Mechanisms, Applications, and Risk Analysis of Surfactant-Enhanced Remediation of Hydrophobic Organic Contaminated Soil
Abstract
:1. Introduction
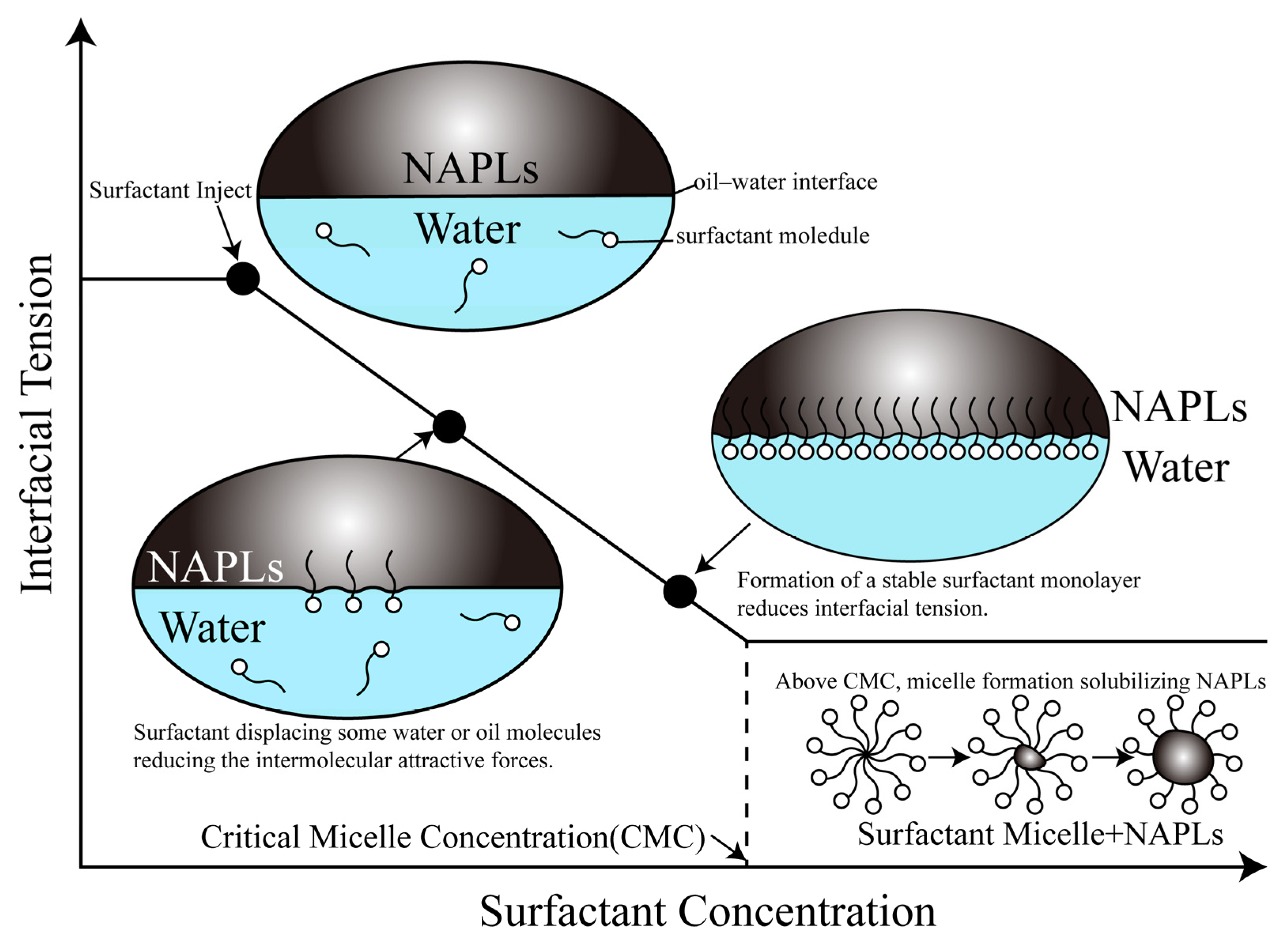
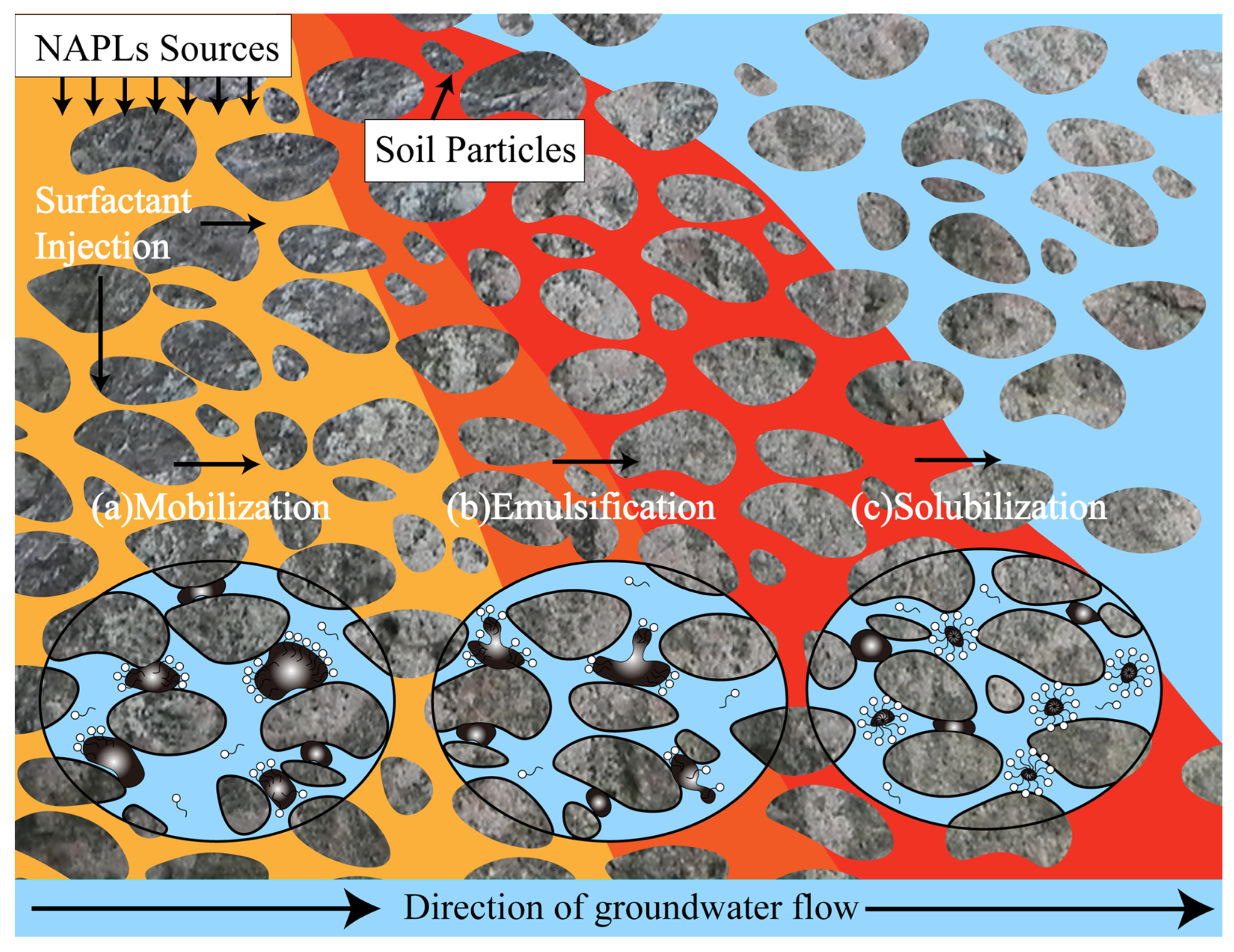
2. Mechanisms of Surfactant-Enhanced Remediation Technologies
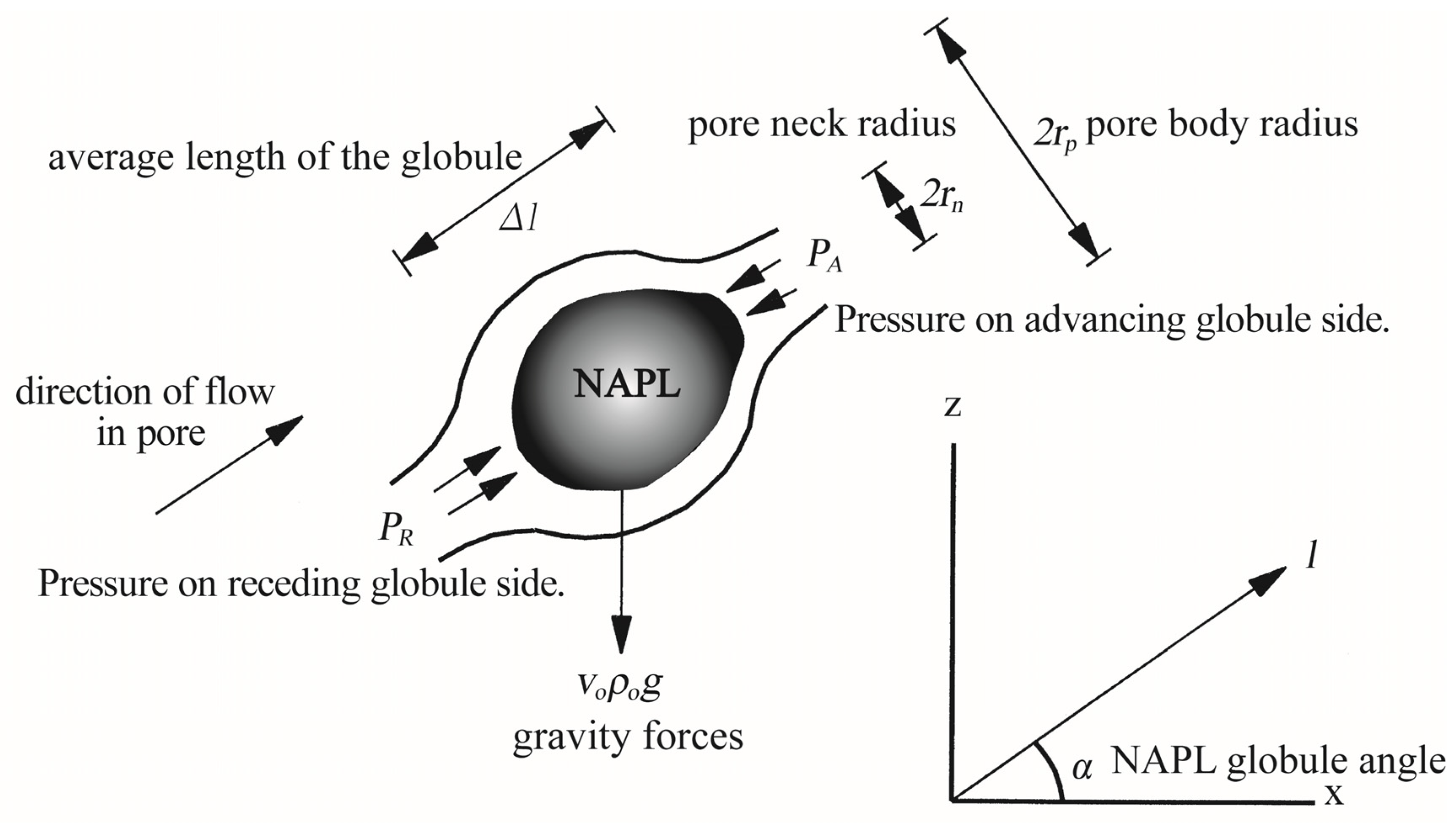
2.1. Mobilization
2.2. Solubilization
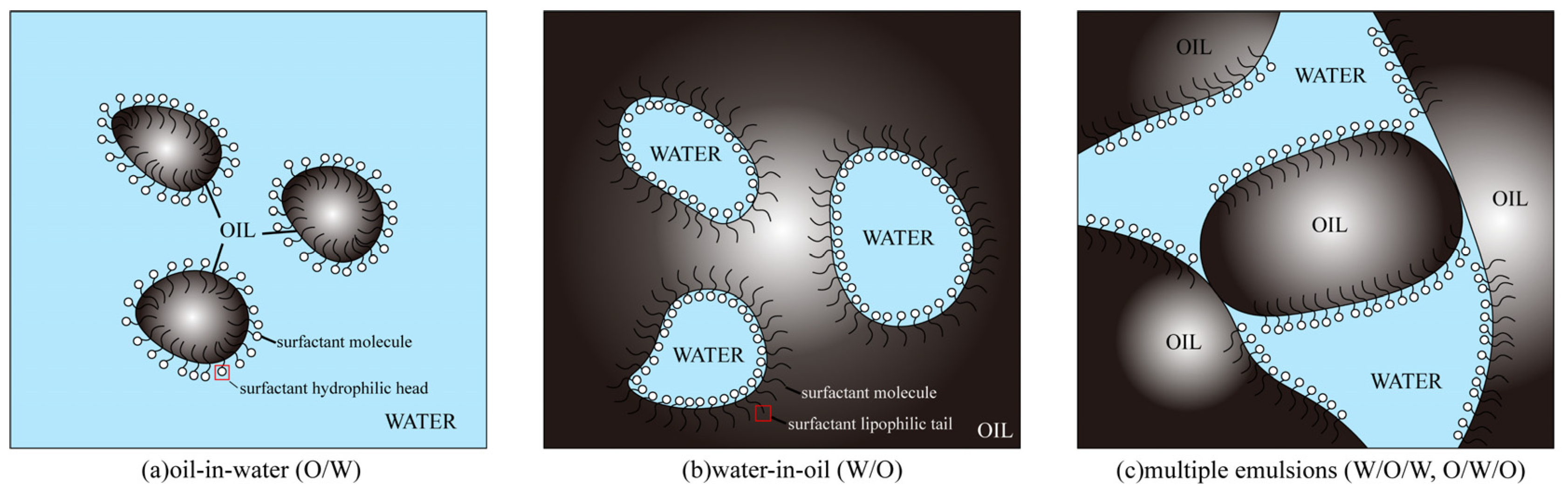
2.3. Emulsification
2.4. Other Mechanisms
3. Surfactant-Enhanced Remediation Techniques
3.1. Surfactant-Enhanced Chemical Oxidative Remediation Technology
3.2. Surfactant-Enhanced Bioremediation Technologies
3.3. Surfactant-Enhanced Physical Remediation Technology
4. Risk Analysis of Surfactant-Enhanced Remediation
5. Conclusions
- Surfactants: The development of new surfactants, such as bilobal surfactants and switchable surfactants, is important in reducing the potential impact while improving the remediation efficiency of NAPLs [67,128]. Another strategy is to integrate various surfactants in the remediation process, leveraging their combined action towards NAPLs. Furthermore, surfactants and reaction intermediates warrant further investigations and ongoing monitoring to ensure SER was implemented with a minimum environmental risk.
- Remediation technology: It is of great importance to further study the synergistic mechanism of SER coupled with different remediation technologies which may surpass the limitations of one single method while concurrently maximizing the outcome, such as with reduced environmental footprint, minimized surfactant usage, and enhanced NAPL removal.
Author Contributions
Funding
Conflicts of Interest
References
- Essaid, H.I.; Bekins, B.A.; Cozzarelli, I.M. Organic contaminant transport and fate in the subsurface: Evolution of knowledge and understanding. Water Resour. Res. 2015, 51, 4861–4902. [Google Scholar] [CrossRef]
- Du Fangzhou, S.X.; Kang, X. Improved Method and Software Development for Assessing NAPL Phase Presence in Contaminated Sites. Saf. Environ. Eng. 2022, 29, 175–182+195. [Google Scholar] [CrossRef]
- Zhang, A.; Cai, W.; Wang, J.; Zhang, M.; Liu, X.; Geng, T. Comparison of Sampling Methods on Groundwater Petroleum Pollution. Saf. Environ. Eng. 2014, 21, 109–113+120. [Google Scholar] [CrossRef]
- Council, N.R. Contaminants in the Subsurface: Source Zone Assessment and Remediation; The National Academies Press: Washington, DC, USA, 2005; p. 370. [Google Scholar]
- Kavanaugh, M.C.; Abriola, L.M.; Newell, C.J. The DNAPL Remediation Challenge: Is There a Case for Source Depletion? United States Environmental Protection Agency: Washington, DC, USA, 2003. [Google Scholar]
- Zhu, H.; Ye, S.; Wu, J.; Xu, H. Characteristics of soil lithology and pollutants in typical contamination sites in China. Earth Sci. Front. 2021, 28, 26–34. [Google Scholar] [CrossRef]
- Ni, G. Research on the Soil/Groundwater Remediation Technologies for the Falling Oil Contaminated Sites in Daqing Oilfield. Ph.D. Thesis, Northeast Petroleum University, Heilongjiang, China, 2020. [Google Scholar] [CrossRef]
- Liang, T.; Zhang, H. Review on Remediation Technology of Chloromethanes Contaminated Soil. In Proceedings of the Chinese Society of Environmental Science 2022 Annual Scientific and Technical Conference—Environmental Engineering Technology Innovation and Application Session, Jiang Xi, Nan Chang, China, 20 August 2022; pp. 769–773+953. [Google Scholar] [CrossRef]
- Saxena, N.; Islam, M.M.; Baliyan, S.; Sharma, D. A comprehensive review on removal of environmental pollutants using a surfactant based remediation process. RSC Sustain. 2023, 1, 2148–2161. [Google Scholar] [CrossRef]
- Peng, S.; Wu, W.; Chen, J. Removal of PAHs with surfactant-enhanced soil washing: Influencing factors and removal effectiveness. Chemosphere 2011, 82, 1173–1177. [Google Scholar] [CrossRef]
- Befkadu, A.A.; Chen, Q. Surfactant-Enhanced Soil Washing for Removal of Petroleum Hydrocarbons from Contaminated Soils: A Review. Pedosphere 2018, 28, 383–410. [Google Scholar] [CrossRef]
- Wang, F.; Chen, J.; Li, Y.; Lu, T.; Chen, W.; Qi, Z.; Wang, X.; Farooq, U. Anionic surfactant-mediated transport of tetracycline antibiotics with different molecular structures in saturated porous media. J. Mol. Liq. 2022, 367, 120402. [Google Scholar] [CrossRef]
- Rosen, M.J.; Kunjappu, J.T. Surfactants and Interfacial Phenomena; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Wei, W.; Ran, Z.; He, H.; Zhou, K.; Huangfu, Z.; Yu, J. Desorption process and morphological analysis of real polycyclic aromatic hydrocarbons contaminated soil by the heterogemini surfactant and its mixed systems. Chemosphere 2020, 254, 126854. [Google Scholar] [CrossRef]
- Davin, M.; Starren, A.; Deleu, M.; Lognay, G.; Colinet, G.; Fauconnier, M.L. Could saponins be used to enhance bioremediation of polycyclic aromatic hydrocarbons in aged-contaminated soils? Chemosphere 2018, 194, 414–421. [Google Scholar] [CrossRef]
- Okuda, I.; McBride, J.F.; Gleyzer, S.N.; Miller, C.T. Physicochemical Transport Processes Affecting the Removal of Residual DNAPL by Nonionic Surfactant Solutions. Environ. Sci. Technol. 1996, 30, 1852–1860. [Google Scholar] [CrossRef]
- Di Trapani, D.; De Marines, F.; Greco Lucchina, P.; Viviani, G. Surfactant-enhanced mobilization of hydrocarbons from soil: Comparison between anionic and nonionic surfactants in terms of remediation efficiency and residual phytotoxicity. Process Saf. Environ. Prot. 2023, 180, 1–9. [Google Scholar] [CrossRef]
- Isaac, O.T.; Pu, H.; Oni, B.A.; Samson, F.A. Surfactants employed in conventional and unconventional reservoirs for enhanced oil recovery—A review. Energy Rep. 2022, 8, 2806–2830. [Google Scholar] [CrossRef]
- Liang, X.; Li, Y.; Bai, J.; Dong, J.; Li, W.; Mo, Y.; Jiang, D.; Zhang, W. Feasibility evaluation of novel anionic-nonionic gemini surfactants for surfactant-enhanced aquifer remediation. J. Clean. Prod. 2023, 393, 136338. [Google Scholar] [CrossRef]
- Kurnia, I.; Fatchurrozi, M.; Anwary, R.G.; Zhang, G. Lessons learned from coreflood experiments with surfactant-polymer and alkali-surfactant-polymer for enhanced oil recovery. Petroleum 2022, 9, 487–498. [Google Scholar] [CrossRef]
- Zhu, L.; Feng, S. Synergistic solubilization of polycyclic aromatic hydrocarbons by mixed anionic–nonionic surfactants. Chemosphere 2003, 53, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Mayer, A.S.; Pope, G.A. The effects of surfactant formulation on nonequilibrium NAPL solubilization. J. Contam. Hydrol. 2003, 60, 55–75. [Google Scholar] [CrossRef] [PubMed]
- Huo, L.; Liu, G.; Li, Y.; Yang, X.; Zhong, H. Solubilization of residual dodecane by surfactants in porous media: The relation between surfactant partition and solubilization. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129421. [Google Scholar] [CrossRef]
- Datta, P.; Tiwari, P.; Pandey, L.M. Oil washing proficiency of biosurfactant produced by isolated Bacillus tequilensis MK 729017 from Assam reservoir soil. J. Pet. Sci. Eng. 2020, 195, 107612. [Google Scholar] [CrossRef]
- Javanbakht, G.; Goual, L. Mobilization and micellar solubilization of NAPL contaminants in aquifer rocks. J. Contam. Hydrol. 2016, 185, 61–73. [Google Scholar] [CrossRef]
- Borkovec, M. From micelles to microemulsion droplets: Size distributions, shape fluctuations, and interfacial tensions. J. Chem. Phys. 1989, 91, 6268–6281. [Google Scholar] [CrossRef]
- Pennell, K.D.; Abriola, L.M.; Weber, W.J. Surfactant-enhanced solubilization of residual dodecane in soil columns. 1. Experimental investigation. Environ. Sci. Technol. 1993, 27, 2332–2340. [Google Scholar] [CrossRef]
- Pennell, K.D.; Pope, G.A.; Abriola, L.M. Influence of Viscous and Buoyancy Forces on the Mobilization of Residual Tetrachloroethylene during Surfactant Flushing. Environ. Sci. Technol. 1996, 30, 1328–1335. [Google Scholar] [CrossRef]
- Das, A.; Nguyen, N.; Farajzadeh, R.; Southwick, J.G.; Vincent-Bonnieu, S.; Khaburi, S.; Al Kindi, A.; Nguyen, Q.P. Experimental study of injection strategy for Low-Tension-Gas flooding in low permeability, high salinity carbonate reservoirs. J. Pet. Sci. Eng. 2020, 184, 106564. [Google Scholar] [CrossRef]
- Ramezanzadeh, M.; Aminnaji, M.; Rezanezhad, F.; Ghazanfari, M.H.; Babaei, M. Dissolution and remobilization of NAPL in surfactant-enhanced aquifer remediation from microscopic scale simulations. Chemosphere 2022, 289, 133177. [Google Scholar] [CrossRef]
- Ramsburg, C.A.; Baniahmad, P.; Muller, K.A.; Robinson, A.D. Emulsion-based recovery of a multicomponent petroleum hydrocarbon NAPL using nonionic surfactant formulations. J. Contam. Hydrol. 2023, 255, 104144. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Han, M.; Chen, S.; Wang, J. Surfactant enhanced imbibition in carbonate reservoirs: Effect of IFT reduction and surfactant partitioning. JCIS Open 2022, 5, 100045. [Google Scholar] [CrossRef]
- Zhao, B.; Zhu, L.; Li, W.; Chen, B. Solubilization and biodegradation of phenanthrene in mixed anionic–nonionic surfactant solutions. Chemosphere 2005, 58, 33–40. [Google Scholar] [CrossRef]
- Ogunmokun, F.A.; Wallach, R. Effect of surfactant surface and interfacial tension reduction on infiltration into hydrophobic porous media. Geoderma 2024, 441, 116735. [Google Scholar] [CrossRef]
- Gardner, K.H.; Arias, M.S. Clay Swelling and Formation Permeability Reductions Induced by a Nonionic Surfactant. Environ. Sci. Technol. 1999, 34, 160–166. [Google Scholar] [CrossRef]
- Zhang, W.; Tang, X.Y.; Weisbrod, N.; Zhao, P.; Reid, B.J. A coupled field study of subsurface fracture flow and colloid transport. J. Hydrol. 2015, 524, 476–488. [Google Scholar] [CrossRef]
- Wang, D.M.; Butler, R.; Zhang, J.; Seright, R. Wettability Survey in Bakken Shale With Surfactant-Formulation Imbibition. SPE Reserv. Eval. Eng. 2012, 15, 695–705. [Google Scholar] [CrossRef]
- Seyedabbasi, M.A.; Farthing, M.W.; Imhoff, P.T.; Miller, C.T. The influence of wettability on NAPL dissolution fingering. Adv. Water Resour. 2008, 31, 1687–1696. [Google Scholar] [CrossRef]
- Alvarez, J.O.; Schechter, D.S. Altering Wettability in Bakken Shale by Surfactant Additives and Potential of Improving Oil Recovery during Injection of Completion Fluids. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 11–13 April 2016. [Google Scholar]
- Li, L.; Chen, J.; Liu, J.; Xu, Z.; Wu, Y.; Zhao, M.; Zhao, G.; Dai, C. Anionic surfactant based on oil-solid interfacial interaction control for efficient residual oil development. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129396. [Google Scholar] [CrossRef]
- Duffield, A.R.; Ramamurthy, R.S.; Campanelli, J.R. Surfactant Enhanced Mobilization of Mineral Oil within Porous Media. Water Air Soil Pollut. 2003, 143, 111–122. [Google Scholar] [CrossRef]
- Bettahar, M.; Ducreux, J.; Schäfer, G.; Van Dorpe, F. Surfactant Enhanced In Situ Remediation of LNAPL Contaminated Aquifers: Large Scale Studies on a Controlled Experimental Site. Transp. Porous Media 1999, 37, 255–276. [Google Scholar] [CrossRef]
- Lowe, D.F.; Oubre, C.L.; Ward, C.H. Surfactants and Cosolvents for NAPL Remediation. A Technologies Practices Manual; Lewis Publications: Boca Raton, FL, USA, 1999. [Google Scholar]
- Harendra, S.; Vipulanandan, C. Effects of Surfactants on Solubilization of Perchloroethylene (PCE) and Trichloroethylene (TCE). Ind. Eng. Chem. Res. 2011, 50, 5831–5837. [Google Scholar] [CrossRef]
- Liang, X.; Dong, J.; Zhang, W.; Mo, Y.; Li, Y.; Bai, J. Solubilization mechanism and mass-transfer model of anionic-nonionic gemini surfactants for chlorinated hydrocarbons. Sep. Purif. Technol. 2024, 330, 125534. [Google Scholar] [CrossRef]
- Barbati, B.; Lorini, L.; Amanat, N.; Bellagamba, M.; Galantini, L.; Petrangeli Papini, M. Enhanced solubilization of strongly adsorbed organic pollutants using synthetic and natural surfactants in soil flushing: Column experiment simulation. J. Environ. Chem. Eng. 2023, 11, 110758. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, Z.; Chen, Y.F. Pore-scale investigation of surfactant-enhanced DNAPL mobilization and solubilization. Chemosphere 2023, 341, 140071. [Google Scholar] [CrossRef]
- Iglesias, O.; Sanromán, M.A.; Pazos, M. Surfactant-Enhanced Solubilization and Simultaneous Degradation of Phenanthrene in Marine Sediment by Electro-Fenton Treatment. Ind. Eng. Chem. Res. 2014, 53, 2917–2923. [Google Scholar] [CrossRef]
- Attwood, D.; Florence, A.T. Surfactant Systems; Springer: Berlin/Heidelberg, Germany, 1983. [Google Scholar]
- Kile, D.E.; Chiou, C.T. Water solubility enhancements of DDT and trichlorobenzene by some surfactants below and above the critical micelle concentration. Environ. Sci. Technol. 2002, 23, 832–838. [Google Scholar] [CrossRef]
- Edwards, D.A.; Luthy, R.G.; Liu, Z. Solubilization of polycyclic aromatic hydrocarbons in micellar nonionic surfactant solutions. Environ. Sci. Technol. 1991, 25, 127–133. [Google Scholar] [CrossRef]
- Buzier, M.; Ravey, J.C. Solubilization properties of nonionic surfactants: I. Evolution of the ternary phase diagrams with temperature, salinity, HLB, and ACN. J. Colloid Interface Sci. 1983, 91, 20–33. [Google Scholar] [CrossRef]
- Masrat, R.; Majid, K. Solubilization of pyrene by mixed polymer-cationic/nonionic surfactant systems: Effect of polymer concentration. Colloids Surf. A Physicochem. Eng. Asp. 2022, 653, 129974. [Google Scholar] [CrossRef]
- Javanbakht, G.; Goual, L. Impact of Surfactant Structure on NAPL Mobilization and Solubilization in Porous Media. Ind. Eng. Chem. Res. 2016, 55, 11736–11746. [Google Scholar] [CrossRef]
- Tick, G.; Slavic, D.R.; Akyol, N.H.; Zhang, Y. Enhanced-solubilization and dissolution of multicomponent DNAPL from homogeneous porous media. J. Contam. Hydrol. 2022, 247, 103967. [Google Scholar] [CrossRef]
- Yang Siyue, S.Y.; Mao, M.; Wang, J.; Zhu, H.; Liu, H.; Yang, S. Experimental Study on Remediation of Petroleum Hydrocarbon Pollution in Subsurface Aquifers Based on SEAR Technology. Res. Environ. Sci. 2023, 36, 954–964. [Google Scholar] [CrossRef]
- Feng, C.; Sun, L.; Liu, W.; Chen, C.; Li, B.; Sun, D. Effects of surfactant on the molecules of different polarity of solubilization: Based on the study of micellar microscopic morphology mechanism. J. Pet. Sci. Eng. 2022, 208, 109563. [Google Scholar] [CrossRef]
- Zhao, X.; Gong, L.; Liao, G.; Luan, H.; Chen, Q.; Liu, D.; Feng, Y. Micellar solubilization of petroleum fractions by heavy alkylbenzene sulfonate surfactant. J. Mol. Liq. 2021, 329, 115519. [Google Scholar] [CrossRef]
- Zhong, H.; Yang, X.; Tan, F.; Brusseau, M.L.; Yang, L.; Liu, Z.; Zeng, G.; Yuan, X. Aggregate-based sub-CMC Solubilization of n-Alkanes by Monorhamnolipid Biosurfactant. New J. Chem. 2016, 40, 2028–2035. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Tan, F.; Zhong, H.; Liu, G.; Ahmad, Z.; Liang, Q. Sub-CMC solubilization of n-alkanes by rhamnolipid biosurfactant: The Influence of rhamnolipid molecular structure. Colloids Surf. B Biointerfaces 2020, 192, 111049. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Jeong, H.Y. Sorption of a nonionic surfactant Tween 80 by minerals and soils. J. Hazard. Mater. 2015, 284, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhu, L. Influence of surfactant sorption on the removal of phenanthrene from contaminated soils. Environ. Pollut. 2008, 152, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Valvatne, P.H.; Blunt, M.J. Predictive pore-scale modeling of two-phase flow in mixed wet media. Water Resour. Res. 2004, 40, W07406. [Google Scholar] [CrossRef]
- Guan, Z.; Tang, X.Y.; Nishimura, T.; Katou, H.; Liu, H.Y.; Qing, J. Surfactant-enhanced flushing enhances colloid transport and alters macroporosity in diesel-contaminated soil. J. Environ. Sci. 2018, 64, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Alexandridis, P.; Holzwarth, J.F.; Hatton, T.A. Thermodynamics of Droplet Clustering in Percolating AOT Water-in-Oil Microemulsions. J. Phys. Chem. 2002, 99, 8222–8232. [Google Scholar] [CrossRef]
- Bodratti, A.M.; Sarkar, B.; Alexandridis, P. Adsorption of poly(ethylene oxide)-containing amphiphilic polymers on solid-liquid interfaces: Fundamentals and applications. Adv. Colloid Interface Sci. 2017, 244, 132–163. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Sun, L.; Liu, X.; Fang, Y. Precipitation-Dissolution switchable surfactants with the potential of simultaneous retrieving of surfactants and hydrophobic organic contaminants from emulsified and micellar eluents. Chem. Eng. J. 2023, 458, 141297. [Google Scholar] [CrossRef]
- Yada, S.; Matsuoka, K.; Nagai Kanasaki, Y.; Gotoh, K.; Yoshimura, T. Emulsification, solubilization, and detergency behaviors of homogeneous polyoxypropylene-polyoxyethylene alkyl ether type nonionic surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2019, 564, 51–58. [Google Scholar] [CrossRef]
- Huang, C.W.; Chang, C.H. A laboratory study on foam-enhanced surfactant solution flooding in removing n-pentadecane from contaminated columns. Colloids Surf. A Physicochem. Eng. Asp. 2000, 173, 171–179. [Google Scholar] [CrossRef]
- Mavaddat, M.; Abdollahi, A.; Mavaddat, Y.; Riahi, S. A New Approach to Use Mixture of Surfactants to Face Salinity Gradient in Microemulsion Flooding. In Proceedings of the 79th EAGE Conference and Exhibition, European Association of Geoscientists & Engineers, Paris, France, 12–15 June 2017. [Google Scholar]
- Heeres, A.S.; Picone, C.S.F.; van der Wielen, L.A.M.; Cunha, R.L.; Cuellar, M.C. Microbial advanced biofuels production: Overcoming emulsification challenges for large-scale operation. Trends Biotechnol. 2014, 32, 221–229. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Food Emulsions; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Salehpour, M.; Sakhaei, Z.; Salehinezhad, R.; Mahani, H.; Riazi, M. Contribution of water-in-oil emulsion formation and pressure fluctuations to low salinity waterflooding of asphaltic oils: A pore-scale perspective. J. Pet. Sci. Eng. 2021, 203, 108597. [Google Scholar] [CrossRef]
- Liu, J.; Zhong, L.; Zewen, Y.; Liu, Y.; Meng, X.; Zhang, W.; Zhang, H.; Yang, G.; Shaojie, W. High-efficiency emulsification anionic surfactant for enhancing heavy oil recovery. Colloids Surf. A Physicochem. Eng. Asp. 2022, 642, 128654. [Google Scholar] [CrossRef]
- Ling, N.N.A.; Haber, A.; Graham, B.F.; Aman, Z.M.; May, E.F.; Fridjonsson, E.O.; Johns, M.L. Quantifying the Effect of Salinity on Oilfield Water-in-Oil Emulsion Stability. Energy Fuels 2018, 32, 10042–10049. [Google Scholar] [CrossRef]
- Umar, A.A.; Saaid, I.B.M.; Sulaimon, A.A. Rheological and stability study of water-in-crude oil emulsions. AIP Conf. Proc. 2016, 1774, 040004. [Google Scholar] [CrossRef]
- Sakhaei, Z.; Riazi, M. In-situ petroleum hydrocarbons contaminated soils remediation by polymer enhanced surfactant flushing: Mechanistic investigation. Process Saf. Environ. Prot. 2022, 161, 758–770. [Google Scholar] [CrossRef]
- Zhao, J.; Torabi, F.; Yang, J. The role of emulsification and IFT reduction in recovering heavy oil during alkaline-surfactant-assisted CO2 foam flooding: An experimental study. Fuel 2022, 313, 122942. [Google Scholar] [CrossRef]
- Wang, K.; Pi, Y.; Wu, Y.; Jiao, G. Research on the Emulsification Performance of ASP Flooding lmpact on Oil Displacement Effect. Sci. Technol. Eng. 2012, 12, 2428–2431. [Google Scholar]
- She, Y.; Zhang, C.; Mahardika, M.A.; Patmonoaji, A.; Hu, Y.; Matsushita, S.; Suekane, T. Pore-scale study of in-situ surfactant flooding with strong oil emulsification in sandstone based on X-ray microtomography. J. Ind. Eng. Chem. 2021, 98, 247–261. [Google Scholar] [CrossRef]
- Urum, K.; Pekdemir, T. Evaluation of biosurfactants for crude oil contaminated soil washing. Chemosphere 2004, 57, 1139–1150. [Google Scholar] [CrossRef] [PubMed]
- Mrozik, A.; Piotrowska-Seget, Z. Bioaugmentation as a strategy for cleaning up of soils contaminated with aromatic compounds. Microbiol. Res. 2010, 165, 363–375. [Google Scholar] [CrossRef]
- Billingsley, K.A.; Backus, S.M.; Wilson, S.; Singh, A.; Ward, O.P. Remediation of PCBs in soil by surfactant washing and biodegradation in the wash by Pseudomonas sp. LB400. Biotechnol. Lett. 2002, 24, 1827–1832. [Google Scholar] [CrossRef]
- Teng Tingting, L.J. Research Progress on Identification of PAHs-Degrading Bacteria and Bioremediation of PAHs-Contaminated Soil. J. Technol. 2022, 22, 16–26. [Google Scholar]
- Perini, B.L.B.; Bitencourt, R.L.; Daronch, N.A.; dos Santos Schneider, A.L.; De Oliveira, D. Surfactant-enhanced in-situ enzymatic oxidation: A bioremediation strategy for oxidation of polycyclic aromatic hydrocarbons in contaminated soils and aquifers. J. Environ. Chem. Eng. 2020, 8, 104013. [Google Scholar] [CrossRef]
- Ghosh, I.; Mukherji, S. Diverse effect of surfactants on pyrene biodegradation by a Pseudomonas strain utilizing pyrene by cell surface hydrophobicity induction. Int. Biodeterior. Biodegrad. 2016, 108, 67–75. [Google Scholar] [CrossRef]
- Wei, K.H.; Ma, J.; Xi, B.D.; Yu, M.D.; Cui, J.; Chen, B.L.; Li, Y.; Gu, Q.B.; He, X.S. Recent progress on in-situ chemical oxidation for the remediation of petroleum contaminated soil and groundwater. J. Hazard. Mater. 2022, 432, 128738. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, L.; Wei, Z.; Zhang, X.; Zhao, C.; Li, H.; Hu, F.; Xu, L. Effects of Sulfate Radical Advanced Oxidation Technology on PAHs Remediation in Contaminated sites. Soils 2020, 52, 532–538. [Google Scholar] [CrossRef]
- Pac, T.J.; Baldock, J.; Brodie, B.; Byrd, J.; Gil, B.; Morris, K.A.; Nelson, D.; Parikh, J.; Santos, P.; Singer, M.; et al. In situ chemical oxidation: Lessons learned at multiple sites. Remediat. J. 2019, 29, 75–91. [Google Scholar] [CrossRef]
- O’Connor, D.; Hou, D.; Ok, Y.S.; Song, Y.; Sarmah, A.K.; Li, X.; Tack, F.M.G. Sustainable in situ remediation of recalcitrant organic pollutants in groundwater with controlled release materials: A review. J. Control. Release 2018, 283, 200–213. [Google Scholar] [CrossRef]
- Besha, A.T.; Bekele, D.N.; Naidu, R.; Chadalavada, S. Recent advances in surfactant-enhanced In-Situ Chemical Oxidation for the remediation of non-aqueous phase liquid contaminated soils and aquifers. Environ. Technol. Innov. 2018, 9, 303–322. [Google Scholar] [CrossRef]
- Wei, K.H.; Zheng, Y.M.; Sun, Y.; Zhao, Z.Q.; Xi, B.D.; He, X.S. Larger aggregate formed by self-assembly process of the mixture surfactants enhance the dissolution and oxidative removal of non-aqueous phase liquid contaminants in aquifer. Sci. Total Environ. 2024, 912, 169532. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Cervilla, R.; Santos, A.; Romero, A.; Lorenzo, D. Abatement of chlorobenzenes in aqueous phase by persulfate activated by alkali enhanced by surfactant addition. J. Environ. Manag. 2022, 306, 114475. [Google Scholar] [CrossRef]
- García-Cervilla, R.; Santos, A.; Romero, A.; Lorenzo, D. Compatibility of nonionic and anionic surfactants with persulfate activated by alkali in the abatement of chlorinated organic compounds in aqueous phase. Sci. Total Environ. 2021, 751, 141782. [Google Scholar] [CrossRef]
- Demiray, Z.; Akyol, N.H.; Akyol, G.; Copty, N.K. Surfactant-enhanced in-situ oxidation of DNAPL source zone: Experiments and numerical modeling. J. Contam. Hydrol. 2023, 258, 104233. [Google Scholar] [CrossRef] [PubMed]
- Nwankwegu, A.S.; Onwosi, C.O. Bioremediation of gasoline contaminated agricultural soil by bioaugmentation. Environ. Technol. Innov. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Ali, M.; Song, X.; Ding, D.; Wang, Q.; Zhang, Z.; Tang, Z. Bioremediation of PAHs and heavy metals co-contaminated soils: Challenges and enhancement strategies. Environ. Pollut. 2022, 295, 118686. [Google Scholar] [CrossRef]
- Mnif, I.; Mnif, S.; Sahnoun, R.; Maktouf, S.; Ayedi, Y.; Ellouze-Chaabouni, S.; Ghribi, D. Biodegradation of diesel oil by a novel microbial consortium: Comparison between co-inoculation with biosurfactant-producing strain and exogenously added biosurfactants. Environ. Sci. Pollut. Res. 2015, 22, 14852–14861. [Google Scholar] [CrossRef]
- Huizenga, J.M.; Schindler, J.; Simonich, M.T.; Truong, L.; Garcia-Jaramillo, M.; Tanguay, R.L.; Semprini, L. PAH bioremediation with Rhodococcus rhodochrous ATCC 21198: Impact of cell immobilization and surfactant use on PAH treatment and post-remediation toxicity. J. Hazard. Mater. 2024, 470, 134109. [Google Scholar] [CrossRef]
- Gan, S.; Lau, E.V.; Ng, H.K. Remediation of soils contaminated with polycyclic aromatic hydrocarbons (PAHs). J. Hazard. Mater. 2009, 172, 532–549. [Google Scholar] [CrossRef]
- Csutak, O.; Corbu, V.M. 17—Bioremediation of oil-contaminated soil by yeast bioaugmentation. In Advances in Yeast Biotechnology for Biofuels and Sustainability; Daverey, A., Dutta, K., Joshi, S., Gea, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 395–447. [Google Scholar]
- Li, X.; Tan, Q.; Zhou, Y.; Chen, Q.; Sun, P.; Shen, G.; Ma, L. Synergic remediation of polycyclic aromatic hydrocarbon-contaminated soil by a combined system of persulfate oxidation activated by biochar and phytoremediation with basil: A compatible, robust, and sustainable approach. Chem. Eng. J. 2023, 452, 139502. [Google Scholar] [CrossRef]
- Lu, H.; Wang, W.; Li, F.; Zhu, L. Mixed-surfactant-enhanced phytoremediation of PAHs in soil: Bioavailability of PAHs and responses of microbial community structure. Sci. Total Environ. 2019, 653, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, C.; Liu, X.; Liang, X.; Wang, Q. Effects of Alkyl Polyglucoside (APG) on Phytoremediation of PAH-Contaminated Soil by an Aquatic Plant in the Yangtze Estuarine Wetland. Water Air Soil Pollut. 2013, 224, 1633. [Google Scholar] [CrossRef]
- Xia, H.; Chi, X.; Yan, Z.; Cheng, W. Enhancing plant uptake of polychlorinated biphenyls and cadmium using tea saponin. Bioresour. Technol. 2009, 100, 4649–4653. [Google Scholar] [CrossRef]
- Labianca, C.; De Gisi, S.; Picardi, F.; Todaro, F.; Notarnicola, M. Remediation of a Petroleum Hydrocarbon-Contaminated Site by Soil Vapor Extraction: A Full-Scale Case Study. Appl. Sci. 2020, 10, 4261. [Google Scholar] [CrossRef]
- Chang YueHua, C.Y.; Yao Meng, Y.M.; Zhao YongSheng, Z.Y. Laboratory study of surfactant-enhanced air sparging remediation-The variation rule of the influence of zone and airflow distribution. China Environ. Sci. 2018, 38, 2585–2592. [Google Scholar] [CrossRef]
- Xu, L.; Yan, L.; Zha, F.; Zhu, F.; Tan, X.; Kang, B.; Yang, C.; Lin, Z. Remediation characteristics of surfactant-enhanced air sparging (SEAS) technology on volatile organic compounds contaminated soil with low permeability. J. Contam. Hydrol. 2022, 250, 104049. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Yuan, Q.; Qu, D.; Liu, W.; Zhao, Y.; Wang, M. Effects of airflow rate distribution and nitrobenzene removal in an aquifer with a low-permeability lens during surfactant-enhanced air sparging. J. Hazard. Mater. 2022, 437, 129383. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Pandit, S. 1.2—Microbial Electrochemical System: Principles and Application. In Microbial Electrochemical Technology; Mohan, S.V., Varjani, S., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 19–48. [Google Scholar]
- Zheng, F.; Gao, B.; Sun, Y.; Shi, X.; Xu, H.; Wu, J.; Gao, Y. Removal of tetrachloroethylene from homogeneous and heterogeneous porous media: Combined effects of surfactant solubilization and oxidant degradation. Chem. Eng. J. 2016, 283, 595–603. [Google Scholar] [CrossRef]
- Risco, C.; López-Vizcaíno, R.; Sáez, C.; Yustres, A.; Cañizares, P.; Navarro, V.; Rodrigo, M.A. Remediation of soils polluted with 2,4-D by electrokinetic soil flushing with facing rows of electrodes: A case study in a pilot plant. Chem. Eng. J. 2016, 285, 128–136. [Google Scholar] [CrossRef]
- Gebregiorgis Ambaye, T.; Vaccari, M.; Franzetti, A.; Prasad, S.; Formicola, F.; Rosatelli, A.; Hassani, A.; Aminabhavi, T.M.; Rtimi, S. Microbial electrochemical bioremediation of petroleum hydrocarbons (PHCs) pollution: Recent advances and outlook. Chem. Eng. J. 2023, 452, 139372. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Latsos, A.; Gidarakos, E. Performance of electroremediation in real contaminated sediments using a big cell, periodic voltage and innovative surfactants. J. Hazard. Mater. 2016, 320, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, A.; Fang, C.; Selvin, J.; Kuppusamy, S.; Ricky Devi, O.; Zhang, F.; Guo, X.; Kadaikunnan, S.; Balu, R.; Liu, X. Plant biomass extracted eco-friendly natural surfactant enhanced bio-electrokinetic remediation of crude oil contaminated soil. Environ. Res. 2024, 245, 117913. [Google Scholar] [CrossRef] [PubMed]
- Hahladakis, J.N.; Calmano, W.; Gidarakos, E. Use and comparison of the non-ionic surfactants Poloxamer 407 and Nonidet P40 with HP-β-CD cyclodextrin, for the enhanced electroremediation of real contaminated sediments from PAHs. Sep. Purif. Technol. 2013, 113, 104–113. [Google Scholar] [CrossRef]
- Denison, S.B.; Da Silva, P.D.; Koester, C.P.; Alvarez, P.J.J.; Zygourakis, K. Clays play a catalytic role in pyrolytic treatment of crude-oil contaminated soils that is enhanced by ion-exchanged transition metals. J. Hazard. Mater. 2022, 437, 129295. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xie, X.; Wu, C. Ball milling enhances pyrolytic remediation of petroleum-contaminated soil and reuse as adsorptive/catalytic materials for wastewater treatment. J. Water Process Eng. 2024, 58, 104894. [Google Scholar] [CrossRef]
- Fu, J.; Cai, P.; Zhan, M.; Xu, X.; Chen, T.; Li, X.; Jiao, W.; Yin, Y. Washing-thermal desorption remediation of paraffin and naphthenic based crude oil contaminated soil. Environ. Eng. 2022, 40, 167–172. [Google Scholar] [CrossRef]
- Cheng, M.; Zeng, G.; Huang, D.; Yang, C.; Lai, C.; Zhang, C.; Liu, Y. Advantages and challenges of Tween 80 surfactant-enhanced technologies for the remediation of soils contaminated with hydrophobic organic compounds. Chem. Eng. J. 2017, 314, 98–113. [Google Scholar] [CrossRef]
- Karthick, A.; Roy, B.; Chattopadhyay, P. A review on the application of chemical surfactant and surfactant foam for remediation of petroleum oil contaminated soil. J. Environ. Manag. 2019, 243, 187–205. [Google Scholar] [CrossRef]
- Zhencheng, Y.; Yifan, S.; Yunfeng, Y. The application of molecular biology-based microbial remediation technologies in petroleum polluted environments. Chin. J. Biotechnol. 2024, 40, 739–757. [Google Scholar] [CrossRef]
- Da Silva, A.F.; Banat, I.M.; Giachini, A.J.; Robl, D. Fungal biosurfactants, from nature to biotechnological product: Bioprospection, production and potential applications. Bioprocess Biosyst. Eng. 2021, 44, 2003–2034. [Google Scholar] [CrossRef] [PubMed]
- Banat, I.M.; Franzetti, A.; Gandolfi, I.; Bestetti, G.; Martinotti, M.G.; Fracchia, L.; Smyth, T.J.; Marchant, R. Microbial biosurfactants production, applications and future potential. Appl. Microbiol. Biotechnol. 2010, 87, 427–444. [Google Scholar] [CrossRef] [PubMed]
- Martienssen, M.; Schirmer, M. Use of Surfactants to Improve the Biological Degradation of Petroleum Hydrocarbons in a Field Site Study. Environ. Technol. 2007, 28, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Dolman, B.M.; Wang, F.; Winterburn, J.B. Integrated production and separation of biosurfactants. Process Biochem. 2019, 83, 1–8. [Google Scholar] [CrossRef]
- Santos, B.L.P.; Vieira, I.M.M.; Ruzene, D.S.; Silva, D.P. Unlocking the potential of biosurfactants: Production, applications, market challenges, and opportunities for agro-industrial waste valorization. Environ. Res. 2024, 244, 117879. [Google Scholar] [CrossRef]
- Dai, C.M.; Tong, W.K.; Hu, J.J.; Duan, Y.P.; Tu, Y.J.; Lai, X.Y.; Liu, S.G.; Li, J.X. Switchable Surfactant-Enhanced Remediation of Polycyclic Aromatic Hydrocarbons in Soil and Groundwater: A Review. Res. Environ. Sci. 2022, 35, 1925–1934. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Zhang, J.; Chen, F.; Li, J.; Wang, W.; Li, S.; Hu, L. Mechanisms, Applications, and Risk Analysis of Surfactant-Enhanced Remediation of Hydrophobic Organic Contaminated Soil. Water 2024, 16, 2093. https://doi.org/10.3390/w16152093
Wu L, Zhang J, Chen F, Li J, Wang W, Li S, Hu L. Mechanisms, Applications, and Risk Analysis of Surfactant-Enhanced Remediation of Hydrophobic Organic Contaminated Soil. Water. 2024; 16(15):2093. https://doi.org/10.3390/w16152093
Chicago/Turabian StyleWu, Lijun, Jieru Zhang, Fenfei Chen, Junjie Li, Wen Wang, Shiyi Li, and Lifang Hu. 2024. "Mechanisms, Applications, and Risk Analysis of Surfactant-Enhanced Remediation of Hydrophobic Organic Contaminated Soil" Water 16, no. 15: 2093. https://doi.org/10.3390/w16152093
APA StyleWu, L., Zhang, J., Chen, F., Li, J., Wang, W., Li, S., & Hu, L. (2024). Mechanisms, Applications, and Risk Analysis of Surfactant-Enhanced Remediation of Hydrophobic Organic Contaminated Soil. Water, 16(15), 2093. https://doi.org/10.3390/w16152093





