Sampling and Analysis of Microplastics in the Coastal Environments of Sri Lanka: Estuaries of the Kelani River to Mahaoya
Abstract
:1. Introduction
2. Materials and Methods
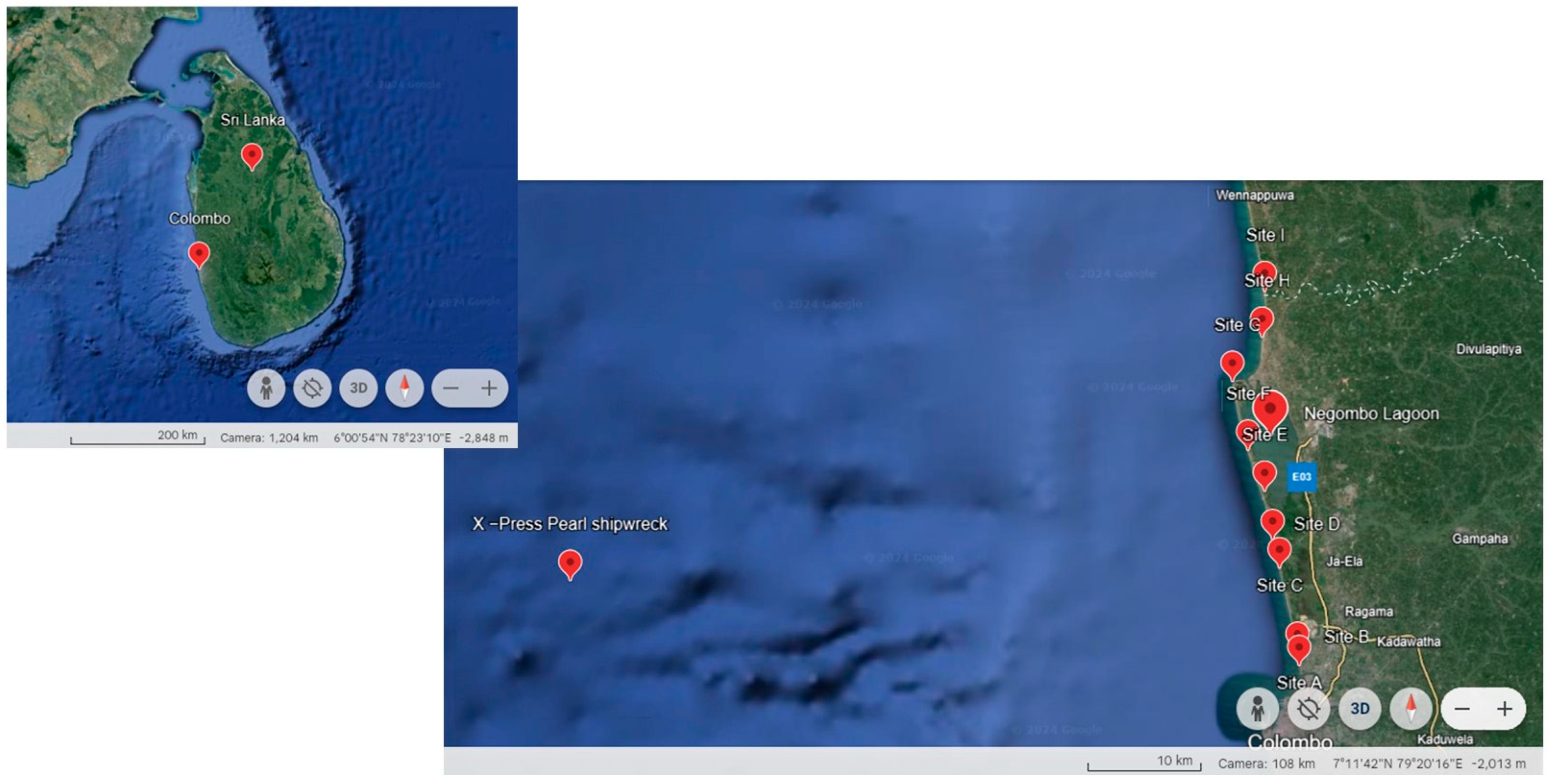
2.1. Study Area and Sample Collection
2.2. Microplastic Analysis: An Optimized NOAA Method
2.2.1. Isolation of MPs
2.2.2. Identification and Characterization of MPs
2.3. Validation of Methodology and Contamination Assessment
2.4. Determination of Pellet Pollution Index (PPI)
2.5. Data Analysis
3. Results
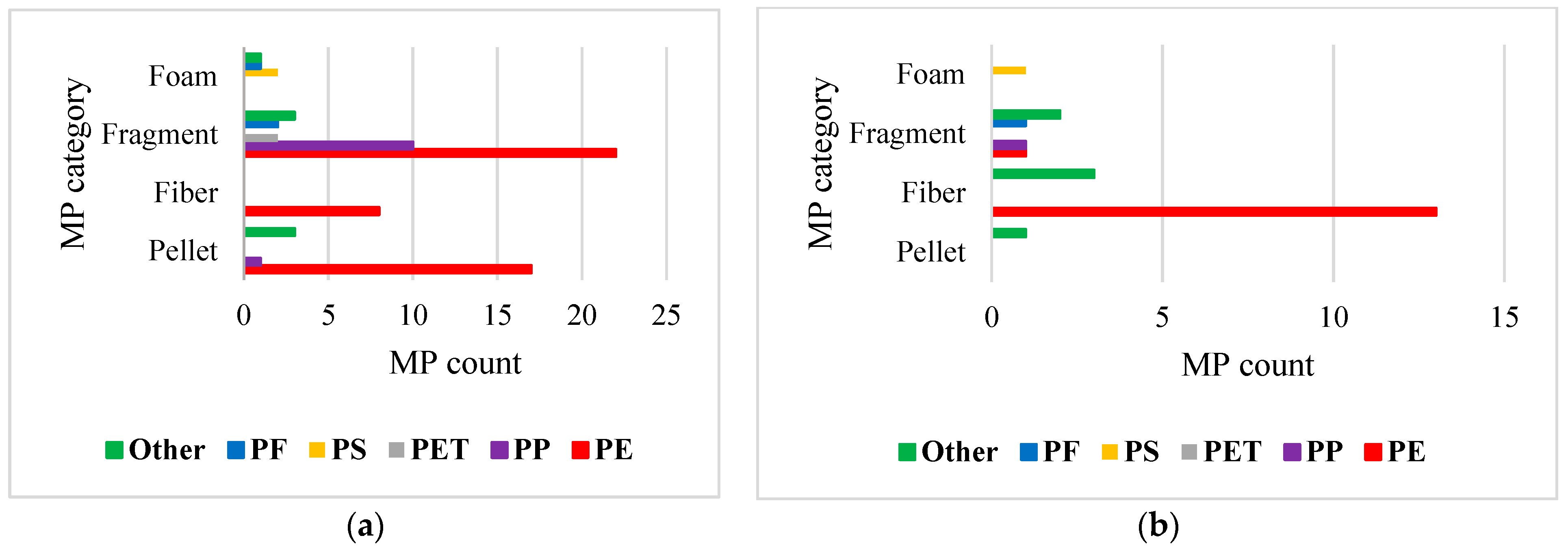
3.1. Morphology of MPs
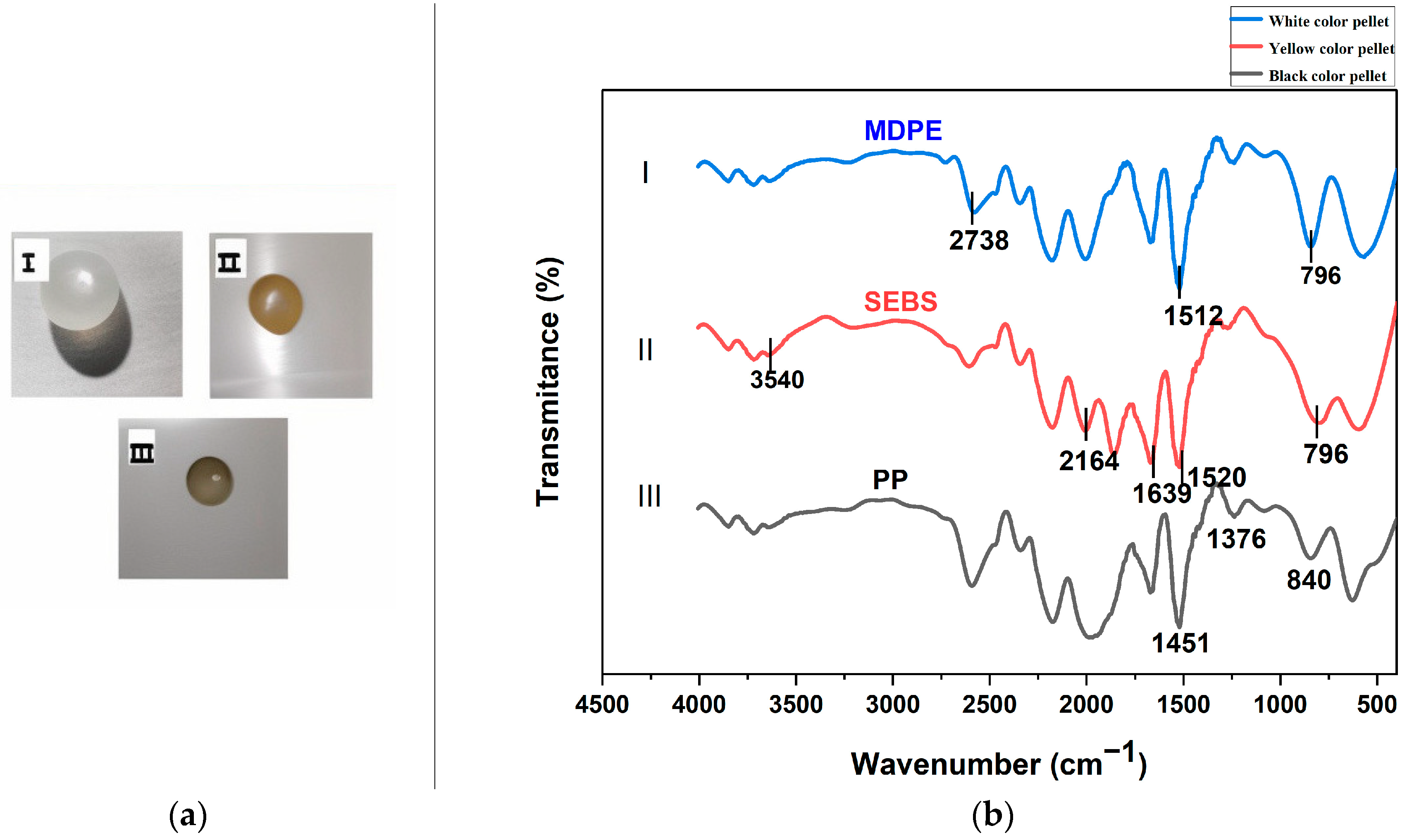
3.2. Polymer Characterization of MPs
3.3. Spatial Distribution of MPs in Sampling Sites
3.4. Pellet Distribution
3.5. Plastic Pellet Pollution Index
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dekiff, J.H.; Remy, D.; Klasmeier, J.; Fries, E. Occurrence and spatial distribution of microplastics in sediments from Norderney. Environ. Pollut. 2014, 186, 248–256. [Google Scholar] [CrossRef]
- IUCN. IUCN Website IUCN Issues Briefs. Available online: www.iucn.org/issues-briefs (accessed on 16 January 2024).
- Yang, L.; Zhang, Y.; Kang, S.; Wang, Z.; Wu, C. Microplastics in soil: A review on methods, occurrence, sources, and potential risk. Sci. Total Environ. 2021, 780, 146546. [Google Scholar] [CrossRef] [PubMed]
- Mariano, S.; Tacconi, S.; Fidaleo, M.; Rossi, M.; Dini, L. Micro and Nanoplastics Identification: Classic Methods and Innovative Detection Techniques. Front. Toxicol. 2021, 3, 636640. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef] [PubMed]
- Claessens, M.; Van Cauwenberghe, L.; Vandegehuchte, M.B.; Janssen, C.R. New techniques for the detection of microplastics in sediments and field collected organisms. Mar. Pollut. Bull. 2013, 70, 227–233. [Google Scholar] [CrossRef]
- Campanale, C.; Massarelli, C.; Bagnuolo, G.; Savino, I.; Uricchio, V.F. The Problem of Microplastics and Regulatory Strategies in Italy. In Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2022; pp. 255–276. [Google Scholar] [CrossRef]
- Ziani, K.; Ioniță-Mîndrican, C.-B.; Mititelu, M.; Neacșu, S.M.; Negrei, C.; Moroșan, E.; Drăgănescu, D.; Preda, O.-T. Microplastics: A Real Global Threat for Environment and Food Safety: A State of the Art Review. Nutrients 2023, 15, 617. [Google Scholar] [CrossRef]
- Park, H.; Park, B. Review of Microplastic Distribution, Toxicity, Analysis Methods, and Removal Technologies. Water 2021, 13, 2736. [Google Scholar] [CrossRef]
- Crawford, C.B.; Quinn, B. Microplastic Identification Techniques. In Microplastic pollutants; Elsevier: Amsterdam, The Netherlands, 2017; pp. 219–267. [Google Scholar] [CrossRef]
- Ateia, M.; Ersan, G.; Alalm, M.G.; Boffito, D.C.; Karanfil, T. Emerging investigator series: Microplastic sources, fate, toxicity, detection, and interactions with micropollutants in aquatic ecosystems—A review of reviews. Environ. Sci. Process. Impacts 2022, 24, 172–195. [Google Scholar] [CrossRef]
- Ali, N.; Liu, W.; Zeb, A.; Shi, R.; Lian, Y.; Wang, Q.; Wang, J.; Li, J.; Zheng, Z.; Liu, J.; et al. Environmental fate, aging, toxicity and potential remediation strategies of microplastics in soil environment: Current progress and future perspectives. Sci. Total Environ. 2024, 906, 167785. [Google Scholar] [CrossRef]
- Cutroneo, L.; Reboa, A.; Besio, G.; Borgogno, F.; Canesi, L.; Canuto, S.; Dara, M.; Enrile, F.; Forioso, I.; Greco, G.; et al. Correction to: Microplastics in seawater: Sampling strategies, laboratory methodologies, and identification techniques applied to port environment. Environ. Sci. Pollut. Res. 2020, 27, 8938–8952. [Google Scholar] [CrossRef] [PubMed]
- Babaremu, K.; Okoya, S.; Hughes, E.; Tijani, B.; Teidi, D.; Akpan, A.; Igwe, J.; Karera, S.; Oyinlola, M.; Akinlabi, E. Sustainable plastic waste management in a circular economy. Heliyon 2022, 8, e09984. [Google Scholar] [CrossRef] [PubMed]
- Watt, E.; Picard, M.; Maldonado, B.; Abdelwahab, M.A.; Mielewski, D.F.; Drzal, L.T.; Misra, M.; Mohanty, A.K. Ocean plastics: Environmental implications and potential routes for mitigation—A perspective. RSC Adv. 2021, 11, 21447–21462. [Google Scholar] [CrossRef] [PubMed]
- Anik, A.H.; Hossain, S.; Alam, M.; Sultan, M.B.; Hasnine, T.; Rahman, M. Microplastics pollution: A comprehensive review on the sources, fates, effects, and potential remediation. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100530. [Google Scholar] [CrossRef]
- Parsai, T.; Figueiredo, N.; Dalvi, V.; Martins, M.; Malik, A.; Kumar, A. Implication of microplastic toxicity on functioning of microalgae in aquatic system. Environ. Pollut. 2022, 308, 119626. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Sinha, J.K.; Ghosh, S.; Vashisth, K.; Han, S.; Bhaskar, R. Microplastics as an Emerging Threat to the Global Environment and Human Health. Sustainability 2023, 15, 10821. [Google Scholar] [CrossRef]
- Caruso, G.; Bergami, E.; Singh, N.; Corsi, I. Plastic occurrence, sources, and impacts in Antarctic environment and biota. Water Biol. Secur. 2022, 1, 100034. [Google Scholar] [CrossRef]
- Cózar, A.; Echevarría, F.; González-Gordillo, J.I.; Irigoien, X.; Úbeda, B.; Hernández-León, S.; Palma, Á.T.; Navarro, S.; García-De-Lomas, J.; Ruiz, A.; et al. Plastic debris in the open ocean. Proc. Natl. Acad. Sci. USA 2014, 111, 10239–10244. [Google Scholar] [CrossRef] [PubMed]
- Saley, A.; Smart, A.; Bezerra, M.; Burnham, T.; Capece, L.; Lima, L.; Carsh, A.; Williams, S.; Morgan, S. Microplastic accumulation and biomagnification in a coastal marine reserve situated in a sparsely populated area. Mar. Pollut. Bull. 2019, 146, 54–59. [Google Scholar] [CrossRef]
- Isobe, A.; Uchiyama-Matsumoto, K.; Uchida, K.; Tokai, T. Microplastics in the Southern Ocean. Mar. Pollut. Bull. 2017, 114, 623–626. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Y.; Tan, W.; Zhang, Z. Microplastics as an Emerging Environmental Pollutant in Agricultural Soils: Effects on Ecosystems and Human Health. Front. Environ. Sci. 2022, 10, 855292. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Zhou, X.; Tian, Y.; Lin, C.; Wang, W.; Zhou, K.; Zhang, Y.; Lin, H. Microplastic abundance, distribution and composition in the mid-west Pacific Ocean. Environ. Pollut. 2020, 264, 114125. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, M.; Lebreton, L.C.M.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic Pollution in the World’s Oceans: More than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea. PLoS ONE 2014, 9, e111913. [Google Scholar] [CrossRef] [PubMed]
- Adamopoulou, A.; Zeri, C.; Garaventa, F.; Gambardella, C.; Ioakeimidis, C.; Pitta, E. Distribution Patterns of Floating Microplastics in Open and Coastal Waters of the Eastern Mediterranean Sea (Ionian, Aegean, and Levantine Seas). Front. Mar. Sci. 2021, 8, 699000. [Google Scholar] [CrossRef]
- de Almeida, M.P.; Gaylarde, C.; Pompermayer, F.C.; Lima, L.d.S.; Delgado, J.d.F.; Scott, D.; Neves, C.V.; Vieira, K.S.; Neto, J.A.B.; Fonseca, E.M. The Complex Dynamics of Microplastic Migration through Different Aquatic Environments: Subsidies for a Better Understanding of Its Environmental Dispersion. Microplastics 2023, 2, 62–77. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, Z.; Guan, D.; Zhao, J.; Zhang, Q.; Liu, Q.; Xie, J.; Sun, Y.; Guo, L. Occurrence Characteristics and Ecotoxic Effects of Microplastics in Environmental Media: A Mini Review. Appl. Biochem. Biotechnol. 2023, 1–24. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. Methods for sampling and detection of microplastics in water and sediment: A critical review. TrAC Trends Anal. Chem. 2019, 110, 150–159. [Google Scholar] [CrossRef]
- Lise, I.; Bråte; Allan, I.J.; Thomas, K.V. Report made for the Norwegian Environment Agency: Microplastics in Marine Environments; Occurrence, Distribution and Effects. Available online: https://www.researchgate.net/publication/273089847 (accessed on 15 January 2023).
- Long, M.; Moriceau, B.; Gallinari, M.; Lambert, C.; Huvet, A.; Raffray, J.; Soudant, P. Interactions between microplastics and phytoplankton aggregates: Impact on their respective fates. Mar. Chem. 2015, 175, 39–46. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef]
- Erni-Cassola, G.; Zadjelovic, V.; Gibson, M.I.; Christie-Oleza, J.A. Distribution of plastic polymer types in the marine environment; A meta-analysis. J. Hazard. Mater. 2019, 369, 691–698. [Google Scholar] [CrossRef]
- Dharmadasa, W.S.; Andrady, A.; Kumara, P.T.P.; Maes, T.; Gangabadage, C. Microplastic pollution in Marine Protected Areas of Southern Sri Lanka. Mar. Pollut. Bull. 2021, 168, 112462. [Google Scholar] [CrossRef] [PubMed]
- Koongolla, J.B.; Andrady, A.; Kumara, P.T.P.; Gangabadage, C. Evidence of microplastics pollution in coastal beaches and waters in southern Sri Lanka. Mar. Pollut. Bull. 2018, 137, 277–284. [Google Scholar] [CrossRef]
- Jang, Y.C.; Ranatunga, R.; Mok, J.Y.; Kim, K.S.; Hong, S.Y.; Choi, Y.R.; Gunasekara, A. Composition and abundance of marine debris stranded on the beaches of Sri Lanka: Results from the first island-wide survey. Mar. Pollut. Bull. 2018, 128, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Weerakoon, W.R.W.M.A.P.; Grøsvik, B.E.; Dalpadado, P.; Wimalasiri, H.B.U.G.M.; Rathnasuriya, M.I.G.; Harischandra, K.A.D.A.T.; Shirantha, R.R.A.R.; Madhushankha, H.M.T.C.; Sampath, W.A.D.; Jayasinghe, R.P.P.K.; et al. Enumeration of microplastics in Sri Lankan waters: Preliminary findings from the R/V Dr. Fridtjof Nansen ecosystem survey, 2018. In Scientific Sessions 2019; National Aquatic Resources Research and Development Agency (NARA): Colombo, Sri Lanka, 2019. [Google Scholar]
- Sewwandi, M.; Hettithanthri, O.; Egodage, S.; Amarathunga, A.; Vithanage, M. Unprecedented marine microplastic contamination from the X-Press Pearl container vessel disaster. Sci. Total Environ. 2022, 828, 154374. [Google Scholar] [CrossRef]
- Sewwandi, M.; Amarathunga, A.; Wijesekara, H.; Mahatantila, K.; Vithanage, M. Contamination and distribution of buried microplastics in Sarakkuwa beach ensuing the MV X-Press Pearl maritime disaster in Sri Lankan sea. Mar. Pollut. Bull. 2022, 184, 114074. [Google Scholar] [CrossRef] [PubMed]
- de Vos, A.; Aluwihare, L.; Youngs, S.; DiBenedetto, M.H.; Ward, C.P.; Michel, A.P.M.; Colson, B.C.; Mazzotta, M.G.; Walsh, A.N.; Nelson, R.K.; et al. The M/V X-Press Pearl Nurdle Spill: Contamination of Burnt Plastic and Unburnt Nurdles along Sri Lanka’s Beaches. ACS Environ. Au 2022, 2, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Partow, H. X-Press Pearl Maritime Disaster Sri Lanka Report of the UN Environmental Advisory Mission; UN: New York, NY, USA, 2021. [Google Scholar]
- Laboratory Methods for the Analysis of Microplastics in the Marine Environment: Recommendations for Quantifying Synthetic Particles in Waters and Sediments; NOAA Marine Debris Division: Silver Spring, MD, USA, 2015.
- Renner, G.; Nellessen, A.; Schwiers, A.; Wenzel, M.; Schmidt, T.C.; Schram, J. Data preprocessing & evaluation used in the microplastics identification process: A critical review & practical guide. TrAC Trends Anal. Chem. 2019, 111, 229–238. [Google Scholar] [CrossRef]
- Bakir, A.; Doran, D.; Silburn, B.; Russell, J.; Archer-Rand, S.; Barry, J.; Maes, T.; Limpenny, C.; Mason, C.; Barber, J.; et al. A spatial and temporal assessment of microplastics in seafloor sediments: A case study for the UK. Front. Mar. Sci. 2023, 9, 1093815. [Google Scholar] [CrossRef]
- Fernandino, G.; Elliff, C.I.; Silva, I.R.; Bittencourt, A.C. How many pellets are too many? The pellet pollution index as a tool to assess beach pollution by plastic resin pellets in Salvador, Bahia, Brazil. J. Integr. Coast. Zone Manag. 2015, 15, 325–332. [Google Scholar] [CrossRef]
- Bolduc, S.; Jung, K.; Venkata, P.; Ashokcline, M.; Jayasinghe, R.; Baillie, C.; Lessard, L. Banana fiber/low-density polyethylene recycled composites for third world eco-friendly construction applications—Waste for life project Sri Lanka. J. Reinf. Plast. Compos. 2018, 37, 1322–1331. [Google Scholar] [CrossRef]
- Jordi Labs LLC. Case Study FTIR for Identification of Contamination; Final Report; Jordi Labs LLC: Mansfield, MA, USA, 2014. [Google Scholar]
- Naranjo, A.; Osswald, T.; Roldán-Alzate, A.; Diego, J.; María, S.; Noriega, D.P. Plastics Testing and Characterization; Carl Hanser Verlag: Munich, Germany, 2008. [Google Scholar]
- Atas, D.D.; Makkonen-CraigSampling, S. Microplastics in Beach Sediments and Analysis Using FTIR Spectroscopy Title: Sampling Microplastics in Beach Sediments and Analysis Using FTIR Spectroscopy. Mater’s Thesis, Arcada University of Applied Sciences, Helsinki, Finland, 2019. [Google Scholar]
- Sunitha, T.; Monisha, V.; Sivanesan, S.; Vasanthy, M.; Prabhakaran, M.; Omine, K.; Sivasankar, V.; Darchen, A. Micro-plastic pollution along the Bay of Bengal coastal stretch of Tamil Nadu, South India. Sci. Total Environ. 2021, 756, 144073. [Google Scholar] [CrossRef] [PubMed]
- Athapaththu, A.; Thushari, G.; Dias, P.; Abeygunawardena, A.; Egodauyana, K.; Liyanage, N.; Pitawala, H.; Senevirathna, J. Plastics in surface water of southern coastal belt of Sri Lanka (Northern Indian Ocean): Distribution and characterization by FTIR. Mar. Pollut. Bull. 2020, 161, 111750. [Google Scholar] [CrossRef]
- Goswami, P.; Vinithkumar, N.V.; Dharani, G. Microplastics particles in seafloor sediments along the Arabian Sea and the Andaman Sea continental shelves: First insight on the occurrence, identification, and characterization. Mar. Pollut. Bull. 2021, 167, 112311. [Google Scholar] [CrossRef] [PubMed]
- Hansani, K.; Thilakarathne, E.; Koongolla, J.B.; Gunathilaka, W.; Perera, B.; Weerasingha, W.; Egodauyana, K. Contamination of microplastics in tropical coral reef ecosystems of Sri Lanka. Mar. Pollut. Bull. 2023, 194, 115299. [Google Scholar] [CrossRef]
- Mayoma, B.S.; Sørensen, C.; Shashoua, Y.; Khan, F.R. Microplastics in beach sediments and cockles (Anadara antiquata) along the Tanzanian coastline. Bull. Environ. Contam. Toxicol. 2020, 105, 513–521. [Google Scholar] [CrossRef]
- Mu, J.; Zhang, S.; Qu, L.; Jin, F.; Fang, C.; Ma, X.; Zhang, W.; Wang, J. Microplastics abundance and characteristics in surface waters from the Northwest Pacific, the Bering Sea, and the Chukchi Sea. Mar. Pollut. Bull. 2019, 143, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Guo, H.; Chen, H.; Wang, S.; Sun, X.; Zou, Q.; Zhang, Y.; Lin, H.; Cai, S.; Huang, J. Microplastics in the Northwestern Pacific: Abundance, distribution, and characteristics. Sci. Total Environ. 2019, 650, 1913–1922. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, C.; Liu, K.; Zhu, L.; Song, Z.; Li, D. Atmospheric microplastic over the South China Sea and East Indian Ocean: Abundance, distribution and source. J. Hazard. Mater. 2020, 389, 121846. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, X.; Liu, K.; Zhu, L.; Wei, N.; Zong, C.; Li, D. Pelagic microplastics in surface water of the Eastern Indian Ocean during monsoon transition period: Abundance, distribution, and characteristics. Sci. Total Environ. 2021, 755, 142629. [Google Scholar] [CrossRef]
- Athawuda, A.M.G.A.D.; Jayasiri, H.B.; Thushari, G.G.N.; Guruge, K.P.G.K.P. Quantification and morphological characterization of plastic litter (0.30–100 mm) in surface waters of off Colombo, west coast of Sri Lanka. Environ. Monit. Assess. 2020, 192, 1–17. [Google Scholar] [CrossRef]
- Li, J.; Green, C.; Reynolds, A.; Shi, H.; Rotchell, J.M. Microplastics in mussels sampled from coastal waters and supermarkets in the United Kingdom. Environ. Pollut. 2018, 241, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Taherdoost. Sampling Methods in Research Methodology; How to Choose a Sampling Technique for Research. 2016. Available online: https://ssrn.com/abstract=3205035 (accessed on 15 January 2023).
- Park, J.; Hong, S.; Shim, W.J.; Khim, J.S.; Park, J. Distribution, compositional characteristics, and historical pollution records of microplastics in tidal flats of South Korea. Mar. Pollut. Bull. 2023, 189, 114741. [Google Scholar] [CrossRef] [PubMed]
- Cocca, M.; Di Pace, E.; Errico, M.E.; Gentile, G.; Montarsolo, A.; Mossotti, R. Springer Water Proceedings of the International Conference on Microplastic Pollution in the Mediterranean Sea. Available online: http://www.springer.com/series/13419 (accessed on 20 February 2023).






| Study Area | Matrix | Abundance (Values Refer to the Same Units) | Abundance (Original Values) | Reference |
|---|---|---|---|---|
| western coastline of Sri Lanka; estuaries of the Kelani River to Mahaoya | Water | - | 2.0 ± 0.6 items/L to 161.0 ± 15.7 items/L | Present study |
| Sediment | - | 3.0 ± 0.3 items/m2 to 656.0 ± 34.5 items/m2 | ||
| 22 beaches along the coastline of Sri Lanka | Sediment | - | 4.1 large (>25 mm) and 158 small (5–25 mm) MPs/m2 | [37] |
| southern coastal region of Sri Lanka | Water | 0.0175 ± 0.0034 items/L | 17.5 ± 3.4 items/m3 | [52] |
| coral reef ecosystems in the eastern coastal region of Sri Lanka | Water | 0.0119 ± 0.002 items/L | 11.9 ± 2.0 items/m3 | [54] |
| Sediment | NA | 42.2 ± 5.9 items/kg | ||
| Bay of Bengal coastal stretch of Tamil Nadu, South India | Water | 0.06–0.82 items/L | 60–820 items/m3 | [51] |
| Sediment | NA | 60–1620 items/kg | ||
| Tanzanian coastline in East Africa | Sediment | NA | 2972 ± 238 particles/kg dry sediment | [55] |
| Chukchi Sea, the Bering Sea, and the Northwest Pacific | Water | 1.3 × 10−4 ± 0.00011 items/L | 0.13 ± 0.11 items/m3 | [56] |
| Mid-west Pacific Ocean | Sediment | 0.03 ± 0.03 items/m2 | 34,039 ± 25,101 pieces/km2 | [25] |
| Northwestern Pacific Ocean | Sediment | 0.01 items/m2 | 1.0 × 104 items/km2 | [57] |
| South China Sea | Water | 0.42 × 10−4 ± 0.000025 items/L | 4.2 ± 2.5 items/100 m3 | [58] |
| East Indian Ocean | Water | 4.0 × 10−6 ± 0.000006 items/L | 0.4 ± 0.6 items/100 m3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weerasekara, P.G.Y.W.; De Silva, D.S.M.; De Silva, R.C.L.; Amarathunga, A.A.D.; Bakir, A.; McGoran, A.R.; Sivyer, D.B.; Reeve, C. Sampling and Analysis of Microplastics in the Coastal Environments of Sri Lanka: Estuaries of the Kelani River to Mahaoya. Water 2024, 16, 1932. https://doi.org/10.3390/w16131932
Weerasekara PGYW, De Silva DSM, De Silva RCL, Amarathunga AAD, Bakir A, McGoran AR, Sivyer DB, Reeve C. Sampling and Analysis of Microplastics in the Coastal Environments of Sri Lanka: Estuaries of the Kelani River to Mahaoya. Water. 2024; 16(13):1932. https://doi.org/10.3390/w16131932
Chicago/Turabian StyleWeerasekara, P. G. Y. W., D. S. M. De Silva, R. C. L. De Silva, A. A. D. Amarathunga, A. Bakir, A. R. McGoran, D. B. Sivyer, and C. Reeve. 2024. "Sampling and Analysis of Microplastics in the Coastal Environments of Sri Lanka: Estuaries of the Kelani River to Mahaoya" Water 16, no. 13: 1932. https://doi.org/10.3390/w16131932





