibility to groundwater contamination in the Panzi sector, Bukavu City, DR Congo). Geo-Eco-Trop 2019, 43, 197–209. [Google Scholar]
- Bäumle, R.; Himmelsbach, T.; Noell, U. Hydrogeology and Geochemistry of a Tectonically Controlled, Deep-Seated and Semi-Fossil Aquifer in the Zambezi Region (Namibia). Hydrogeol. J. 2019, 27, 885–914. [Google Scholar] [CrossRef]
- Mokhtar, K.; Boualem, B.; Layachi, G. Hydrochemical Characterization and Water Quality of the Continental Intercalare Aquifer in the Ghardaïa Region (Algerian Sahara). J. Ecol. Eng. 2021, 22, 152–162. [Google Scholar] [CrossRef]
- Rahal, O.; Gouaidia, L.; Fidelibus, M.D.; Marchina, C.; Natali, C.; Bianchini, G. Hydrogeological and Geochemical Characterization of Groundwater in the F’Kirina Plain (Eastern Algeria). Appl. Geochem. 2021, 130, 104983. [Google Scholar] [CrossRef]
- Kamal, S.; Sefiani, S.; Laftouhi, N.-E.; El Mandour, A.; Moustadraf, J.; Elgettafi, M.; Himi, M.; Casas, A. Hydrochemical and Isotopic Assessment for Characterizing Groundwater Quality and Recharge Processes under a Semi Arid Area: Case of the Haouz Plain Aquifer (Central Morocco). J. Afr. Earth Sci. 2021, 174, 104077. [Google Scholar] [CrossRef]
- Hakimi, Y.; Orban, P.; Deschamps, P.; Brouyere, S. Hydrochemical and Isotopic Characteristics of Groundwater in the Continental Intercalaire Aquifer System: Insights from Mzab Ridge and Surrounding Regions, North of the Algerian Sahara. J. Hydrol. Reg. Stud. 2021, 34, 100791. [Google Scholar] [CrossRef]
- Allen, S.T.; Sprenger, M.; Bowen, G.J.; Brooks, J.R. Spatial and Temporal Variations in Plant Source Water. In Stable Isotopes in Tree Rings: Inferring Physiological, Climatic and Environmental Responses; Springer International Publishing: Cham, Switzerland, 2022; pp. 501–535. [Google Scholar]
- Fantong, W.Y.; Chounna, G.; Nenkam, T.L.L.J.; Fouepe, A.T.; Fru, E.C.; Vassolo, S.; Montcoudiol, N.; Fodoue, Y.; Haman, J.B.D.; Carlier, C.; et al. Hydrogeochemistry of Low Agricultural Soil Yield in Sahelian and Sub-Tropical Watersheds, Northern Cameroon. J. Afr. Earth Sci. 2023, 199, 104823. [Google Scholar] [CrossRef]
- Javed, T.; Ahmad, N.; Ahmad, S.R. Coupling Hydrogeochemistry and Stable Isotopes (δ2H, δ18O and δ13C) to Identify Factors Affecting Arsenic Enrichment of Surface Water and Groundwater in Precambrian Sedimentary Rocks, Eastern Salt Range, Punjab, Pakistan. Environ. Geochem. Health 2023, 45, 6643–6673. [Google Scholar] [CrossRef] [PubMed]
- Zouari, K.; Trabelsi, R.; Araguás Araguás, L.L.; Hussaini, S.; Rabe, S.; Alassane, A. Use of Hydrochemical and Isotopic Tracers to Investigate the Groundwater Quality and Recharge Processes of the Shared Iullemeden Aquifer System in the Sahel Region (Western Africa). Hydrogeol. J. 2024, 32, 219–240. [Google Scholar] [CrossRef]
- Lindsey, B.D.; Jurgens, B.C.; Belitz, K. Tritium as an Indicator of Modern, Mixed, and Premodern Groundwater Age; US Geological Survey: Reston, VA, USA, 2019.
- Huneau, F.; Dakoure, D.; Celle-Jeanton, H.; Vitvar, T.; Ito, M.; Traore, S.; Compaore, N.F.; Jirakova, H.; Le Coustumer, P. Flow Pattern and Residence Time of Groundwater within the South-Eastern Taoudeni Sedimentary Basin (Burkina Faso, Mali). J. Hydrol. 2011, 409, 423–439. [Google Scholar] [CrossRef]
- Taupin, J.D. Gestion Intégrée et Durable des Systèmes Aquifères et des Bassins Partagés de la Région du Sahel. RAF/7/011. Bassin de Taoudéni; AIEA: Vienne, Austria, 2017. [Google Scholar]
- Ouedraogo, C. Synthèse Géologique de la Région Ouest du Burkina Faso; Programme de Valorisation des Ressources en Eau de l’Ouest (P/VREO); Ministère de l’Agriculture, de l’Hydraulique et des Ressources Halieutiques: Ouagadougou, Burkina Faso, 2006.
- du Bois, K.; Boyer, P.; Fiévet, B.; Garnier-Laplace, J.; Gurriaran, R.; Le Dizes-Maurel, S.; Maro, D.; Masson, M.; Pierrard, O.; Renaud, P. Inventaire Des Sources de Tritium et Synthèse Bibliographique. In Livre Blanc du Tritium: Groupes de Réflexion Menés de Mai 2008 à Avril 2010 Sous l’égide de l’ASN et Bilan Annuel des Rejets de Tritium Pour les Installations Nucléaire de Base de 2015 à 2019; Autorité de Sûreté Nucléaire: Montrouge, France, 2010; pp. 44–110. [Google Scholar]
- Gombert, P. Synthèse sur la Géologie et l’hydrogéologie de la Formation Sédimentaire des Grès Inferieurs; 1998; p. 29. [Google Scholar]
- Dipama, J.M. Le Mécanisme Général de La Genèse des Pluies et Leur Répartition au Burkina-Faso; Espace Scientifique: Rennes, France, 2005. [Google Scholar]
- Derouane, J. Modélisation Hydrogéologique du Bassin Sédimentaire; Programme de Valorisation des Ressources en Eau de l’Ouest (VREO); Direction Générale des Ressources en Eau. Inistère de l’Agriculture, de l’Hydraulique et des Ressources Halieutiques: Ouagadougou, Burkina Faso, 2008; p. 101.
- Trompette, R. Le Précambrien Supérieur et le Paléozoïque Inférieur de l’Adrar de Mauritanie (Bordure Occidentale du Bassin de Taoudeni, Afrique de l’Ouest), un Exemple de Sédimentation de Craton. Étude Stratigraphique et Sédimentologique—TOME 2 (Séries 2 et 3). Ph.D. Thesis, Université de Provence—Aix-Marseille I, Marseille, France, 1973. [Google Scholar]
- Bronner, G.; Roussel, J.; Trompette, R.; Clauer, N. Genesis and Geodynamic Evolution of the Taoudeni Cratonic Basin (Upper Precambrian and Paleozoic), Western Africa. Dyn. Plate Inter. 1980, 1, 81–90. [Google Scholar]
- Ouedraogo, C. Étude Géologique des Formations Sédimentaires du Bassin Précambrien Supérieur et Paléozoïque de Taoudéni en Haute-Volta. Ph.D. Thesis, Laboratoire de pétrologie de la surface, Université de Poitiers, Poitiers, France, 1983. [Google Scholar]
- Kruserman, G.; de Ridder, N. Analysis and Evaluation of Pumping Test Data, 2nd ed.; International Institute for Land Reclamation and Improvement (ILRI); Veenman drukers: Wageningen, The Netherlands, 1994. [Google Scholar]
- Castaing, C.; Metour, J.L.; Billa, M.; Donzeau, P.; Chevremont, P.; Egal, E.; Zida, B. Carte Géologique et Minière du Burkina à 1/1 000 000 du Burkina Faso; BRGM: Ouagadougou, Burkina Faso, 2003. [Google Scholar]
- Appelo, C.A.J.; Postma, D. Geochemistry, Groundwater and Pollution; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Sundaram, B.; Feitz, A.J.; de Caritat, P.; Plazinska, A.; Brodie, R.S.; Coram, J.; Ransley, T. Groundwater Sampling and Analysis—A Field Guide. Geosci. Aust. Rec. 2009, 27, 104. [Google Scholar]
- Mouhri, A.; Flipo, N.; Rejiba, F.; de Fouquet, C.; Bodet, L.; Kurtulus, B.; Tallec, G.; Durand, V.; Jost, A.; Ansart, P.; et al. Designing a Multi-Scale Sampling System of Stream—Aquifer Interfaces in a Sedimentary Basin. J. Hydrol. 2013, 504, 194–206. [Google Scholar] [CrossRef]
- Hiscock, K.M.; Bense, V.F. Hydrogeology: Principles and Practice; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- WHO. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating First Addendum, 4th ed.; 1st add.; World Health Organization: Geneva, Switzerland, 2017; ISBN 978-92-4-154995-0. [Google Scholar]
- Ndembo, L. Apport des Outils Hydrogéochimiques et Isotopiques à la Gestion de l’aquifère du Mont Amba. Ph.D. Thesis, Université de Kinshasa, Université de Avignon et Pays de Vaucluse, Vaucluse, France, 2009. [Google Scholar]
- Asmael, N. Hydrochemistry, Isotopes and Groundwater Modeling to Characterize Multi-Layered Aquifers Flow System in the Upper Part of Awaj River—Damascus Basin (Syria). Ph.D. Dissertation, Université Michel de Montaigne, Bordeaux, France, 2015. [Google Scholar]
- Plummer, L.N.; Jones, B.F.; Truesdell, A.H. WATEQF—A FORTRAN IV Version of WATEQ: A Computer Program for Calculating Chemical Equilibrium of Natural Waters; Department of the Interior, Geological Survey, Water Resources Division: Reston, VA, USA, 1976.
- Alley, W.M. Regional Ground-Water Quality; Van Nostrand Reinhold: New York, NY, USA, 1993; ISBN 978-0-442-00937-3. [Google Scholar]
- Parkhust, D.; Appelo, C. User’s Guide to PHREEQC (Version 2): A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations; U.S. Geological Survey, Earth Science Information Center: Reston, VA, USA, 1999.
- Similer, R. Diagrammes. Manuel d’Utilisation 2014. Available online: https://terre-et-eau.univ-avignon.fr/equipements-de-terrain-et-de-laboratoire/logiciels/ (accessed on 14 May 2023).
- Clark, I.D.; Fritz, P. Environmental Isotopes in Hydrogeology; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Aggarwal, P.K.; Araguás-Araguás, L.J.; Groening, M.; Kulkarni, K.M.; Kurttas, T.; Newman, B.D.; Vitvar, T. Global Hydrological Isotope Data and Data Networks. In Isoscapes: Understanding Movement, Pattern, and Process on Earth through Isotope Mapping; Springer: Berlin/Heidelberg, Germany, 2010; pp. 33–50. [Google Scholar]
- Cook, P. Introduction to Isotopes and Environmental Tracers as Indicators of Groundwater Flow; The Groundwater Project: Guelph, ON, Canada, 2020; ISBN 978-1-77705-418-2. [Google Scholar]
- Hubert, P.; Olive, P.; Ravailleau, S. Estimation pratique de l’âge des eaux souterraines par le tritium. Rev. Sci. L’eau 1996, 9, 523–533. [Google Scholar] [CrossRef]
- Edmunds, W.M.; Smedley, P.L. Groundwater Geochemistry and Health: An Overview. Geol. Soc. Lond. Spec. Publ. 1996, 113, 91–105. [Google Scholar] [CrossRef]
- Naidu, S.; Gupta, G.; Singh, R.; Tahama, K.; Erram, V.C. Hydrogeochemical Processes Regulating the Groundwater Quality and Its Suitability for Drinking and Irrigation Purpose in Parts of Coastal Sindhudurg District, Maharashtra. J. Geol. Soc. India 2021, 97, 173–185. [Google Scholar] [CrossRef]
- Bieupoude, G. Pascal Mapping Groundwater Intrinsic Vulnerability Using a New Physically Based Modeling in Kou Basin Bobo-Dioulasso/Burkina Faso. Ph.D. Dissertation, Institut International d’Ingénierie de l’Eau et de l’Environnement (2iE), Ouagadougou, Burkina Faso, 2008. [Google Scholar]
- Samara, T.; Yoxas, G. Drastic Method to Map Groundwater Vulnerability to Pollution Using Nitrate Measurements in Agricultural Areas. Bull. Geol. Soc. Greece 2013, 47, 981–991. [Google Scholar] [CrossRef]
- Bambara, A.; Orban, P.; Therrien, R.; Hallot, E.; Brouyère, S. Modelling Exchanges between Surface Water Reservoirs and Groundwater in Basement Areas: Case of Kierma (Burkina Faso). In Proceedings of the 48ème Congrès Annuel de l’Association Internationale des Hydrogéologues, Bruxelles, Belgium, 6–10 September 2021. [Google Scholar]
- Hassoune, E.M.; Koulali, Y.; Hadarbach, D.; Bouzidi, A. Effets Des Rejets Liquides Domestiques et Industriels Sur La Qualité Des Eaux Souterraines Au Nord de La Ville de Settat (Maroc). Bull. L’institut Sci. Rabat 2006, 28, 61–71. [Google Scholar]
- Asslouj, J.; Kholtei, S.; Amrani-Paaza, N.; Hilali, A. Impact des activités anthropiques sur la qualité des eaux souterraines de la communauté Mzamza (Chaouia, Maroc). Rev. Sci. L’eau 2007, 20, 309–321. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Q. Research Advances in Identifying Sulfate Contamination Sources of Water Environment by Using Stable Isotopes. Int. J. Env. Res. Public Health 2019, 16, 1914. [Google Scholar] [CrossRef] [PubMed]
- Demlie, M.; Titus, R. Hydrogeological and Hydrogeochemical Characteristics of the Natal Group Sandstone, South Africa. S. Afr. J. Geol. 2015, 118, 33–44. [Google Scholar] [CrossRef]
- Fetter, C.W. Applied Hydrogeology, 4th ed.; Prentice-Hall, Inc.: Hoboken, NJ, USA, 2001; ISBN 0-13-088239-9. [Google Scholar]
- Gourcy, L.; Aranyossy, J.-F.; Olivry, J.-C.; Zuppi, G.M. Évolution Spatio-Temporelle Des Teneurs Isotopiques (δ2H–δ18O) Des Eaux de La Cuvette Lacustre Du Fleuve Niger (Mali). Comptes Rendus L’académie Sci. Ser. IIA-Earth Planet. Sci. 2000, 331, 701–707. [Google Scholar] [CrossRef]
- Mathieu, R.; Bariac, T.; Fouillac, C.; Guillot, B.; Mariotti, A. Variation En Isotopes Stables Dans Les Précipitations En 1988 et 1989 Au Burkina Faso; Apports de La Météorologie Régionale [Stable Isotopes Variations in Rainfall in 1988 and 189 in Burkina-Faso: Contributions of Regional Meteorology]. ORSTOM-METEO FRANCE Veill. Clim. Satellitaire 1993, 45, 47–64. [Google Scholar]
- Craig, H. Standard for Reporting Concentrations of Deuterium and Oxygen-18 in Natural Waters. Science 1961, 133, 1833–1834. [Google Scholar] [CrossRef]
- Fontes, J.-C. Les Isotopes Du Milieu Dans Les Eaux Naturelles. La Houille Blanche 1976, 205–221. [Google Scholar] [CrossRef]
- Kafando, M.B.; Koïta, M.; Le Coz, M.; Yonaba, O.R.; Fowe, T.; Zouré, C.O.; Faye, M.D.; Leye, B. Use of Multidisciplinary Approaches for Groundwater Recharge Mechanism Characterization in Basement Aquifers: Case of Sanon Experimental Catchment in Burkina Faso. Water 2021, 13, 3216. [Google Scholar] [CrossRef]
- Fetter, C.W.; Boving, T.; Kreamer, D. Contaminant Hydrogeology, 3rd ed.; Waveland Press: Long Grove, IL, USA, 2017; ISBN 978-1-4786-3650-2. [Google Scholar]
- Eymold, W.K.; Swana, K.; Moore, M.T.; Whyte, C.J.; Harkness, J.S.; Talma, S.; Murray, R.; Moortgat, J.B.; Miller, J.; Vengosh, A.; et al. Hydrocarbon-Rich Groundwater above Shale-Gas Formations: A Karoo Basin Case Study. Groundwater 2018, 56, 204–224. [Google Scholar] [CrossRef] [PubMed]
- Hamed, Y.; Ahmadi, R.; Demdoum, A.; Bouri, S.; Gargouri, I.; Dhia, H.B.; Al-Gamal, S.; Laouar, R.; Choura, A. Use of Geochemical, Isotopic, and Age Tracer Data to Develop Models of Groundwater Flow: A Case Study of Gafsa Mining Basin-Southern Tunisia. J. Afr. Earth 2014, 100, 418–436. [Google Scholar] [CrossRef]
- Goodman, W.; Phillips, S.; Dyk, D.V.; Grobler, N. Conceptual Isotope Hydrostratigraphic Models for North American Sedimentary Basins: Understanding Water Sources in Deep Subsurface Mines. In Proceedings of the International Mine Water Association 2019 Meeting, Perm, Russia, 15–19 July 2019. [Google Scholar]
- Guetat, P.; Douche, C.; Hubinois, J.C. Le Tritium et l’environnement: Sources, Mesures et Transferts. Radioprotection 2008, 43, 547–569. [Google Scholar] [CrossRef]
- Healy, R.W. Estimating Groundwater Recharge; Cambridge University Press: Cambridge, UK, 2010; ISBN 978-0-521-86396-4. [Google Scholar]
- Scanlon, B.R.; Healy, R.W.; Cook, P.G. Choosing Appropriate Techniques for Quantifying Groundwater Recharge. Hydrogeol. J. 2002, 10, 18–39. [Google Scholar] [CrossRef]
- Jung, Y.-Y.; Koh, D.-C.; Yoon, Y.-Y.; Kwon, H.-I.; Heo, J.; Ha, K.; Yun, S.-T. Using Stable Isotopes and Tritium to Delineate Groundwater Flow Systems and Their Relationship to Streams in the Geum River Basin, Korea. J. Hydrol. 2019, 573, 267–280. [Google Scholar] [CrossRef]
- Ouedraogo, I.; Girard, A.; Vanclooster, M.; Jonard, F. Modelling the Temporal Dynamics of Groundwater Pollution Risks at the African Scale. Water 2020, 12, 1406. [Google Scholar] [CrossRef]
- Carrey, R.; Ballesté, E.; Blanch, A.R.; Lucena, F.; Pons, P.; López, J.M.; Rull, M.; Solà, J.; Micola, N.; Fraile, J.; et al. Combining Multi-Isotopic and Molecular Source Tracking Methods to Identify Nitrate Pollution Sources in Surface and Groundwater. Water Res. 2021, 188, 116537. [Google Scholar] [CrossRef]
- Telloli, C.; Rizzo, A.; Salvi, S.; Pozzobon, A.; Marrocchino, E.; Vaccaro, C. Characterization of Groundwater Recharge through Tritium Measurements. In Proceedings of the Advances in Geosciences; Copernicus GmbH: Göttingen, Germany, 2022; Volume 57, pp. 21–36. [Google Scholar]
- Heine, F.; Einsiedl, F. Groundwater Dating with Dissolved Organic Radiocarbon: A Promising Approach in Carbonate Aquifers. Appl. Geochem. 2021, 125, 104827. [Google Scholar] [CrossRef]
- Ram, R.; Burg, A.; Zappala, J.C.; Yokochi, R.; Yechieli, Y.; Purtschert, R.; Jiang, W.; Lu, Z.T.; Mueller, P.; Bernier, R.; et al. Identifying Recharge Processes into a Vast “Fossil” Aquifer Based on Dynamic Groundwater 81Kr Age Evolution. J. Hydrol. 2020, 587, 124946. [Google Scholar] [CrossRef]
- Bäumle, R.; Purtschert, R.; Mueller, P.; Krekeler, T.; Zappala, J.C.; Matsumoto, T.; Gröger-Trampe, J.; Koeniger, P.; Vockenhuber, C.; Romeo, N.; et al. New Insights into the Flow Dynamics of a Deep Freshwater Aquifer in the Semi-Arid and Saline Cuvelai-Etosha Basin, Northern Namibia: Results of a Multi. J. Hydrol. 2024, 52, 101721. [Google Scholar] [CrossRef]
- Sogreah Ingénierie Etude ses Ressources en eau Souterraine de la Zone Sédimentaire de la Région de Bobo Dioulasso; Direction des Etudes et de la Planification; Ministère de l’Eau: Ouagadougou, Burkina Faso, 1994.








| Lithology | Hydrogeochemistry | Stable Isotopes | Tritium |
|---|---|---|---|
| CT | 24 | 12 | 6 |
| GFB | 6 | 39 | 22 |
| SQ | 7 | 4 | 1 |
| GFG | 47 | 17 | 5 |
| GFR | 13 | 8 | 5 |
| GGQ | 74 | 11 | 9 |
| GKS | 15 | 2 | 1 |
| SAC1 | 65 | 6 | 1 |
| SAC2 | 86 | 15 | 7 |
| Surface Water (SW) | 18 | 17 | 7 |
| Total | 347 | 149 | 72 |
| Statistics | ||||||||
|---|---|---|---|---|---|---|---|---|
| No. of samples | 347.00 | 347.00 | 347.00 | 347.00 | 347.00 | 347.00 | 347.00 | 347.00 |
| Average | 7.42 | 9.92 | 23.96 | 14.93 | 12.41 | 133.22 | 15.10 | 10.95 |
| Standard deviation | 13.64 | 21.92 | 29.65 | 21.81 | 56.87 | 132.45 | 55.90 | 37.62 |
| Min | 0.09 | 0.10 | 0.10 | 0.05 | 0.01 | 0.10 | 0.01 | 0.10 |
| First quantile | 1.675 | 2 | 3.89 | 1.615 | 0.44 | 25.56 | 0.3 | 0.42 |
| Median | 3.20 | 4.00 | 14.06 | 7.20 | 2.00 | 86.60 | 2.00 | 2.29 |
| Third quantile | 7.03 | 10.82 | 36 | 20.86 | 4.5 | 209.84 | 4.16 | 10.39 |
| Max | 167.92 | 297.83 | 239.20 | 210.60 | 859.70 | 819.80 | 606.07 | 640.63 |
| Variation coeff. (%) | 183.89 | 220.84 | 123.74 | 146.08 | 458.44 | 99.42 | 370.14 | 343.62 |
| Reservoirs | Geology |
|---|---|
| Sandstone IC | GI, GKS, GFG, GGQ, GFR, SQ, GFB |
| Clayey-carbonate IC | SAC1 and SAC2 |
| CT | CT+ Surface Water (SW) |
| Reservoirs | Number of Samples | HCO3-Ca (%) | HCO3-Ca-Mg (%) | HCO3-Na-K (%) | SO4-Cl-Na-K (%) | SO4-Cl-Ca-Mg (%) |
|---|---|---|---|---|---|---|
| Sandstone IC | 155 | 10.97 | 49.68 | 20.00 | 7.10 | 12.26 |
| Clayey-carbonate IC | 150 | 14.67 | 65.33 | 10.00 | 2.67 | 7.33 |
| CT | 24 | 8.33 | 62.50 | 4.17 | 0.00 | 25.00 |
| SW | 18 | 5.56 | 61.11 | 27.78 | 5.56 | 0.00 |
| Total | 347 | 12.10 | 57.93 | 14.99 | 4.61 | 10.37 |
| GNIP Stations | Follow-Up Period |
|---|---|
| Barogo | 1988–1989 |
| Bobo Dioulasso | 1997–2016 |
| Houndé | 2004–2005 |
| Nasso | 2004–2005 |
| Ouagadougou | 2004–2019 |
| Statistics | No. of Samples | Average 1 | Minimum | Maximum | |
|---|---|---|---|---|---|
| Rainwater | 2H (‰ vs. SMOW) | 72 | −24.82 | −68.74 | 9.5 |
| 18O (‰ vs. SMOW) | 72.00 | −4.30 | −9.63 | 0.81 | |
| d (‰ vs. SMOW) 3H (T.U) | 72.00 65.00 | 9.65 3.58 | 0.08 1.95 | 9.13 5.37 | |
| Surface water | 2H (‰ vs. SMOW) | 17.00 | −22.70 | −31.46 | −0.81 |
| 18O (‰ vs. SMOW) | 17.00 | −3.53 | −5.07 | 1.34 | |
| d (‰ vs. SMOW) | 17.00 | 5.51 | −11.53 | 14.06 | |
| Groundwater | 2H (‰ vs. SMOW) | 133.00 | −29.21 | −39.30 | −9.90 |
| 18O (‰ vs. SMOW) | 133.00 | −4.66 | −6.48 | −1.47 | |
| d (‰ vs. SMOW) 3H (T.U) | 133.00 71.00 | 8.11 0.01 | −4.93 1.54 | 13.84 8.7 |
| Proposed Aquifer’s (Water Table) Name | Lithology |
|---|---|
| CT | CT |
| Upper sandstone IC | GFB |
| SQ | |
| SAC2 | SAC2 |
| Middle sandstone IC | GFR |
| SAC1 | SAC1 |
| Lower sandstone IC | GGQ |
| GFG | |
| GKS | |
| GI |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kutangila, S.M.; Kafando, M.B.; Keita, A.; Mounirou, L.A.; Yonaba, R.; Ouedraogo, M.; Koita, M. Subsurface Hydrodynamics of the Southeastern Taoudéni Basin (West Africa) through Hydrogeochemistry and Isotopy. Water 2024, 16, 1922. https://doi.org/10.3390/w16131922
Kutangila SM, Kafando MB, Keita A, Mounirou LA, Yonaba R, Ouedraogo M, Koita M. Subsurface Hydrodynamics of the Southeastern Taoudéni Basin (West Africa) through Hydrogeochemistry and Isotopy. Water. 2024; 16(13):1922. https://doi.org/10.3390/w16131922
Chicago/Turabian StyleKutangila, Succès Malundama, Moussa Bruno Kafando, Amadou Keita, Lawani Adjadi Mounirou, Roland Yonaba, Mahamadi Ouedraogo, and Mahamadou Koita. 2024. "Subsurface Hydrodynamics of the Southeastern Taoudéni Basin (West Africa) through Hydrogeochemistry and Isotopy" Water 16, no. 13: 1922. https://doi.org/10.3390/w16131922
APA StyleKutangila, S. M., Kafando, M. B., Keita, A., Mounirou, L. A., Yonaba, R., Ouedraogo, M., & Koita, M. (2024). Subsurface Hydrodynamics of the Southeastern Taoudéni Basin (West Africa) through Hydrogeochemistry and Isotopy. Water, 16(13), 1922. https://doi.org/10.3390/w16131922







