Mesocosm Experiment to Evaluate Relations between Chlorophyll-a Concentration and Water Surface Reflectance in an Anthropogenic Reservoir
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Description of the Experiment
- Total phosphorus—by optical emission spectrometry according to the standard PN ISO 11885:2009 [30];
- Total nitrogen—by high-temperature combustion with IR detection according to the standard PN-EN 12260:2004 [31];
- Chemical oxygen demand—spectrophotometric method according to the standard PN-ISO 15705:2005 [32];
- Conductivity—conductometric method according to the standard PN-EN 27888-1999 [33];
- pH—potentiometric method according to the standard PN-EN ISO 10523:2021 [34];
- Oxygen—electrochemical method according to the standard PN-EN ISO 5814:2013-04 [35];
- Secchi depth—measuring the optical properties of the water column using a disc in accordance with the standard EN ISO/IEC 17025 [36];
- Turbidity (NTU)—using nephelometers according to standards (PN-EN ISO 7027-1:2016-09) [37];
- Total organic carbon (TOC) and dissolved organic carbon (DOC)—by oxidation according to PN-EN 1484:1999 [38];
2.3. Methodology of Data Analyzing
3. Results
4. Discussion
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adjovu, G.E.; Stephen, H.; James, D.; Ahmad, S. Overview of the Application of Remote Sensing in Effective Monitoring of Water Quality Parameters. Remote Sens. 2023, 15, 1938. [Google Scholar] [CrossRef]
- Blackwell, R.J.; Boland, D.H.P. Trophic classification of selected Colorado lakes. In Trophic State of Lakes and Reservoirs; National Aeronautics and Space Administration; U.S. Environmental Protection Agency: Washington, DC, USA, 1979. [Google Scholar]
- Caselles, V.; López, M.J.; Soria, J.M. Estudio del estado trófico del lago de la Albufera (Valencia)a partir de imágenes Landsat-5 (TM). In Comunicaciones de la I Reunión Científica del Grupo de Trabajo de Teledetección; Clotet, N., Sole, L., Eds.; Industrias Gráficas Miba: Barcelona, Spain, 1987. [Google Scholar]
- Gómez, J.A.D.; Alonso, C.A.; García, A.A. Remote sensing as a tool for monitoring water quality parameters for Mediterranean Lakes 6. Environ. Monit. Assess. 2011, 181, 317–334. [Google Scholar] [CrossRef]
- Ortiz, J.L.; Peña, R. Aplicación de imágenes multiespectrales en cartografía de embalses. In Coloquio Hispano-Francésso Bretelle Detección y Aplicación Integrada del Territorio; MOPU, Ed.; MOPU: Madrid, Spain, 1988. [Google Scholar]
- Barrett, D.C.; Frazier, A.E. Automated method for monitoring water quality using Landsat imagery. Water 2016, 8, 257. [Google Scholar] [CrossRef]
- Allan, M.G.; Hamilton, D.P.; Hicks, B.J.; Brabyn, L. Landsat remote sensing of chlorophyll a concentrations in central North Island lakes of New Zealand. Int. J. Remote Sens. 2011, 32, 2037–2055. [Google Scholar] [CrossRef]
- Kneubühler, M.; Frank, T.; Kellenberger, T.; Pasche, N.; Schmid, M.; Lacoste, H.; Ouwehand, L. Mapping chlorophyll-a in Lake Kivu with remote sensing methods. In Proceedings of the Envisat Symposium 2007, Montreux, Switzerland, 23–27 April 2007. ESA SP-636. [Google Scholar]
- Song, Y.; Song, X.D.; Jiang, H.; Guo, Z.B.; Guo, Q.H. Quantitative remote sensing retrieval for algae in inland waters. Spectrosc. Spectr. Anal. 2010, 30, 1075–1079. [Google Scholar]
- Tebbs, E.J.; Remedios, J.J.; Harper, D.M. Remote sensing of chlorophyll-a as a measure of cyanobacterial biomass in Lake Bogoria, a hypertrophic, saline–alkaline, flamingo lake, using Landsat ETM+. Remote Sens. Environ. 2013, 135, 92–106. [Google Scholar] [CrossRef]
- Kutser, T.; Paavel, B.; Verpoorter, C.; Kauer, T.; Vahtmäe, E. Remote sensing of water quality in optically complex lakes. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2012, 39, 165–169. [Google Scholar] [CrossRef]
- Abbas, M.; Alameddine, I. Predicting water quaily variability in a Mediterranean hypereutrophic monomictic reservoir using Sentinel 2 MSI: The importance of considering model functional form. Environ. Monit. Assess. 2023, 195, 923. [Google Scholar] [CrossRef] [PubMed]
- Sodré, E.D.O.; Langlais-Bourassa, A.; Pollard, A.I.; Beisner, B.E. Functional and taxonomic biogeography of phytoplankton and zooplankton communities in relation to environmental variation across the contiguous USA. J. Plankton Res. 2020, 42, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Bracher, A.; Bouman, H.A.; Brewin, R.J.W.; Bricaud, A.; Brotas, V.; Ciotti, A.M. Obtaining phytoplankton diversity from ocean color: A scientific roadmap for future development. Front. Mar. Sci. 2017, 4, 55. [Google Scholar] [CrossRef]
- Mouw, C.B.; Hardman-Montford, N.; Alvain, S.; Bracher, A.; Brewin, R.J.W.; Bricaud, A.; Ciotti, A.M.; Devred, E.; Fujiwara, A.; Hirata, T.; et al. A consumer’s guide to satellite remote sensing of multiple phytoplankton groups in the global ocean. Front. Mar. Sci. 2017, 4, 41. [Google Scholar] [CrossRef]
- Gitelson, A.; Mayo, M.; Yacobi, Y.Z. Signature analysis of reflectance spectra and its application for remote observations of the phytoplankton distribution in Lake Kinneret. In Proceedings of the Mesures Physiques et Signatures en Teledetection, ISPRS 6th International Symposium, Val d’Isere, France, 17–21 January 1994; pp. 277–283. [Google Scholar]
- Arenz, J.R.R.F.; Lewis, J.R.W.M.; Saunders, J.F., III. Determination of chlorophyll and dissolved organic carbon from reflectance data for Colorado reservoirs. Int. J. Remote Sens. 1996, 17, 1547–1565. [Google Scholar] [CrossRef]
- Gitelson, A.; Yacobi, Y.Z.; Karnieli, A.; Kress, N. Reflectance spectra of polluted marine waters in Haifa Bay, southeastern Mediterranean: Features and application for remote estimation of chlorophyll concentrations. Isr. J. Earth Sci. 1996, 45, 127–136. [Google Scholar]
- Cândido, A.K.A.A.; Filho, A.C.P.; Haupenthal, M.R.; da Silva, N.M.; de Sousa Correa, J.; Ribeiro, M.L. Water quality and chlorophyll measurement through vegetation indices generated from orbital and suborbital images. Water Air Soil Pollut. 2016, 227, 224. [Google Scholar] [CrossRef]
- Moss, B. Shallow Lakes Biomanipulation and Eutrophication. Scope Newsl. 1998, 29, 2–45. [Google Scholar]
- Pierzchała, Ł.; Sierka, E. Do submerged plants improve the water quality in mining subsidence reservoirs? Appl. Ecol. Environ. Res. 2020, 18, 5661–5672. [Google Scholar] [CrossRef]
- Rodgers, E.M. Adding climate change to the mix: Responses of aquatic ectotherms to the combined effects of eutrophication and warming. Biol. Lett. 2021, 17, 20210442. [Google Scholar] [CrossRef]
- Guo, H.; Zhu, X.; Huang, J.J.; Zhang, Z.; Tian, S.; Chen, Y. An enhanced deep learning approach to assessing inland lake water quality and its response to climate and anthropogenic factors. J. Hydrol. 2023, 620, 129466. [Google Scholar] [CrossRef]
- Vallino, J. Improving marine ecosystem models: Use of data assimilation and mesocosm experiments. J. Mar. Res. 2000, 58, 117–164. [Google Scholar] [CrossRef]
- Raygosa-Barahona, R.R.; Putzeys, S.; Herrera, J.; Pech, D. Low Cost Mesocosms Design for Studies of Tropical Marine Environments. Biogeosci. Discuss. 2019, 2019, 1–15. [Google Scholar]
- Peperzak, L.; Timmermans, K.R.; Wernand, M.R.; Oosterhuis, S.; Van der Woerd, H.J. A mesocosm tool to optically study phytoplankton dynamics. Limnol. Oceanogr. Methods 2011, 9, 232–244. [Google Scholar] [CrossRef]
- Chen, C.C.; Petersen, J.E.; Kemp, W.M. Spatial and temporal scaling of periphyton growth on walls of estuarine mesocosms. Mar. Ecol. Prog. Ser. 1997, 155, 1–15. [Google Scholar] [CrossRef]
- PN-ISO 10260:2002; Measurement of Biochemical Parameters—Spectrometric Determination of the Chlorophyll-a Concentration. Polish Committee for Standardization: Warsaw, Poland, 2002.
- Zhang, Y.; Qin, B.; Zhang, L.; Zhu, G.; Chen, W. Spectral absorption and fluorescence of chromophoric dissolved organic matter in shallow lakes in the middle and lower reaches of the Yangtze River. J. Freshw. Ecol. 2005, 20, 451–459. [Google Scholar] [CrossRef]
- ISO 11885:2007; Water Quality—Determination of Selected Elements by Inductively Coupled Plasma Optical Emission Spectrometry. International Organization for Standardization: Geneva, Switzerland, 2007.
- EN 12260:2004; Water Quality—Determination of Nitrogen—Determination of Bound Nitrogen (TNb), Following Oxidation to Nitrogen Oxides. Association Francaise de Normalisation: La Plaine Saint-Denis, France, 2004.
- PN-ISO 15705:2005; Water Quality—Specifies a Method for the Determination of the Chemical Oxygen Demand (ST-COD) Using the Sealed Tube Method. Polish Committee for Standardization: Warsaw, Poland, 2005.
- PN-EN 27888-1999; Water Quality—Determination of Electrical Conductivity. Polish Committee for Standardization: Warsaw, Poland, 1999.
- PN-EN ISO 10523:2021; Water Quality—Determination of pH. International Organization for Standardization: Geneva, Switzerland, 2021.
- PN-EN ISO 5814:2013-04; Water Quality—Determination of Dissolved Oxygen—Electrochemical Probe Method. International Organization for Standardization: Geneva, Switzerland, 2013.
- EN ISO/IEC 17025:2017; General Requirements for the Competence of Testing and Calibration Laboratories. British Standards Institution: London, UK, 2017.
- ISO 7027-1:2016; Waterquality—Determination of Turbidity—Part 1: Quantitative Methods. International Organization for Standardization: Geneva, Switzerland, 2016.
- PN-EN 1484:1999; Water Analysis—Guidelines for the Determination of Total Organic Carbon (TOC) and Dissolved Organic Carbon (DOC). Polish Committee for Standardization: Warsaw, Poland, 1999.
- PN-EN 872:2007; Water Quality—Determination of Suspended Solids—Method by Filtration through Glass Fibre Filters. Polish Committee for Standardization: Warsaw, Poland, 2007.
- PN-C-04559-02:1972; Testing of the Suspended Solids Content—Determination of Total Suspended Solids, Mineral Suspended Solids and Volatile Suspended Solids by Mass. Polish Committee for Standardization: Warsaw, Poland, 1972.
- Schalles, J.F.; Gitelson, A.A.; Yacobi, Y.Z.; Kroenke, A.E. Estimation of chlorophyll a from time series measurements of high spectral resolution reflectance in an eutrophic lake. J. Phycol. 1998, 34, 383–390. [Google Scholar] [CrossRef]
- Osińska-Skotak, K. Możliwość teledetekcyjnego monitorowania zawartości chlorofilu-a w wodach śródlądowych. Teledetekcja Srodowiska 2009, 42, 59–68. [Google Scholar]
- Carpenter, S.R. Microcosm experiments have limited relevance for community and ecosystem ecology. Ecology 1996, 77, 677–680. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, W.; Tian, Y.Q.; Yu, Q.; Zheng, Y.; Huang, L. Remote estimation of colored dissolved organic matter and chlorophyll-a in Lake Huron using Sentinel-2 measurements. J. Appl. Remote Sens. 2017, 11, 036007. [Google Scholar] [CrossRef]
- Pierzchała, Ł. Assessment of the possibility of using remote sensing methods for measuring eutrophication of inland water reservoirs. Ecol. Eng. Environ. Technol. 2020, 21, 27–32. [Google Scholar]
- Dzierzbicka-Glowacka, L.; Kulinski, K.; Maciejewska, A.; Jakucki, J.; Pempkowiak, J. Particulate organic carbon in the southern Baltic Sea: Numerical simulations and experimental data. Oceanologia 2010, 52, 621–648. [Google Scholar] [CrossRef]
- Winogradow, A.; Mackiewicz, A.; Pempkowiak, J. Seasonal changes in particulate organic matter (POM) concentrations and properties measured from deep areas of the Baltic Sea. Oceanologia 2019, 61, 505–521. [Google Scholar] [CrossRef]
- Scheffer, M. Ecology of Shallow Lakes; Chapman & Hall: London, UK, 1998; p. 64. [Google Scholar]
- Ficek, D.; Kaczmarek, S.; Ston-Egiert, J.; Wozniak, B.; Majchrowski, R.; Dera, J. Spectra of light absorption by phytoplankton pigments in the Baltic; conclusions to be drawn from a Gaussian analysis of empirical data. Oceanologia 2004, 46, 533–555. [Google Scholar]
- Agatova, A.I.; Lapina, N.M.; Torgunova, N.I.; Sapozhnikov, V.V.; Milovskaya, L.V. Organic matter and its rate of transformation in spawning and feeding lakes of Kamchatka. Water Resour. 2004, 31, 691–701. [Google Scholar] [CrossRef]
- Wozniak, B.; Wozniak, S.B.; Tyszka, K.; Ostrowska, M.; Majchrowski, R.; Ficek, D.; Dera, J. Modelling the light absorption properties of particulate matter forming organic particles suspended in seawater. Part 2. Modelling results. Oceanologia 2005, 47, 621–662. [Google Scholar]
- Madonia, A.; Caruso, G.; Piazzolla, D.; Bonamano, S.; Piermattei, V.; Zappalà, G.; Marcelli, M. Chromophoric Dissolved Organic Matter as a Tracer of Fecal Contamination for Bathing Water Quality Monitoring in the Northern Tyrrhenian Sea (Latium, Italy). J. Mar. Sci. Eng. 2020, 8, 430. [Google Scholar] [CrossRef]
- Woźniak, S.B.; Meler, J.; Lednicka, B.; Zdun, A.; Stoń-Egiert, J. Inherent optical properties of suspended particulate matter in the southern Baltic Sea. Oceanologia 2011, 53, 691–729. [Google Scholar]
- Sun, D.; Hu, C.; Qiu, Z.; Cannizzaro, J.P.; Barnes, B.B. Influence of a red band-based water classification approach on chlorophyll algorithms for optically complex estuaries. Remote Sens. Environ. 2014, 155, 289–302. [Google Scholar] [CrossRef]
- Zhu, W.; Yu, Q.; Tian, Y.Q.; Becker, B.L.; Zheng, T.; Carrick, H.J. An assessment of remote sensing algorithms for colored dissolved organic matter in complex freshwater environments. Remote Sens. Environ. 2014, 140, 766–778. [Google Scholar] [CrossRef]
- Brezonik, P.L.; Olmanson, L.G.; Finlay, J.C.; Bauer, M.E. Factors affecting the measurement of CDOM by remote sensing of optically complex inland waters. Remote Sens. Environ. 2015, 157, 199–215. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Qin, B.Q.; Ma, R.H.; Zhu, G.W.; Zhang, L.; Chen, W.M. Chromophoric dissolved organic matter absorption characteristics with relation to fluorescence in typical macrophyte, algae lake zones of Lake Taihu. Huan Jing Ke Xue = Huanjing Kexue 2005, 26, 142–147. [Google Scholar]
- Kutser, T.; Paavel, B.; Verpoorter, C.; Ligi, M.; Soomets, T.; Toming, K.; Casal, G. Remote sensing of black lakes and using 810 nm reflectance peak for retrieving water quality parameters of optically complex waters. Remote Sens. 2016, 8, 497. [Google Scholar] [CrossRef]
- Mackay, E.B.; Feuchtmayr, H.; De Ville, M.M.; Thackeray, S.J.; Callaghan, N.; Marshall, M.; Maberly, S.C. Dissolved organic nutrient uptake by riverine phytoplankton varies along a gradient of nutrient enrichment. Sci. Total Environ. 2020, 722, 137837. [Google Scholar] [CrossRef] [PubMed]
- Reinl, K.L.; Harris, T.D.; Elfferich, I.; Coker, A.; Zhan, Q.; Domis, L.N.D.S.; Sweetman, J.N. The role of organic nutrients in structuring freshwater phytoplankton communities in a rapidly changing world. Water Res. 2022, 219, 118573. [Google Scholar] [CrossRef] [PubMed]




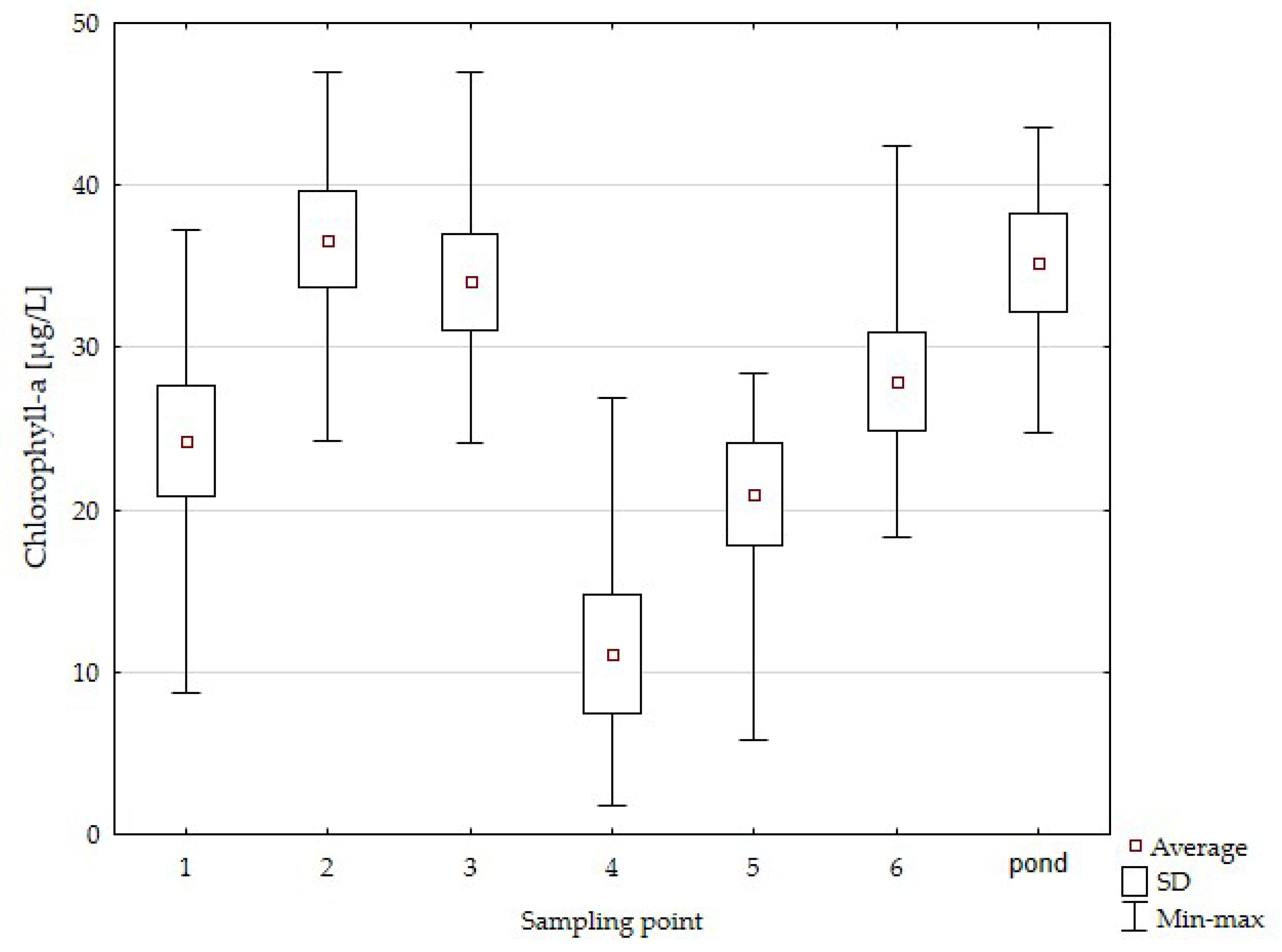
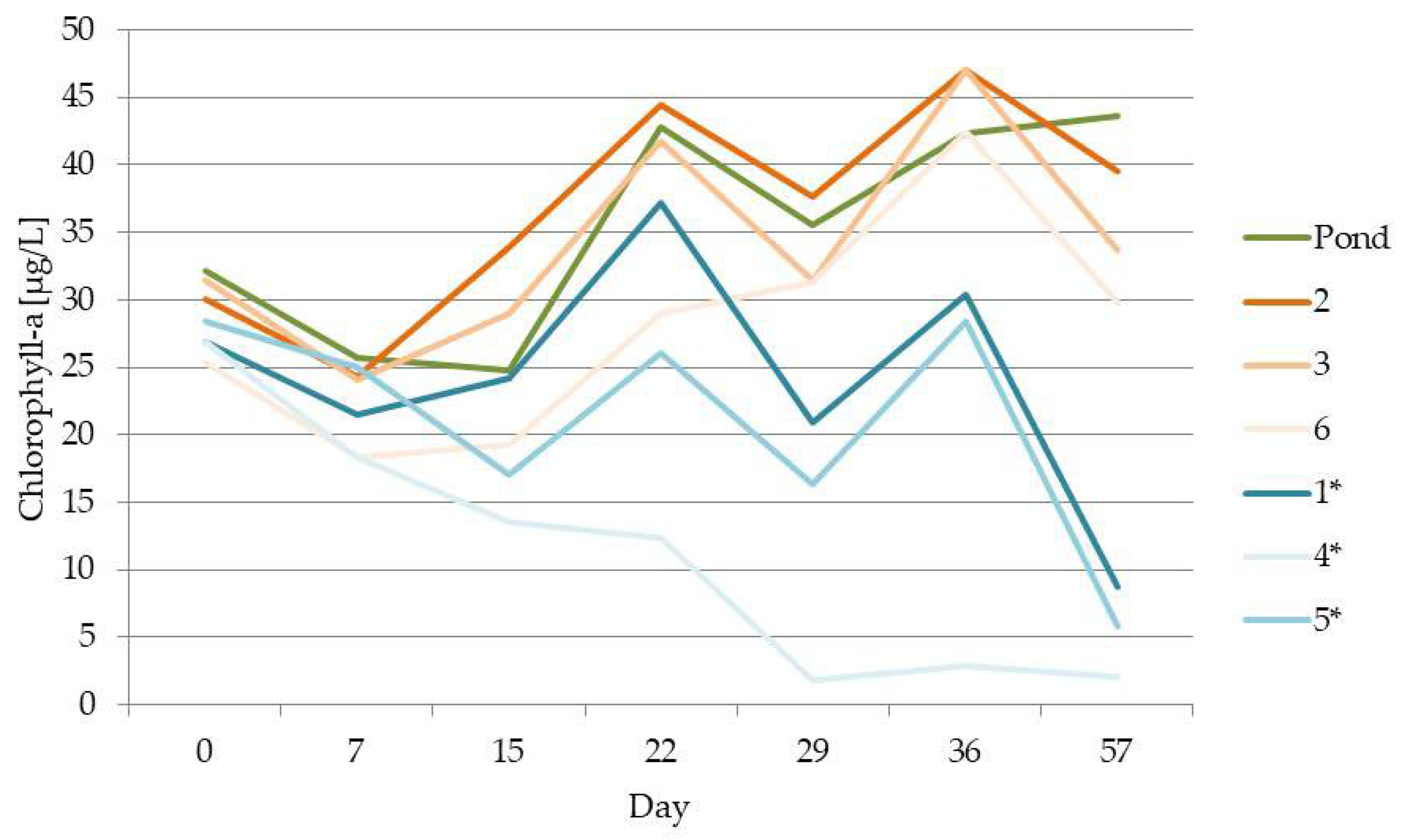

| Parameater | Mean Value | Standard Deviation |
|---|---|---|
| Chlorophill a [µg/L] | 35.27 | 8.02 |
| Secchi disk [m] | 0.76 | 0.09 |
| Chemical oxygen demand [mg/L] | 28 | 2.53 |
| Total organic carbon, TOC [mg/L] | 7.44 | 0.90 |
| Dissolved organic carbon, DOC [mg/L] | 3.82 | 1.34 |
| Total phosphors [mg/L] | 0.026 | 0.004 |
| Total nitrogen [mg/L] | 0.79 | 0.29 |
| Conductivity [μS/cm] | 596.50 | 9.65 |
| pH | 8.34 | 0.19 |
| Oxygen [mg/L] | 8.57 | 2.26 |
| Name of Data | Index | Range of Chl-a Concentration [µg/L] | Correlation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chl-a | Range of Turbity [NTU] | Turbidity [NTU] | Range of POM [mg/L] | POM [mg/L] | Range of TOC [mg/L] | TOC [mg/L] | Range of DOC [mg/L] | DOC [mg/L] | |||
| Pond, n = 6 | R706/674 | 24.8–43.6 | −0.59 | 7.5–37 | 0.71 | 7–13 | −0.34 | 6.1–8.8 | −0.91 | <0.5–5.07 | −0.18 |
| R450/531 | −0.79 | 7.5–37 | 0.46 | 7–13 | −0.47 | 6.1–8.8 | −0.68 | 0.02 | |||
| Box 1, n = 6 | R706/674 | 8.7–37.2 | 0.23 | 2.3–31 | 0.73 | 2.8–9.4 | 0.53 | 6.7–8.6 | −0.71 | 1.83–8.28 | −0.66 |
| R450/531 | −0.01 | 2.3–31 | 0.44 | 2.8–9.4 | 0.35 | 6.7–8.6 | −0.44 | −0.42 | |||
| Box 2, n = 6 | R706/674 | 24.3–47 | −0.52 | 6.5–36 | 0.76 | 6.8–16 | 0.13 | 6.3–9 | −0.89 | <0.5–6.56 | −0.41 |
| R450/531 | −0.90 | 6.5–36 | 0.31 | 6.8–16 | −0.48 | 6.3–9 | −0.63 | 0.13 | |||
| Box 3, n = 6 | R706/674 | 24.1–47 | −0.49 | 5.7–33 | 0.75 | 6.6–16 | 0.56 | 7.4–9.6 | −0.81 | <0.5–6.76 | −0.77 |
| R450/531 | −0.76 | 5.7–33 | 0.43 | 6.6–16 | 0.21 | 7.4–9.6 | −0.80 | −0.37 | |||
| Box 4, n = 6 | R706/674 | 1.8–26.9 | 0.97 | 0.72–23 | 0.88 | 4–8.4 | 0.89 | 6.7–7.8 | −0.48 | 3.84–11.24 | −0.96 |
| R450/531 | 0.66 | 0.72–23 | 0.55 | 4–8.4 | 0.86 | 6.7–7.8 | −0.08 | −0.85 | |||
| Box 5, n = 6 | R706/674 | 5.8–28.4 | 0.43 | 2–27 | 0.78 | 4–7.8 | 0.61 | 6.4–8.4 | −0.86 | 3.84–12.86 | −0.73 |
| R450/531 | 0.30 | 2–27 | 0.47 | 4–7.8 | 0.59 | 6.4–8.4 | −0.55 | −0.61 | |||
| Box 6, n = 6 | R706/674 | 18.3–42.4 | −0.68 | 5–26 | 0.83 | 5.8–9 | −0.70 | 6.5–8.2 | −0.85 | 3.14–6.13 | −0.07 |
| R450/531 | −0.84 | 5–26 | 0.62 | 5.8–9 | −0.83 | 6.5–8.2 | −0.57 | 0.39 | |||
| Name of Data | Index | Range of Chl-a Concentration [µg/L] | Correlation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chl-a | Range of Turbidity [NTU] | Turbidity [NTU] | Range of POM [mg/L] | POM [mg/L] | Range of TOC [mg/L] | TOC [mg/L] | Range DOC [mg/L] | DOC [mg/L] | |||
| Range 1 (from all, n = 14) | R706/674 | 1.8–21.5 | 0.63 | 0.72–25 | 0.90 | 1–8.4 | 0.79 | 7–8.6 | −0.34 | 3.84–12.36 | −0.90 |
| R450/531 | 0.40 | 0.72–25 | 0.56 | 1–8.4 | 0.59 | 7–8.6 | −0.34 | −0.63 | |||
| Range 2 (from all, n = 30) | R706/674 | 24.1–47 | −0.36 | 4.8–37 | 0.70 | 5.4–16 | 0.15 | 6.1–9.6 | −0.67 | <0.5–6.76 | −0.43 |
| R450/531 | −0.79 | 4.8–37 | 0.38 | 5.4–16 | −0.33 | 6.1–9.6 | −0.64 | 0.03 | |||
| Range 3 (from all, n =24) | R706/674 | 1.8–26.9 | 0.81 | 0.72–27 | 0.88 | 1–9 | 0.84 | 6.5–8.6 | −0.67 | 2.66–12.36 | −0.85 |
| R450/531 | 0.42 | 0.72–27 | 0.51 | 1–9 | 0.61 | 6.5–8.6 | −0.34 | −0.57 | |||
| Range 4 (from all, n = 25) | R706/674 | 28.4–47 | −0.17 | 4.8–37 | 0.72 | 5.4–16 | 0.39 | 6.1–9.6 | −0.62 | <0.5–6.76 | 0.22 |
| R450/531 | −0.70 | 4.8–37 | 0.35 | 5.4–16 | −0.14 | 6.1–9.6 | −0.71 | 0.31 | |||
| Range 5 (all, n = 44) | R706/674 | 1.8 −47 | 0.23 | 1–16 | 0.79 | 19–35 | −0.40 | 6.1–9.6 | −0.60 | <0.5–12.36 | −0.64 |
| R450/531 | −0.47 | 1–16 | 0.33 | 19–35 | −0.03 | 6.1–9.6 | −0.58 | 0.01 | |||
| Variables | R706/674 | R450/531 | Chl-a | NTU | POM | TOC | DOC |
|---|---|---|---|---|---|---|---|
| R706/674 | 1 | 0.54 | 0.23 | 0.79 | 0.45 | −0.60 | −0.64 |
| R450/531 | 0.54 | 1 | −0.47 | 0.33 | −0.23 | −0.58 | 0.01 |
| Chl-a | 0.23 | −0.47 | 1 | 0.28 | 0.79 | 0.19 | −0.76 |
| NTU | 0.79 | 0.33 | 0.28 | 1 | 0.52 | −0.51 | −0.69 |
| POM | 0.45 | −0.23 | 0.79 | 0.52 | 1 | 0.09 | −0.91 |
| TOC | −0.6 | −0.58 | 0.19 | −0.51 | 0.09 | 1 | 0.24 |
| DOC | −0.64 | 0.01 | −0.76 | −0.6 | −0.91 | 0.24 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pierzchała, Ł. Mesocosm Experiment to Evaluate Relations between Chlorophyll-a Concentration and Water Surface Reflectance in an Anthropogenic Reservoir. Water 2024, 16, 1926. https://doi.org/10.3390/w16131926
Pierzchała Ł. Mesocosm Experiment to Evaluate Relations between Chlorophyll-a Concentration and Water Surface Reflectance in an Anthropogenic Reservoir. Water. 2024; 16(13):1926. https://doi.org/10.3390/w16131926
Chicago/Turabian StylePierzchała, Łukasz. 2024. "Mesocosm Experiment to Evaluate Relations between Chlorophyll-a Concentration and Water Surface Reflectance in an Anthropogenic Reservoir" Water 16, no. 13: 1926. https://doi.org/10.3390/w16131926




