Distribution of Natural Trace Elements in the Drinking Water Sources of Hungary
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sampling
2.3. Chemical Analysis
2.4. Statistical and Geospatial Methods
3. Results
3.1. Prevalence and Distribution of Natural Elements
3.2. Water Supply System Clusters by Water Composition
3.3. Spatial Distribution
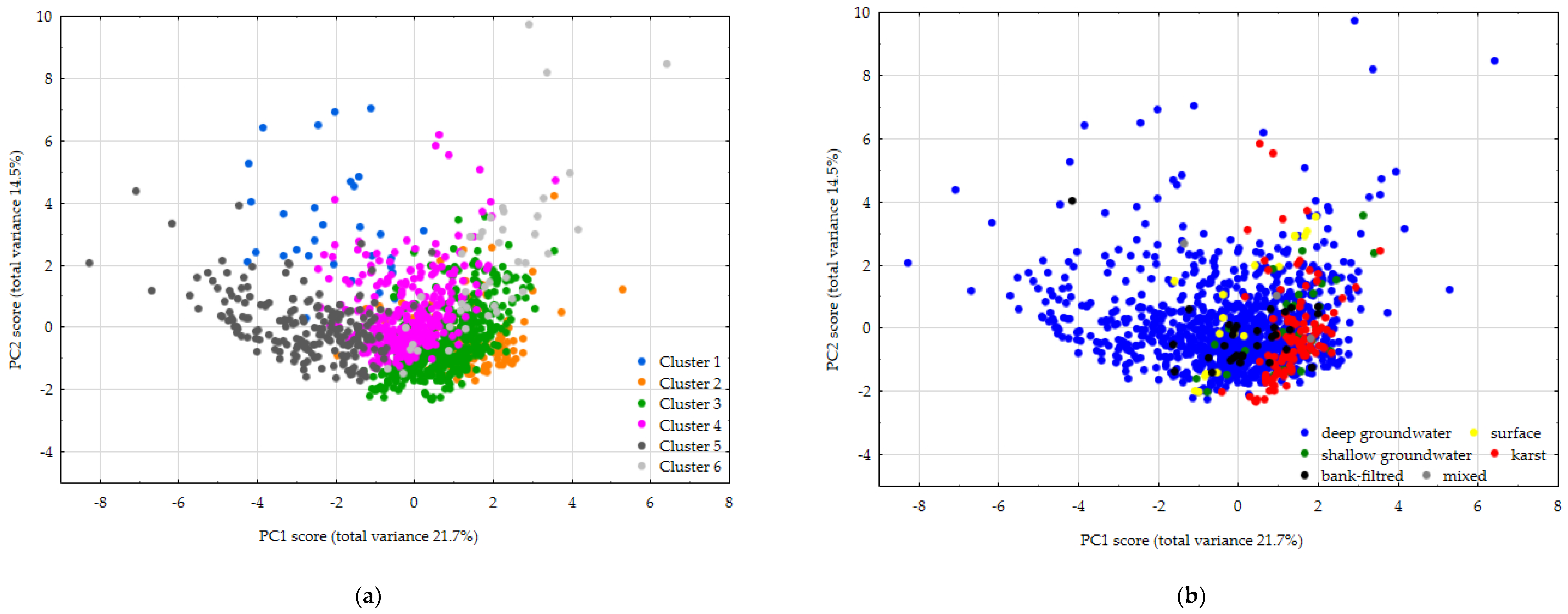
3.4. Principal Component Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The European Parliament; The Council of the European Union. Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the Quality of Water Intended for Human Consumption. 2020. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32020L2184 (accessed on 1 March 2024).
- World Health Organization. Water Safety Plan Manual Step-by-Step Risk Management for Drinking-Water Suppliers, 2nd ed.; World Health Organization: Geneva, Switzerland, 2023; Available online: https://iris.who.int/bitstream/handle/10665/366148/9789240067691-eng.pdf?sequence=1 (accessed on 1 March 2024).
- Council of the European Union. Council Directive 98/83/EC of 3 November 1998 on the Quality of Water Intended for Human Consumption. 1998. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:31998L0083 (accessed on 1 March 2024).
- World Health Organization. Guidelines for Drinking-Water Quality, 4th ed.; Incorporating the First and Second Addenda; World Health Organization: Geneva, Switzerland, 2022; Available online: https://www.who.int/publications/i/item/9789241549950 (accessed on 1 March 2024).
- European Food Safety Authority (EFSA). Uranium in foodstuffs, in particular mineral water. EFSA 2009, 7, 1018. [Google Scholar] [CrossRef]
- Nriagu, J.; Nam, D.-H.; Ayanwola, T.A.; Dinh, H.; Erdenechimeg, E.; Ochir, C.; Bolormaa, T.-A. High levels of uranium in groundwater of Ulaanbaatar, Mongolia. Sci. Total Environ. 2012, 414, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Cicchella, D.; Albanese, S.; De Vivo, B.; Dinelli, E.; Giaccio, L.; Lima, A.; Valera, P. Trace elements and ions in Italian bottled mineral waters: Identification of anomalous values and human health related effects. J. Geochem. Explor. 2010, 107, 336–349. [Google Scholar] [CrossRef]
- Frengstad, B.; Skrede, A.K.M.; Banks, D.; Krog, J.R.; Siewers, U. The chemistry of Norwegian groundwaters: III. The distribution of trace elements in 476 crystalline bedrock groundwaters, as analysed by ICP-MS techniques. Sci. Total Environ. 2000, 246, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Y.; Xie, X. Occurrence, behavior and distribution of high levels of uranium in shallow groundwater at Datong basin, northern China. Sci. Total Environ. 2014, 472, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Lapworth, D.J.; Brauns, B.; Chattopadhyay, S.; Gooddy, D.C.; Loveless, S.E.; MacDonald, A.M.; McKenzie, A.A.; Muddu, S.; Nara, S.N. Elevated uranium in drinking water sources in basement aquifers of southern India. Appl. Geochem. 2021, 133, 105092. [Google Scholar] [CrossRef]
- Cinti, D.; Poncia, P.; Brusca, L.; Tassi, F.; Quattrocchi, F.; Vaselli, O. Spatial distribution of arsenic, uranium and vanadium in the volcanic-sedimentary aquifers of the Vicano–Cimino Volcanic District (Central Italy). J. Geochem. Explor. 2015, 152, 123–133. [Google Scholar] [CrossRef]
- Jiang, L.; He, P.; Chen, J.; Liu, Y.; Liu, D.; Qin, G.; Tan, N. Magnesium Levels in Drinking Water and Coronary Heart Disease Mortality Risk: A Meta-Analysis. Nutrients 2016, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Catling, L.A.; Abubakar, I.; Lake, I.R.; Swift, L.; Hunter, P.R. A systematic review of analytical observational studies investigating the association between cardiovascular disease and drinking water hardness. J. Water Health 2008, 6, 433–442. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Calcium and Magnesium in Drinking Water: Public Health Significance (Beneficial Impacts on Health); WHO: Geneva, Switzerland, 2009; p. 180. [Google Scholar]
- Gianfredi, V.; Bragazzi, N.L.; Nucci, D.; Villarini, M.; Moretti, M. Cardiovascular diseases and hard drinking waters: Implications from a systematic review with meta-analysis of case-control studies. J. Water Health 2017, 15, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Kozisek, F. Regulations for calcium, magnesium or hardness in drinking water in the European Union member states. Regul. Toxicol. Pharmacol. 2020, 112, 104589. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.; Sciacca, S.; I La Milia, D.; Poscia, A.; Moscato, U. Vanadium in drinking water: Toxic or therapeutic?! Systematic literature review and analysis of the population exposure in an Italian volcanic region. Eur. J. Public Health 2014, 24 (Suppl. S2), cku162-080. [Google Scholar] [CrossRef]
- Ferrante, M.; Conti, G.O.; Rasic-Milutinovic, Z.; Jovanovic, D. Health effects of metals and related substances in drinking water. In Metals and Related Substances in Drinking Water; IWA Publishing: London, UK, 2014. [Google Scholar]
- Vasseghian, Y.; Rad, S.S.; Vilas–Boas, J.A.; Khataee, A. A global systematic review, meta-analysis, and risk assessment of the concentration of vanadium in drinking water resources. Chemosphere 2021, 267, 128904. [Google Scholar] [CrossRef] [PubMed]
- Kabacs, N.; Memon, A.; Obinwa, T.; Stochl, J.; Perez, J. Lithium in drinking water and suicide rates across the East of England. Br. J. Psychiatry 2011, 198, 406–407. [Google Scholar] [CrossRef] [PubMed]
- Liaugaudaite, V.; Mickuviene, N.; Raskauskiene, N.; Naginiene, R.; Sher, L. Lithium levels in the public drinking water supply and risk of suicide: A pilot study. J. Trace Elem. Med. Biol. 2017, 43, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Pompili, M.; Vichi, M.; Dinelli, E.; Pycha, R.; Valera, P.; Albanese, S.; Lima, A.; De Vivo, B.; Cicchella, D.; Fiorillo, A.; et al. Relationships of local lithium concentrations in drinking water to regional suicide rates in Italy. World J. Biol. Psychiatry 2015, 16, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Giotakos, O.; Nisianakis, P.; Tsouvelas, G.; Giakalou, V.-V. Lithium in the Public Water Supply and Suicide Mortality in Greece. Biol. Trace Element Res. 2013, 156, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Kapusta, N.D.; Mossaheb, N.; Etzersdorfer, E.; Hlavin, G.; Thau, K.; Willeit, M.; Praschak-Rieder, N.; Sonneck, G.; Leithner-Dziubas, K. Lithium in drinking water and suicide mortality. Br. J. Psychiatry 2011, 198, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; He, M.-Y.; Li, B.; Wen, X.; Zhou, J.; Cheng, Y.; Zhang, N.; Deng, L. Multi-isotopic composition (Li and B isotopes) and hydrochemistry characterization of the Lakko Co Li-rich salt lake in Tibet, China: Origin and hydrological processes. J. Hydrol. 2024, 630, 130714. [Google Scholar] [CrossRef]
- Izsak, B.; Hidvegi, A.; Balint, L.; Malnasi, T.; Vargha, M.; Pandics, T.; Rimer, Z.; Dome, P. Investigation of the association between lithium levels in drinking water and suicide mortality in Hungary. J. Affect. Disord. 2022, 298, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Barjasteh-Askari, F.; Davoudi, M.; Amini, H.; Ghorbni, M.; Yaseri, M.; Yunesian, M.; Mahvi, A.H.; Lester, D. Relationship between suicide mortality and lithium in drinking water: A systematic review and meta-analysis. J. Affect. Disord. 2020, 264, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Memon, A.; Rogers, I.; Fitzsimmons, S.M.D.D.; Cater, B.; Strawbridge, R.; Hidalgo-Mazzei, D.; Young, A.H. Association between naturally occurring lithium in drinking water and suicide rates: Systematic review and meta-analysis of ecological studies. Br. J. Psychiatry 2020, 217, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Eyre-Watt, B.; Mahendran, E.; Suetani, S.; Firth, J.; Kisely, S.; Siskind, D. The association between lithium in drinking water and neuropsychiatric outcomes: A systematic review and meta-analysis from across 2678 regions containing 113 million people. Aust. N. Z. J. Psychiatry 2021, 55, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Abejón, R. A Bibliometric Analysis of Research on Selenium in Drinking Water during the 1990–2021 Period: Treatment Options for Selenium Removal. Int. J. Environ. Res. Public Health 2022, 19, 5834. [Google Scholar] [CrossRef] [PubMed]
- Barron, E.; Migeot, V.; Rabouan, S.; Potin-Gautier, M.; Séby, F.; Hartemann, P.; Lévi, Y.; Legube, B. The case for re-evaluating the upper limit value for selenium in drinking water in Europe. J. Water Health 2009, 7, 630–641. [Google Scholar] [CrossRef]
- Godebo, T.R.; Stoner, H.; Kodsup, P.; Stoltzfus, M.; Nyachoti, S.; Atkins, S.; Jeuland, M. Selenium in drinking water and cereal grains, and biomarkers of Se status in urine and fingernails of the Main Ethiopian Rift Valley population. J. Trace Elem. Med. Biol. 2023, 77, 127137. [Google Scholar] [CrossRef] [PubMed]
- National Center for Public Health and Pharmacy. Magyarország Ivóvizének Minősége, 2022. 2023. Available online: https://www.nnk.gov.hu/attachments/article/726/2023_9_Ivo%CC%81vi%CC%81zmino%CC%8Bse%CC%81g%202022.pdf (accessed on 1 March 2024).
- Erőss, A.; Csondor, K.; Izsák, B.; Vargha, M.; Horváth, Á.; Pándics, T. Uranium in groundwater—The importance of hydraulic regime and groundwater flow system’s understanding. J. Environ. Radioact. 2018, 195, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Csondor, K.; Baják, P.; Surbeck, H.; Izsák, B.; Horváth, Á.; Vargha, M.; Erőss, A. Transient nature of riverbank filtered drinking water supply systems—A new challenge of natural radioactivity assessment. J. Environ. Radioact. 2020, 211, 106072. [Google Scholar] [CrossRef] [PubMed]
- Baják, P.; Hegedűs-Csondor, K.; Tiljander, M.; Korkka-Niemi, K.; Surbeck, H.; Izsák, B.; Vargha, M.; Horváth, Á.; Pándics, T.; Erőss, A. Integration of a Shallow Soda Lake into the Groundwater Flow System by Using Hydraulic Evaluation and Environmental Tracers. Water 2022, 14, 951. [Google Scholar] [CrossRef]
- Baják, P.; Molnár, B.; Hegedűs-Csondor, K.; Tiljander, M.; Jobbágy, V.; Kohuth-Ötvös, V.; Izsák, B.; Vargha, M.; Horváth, Á.; Csipa, E.; et al. Natural Radioactivity in Drinking Water in the Surroundings of a Metamorphic Outcrop in Hungary: The Hydrogeological Answer to Practical Problems. Water 2023, 15, 1637. [Google Scholar] [CrossRef]
- ISO 5667-11:2009; Water Quality—Sampling Part 11: Guidance on Sampling of Groundwaters. International Organization for Standardization: Geneva, Switzerland, 2009.
- ISO 17294-2:2016; Water Quality—Application of Inductively Coupled Plasma Mass Spectrometry (ICP-MS) Part 2: Determination of Selected Elements Including Uranium Isotopes. International Organization for Standardization: Geneva, Switzerland, 2016.
- Croghan, C.W.; Egeghy, P.P. Methods of Dealing with Values Below the Limit of Detection Using SAS; US-EPA: Washington, DC, USA, 2003. [Google Scholar]
- Everitt, B.S.; Landau, S.; Leese, M.; Stahl, D. Cluster Analysis, 1st ed.; Wiley Series in Probability and Statistics; John Wiley & Sons Ltd.: West Sussex, UK, 2011. [Google Scholar] [CrossRef]
- Wold, S.; Esbensen, K.; Geladi, P. Principal component analysis. Chemom. Intell. Lab. Syst. 1987, 2, 37–52. [Google Scholar] [CrossRef]
- Bufa-Dőrr, Z.; Sebestyén, Á.; Izsák, B.; Schmoll, O.; Pándics, T.; Vargha, M. Dual system of water safety plan auditing in Hungary: Benefits and lessons learnt. J. Water Health 2023, 21, 1663–1675. [Google Scholar] [CrossRef] [PubMed]
- Tóth, Á.; Baják, P.; Szijártó, M.; Tiljander, M.; Korkka-Niemi, K.; Hendriksson, N.; Mádl-Szőnyi, J. Multimethodological Revisit of the Surface Water and Groundwater Interaction in the Balaton Highland Region—Implications for the Overlooked Groundwater Component of Lake Balaton, Hungary. Water 2023, 15, 1006. [Google Scholar] [CrossRef]
- Ingebritsen, S.E.; Sanford, W.E.; Neuzil, C.E. Groundwater in Geologic Processes, 2nd ed.; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar] [CrossRef]
- Tóth, J. Gravitational Systems of Groundwater Flow: Theory, Evaluation, Utilization; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Czauner, B.; Erőss, A.; Szkolnikovics-Simon, S.; Markó, Á.; Baják, P.; Trásy-Havril, T.; Szijártó, M.; Szabó, Z.; Hegedűs-Csondor, K.; Mádl-Szőnyi, J. From basin-scale groundwater flow to integrated geofluid research in the hydrogeology research group of Eötvös Loránd University, Hungary. J. Hydrol. X 2022, 17, 100142. [Google Scholar] [CrossRef]
- Tóth, J. Groundwater as a geologic agent: An overview of the causes, processes, and manifestations. Hydrogeol. J. 1999, 7, 1–14. [Google Scholar] [CrossRef]
- Lenkey, L.; Zsemle, F.; Mádl-Szőnyi, J.; Dövényi, P.; Rybach, L. Possibilities and limitations in the utilization of the Neogene geothermal reservoirs in the Great Hungarian Plain, Hungary. Central Eur. Geol. 2008, 51, 241–252. [Google Scholar] [CrossRef]
- Csondor, K.; Czauner, B.; Csobaji, L.; Győri, O.; Erőss, A. Characterization of the regional groundwater flow systems in south Transdanubia (Hungary) to understand karst evolution and development of hydrocarbon and geothermal resources. Hydrogeol. J. 2020, 28, 2803–2820. [Google Scholar] [CrossRef]
- Tóth, J.; Almási, I. Interpretation of observed fluid potential patterns in a deep sedimentary basin under tectonic compression: Hungarian Great Plain, Pannonian Basin. Geofluids 2001, 1, 11–36. [Google Scholar] [CrossRef]
- Hungarian Academy of Sciences. National Atlas of Hungary—Natural Environment; Hungarian Academy of Sciences, Research Centre for Astronomy and Earth Sciences, Geographical Institute: Budapest, Hungary, 2018. [Google Scholar]
- Dura, G.; Rudnai, P.; Kádár, M.; Vargha, M. The health risks of consuming drinking water with elevated arsenic content of geochemical origin. Central Eur. Geol. 2014, 57, 307–316. [Google Scholar] [CrossRef]
- Smedley, P.; Kinniburgh, D. Uranium in natural waters and the environment: Distribution, speciation and impact. Appl. Geochem. 2023, 148, 105534. [Google Scholar] [CrossRef]
- Vengosh, A.; Coyte, R.M.; Podgorski, J.; Johnson, T.M. A critical review on the occurrence and distribution of the uranium- and thorium-decay nuclides and their effect on the quality of groundwater. Sci. Total Environ. 2022, 808, 151914. [Google Scholar] [CrossRef] [PubMed]
- Baják, P.; Csondor, K.; Pedretti, D.; Muniruzzaman, M.; Surbeck, H.; Izsák, B.; Vargha, M.; Horváth, Á.; Pándics, T.; Erőss, A. Refining the conceptual model for radionuclide mobility in groundwater in the vicinity of a Hungarian granitic complex using geochemical modeling. Appl. Geochem. 2022, 137, 105201. [Google Scholar] [CrossRef]
- Gjengedal, E.L.F.; Joudi, B.; Heim, M.; Steffensen, I.-L.; Lund, V. Uranium and lanthanum in Norwegian drinking water—Is there cause for concern? Sci. Total Environ. 2023, 889, 164287. [Google Scholar] [CrossRef] [PubMed]
- National Center for Public Health and Pharmacy. Módszertani Útmutató Ivóvizek Radiológiai Paramétereinek Vizsgálatához és Értékeléséhez. 2023. Available online: https://www.nnk.gov.hu/attachments/article/726/2023_8_M%C3%B3dsz.%C3%BAtm._Iv%C3%B3viz_radiologiai%20vizsg..pdf (accessed on 1 March 2024).
- Ćurković, M.; Sipos, L.; Puntarić, D.; Dodig-Ćurković, K.; Pivac, N.; Kralik, K. Arsenic, Copper, Molybdenum, and Selenium Exposure through Drinking Water in Rural Eastern Croatia. Pol. J. Environ. Stud. 2016, 25, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Smedley, P.L.; Cooper, D.M.; Lapworth, D.J. Molybdenum distributions and variability in drinking water from England and Wales. Environ. Monit. Assess. 2014, 186, 6403–6416. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Westerhoff, P.; Zeng, C. Lithium occurrence in drinking water sources of the United States. Chemosphere 2022, 305, 135458. [Google Scholar] [CrossRef] [PubMed]
- Abdo, N.; Alhamid, A.; Abu Dalo, M.; Graboski-Bauer, A.; Al Harahsheh, M. Potential health risk assessment of mixtures of heavy metals in drinking water. Groundw. Sustain. Dev. 2024, 25, 101147. [Google Scholar] [CrossRef]
- Radulescu, C.; Bretcan, P.; Pohoata, A.; Tanislav, D.; Stirbescu, R.M. Assessment of drinking water quality using statistical analysis: A case study. J. Phys. 2016, 61, 1604–1616. [Google Scholar]
- Ahada, C.P.S.; Suthar, S. Hydrochemistry of groundwater in North Rajasthan, India: Chemical and multivariate analysis. Environ. Earth Sci. 2017, 76, 203. [Google Scholar] [CrossRef]
- Priya, K.; Arulraj, G.P. A correlation–regression model for the physicochemical parameters of the groundwater in Coimbatore city, India. Environ. Technol. 2011, 32, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Figueroa, C.; Chalá, D.C.; Gutiérrez-Ribon, G.; Quiñones-Bolaños, E. A Framework to Evaluate Groundwater Quality and the Relationship between Rock Weathering and Groundwater Hydrogeochemistry in the Tropical Zone: A Case Study of Coastal Aquifer Arroyo Grande, in the Caribbean Region of Colombia. Water 2024, 16, 1650. [Google Scholar] [CrossRef]



| Variable | Dimension | LOQ | n | Minimum | Lower Quartile | Mean | Std. Dev. | Median | Upper Quartile | Maximum | % of Samples <LOQ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| B | µg/L | 10 | 1256 | <LOQ | 13 | 84 | 185 | 25 | 62 | 2570 | 16 |
| Ba | µg/L | 10 | 1256 | <LOQ | 54 | 120 | 83 | 103 | 170 | 551 | 2 |
| Be | µg/L | 1.0 | 1256 | <LOQ | <LOQ | <LOQ | 0 | <LOQ | <LOQ | <LOQ | 100 |
| Ca | mg/L | 0.50 | 1256 | 2.5 | 40 | 64 | 31 | 63 | 85 | 174 | 0 |
| Co | µg/L | 1.0 | 1256 | <LOQ | <LOQ | <LOQ | 0.42 | <LOQ | <LOQ | 11 | 99 |
| K | mg/L | 0.50 | 1256 | <LOQ | 1.1 | 1.9 | 1.9 | 1.4 | 2.0 | 32 | 2 |
| Li | µg/L | 1.0 | 1256 | <LOQ | 5.1 | 16 | 25 | 10 | 17 | 265 | 2 |
| Mg | mg/L | 0.50 | 1256 | 0.55 | 15 | 25 | 14 | 23 | 35 | 100 | 0 |
| Mo | µg/L | 1.0 | 1256 | <LOQ | <LOQ | 2.4 | 6.6 | <LOQ | 1.6 | 103 | 58 |
| Na | mg/L | 0.50 | 1256 | <LOQ | 15 | 40 | 40 | 25 | 50 | 252 | 0 |
| Se | µg/L | 1.0 | 1256 | <LOQ | <LOQ | <LOQ | 0.46 | <LOQ | <LOQ | 7.0 | 86 |
| Sr | µg/L | 10 | 1256 | 21 | 241 | 448 | 354 | 366 | 519 | 3310 | 0 |
| Ti | µg/L | 1.0 | 1256 | <LOQ | <LOQ | 8.4 | 14 | 2.9 | 7.3 | 83 | 31 |
| U | µg/L | 1.0 | 1256 | <LOQ | <LOQ | 1.5 | 2.4 | <LOQ | 1.3 | 41 | 72 |
| V | µg/L | 1.0 | 1256 | <LOQ | <LOQ | <LOQ | 0.85 | <LOQ | <LOQ | 17 | 91 |
| Variable | B | Ba | Ca | K | Li | Mg | Mo | Na | Se | Sr | Ti | U | V |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | 1.000 | ||||||||||||
| Ba | 0.062 | 1.000 | |||||||||||
| Ca | −0.342 | −0.08 | 1.000 | ||||||||||
| K | 0.088 | 0.040 | 0.131 | 1.000 | |||||||||
| Li | 0.224 | 0.084 | −0.081 | 0.401 | 1.000 | ||||||||
| Mg | −0.275 | 0.061 | 0.556 | 0.147 | −0.024 | 1.000 | |||||||
| Mo | 0.317 | −0.023 | −0.203 | −0.007 | 0.041 | −0.159 | 1.000 | ||||||
| Na | 0.537 | 0.213 | −0.579 | 0.061 | 0.261 | −0.361 | 0.301 | 1.000 | |||||
| Se | −0.061 | −0.081 | 0.112 | 0.082 | 0.041 | 0.186 | −0.033 | −0.084 | 1.000 | ||||
| Sr | −0.098 | 0.354 | 0.165 | 0.193 | 0.190 | 0.462 | −0.091 | 0.002 | 0.062 | 1.000 | |||
| Ti | −0.080 | −0.062 | 0.330 | −0.023 | −0.048 | 0.300 | −0.071 | −0.119 | −0.070 | 0.032 | 1.000 | ||
| U | −0.082 | −0.153 | 0.120 | 0.136 | 0.045 | 0.298 | 0.020 | −0.106 | 0.433 | 0.107 | −0.006 | 1.000 | |
| V | −0.035 | −0.131 | 0.017 | 0.199 | −0.047 | 0.032 | 0.021 | −0.081 | 0.046 | −0.050 | −0.023 | 0.133 | 1.000 |
| Cluster Number | Characteristic Element | Σn | Distribution by Water Type | |||||
|---|---|---|---|---|---|---|---|---|
| Deep Groundw. | Karst w. | Shallow Groundw. | Surface w. | Bank-Filtered w. | Mixed w. | |||
| Cluster 1 | Li, B, Na, Mo, K | 31 | 27 | 1 | 1 | 1 | 1 | 0 |
| Cluster 2 | Ti, Ca, Mg | 151 | 138 | 5 | 2 | 0 | 5 | 1 |
| Cluster 3 | Ca | 488 | 333 | 101 | 20 | 12 | 20 | 1 |
| Cluster 4 | Ba, Na, Sr | 343 | 340 | 3 | 0 | 0 | 0 | 0 |
| Cluster 5 | Mo, B, Na | 200 | 196 | 0 | 0 | 0 | 2 | 1 |
| Cluster 6 | Mg, K, U, Se, V | 43 | 27 | 1 | 6 | 5 | 4 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izsák, B.; Hegedűs-Csondor, K.; Baják, P.; Erőss, A.; Erdélyi, N.; Vargha, M. Distribution of Natural Trace Elements in the Drinking Water Sources of Hungary. Water 2024, 16, 2122. https://doi.org/10.3390/w16152122
Izsák B, Hegedűs-Csondor K, Baják P, Erőss A, Erdélyi N, Vargha M. Distribution of Natural Trace Elements in the Drinking Water Sources of Hungary. Water. 2024; 16(15):2122. https://doi.org/10.3390/w16152122
Chicago/Turabian StyleIzsák, Bálint, Katalin Hegedűs-Csondor, Petra Baják, Anita Erőss, Norbert Erdélyi, and Márta Vargha. 2024. "Distribution of Natural Trace Elements in the Drinking Water Sources of Hungary" Water 16, no. 15: 2122. https://doi.org/10.3390/w16152122
APA StyleIzsák, B., Hegedűs-Csondor, K., Baják, P., Erőss, A., Erdélyi, N., & Vargha, M. (2024). Distribution of Natural Trace Elements in the Drinking Water Sources of Hungary. Water, 16(15), 2122. https://doi.org/10.3390/w16152122






