A Novel Coupling of a Biological Aerated Filter with Al3+ Addition-O3/H2O2 with Microbubble for the Advanced Treatment of Proprietary Chinese Medicine Secondary Effluent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Features of PCMSE (or WSPTA)
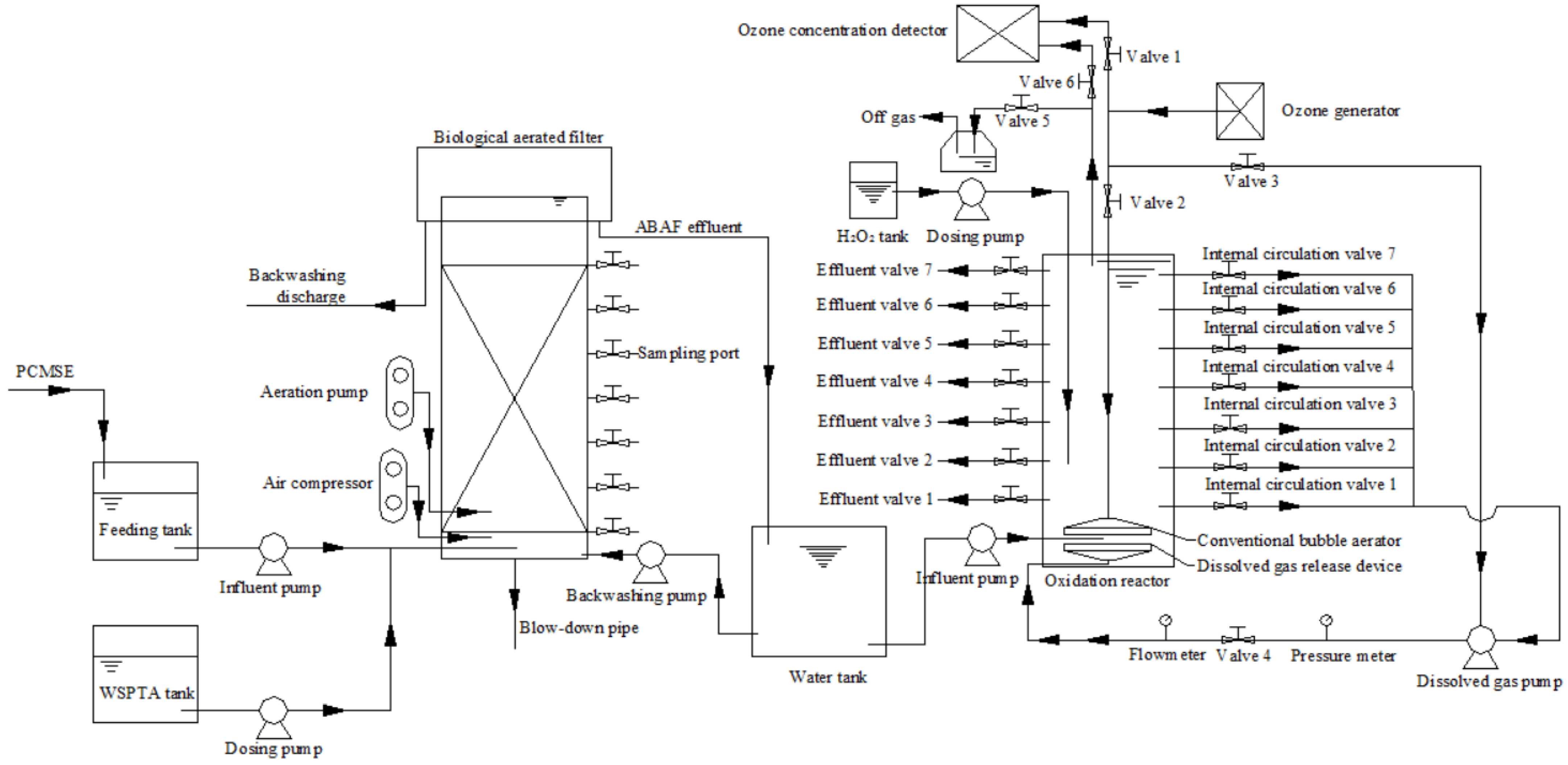
2.2. Pilot Device and Experiment Arrangement
2.2.1. Pilot Device
2.2.2. Experiment Arrangement
- (1).
- (2).
- (3).
- (4).
- Apart from this, to facilitate comparisons, the performance of OHOMB and OHOCB in treating BAFA effluent was respectively investigated under the same condition (namely, the optimum oxidation condition examined in step No. 3) (Figure S4 and Table S7). The oxidation reactor in Figure S4 was operated in continuous mode with internal circulation. Correspondingly, the optimum operation condition for OHOMB was defined, and is given in the Section 3.2;
- (5).
- Then, the performances of these oxidation systems (i.e., ozonation alone with microbubble, ozonation alone with conventional bubble, and H2O2 oxidation alone) in treating BAFA effluent were investigated, respectively, under the corresponding condition (Figure S5 and Table S7), and the experiment was executed with the same operation mode as step No. 4;
- (6).
- Finally, the removal of organics and TP in PCMSE by these combined processes (i.e., CBAFAOHOMB, CBAFAOHOCB, COHOMBBAFA) was assessed under the corresponding conditions (Figure S6). During this period, the pilot device was operated in semi-batch mode.
2.3. Analysis Method
2.3.1. Wastewater Sample
2.3.2. Analysis Method
2.4. SOUR Determination
2.5. Filtration Test
3. Results
3.1. BAFA Performance
3.1.1. TP and SS Elimination
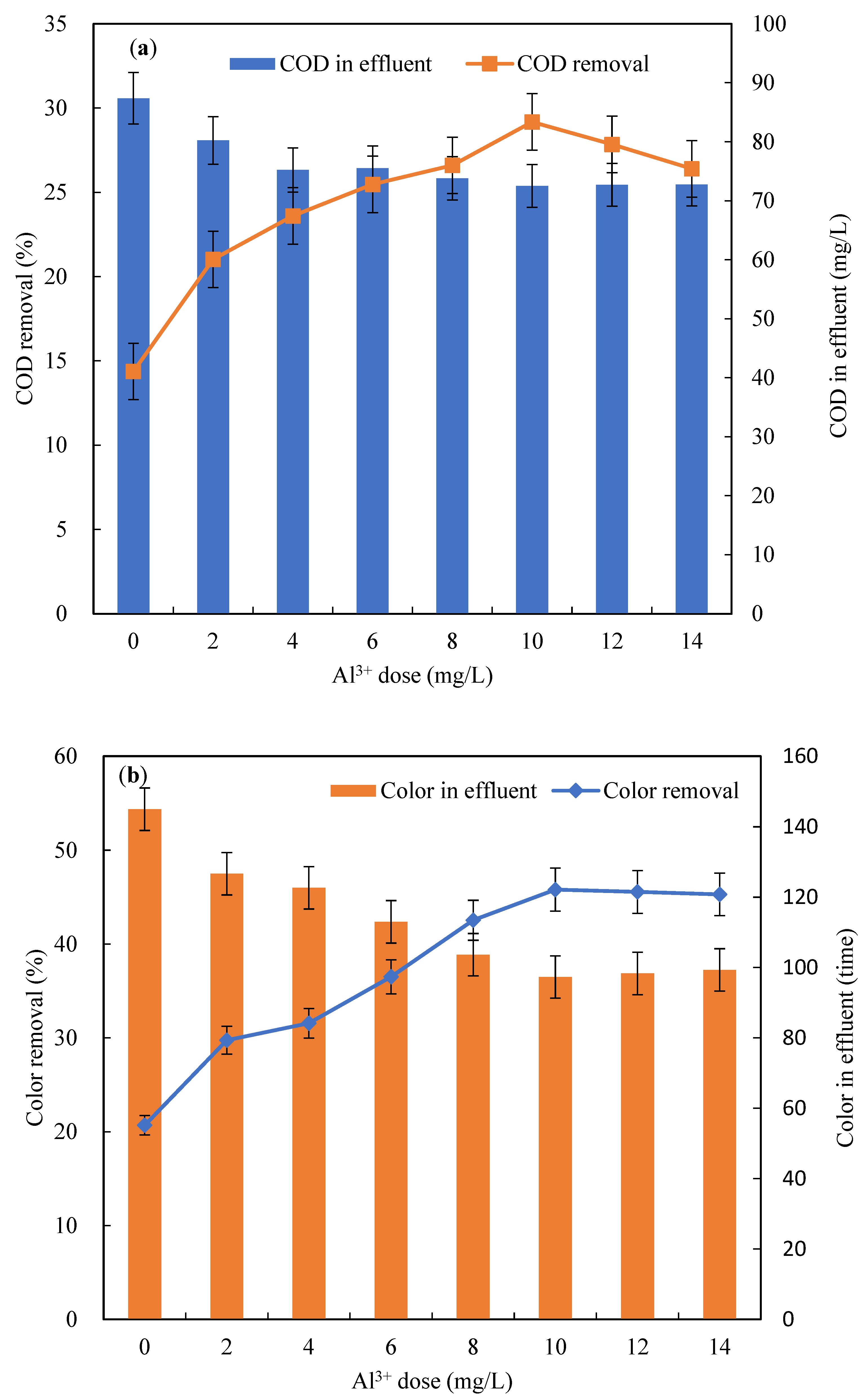
3.1.2. COD and Color Removal
3.1.3. Impact of Al3+ Addition on Biofilm Activity
3.2. Performance at OHOMB Stage
3.2.1. Organic Removal
3.2.2. Cost Evaluation
3.3. Performance of CBAFAOHOMB
4. Conclusions
- (1)
- CBAFAOHOMB markedly exerted the merits of BAFA and OHOMB, and was an efficient process for advanced treatment of PCMSE.
- (2)
- TP elimination depended on BAFA, and organic reduction was mainly owed to BAFA and OHOMB.
- (3)
- The addition of Al3+ into the biological-aerated filter significantly enhanced TP removal and relatively markedly improved organic elimination; bio-film activity in BAFA was not affected under a 10 mg/L Al3+ dose.
- (4)
- During O3/H2O2 oxidation, microbubble aeration more efficiently enhanced organic removal than conventional bubble aeration.
- (5)
- SS lowered oxidation performance relatively significantly and increased the operation cost during OHOMB oxidation of PCMSE.
- (6)
- Reuse of WSPTA was feasible, and this reuse not only saved PCMSE treatment costs but also recycled Al3+ resources.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PCMSE | Proprietary Chinese medicine secondary effluent |
| BAFA | Biological-aerated filter with Al3+ addition |
| OHOMB | O3/H2O2 with microbubble |
| CBAFAOHOMB | Coupling of BAFA-OHOMB |
| TP | Total phosphorus |
| COD | Chemical oxygen demand |
| SS | Suspended solid |
| WSPTA | Wastewater from soaking of Pinellia Ternata with alumen |
| OHOCB | O3/H2O2 with conventional bubble |
| CBAFAOHOCB | Coupling of BAFA-OHOCB |
| COHOMBBAFA | Coupling of OHOMB-BAFA |
| UV280 | Ultraviolet absorbance at 280 nm |
| SOUR | Specific oxygen uptake rate |
References
- Lv, L.; Li, Y.; Meng, L.; Qin, W.; Wu, C. Predicting acute toxicity of traditional Chinese medicine wastewater using UV absorption and volatile fatty acids as surrogates. Chemosphere 2017, 194, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lv, L.; Gong, X.; Qin, W.; Wu, C.; Meng, L. Performance evaluation and hydraulic characteristics of an innovative controlled double circle anaerobic reactor for treating traditional Chinese medicine wastewater. Biochem. Eng. J. 2017, 128, 186–194. [Google Scholar] [CrossRef]
- Lv, L.; Li, W.; Wu, C.; Meng, L.; Qin, W. Microbial community composition and function in a pilot-scale anaerobic-anoxic-aerobic combined process for the treatment of traditional Chinese medicine wastewater. Bioresour. Technol. 2017, 240, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Deng, Q.; Lu, Y.; Qin, R.; Chen, S.; Wei, J.; Chen, M.; Huang, Z. Effects of hydraulic retention time on the performance and microbial community of an anaerobic baffled reactor-bioelectricity Fenton coupling reactor for treatment of traditional Chinese medicine wastewater. Bioresour. Technol. 2019, 288, 121508. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Lu, Y.; Deng, Q.; Chen, S.; Pang, G.; Chen, W.; Chen, M.; Huang, Z. Performance of a novel ABR-bioelectricity-Fenton coupling reactor for treating traditional Chinese medicine wastewater containing catechol. Ecoto Environ. Safe 2019, 177, 39–46. [Google Scholar] [CrossRef]
- GB21906-2008; Discharge Standard of Water Pollutants for Pharmaceutical Industry Chinese Traditional Medicine Category. China Environmental Science Press: Beijing, China, 2008.
- Wu, Q.; Li, W.; Yu, W.; Li, Y.; Li, A. Removal of fluorescent dissolved organic matter in biologically treated textile wastewater by ozonation-biological aerated filter. J. Taiwan Inst. Chem. E 2016, 59, 359–364. [Google Scholar] [CrossRef]
- Zheng, J.; Lin, T.; Chen, W.; Tao, H.; Tan, Y.; Ma, B. Removal of precursors of typical nitrogenous disinfection byproducts in ozonation integrated with biological activated carbon (O3/BAC). Chemosphere 2018, 209, 68–77. [Google Scholar] [CrossRef]
- Ding, P.; Chu, L.; Wang, J. Advanced treatment of petrochemical wastewater by combined ozonation and biological aerated filter. Environ. Sci. Pollut. Res. 2018, 25, 9673–9682. [Google Scholar] [CrossRef]
- Yuan, Z.; Sui, M.; Yuan, B.; Li, P.; Wang, J.; Qin, J.; Xu, G. Degradation of ibuprofen using ozone combined with peroxymonosulfate. Environ. Sci. Water Res. Technol. 2017, 3, 960–969. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, H.; Zhang, Y.; Deng, Q.; Zhang, J. Degradation of Bisphenol a using ozone/persulfate process: Kinetics and mechanism. Water Air Soil Pollut. 2016, 52, 227. [Google Scholar] [CrossRef]
- Shen, Y.; Xu, Q.; Gao, D. Degradation of an anthraquinone dye by ozone/Fenton: Response surface approach and degradation pathway. Ozone Sci. Eng. 2017, 39, 219–232. [Google Scholar] [CrossRef]
- Ji, F.; Zhang, H.; Li, J.; Lai, B. Treatment of reverse osmosis (RO) concentrate from an old landfill site by Fe0/PS/O3 process. J. Chem. Technol. Biot. 2017, 92, 2616–2625. [Google Scholar] [CrossRef]
- Adil, S.; Maryam, B.; Kim, E.J. Individual and simultaneous degradation of sulfamethoxazole and trimethoprim by ozone, ozone/hydrogen peroxide and ozone/persulfate processes: A comparative study. Environ. Res. Sect. A 2020, 189, 109889. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Luo, Y.; Ran, G. An investigation of refractory organics in membrane bioreactor effluent following the treatment of landfill leachate by the O3/H2O2 and MW/PS processes. Waste Manag. 2019, 97, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Li, L.; Song, J.; Wu, M.; Zhou, S. Advanced treatment of bio-treated wastewater with combined O3/H2O2-BAF process in paper mill. China Pulp Pap. 2023, 11, 91–95. (In Chinese) [Google Scholar]
- Tang, G.; Wei, Y.; Qin, J.; Zhang, Z.; Qian, D.; Yu, X. Combined coagulation/decantation-ozone/hydrogen peroxide-biological aerated filter process for advanced treatment of bio-treated leachate from Chinese herbal medicine residue. Environ. Sci. Pollut. R 2021, 28, 37627–37635. [Google Scholar] [CrossRef] [PubMed]
- Zucker, I.; Lester, Y.; Avisar, D.; Hubner, U.; Jekel, M.; Weinberger, Y.; Mamane, H. Influence of wastewater particles on ozone degradation of trace organic contaminants. Environ. Sci. Technol. 2016, 49, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhou, Y.; Wang, Y.; Guo, M. Innovative combination of Fe2+-BAF and ozonation for enhancing organic micropollutants removal treating petrochemical secondary effluent. J. Hazard. Mater. 2017, 323, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y. Research on Chemically Enhanced Phosphorus Removal by UBAF. Master’s Dissertation, Harbin Institute of Technology, Harbin, China, 2011. (In Chinese). [Google Scholar]
- Liu, Y.; Wang, X.; Wang, X.; Chen, R. Influence of aluminum salt on microbial community in activated sludge and pollutant removal. China Water Wastewater 2012, 28, 128–131. (In Chinese) [Google Scholar]
- Petroula, S.; Nicolas, K. Disinfection applications of ozone micro- and Nanobubbles. Environ. Sci. Nano 2021, 8, 3493. [Google Scholar] [CrossRef]
- Kim, I.; Lee, J. Comparison of ozonation removal for PPCPs in secondary treated sewage by microbubble generator and ejector. Environ. Eng. Res. 2021, 27, 200163. [Google Scholar] [CrossRef]
- Kim, T.K.; Kim, T.; Lee, I.; Choi, K.; Zoh, K.D. Removal of tetramethylammonium hydroxide (TMAH) in semiconductor wastewater using the nano-ozone H2O2 process. J. Hazard. Mater. 2021, 409, 123759. [Google Scholar] [CrossRef]
- Zheng, T.; Wang, Q.; Zhang, T.; Shi, Z.; Tian, Y.; Shi, S.; Smaled, N.; Wang, J. Microbubble enhanced ozonation process for advanced treatment of wastewater produced in acrylic fiber manufacturing industry. J. Hazard. Mater. 2015, 287, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Azuma, T.; Otomo, K.; Kunitou, M.; Shimizu, M.; Hosomaru, K.; Mikata, S.; Mino, Y.; Hayashi, T. Removal of pharmaceuticals in water by introduction of ozonated microbubbles. Sep. Purif. Technol. 2019, 212, 483–489. [Google Scholar] [CrossRef]
- Environment Protection Bureau of China. Analytical Methods of Water and Wastewater, 4th ed.; China Environment Science Press: Beijing, China, 2012. (In Chinese) [Google Scholar]
- Park, S.; Cho, K.; Lee, T.; Lee, E.; Bae, H. Improved insights into the adaptation and selection of Nitrosomonas spp. for partial nitritation under saline conditions based on specific oxygen uptake rates and next generation sequencing. Sci. Total Environ. 2022, 822, 153644. [Google Scholar] [CrossRef]
- Wei, N.; Zhang, G.; Liu, D.; Wu, Y.; Wang, J. Coagulation behavior of polyaluminum chloride: Effects of pH and coagulant dosage. Chin. J. Chem. Eng. 2015, 6, 1041–1046. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhao, X.; Yang, C.; Hong, S.; Zhou, Z.; Zhang, C. Experimental study on treatment technology of cleaning wastewater from gas turbine compressor by Fenton-coagulation combined process. Therm. Power Gener. 2022, 51, 133–140. (In Chinese) [Google Scholar]
- Zhang, Z.; Wang, Y.; Leslie, G.L.; Waite, T.D. Effect of ferric and ferrous iron addition on phosphorus removal and fouling in submerged membrane bioreactors. Water Res. 2015, 69, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Altmann, J.; Rehfeld, R.; Träder, K.; Sperlich, A.; Jekel, M. Combination of granular activated carbon adsorption and deep-bed filtration as a single advanced wastewater treatment step for organic micropollutant and phosphorus removal. Water Res. 2016, 92, 131–139. [Google Scholar] [CrossRef]
- Yan, M.; Wang, D.; Ni, J.; Qu, J.; Chow, C.W.K.; Liu, H. Mechanism of natural organic matter removal by polyaluminum chloride: Effect of coagulant particle size and hydrolysis kinetics. Water Res. 2008, 42, 3361–3370. [Google Scholar] [CrossRef]
- Cui, X. Effects and Mechanisms of Residual Al-Coagulant in Reclaimed Wastewater on Bioclogging and Biofilm Formation. Ph.D. Dissertation, Northeast Normal University, Changchun, China, 2017. (In Chinese). [Google Scholar]
- Yu, J.; Ma, T.; Liu, Z.; Li, Y. Effect on microorganism activity of adding PAC in advanced wastewater treatment. J. Hohai Univ. 2012, 40, 451–454. (In Chinese) [Google Scholar] [CrossRef]
- Wang, W.; Cai, Y.; Hu, H.; Chen, J.; Wang, J.; Wu, Q. Advanced treatment of bio-treated dyeing and finishing wastewater using ozone-biological activated carbon: A study on the synergistic effects. Chem. Eng. J. 2019, 359, 168–175. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M.; Gohari, F. Degradation of 2, 4, 6-trichlorophenol in aqueous solutions using peroxymonosulfate/activated carbon/UV process via sulfate and hydroxyl radicals. J. Water Process Eng. 2016, 9, 22–28. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, X.; Li, J. Advanced treatment of oilfield production wastewater by an integration of coagulation/flotation, catalytic ozonation and biological processes. Environ. Technol. 2016, 37, 2536–2544. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wu, C.; Zhou, Y.; Wang, Y.; He, X. Effect of wastewater particles on catalytic ozonation in the advanced treatment of petrochemical secondary effluent. Chem. Eng. J. 2018, 345, 280–289. [Google Scholar] [CrossRef]
- Oh, B.T.; Seo, Y.S.; Sudhakar, D.; Choe, J.H.; Lee, S.M.; Park, Y.J.; Cho, M. Oxidative degradation of endotoxin by advanced oxidation process (O3/H2O2 & UV/H2O2). J. Hazard. Mater. 2017, 279, 105–110. [Google Scholar] [CrossRef]
- Fu, L.; Wu, C.; Zhou, Y.; Zuo, J.; Song, G.; Tan, Y. Ozonation reactivity characteristics of dissolved organic matter in secondary petrochemical wastewater by single ozone, ozone/H2O2, and ozone/catalyst. Chemosphere 2019, 233, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, G.; Liu, C.; Zhang, R.; Chen, X.; Zhang, L. Synergistic effect of microbubbles and activated carbon on the ozonation treatment of synthetic dyeing wastewater. Sep. Purif. Technol. 2018, 201, 10–18. [Google Scholar] [CrossRef]
- Wu, C.; Li, P.; Xia, S.; Wang, S.; Wang, Y.; Hu, J.; Liu, Z.; Yu, S. The role of interface in microbubble ozonation of aromatic compounds. Chemosphere 2019, 220, 1067–1074. [Google Scholar] [CrossRef]
- Zimmermann, S.G.; Wittenwiler, M.; Hollender, J.; Krauss, M.; Ort, C.; Siegrist, H.; von Gunten, U. Kinetic assessment and modeling of an ozonation step for full-scale municipal wastewater treatment: Micropollutant oxidation, by-product formation and disinfection. Water Res. 2011, 45, 605–617. [Google Scholar] [CrossRef]
- Hilles, A.H.; Abu Amr, S.S.; Hussein, R.A.; El-Sebaie, O.D.; Arafa, A.I. Performance of combined sodium persulfate/H2O2 based advanced oxidation process in stabilized landfill leachate treatment. J. Environ. Manag. 2016, 166, 493–498. [Google Scholar] [CrossRef] [PubMed]


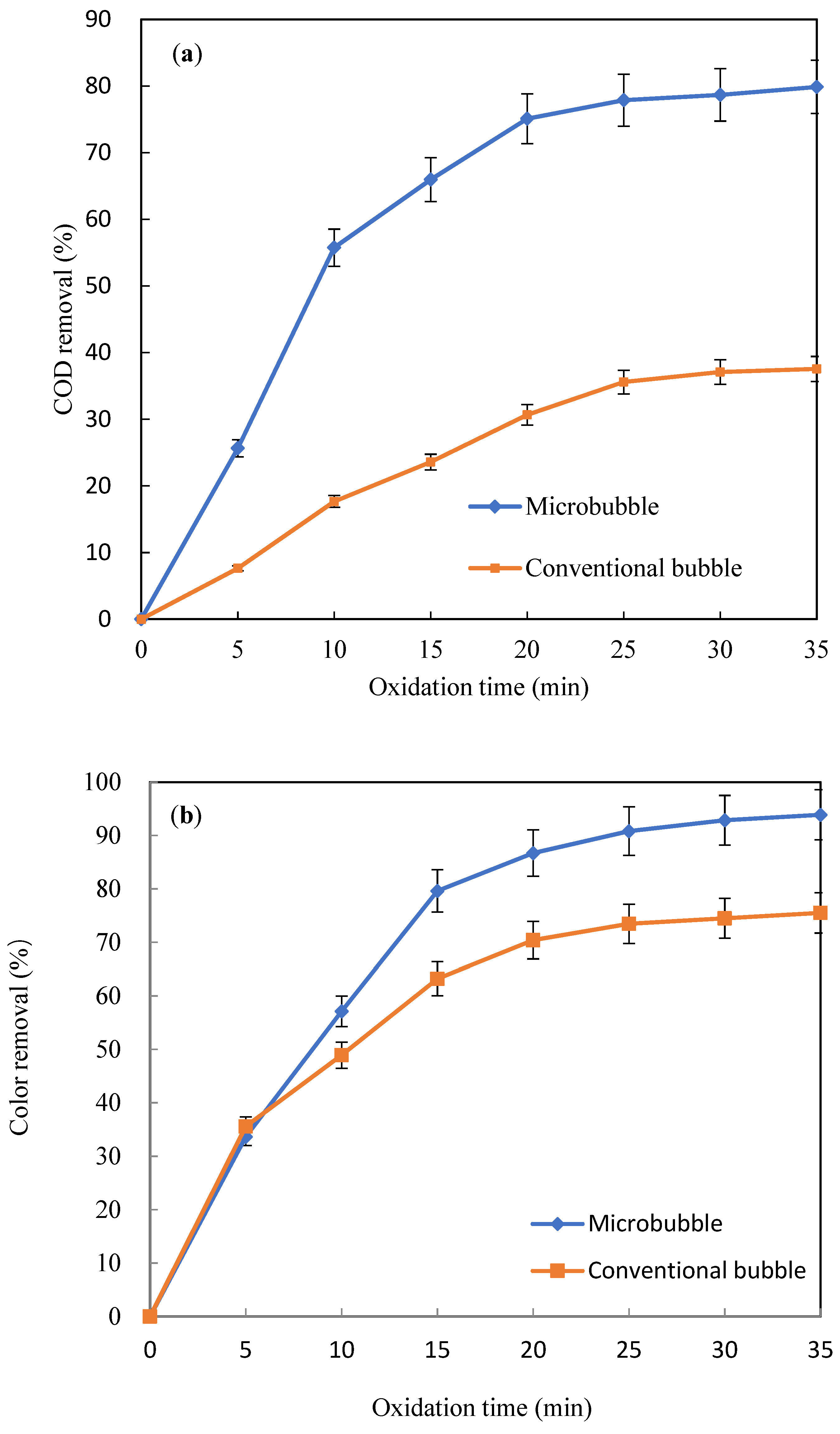

| Process | COD | Color |
|---|---|---|
| OHOMB | k = 0.24 R2 = 0.902 | k = 0.42 R2 = 0.963 |
| OHOCB | k = 0.11 R2 = 0.936 | k = 0.21 R2 = 0.903 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, S.; Tai, Y.; Wang, R.; Jiang, Y.; Zhou, L.; Tang, G.; Xv, Y.; Hua, C.; Yue, X. A Novel Coupling of a Biological Aerated Filter with Al3+ Addition-O3/H2O2 with Microbubble for the Advanced Treatment of Proprietary Chinese Medicine Secondary Effluent. Water 2024, 16, 2030. https://doi.org/10.3390/w16142030
Yi S, Tai Y, Wang R, Jiang Y, Zhou L, Tang G, Xv Y, Hua C, Yue X. A Novel Coupling of a Biological Aerated Filter with Al3+ Addition-O3/H2O2 with Microbubble for the Advanced Treatment of Proprietary Chinese Medicine Secondary Effluent. Water. 2024; 16(14):2030. https://doi.org/10.3390/w16142030
Chicago/Turabian StyleYi, Shilin, Yan Tai, Rui Wang, Yuehan Jiang, Luwei Zhou, Guomin Tang, Ying Xv, Chengwei Hua, and Xuemin Yue. 2024. "A Novel Coupling of a Biological Aerated Filter with Al3+ Addition-O3/H2O2 with Microbubble for the Advanced Treatment of Proprietary Chinese Medicine Secondary Effluent" Water 16, no. 14: 2030. https://doi.org/10.3390/w16142030




