Comparison of Hexavalent Chromium Adsorption Behavior on Conventional and Biodegradable Microplastics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Batch Adsorption Experiments
2.3. Desorption Experiments
2.4. Characterization of MPs
2.5. Analytical Methods
3. Results and Discussion
3.1. Characterization of the Representative Microplastics
3.1.1. Results of SEM
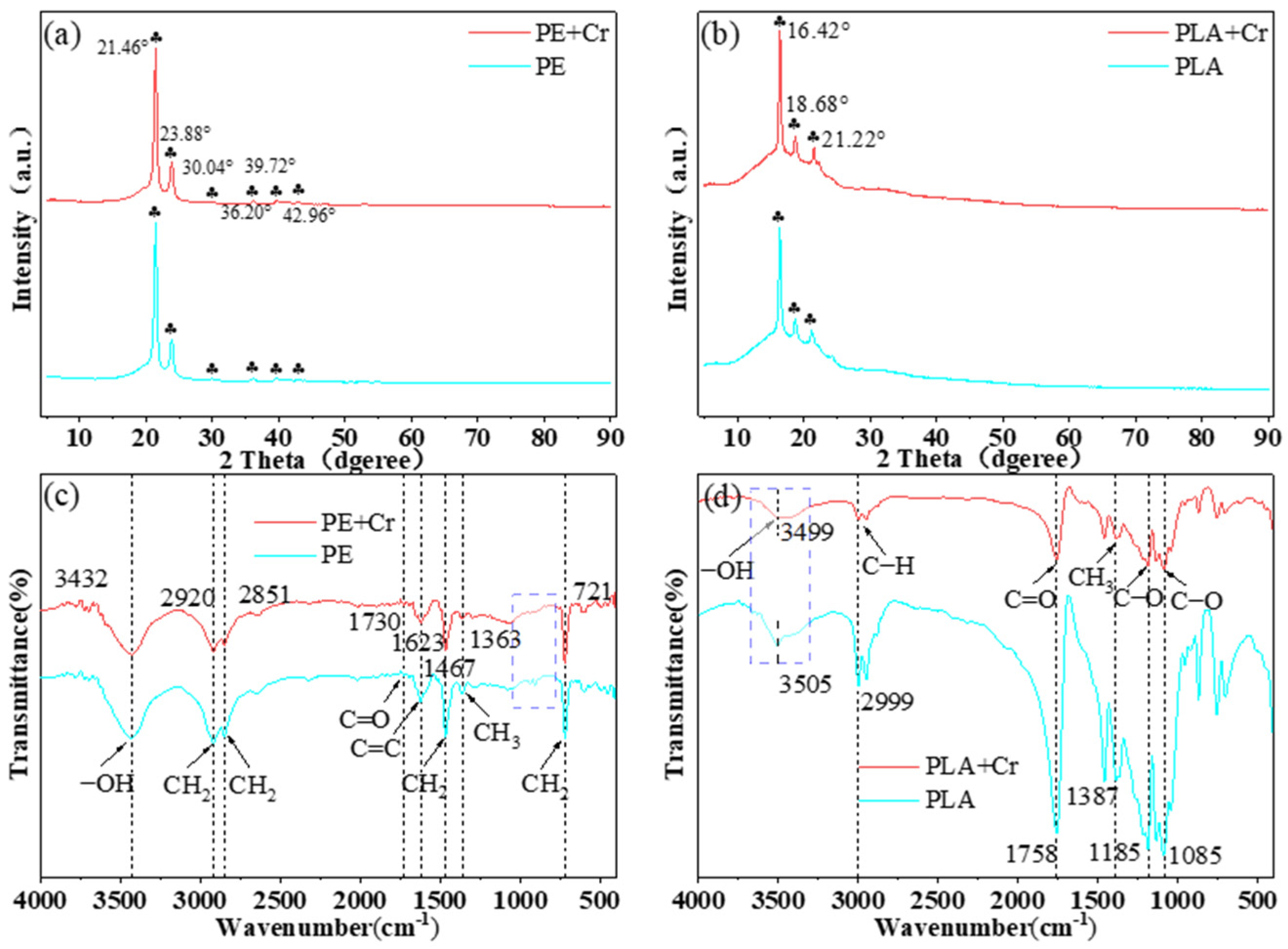
3.1.2. Results from XRD and FTIR Analysis
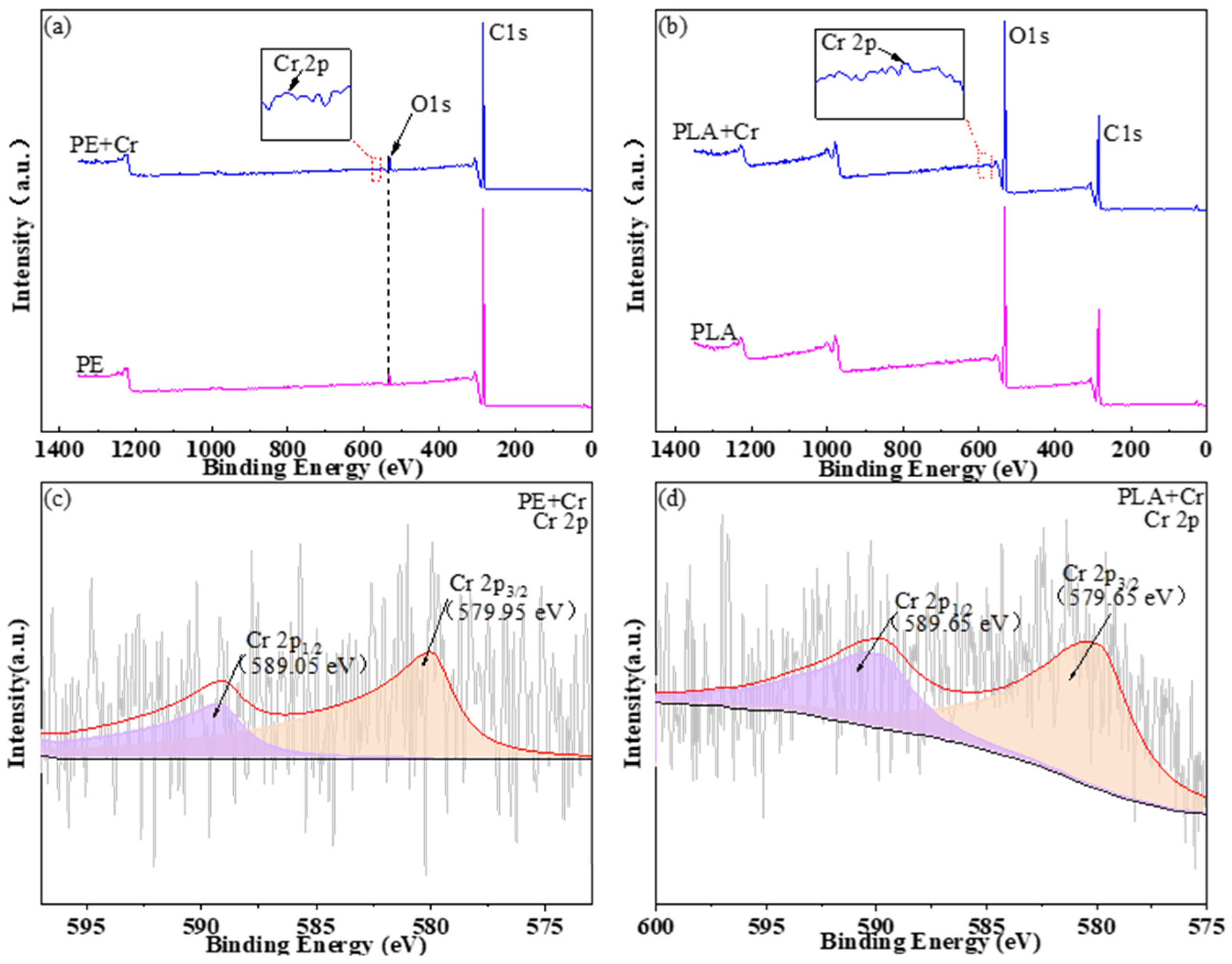
3.1.3. Results of XPS Analysis
3.2. Effect of MP Dose on Adsorption
3.3. Adsorption Kinetics of Cr(VI) onto the Representative Microplastics
3.4. Adsorption Isotherms of Cr(VI) Onto the Representative Microplastics
3.5. The Influence of Environmental Factors on the Adsorption of Cr(VI) Onto PE and PLA
3.5.1. The Influence of pH and Salinity
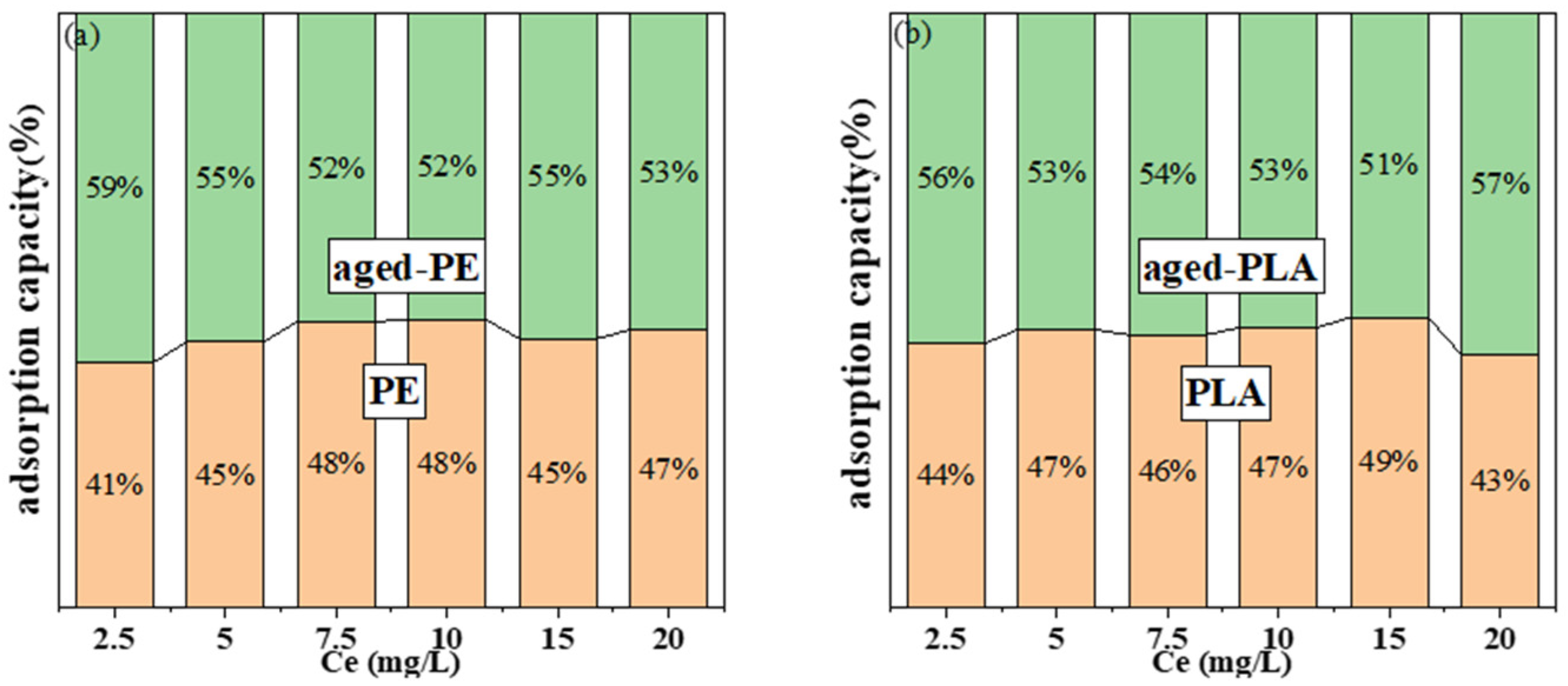
3.5.2. The Influence of Photoaging
3.5.3. The Influence of the Presence of Surfactants and Cu2+
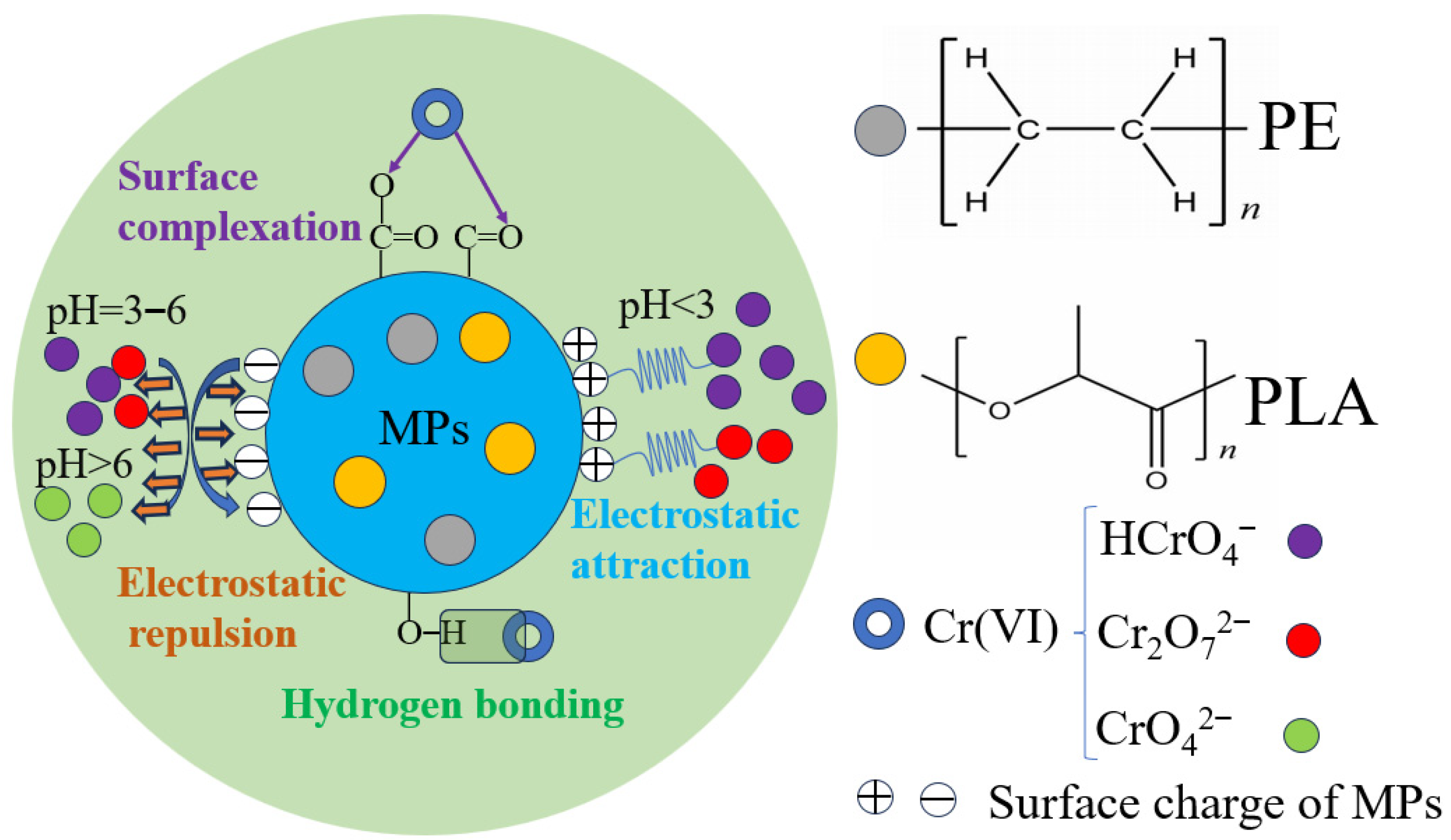
3.6. Adsorption Mechanisms
3.7. Desorption of Cr(VI)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ghaffar, I.; Rashid, M.; Akmal, M.; Hussain, A. Plastics in the Environment as Potential Threat to Life: An Overview. Environ. Sci. Pollut. Res. 2022, 29, 56928–56947. [Google Scholar] [CrossRef] [PubMed]
- Cózar, A.; Echevarría, F.; González-Gordillo, J.I.; Irigoien, X.; Úbeda, B.; Hernández-León, S.; Palma, Á.T.; Navarro, S.; García-de-Lomas, J.; Ruiz, A.; et al. Plastic Debris in the Open Ocean. Proc. Natl. Acad. Sci. USA 2014, 111, 10239–10244. [Google Scholar] [CrossRef] [PubMed]
- Barros, J.; Seena, S. Plastisphere in Freshwaters: An Emerging Concern. Environ. Pollut. 2021, 290, 118123. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Chen, X.; Xu, C.; Zhou, C.; Wang, C. Internal Interaction between Chemically-Pretreated Polypropylene Microplastics and Floc Growth during Flocculation: Critical Effect on Floc Properties and Flocculation Mechanisms. Sep. Purif. Technol. 2023, 306, 122710. [Google Scholar] [CrossRef]
- He, W.; Chen, X.; Xu, C.; Zhou, C.; Luo, J. Effect of Microplastic Aging Degree on Filter Cake Formation and Membrane Fouling Characteristics in Ultrafiltration Process with Pre-Coagulation. Sep. Purif. Technol. 2023, 310, 123221. [Google Scholar] [CrossRef]
- He, W.; Zheng, S.; Chen, X.; Lu, D.; Zeng, Z. Alkaline Aging Significantly Affects Mn(II) Adsorption Capacity of Polypropylene Microplastics in Water Environments: Critical Roles of Natural Organic Matter and Colloidal Particles. J. Hazard. Mater. 2022, 438, 129568. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Sun, H.; Wang, D.; Wang, S. Enhanced Pb2+ Adsorption Using Recyclable Magnetic Sodium Alginate in a Network Structure for High Renewable Capacity. J. Polym. Eng. 2024, 44, 354–363. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Yang, A.; Zhu, Q.; Sun, H.; Sun, P.; Yao, B.; Zang, Y.; Du, X.; Dong, L. Xanthate-Modified Magnetic Fe3O4@SiO2-Based Polyvinyl Alcohol/Chitosan Composite Material for Efficient Removal of Heavy Metal Ions from Water. Polymers 2022, 14, 1107. [Google Scholar] [CrossRef]
- Dong, L.; Shan, C.; Liu, Y.; Sun, H.; Yao, B.; Gong, G.; Jin, X.; Wang, S. Characterization and Mechanistic Study of Heavy Metal Adsorption by Facile Synthesized Magnetic Xanthate-Modified Chitosan/Polyacrylic Acid Hydrogels. Int. J. Environ. Res. Public Health 2022, 19, 11123. [Google Scholar] [CrossRef]
- Xiong, X.; Wang, J.; Liu, J.; Xiao, T. Microplastics and Potentially Toxic Elements: A Review of Interactions, Fate and Bioavailability in the Environment. Environ. Pollut. 2024, 340, 122754. [Google Scholar] [CrossRef] [PubMed]
- Vaiopoulou, E.; Gikas, P. Regulations for Chromium Emissions to the Aquatic Environment in Europe and Elsewhere. Chemosphere 2020, 254, 126876. [Google Scholar] [CrossRef] [PubMed]
- Monga, A.; Fulke, A.B.; Dasgupta, D. Recent Developments in Essentiality of Trivalent Chromium and Toxicity of Hexavalent Chromium: Implications on Human Health and Remediation Strategies. J. Hazard. Mater. Adv. 2022, 7, 100113. [Google Scholar] [CrossRef]
- Lu, X.; Zeng, F.; Wei, S.; Gao, R.; Abdurahman, A.; Wang, H.; Liang, W. Effects of Humic Acid on Pb2+ Adsorption onto Polystyrene Microplastics from Spectroscopic Analysis and Site Energy Distribution Analysis. Sci. Rep. 2022, 12, 8932. [Google Scholar] [CrossRef]
- Acosta-Coley, I.; Mendez-Cuadro, D.; Rodriguez-Cavallo, E.; De La Rosa, J.; Olivero-Verbel, J. Trace Elements in Microplastics in Cartagena: A Hotspot for Plastic Pollution at the Caribbean. Mar. Pollut. Bull. 2019, 139, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Holmes, L.A.; Turner, A.; Thompson, R.C. Adsorption of Trace Metals to Plastic Resin Pellets in the Marine Environment. Environ. Pollut. 2012, 160, 42–48. [Google Scholar] [CrossRef]
- Guo, X.; Wang, J. The Chemical Behaviors of Microplastics in Marine Environment: A Review. Mar. Pollut. Bull. 2019, 142, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Valenzuela, A.P.; Troncoso-Rojas, R.; Islas-Rubio, A.R.; Samsudin, H.; Peralta, E.; Soto-Valdez, H. Replacement of Conventional Packaging with Sustainable Materials for Corn Tortillas. Food Packag. Shelf Life 2023, 40, 101207. [Google Scholar] [CrossRef]
- Yao, Z.; Seong, H.J.; Jang, Y.-S. Environmental Toxicity and Decomposition of Polyethylene. Ecotoxicol. Environ. Saf. 2022, 242, 113933. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Cao, W.; Hu, Y.; Shen, W. The Influence of Pb(II) Adsorption on (Non) Biodegradable Microplastics by UV/O3 Oxidation Treatment. J. Environ. Chem. Eng. 2022, 10, 108615. [Google Scholar] [CrossRef]
- Wang, L.; Guo, C.; Qian, Q.; Lang, D.; Wu, R.; Abliz, S.; Wang, W.; Wang, J. Adsorption Behavior of UV Aged Microplastics on the Heavy Metals Pb(II) and Cu(II) in Aqueous Solutions. Chemosphere 2023, 313, 137439. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Gong, K.; Shao, X.; Liang, W.; Zhang, W.; Peng, C. Effect of Polyethylene, Polyamide, and Polylactic Acid Microplastics on Cr Accumulation and Toxicity to Cucumber (Cucumis sativus L.) in Hydroponics. J. Hazard. Mater. 2023, 450, 131022. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Kumar, S.; Wang, Q.; Wang, M.; Fahad, S.; Nizamani, M.M.; Chang, K.; Khan, S.; Huang, Q.; Zhu, G. Influence of Polyvinyl Chloride Microplastic on Chromium Uptake and Toxicity in Sweet Potato. Ecotoxicol. Environ. Saf. 2023, 251, 114526. [Google Scholar] [CrossRef] [PubMed]
- Ainali, N.M.; Kalaronis, D.; Evgenidou, E.; Kyzas, G.Z.; Bobori, D.C.; Kaloyianni, M.; Yang, X.; Bikiaris, D.N.; Lambropoulou, D.A. Do Poly(Lactic Acid) Microplastics Instigate a Threat? A Perception for Their Dynamic towards Environmental Pollution and Toxicity. Sci. Total Environ. 2022, 832, 155014. [Google Scholar] [CrossRef] [PubMed]
- Luangrath, A.; Na, J.; Kalimuthu, P.; Song, J.; Kim, C.; Jung, J. Ecotoxicity of Polylactic Acid Microplastic Fragments to Daphnia Magna and the Effect of Ultraviolet Weathering. Ecotoxicol. Environ. Saf. 2024, 271, 115974. [Google Scholar] [CrossRef]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation Rates of Plastics in the Environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, Y.; Zhang, Z.; Pan, Y.; Li, X.; Zhuang, Z.; Li, J.; Luo, Q.; Chen, X. Enhanced Reproductive Toxicity of Photodegraded Polylactic Acid Microplastics in Zebrafish. Sci. Total Environ. 2024, 912, 168742. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, X.; Xia, S.; Zhao, J. Cu(II) Adsorption on Poly(Lactic Acid) Microplastics: Significance of Microbial Colonization and Degradation. Chem. Eng. J. 2022, 429, 132306. [Google Scholar] [CrossRef]
- Tian, X.; Weixie, L.; Wang, S.; Zhang, Y.; Xiang, Q.; Yu, X.; Zhao, K.; Zhang, L.; Penttinen, P.; Gu, Y. Effect of Polylactic Acid Microplastics and Lead on the Growth and Physiological Characteristics of Buckwheat. Chemosphere 2023, 337, 139356. [Google Scholar] [CrossRef] [PubMed]
- Shang, G.; Zhai, J.; Xu, G.; Wang, L.; Wang, X. Ecotoxicological Effects of Co-Exposure Biodegradable Microplastics Polylactic Acid with Cadmium Are Higher than Conventional Microplastics Polystyrene with Cadmium on the Earthworm. Sci. Total Environ. 2023, 903, 166953. [Google Scholar] [CrossRef]
- Shen, M.; Song, B.; Zeng, G.; Zhang, Y.; Teng, F.; Zhou, C. Surfactant Changes Lead Adsorption Behaviors and Mechanisms on Microplastics. Chem. Eng. J. 2021, 405, 126989. [Google Scholar] [CrossRef]
- Shao, X.; Zhang, Q.; Liang, W.; Gong, K.; Fu, M.; Saif, S.; Peng, C.; Zhang, W. Polyamide Microplastics as Better Environmental Vectors of Cr(VI) in Comparison to Polyethylene and Polypropylene Microplastics. Mar. Pollut. Bull. 2023, 186, 114492. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.; Benya, A.; Hota, S.; Kumar, M.S.; Singh, S. Eco-Toxicity of Hexavalent Chromium and Its Adverse Impact on Environment and Human Health in Sukinda Valley of India: A Review on Pollution and Prevention Strategies. Environ. Chem. Ecotoxicol. 2023, 5, 46–54. [Google Scholar] [CrossRef]
- Assefa, H.; Singh, S.; Olu, F.E.; Dhanjal, D.S.; Mani, D.; Khan, N.A.; Singh, J.; Ramamurthy, P.C. Advances in Adsorption Technologies for Hexavalent Chromium Removal: Mechanisms, Materials, and Optimization Strategies. Desalination Water Treat. 2024, 319, 100576. [Google Scholar] [CrossRef]
- Lv, Y.; Huang, Y.; Kong, M.; Yang, Q.; Li, G. Multivariate Correlation Analysis of Outdoor Weathering Behavior of Polypropylene under Diverse Climate Scenarios. Polym. Test. 2017, 64, 65–76. [Google Scholar] [CrossRef]
- Fan, X.; Zou, Y.; Geng, N.; Liu, J.; Hou, J.; Li, D.; Yang, C.; Li, Y. Investigation on the Adsorption and Desorption Behaviors of Antibiotics by Degradable MPs with or without UV Ageing Process. J. Hazard. Mater. 2021, 401, 123363. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, N.; Hou, L.; Li, C.; Li, C. Adsorption Behaviors of Chlorpyrifos on UV Aged Microplastics. Mar. Pollut. Bull. 2023, 190, 114852. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xue, B.; Lin, H.; Lan, W.; Wang, X.; Wei, J.; Li, M.; Li, M.; Duan, Y.; Lv, J.; et al. The Adsorption and Release Mechanism of Different Aged Microplastics toward Hg(II) via Batch Experiment and the Deep Learning Method. Chemosphere 2024, 350, 141067. [Google Scholar] [CrossRef]
- Jiang, Y.; Qin, Z.; Fei, J.; Ding, D.; Sun, H.; Wang, J.; Yin, X. Surfactant-Induced Adsorption of Pb(II) on the Cracked Structure of Microplastics. J. Colloid Interface Sci. 2022, 621, 91–100. [Google Scholar] [CrossRef]
- Lv, M.; Zhang, T.; Ya, H.; Xing, Y.; Wang, X.; Jiang, B. Effects of Heavy Metals on the Adsorption of Ciprofloxacin on Polyethylene Microplastics: Mechanism and Toxicity Evaluation. Chemosphere 2023, 315, 137745. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Su, F.; Wang, Y.; Peng, L.; Liu, D. Adsorption Behaviour of Microplastics on the Heavy Metal Cr(VI) before and after Ageing. Chemosphere 2022, 302, 134865. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhang, J.; Zhang, Z.; Gao, H.; Xu, W.; Xia, X. Insights into Adsorption Behavior and Mechanism of Cu(II) onto Biodegradable and Conventional Microplastics: Effect of Aging Process and Environmental Factors. Environ. Pollut. 2024, 342, 123061. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Ran, Z.; Cang, Y.; Chen, X.; Shaaban, M.; Peng, Q.-A. Efficient Removal of Cr(VI) and As(V) from an Aquatic System Using Iron Oxide Supported Typha Biochar. Environ. Res. 2023, 225, 115588. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Tao, R.; Cheng, S.; Xing, B.; Meng, W.; Nie, Y.; Zhang, C.; Yu, J. Microwave-Assisted One-Pot Method Preparation of ZnO Decorated Biochar for Levofloxacin and Cr(VI) Removal from Wastewater. Ind. Crops Prod. 2024, 208, 117863. [Google Scholar] [CrossRef]
- Li, H.; Wang, F.; Li, J.; Deng, S.; Zhang, S. Adsorption of Three Pesticides on Polyethylene Microplastics in Aqueous Solutions: Kinetics, Isotherms, Thermodynamics, and Molecular Dynamics Simulation. Chemosphere 2021, 264, 128556. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, R.; Li, Z.; Yan, B. Adsorption Properties and Influencing Factors of Cu(II) on Polystyrene and Polyethylene Terephthalate Microplastics in Seawater. Sci. Total Environ. 2022, 812, 152573. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, X.; Liu, W.; Li, M.; Chen, Y.; Yang, Y.; Yang, S. Simple Synthesis, Characterization and Mechanism of Fe/Zr Bimetallic-Organic Framework for Cr (VI) Removal from Wastewater. J. Environ. Chem. Eng. 2024, 12, 112040. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, L.; Hua, T.; Li, Y.; Zhou, X.; Wang, W.; You, Z.; Wang, H.; Li, M. The Mechanism for Adsorption of Cr(VI) Ions by PE Microplastics in Ternary System of Natural Water Environment. Environ. Pollut. 2020, 257, 113440. [Google Scholar] [CrossRef]
- Plohl, O.; Erjavec, A.; Fras Zemljič, L.; Vesel, A.; Čolnik, M.; Škerget, M.; Fan, Y.V.; Čuček, L.; Trimmel, G.; Volmajer Valh, J. Morphological, Surface and Thermal Properties of Polylactic Acid Foils, Melamine-Etherified Resin, and Polyethylene Terephthalate Fabric during (Bio)Degradation in Soil. J. Clean. Prod. 2023, 421, 138554. [Google Scholar] [CrossRef]
- Huang, W.; Deng, J.; Liang, J.; Xia, X. Comparison of Lead Adsorption on the Aged Conventional Microplastics, Biodegradable Microplastics and Environmentally-Relevant Tire Wear Particles. Chem. Eng. J. 2023, 460, 141838. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Li, Y.; Li, J.; Liu, Y.; Xia, S.; Zhao, J. Effects of Exposure of Polyethylene Microplastics to Air, Water and Soil on Their Adsorption Behaviors for Copper and Tetracycline. Chem. Eng. J. 2021, 404, 126412. [Google Scholar] [CrossRef]
- Lin, Z. Comparative Analysis of Kinetics and Mechanisms for Pb(II) Sorption onto Three Kinds of Microplastics. Ecotoxicol. Environ. Saf. 2021, 208, 111451. [Google Scholar] [CrossRef]
- You, H.; Cao, C.; Sun, X.; Huang, B.; Qian, Q.; Chen, Q. Microplastics as an Emerging Vector of Cr(VI) in Water: Correlation of Aging Properties and Adsorption Behavior. Sci. Total Environ. 2023, 904, 166480. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.; Sun, X.; Tang, S.; Zhang, Y.; Li, M.; Wang, X. Adsorption Mechanism of Cadmium on Polystyrene Microplastics Containing Hexabromocyclododecane. Environ. Technol. Innov. 2021, 24, 102036. [Google Scholar] [CrossRef]
- Gao, Z.; Cizdziel, J.V.; Wontor, K.; Olubusoye, B.S. Adsorption/Desorption of Mercury (II) by Artificially Weathered Microplastics: Kinetics, Isotherms, and Influencing Factors. Environ. Pollut. 2023, 337, 122621. [Google Scholar] [CrossRef]
- He, W.; Wang, X.; Zhang, Y.; Zhu, B.; Wu, H. Adsorption Behavior of Aged Polystyrene Microplastics (PSMPs) for Manganese in Water: Critical Role of Hydrated Functional Zone Surrounding the Microplastic Surface. J. Environ. Chem. Eng. 2022, 10, 109040. [Google Scholar] [CrossRef]
- Yu, X.; Wang, B.; Han, C.; Liu, L.; Han, X.; Zheng, B.; Zhang, B.; Sun, J.; Zhang, Z.; Ma, W.; et al. Physicochemical and Biological Changes on Naturally Aged Microplastic Surfaces in Real Environments over 10 Months. Environ. Pollut. 2023, 337, 122522. [Google Scholar] [CrossRef]
- Brandon, J.; Goldstein, M.; Ohman, M.D. Long-Term Aging and Degradation of Microplastic Particles: Comparing In Situ Oceanic and Experimental Weathering Patterns. Mar. Pollut. Bull. 2016, 110, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Huang, J.; Zhang, W.; Shi, L.; Yi, K.; Zhang, C.; Pang, H.; Li, J.; Li, S. Investigation of the Adsorption Behavior of Pb(II) onto Natural-Aged Microplastics as Affected by Salt Ions. J. Hazard. Mater. 2022, 431, 128643. [Google Scholar] [CrossRef]
- Yu, Y.; Ding, Y.; Zhou, C.; Ge, S. Aging of Polylactic Acid Microplastics during Hydrothermal Treatment of Sewage Sludge and Its Effects on Heavy Metals Adsorption. Environ. Res. 2023, 216, 114532. [Google Scholar] [CrossRef]
- Wang, K.; Kou, Y.; Guo, C.; Wang, K.; Li, J.; Schmidt, J.; Wang, M.; Liang, S.; Wang, W.; Lu, Y.; et al. Comparison of Rhodamine B Adsorption and Desorption on the Aged Non-Degradable and Degradable Microplastics: Effects of Charge-Assisted Hydrogen Bond and Underline Mechanism. Environ. Technol. Innov. 2024, 35, 103739. [Google Scholar] [CrossRef]








| Model | Parameter | PE | PLA |
|---|---|---|---|
| Pseudo-first-order model | K1 (h−1) | 0.198 | 0.391 |
| qe (mg/g) | 0.339 | 0.397 | |
| R2 | 0.989 | 0.993 | |
| Pseudo-second-order model | K2 (g/(mg·h)) | 0.688 | 1.464 |
| qe (mg/g) | 0.384 | 0.428 | |
| R2 | 0.985 | 0.992 | |
| Elovich model | α | 0.276 | 4.001 |
| β | 13.979 | 18.919 | |
| R2 | 0.953 | 0.961 |
| Model | Temperature | Parameter | PE | PLA |
|---|---|---|---|---|
| Langmuir model | 288 K | KL (L/mg) | 0.069 | 0.063 |
| qm (mg/g) | 0.559 | 0.884 | ||
| R2 | 0.975 | 0.988 | ||
| 298 K | KL (L/mg) | 0.144 | 0.095 | |
| qm (mg/g) | 0.465 | 0.794 | ||
| R2 | 0.912 | 0.975 | ||
| 308 K | KL (L/mg) | 0.092 | 0.086 | |
| qm (mg/g) | 0.556 | 0.806 | ||
| R2 | 0.875 | 0.929 | ||
| Freundlich model | 288 K | Kf [(mg/g)·(L/mg)1/n] 1/n | 0.053 0.612 | 0.078 0.621 |
| R2 | 0.943 | 0.985 | ||
| 298 K | Kf [(mg/g)·(L/mg)1/n] 1/n | 0.093 0.451 | 0.110 0.528 | |
| R2 | 0.845 | 0.996 | ||
| 308 K | Kf [(mg/g)·(L/mg)1/n] 1/n | 0.071 0.554 | 0.100 0.553 | |
| R2 | 0.839 | 0.949 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, Z.; Wang, Z.; Tang, H.; Hursthouse, A. Comparison of Hexavalent Chromium Adsorption Behavior on Conventional and Biodegradable Microplastics. Water 2024, 16, 2050. https://doi.org/10.3390/w16142050
Fang Z, Wang Z, Tang H, Hursthouse A. Comparison of Hexavalent Chromium Adsorption Behavior on Conventional and Biodegradable Microplastics. Water. 2024; 16(14):2050. https://doi.org/10.3390/w16142050
Chicago/Turabian StyleFang, Zongzhi, Zhenghua Wang, Han Tang, and Andrew Hursthouse. 2024. "Comparison of Hexavalent Chromium Adsorption Behavior on Conventional and Biodegradable Microplastics" Water 16, no. 14: 2050. https://doi.org/10.3390/w16142050






