Defluoridation of Water Using Cu-Mg-Binary-Metal-Oxide-Coated Sand
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Coated Sand Sorbent
2.3. Characterization of Coated Sand
2.4. Adsorption Experiments
2.5. Zeta Potential Analysis
2.6. Effect of pH on Fluoride Adsorption
2.7. Effect of Co-Existing Ions on Fluoride Adsorption
2.8. Adsorbent Recycling and Re-Use Experiments
3. Results
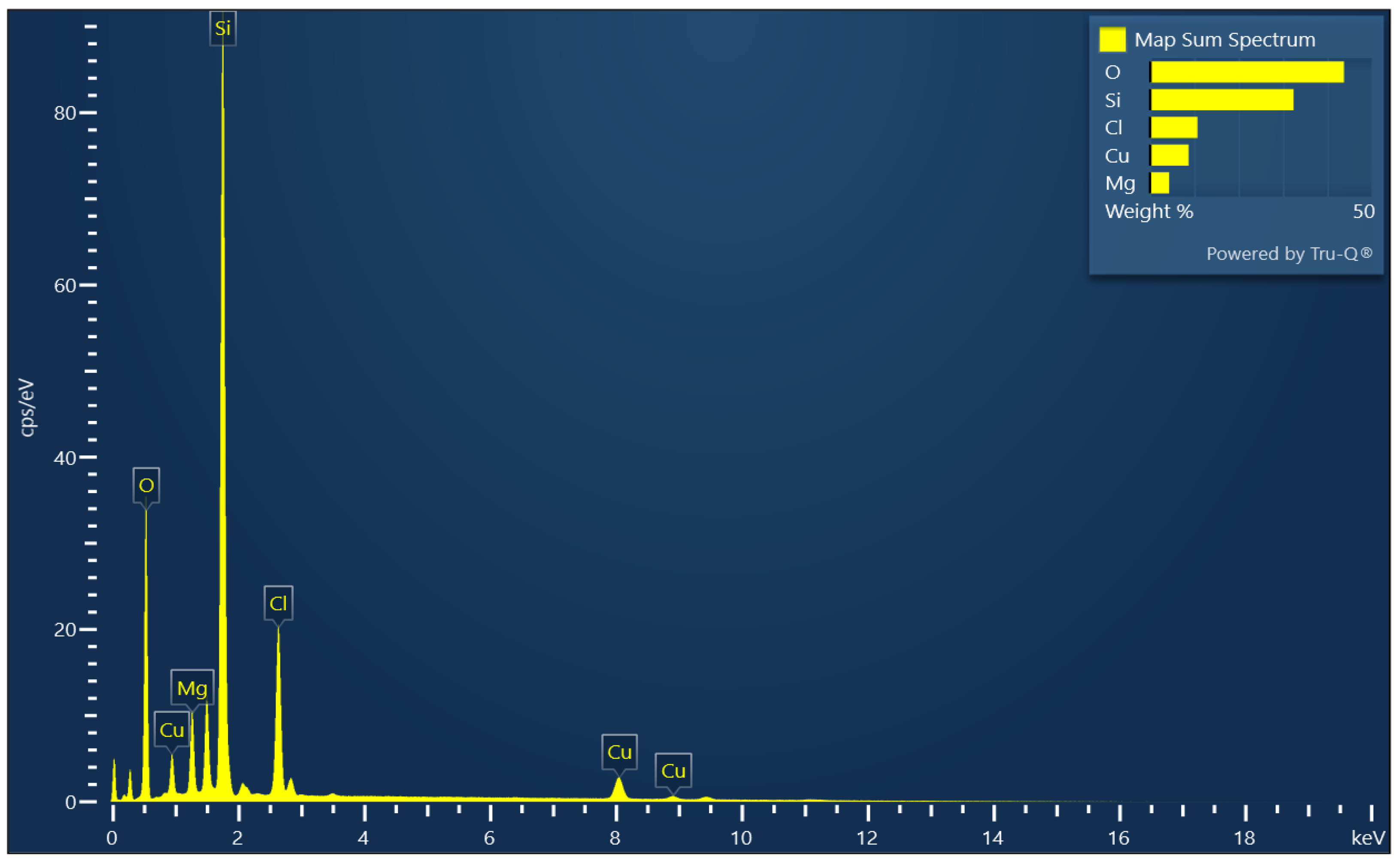
3.1. Characterization of CMCS Sorbent
3.2. Adsorption Kinetics Experiments
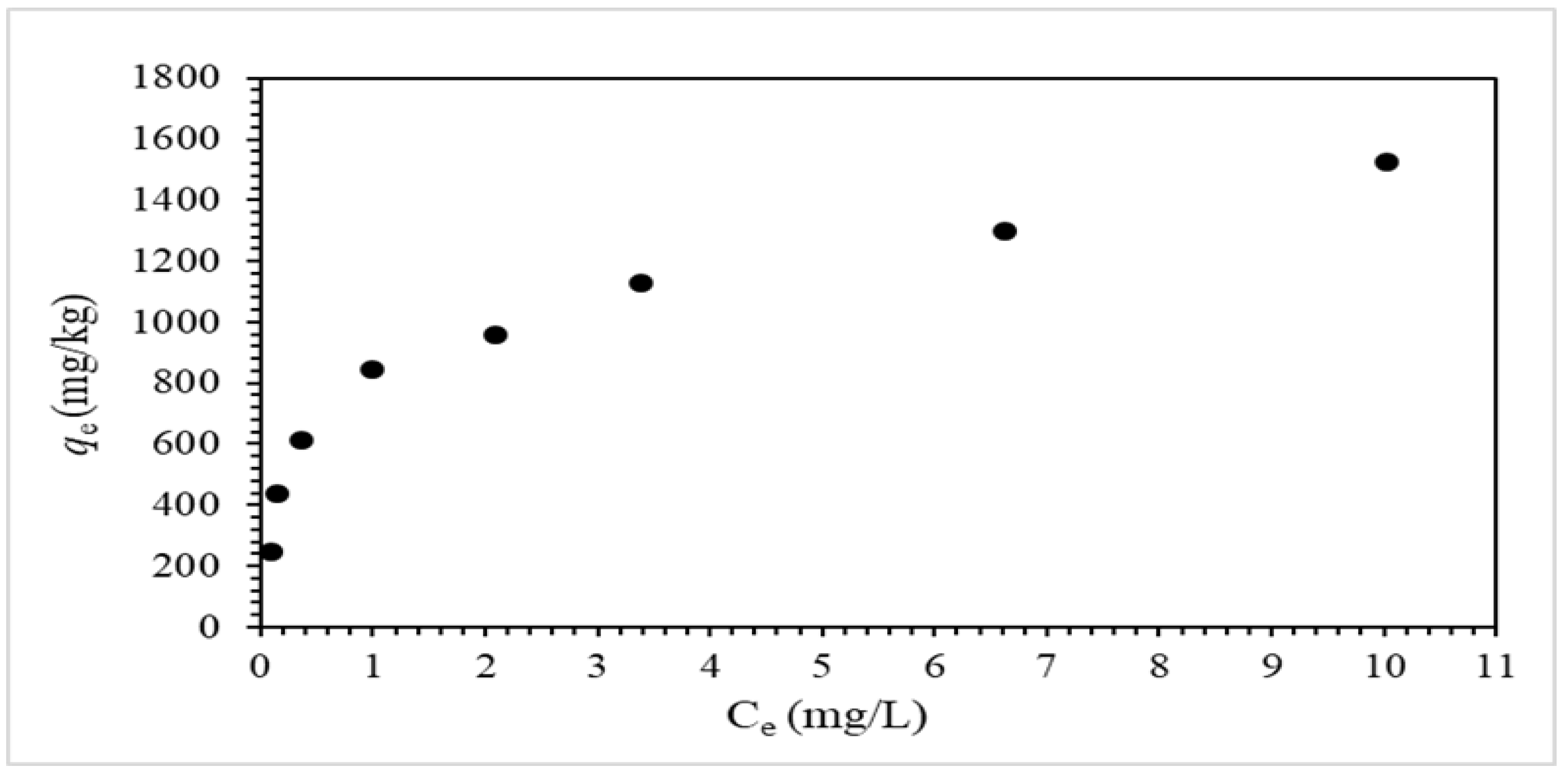
3.3. Adsorption Equilibrium Experiments
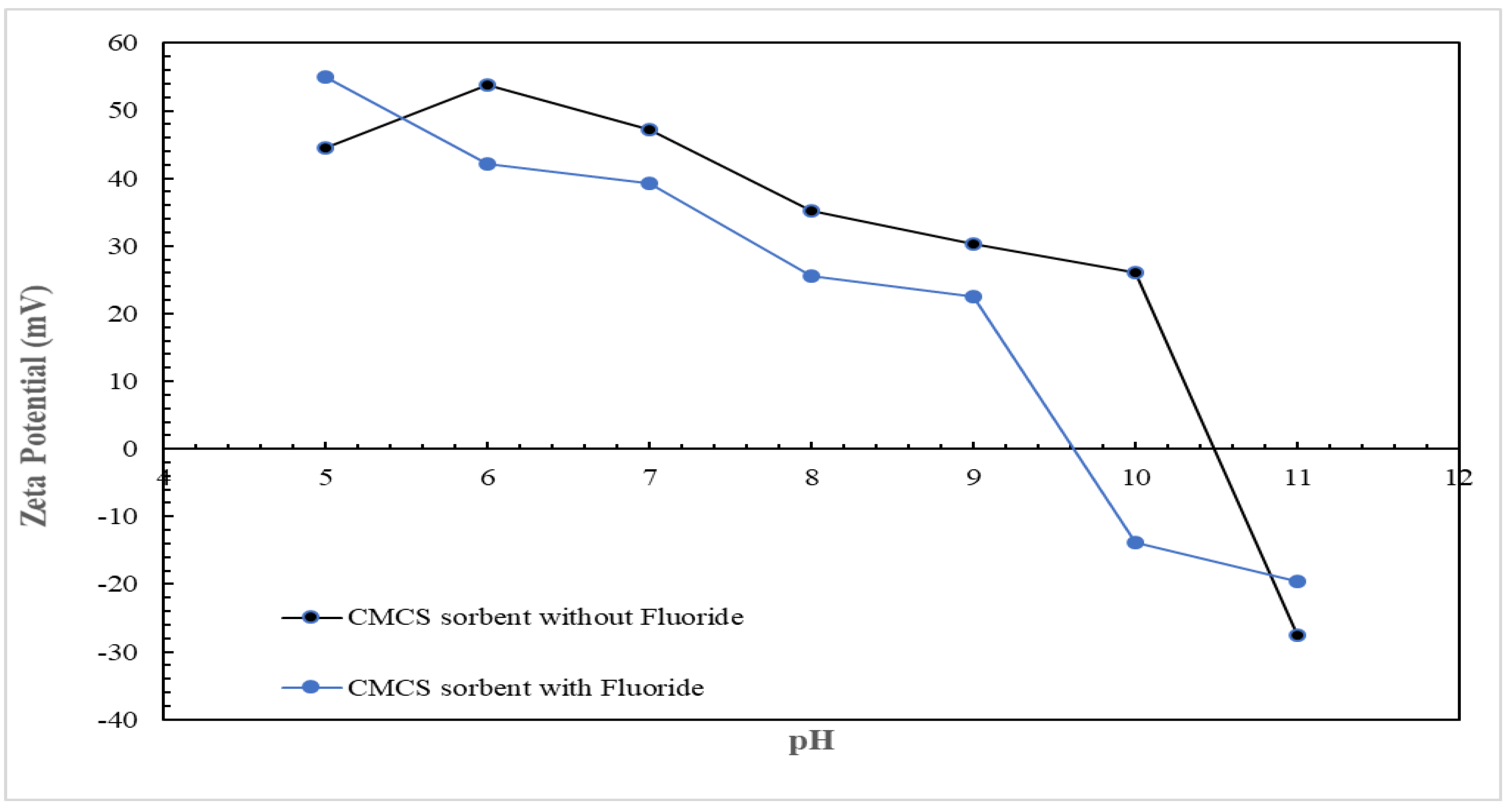
3.4. Surface Charge Analysis of Adsorbent
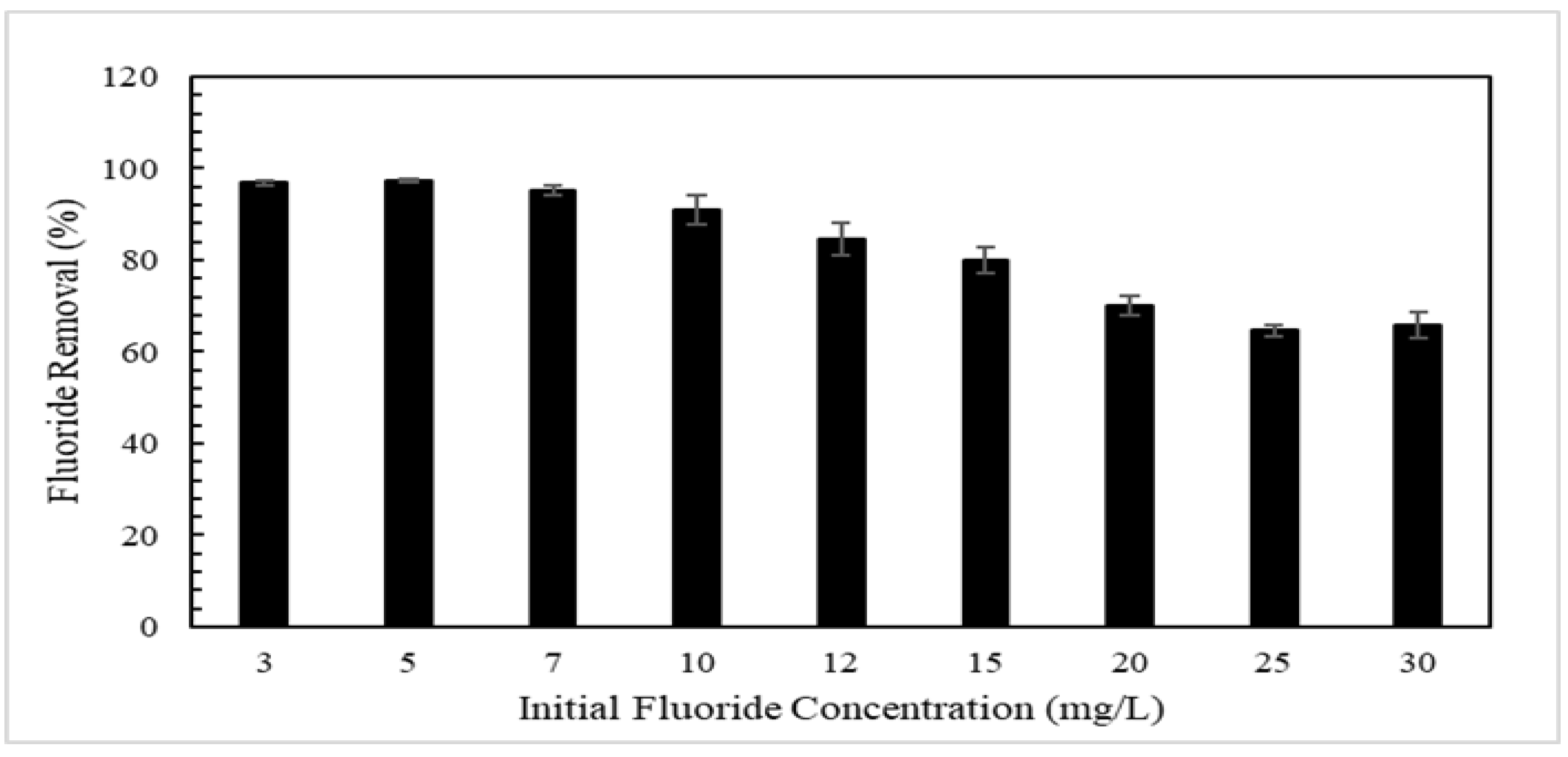
3.5. Effect of pH on Fluoride Adsorption
3.6. Adsorption Mechanism
3.7. Effect of Co-Existing Ions on Fluoride Adsorption
3.8. Re-Coating and Reuse of the CMCS Sorbent
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghosh, A.; Mukherjee, K.; Ghosh, S.K.; Saha, B. Sources and toxicity of fluoride in the environment. Res. Chem. Intermed. 2013, 39, 2881–2915. [Google Scholar] [CrossRef]
- Peterson, P.J. Assessment of exposure to chemical contaminants in water and food. Sci. Total Environ. 1995, 168, 123–129. [Google Scholar] [CrossRef] [PubMed]
- UNESCO World Water Assessment Program. The United Nations World Water Development Report 2021: Valuing Water; UNESCO: Paris, France, 2021; pp. 1–187. Available online: https://unesdoc.unesco.org/notice?id=p::usmarcdef_0000375724 (accessed on 9 October 2023).
- The United Nations Educational, Scientific and Cultural Organization. The United Nations World Water Development Report 2015; United Nations: New York, NY, USA, 2015. [Google Scholar] [CrossRef]
- WHO. International Program on Chemical Safety 1984 Fluorine and Fluorides; World Health Organization: Geneva, Switzerland, 1984. [Google Scholar]
- US EPA. ALSA Tech, LLC, and Battelle. Design Manual: Removal of Fluoride from Drinking Water Supplies by Activated Alumina. (Report No. EPA/600/R-14/236); The Water Supply and Water Resources Division, National Risk Management Research Laboratory, Office of Research and Development, U.S. Environmental Protection Agency: Cincinnati, OH, USA, 2014.
- U.S. Department of Health and Human Services Federal Panel on Community Water Fluoridation. U.S. Public Health Service Recommendation for Fluoride Concentration in Drinking Water for the Prevention of Dental Caries. Public Health Rep. 2015, 130, 318–331. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Fluoride in Drinking Water: A Scientific Review of EPA’s Standards; The National Academies Press: Washington, DC, USA, 2006.
- World Health Organization. Guidelines for Drinking-Water Quality, Third Edition, Incorporating the First and Second Addenda. Volume 1: Recommendations; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Ahmad, S.; Singh, R.; Arfin, T.; Neeti, K. Fluoride contamination, consequences and removal techniques in water: A review. Environ. Sci. Adv. 2022, 1, 620–661. [Google Scholar] [CrossRef]
- Arora, M.; Maheshwari, R.C.; Jain, S.K.; Gupta, A. Use of membrane technique for potable water production. Desalination 2004, 170, 105–112. [Google Scholar] [CrossRef]
- Meenakshi; Maheshwari, R.C. Fluoride in drinking water and its removal. J. Hazard. Mater. 2006, 137, 456–463. [Google Scholar] [CrossRef]
- Tang, Y.; Guan, X.; Wang, J.; Gao, N.; McPhail, M.R.; Chusuei, C.C. Fluoride adsorption onto granular ferric hydroxide: Effects of ionic strength, pH, surface loading, and major co-existing anions. J. Hazard. Mater. 2009, 171, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A.; Kumar, E.; Sillanpää, M. Fluoride removal from water by adsorption—A review. Chem. Eng. J. 2011, 171, 811–840. [Google Scholar] [CrossRef]
- Habuda-Stanić, M.; Ravančić, M.; Flanagan, A. A review on adsorption of fluoride from aqueous solution. Materials 2014, 7, 6317–6366. [Google Scholar] [CrossRef]
- Velazquez-Jimenez, L.H.; Vences-Alvarez, E.; Flores-Arciniega, J.L.; Flores-Zuñiga, H.; Rangel-Mendez, J.R. Water Defluoridation with Special Emphasis on Adsorbents-Containing Metal Oxides and/or Hydroxides: A Review. Sep. Purif. Technol. 2015, 150, 292–307. [Google Scholar] [CrossRef]
- Yadav, K.K.; Gupta, N.; Kumar, V.; Khan, S.A.; Kumar, A. A review of emerging adsorbents and current demand for defluoridation of water: Bright future in water sustainability. Environ. Int. 2018, 111, 80–108. [Google Scholar] [CrossRef] [PubMed]
- Ku, Y.; Chiou, H.-M. The adsorption of fluoride ion from aqueous solution by activated alumina. Water Air Soil Pollut. 2002, 133, 349–361. [Google Scholar] [CrossRef]
- Ghorai, S.; Pant, K.K. Investigations on the column performance of fluoride adsorption by activated alumina in a fixed-bed. Chem. Eng. J. 2004, 98, 165–173. [Google Scholar] [CrossRef]
- López Valdivieso, A.; Reyes Bahena, J.L.; Song, S.; Herrera Urbina, R. Temperature effect on the zeta potential and fluoride adsorption at the α-Al2O3/aqueous solution interface. J. Colloid Interface Sci. 2006, 298, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kamble, S.P.; Deshpande, G.; Barve, P.P.; Rayalu, S.; Labhsetwar, N.K.; Malyshew, A.; Kulkarni, B.D. Adsorption of fluoride from aqueous solution by alumina of alkoxide nature: Batch and continuous operation. Desalination 2010, 264, 15–23. [Google Scholar] [CrossRef]
- Salifu, A.; Petrusevski, B.; Ghebremichael, K.L.; Modestus, L.; Buamah, R.; Aubry, C.; Amy, G.L. Aluminum (hydr)oxide coated pumice for fluoride removal from drinking water: Synthesis, equilibrium, kinetics and mechanism. Chem. Eng. J. 2013, 228, 63–74. [Google Scholar] [CrossRef]
- Modaresahmadi, K.; Khodadoust, A.P.; Wescott, J. Adsorption of fluoride from water using aluminum coated sand: Kinetics, equilibrium, effect of pH, and coexisting ions. J. Geosci. Environ. Prot. 2022, 10, 224–241. [Google Scholar] [CrossRef]
- Loganathan, P.; Vigneswaran, S.; Kandasamy, J.; Naidu, R. Defluoridation of drinking water using adsorption processes. J. Hazard. Mater. 2013, 248–249, 1–19. [Google Scholar] [CrossRef]
- Modaresahmadi, K.; Khodadoust, A.P.; Wescott, J. Adsorption of fluoride from water using Al–Mg–Ca ternary metal oxide-coated sand. Water Supply 2023, 23, 4699–4713. [Google Scholar] [CrossRef]
- Dhillon, A.; Soni, S.K.; Kumar, D. Enhanced fluoride removal performance by Ce–Zn binary metal oxide: Adsorption characteristics and mechanism. J. Fluor. Chem. 2017, 199, 67–76. [Google Scholar] [CrossRef]
- Swaina, S.K.; Patnaik, T.; Patnaik, P.C.; Jha, U.; Dey, R.K. Development of new alginate entrapped Fe(III)-Zr(IV) binary mixed oxide for removal of fluoride from water bodies. Chem. Eng. J. 2013, 215–216, 763–771. [Google Scholar] [CrossRef]
- Thakre, D.; Jagtap, S.; Sakhare, N.; Labhsetwar, N.; Meshram, S.; Rayalu, S. Chitosan based mesoporous Ti-Al binary metal oxide supported beads for defluoridation of water. Chem. Eng. J. 2010, 158, 315–324. [Google Scholar] [CrossRef]
- Zhu, J.; Lin, X.; Wu, P.; Zhou, Q.; Luo, X. Fluoride removal from aqueous solution by Al(III)-Zr(IV) binary oxide adsorbent. Appl. Surf. Sci. 2015, 357, 91–100. [Google Scholar] [CrossRef]
- Sundaram, C.S.; Viswanathan, N.; Meenakshi, S. Defluoridation of water using magnesia/chitosan composite. J. Hazard. Mater. 2009, 163, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Oladoja, N.A.; Chen, S.; Drewes, J.E.; Helmreich, B. Characterization of granular matrix supported nano magnesium oxide as an adsorbent for defluoridation of groundwater. Chem. Eng. J. 2015, 281, 632–643. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency; Antimicrobial Copper Alloys Group. Available online: https://www3.epa.gov/pesticides/chem_search/ppls/082012-00002-20101110.pdf (accessed on 28 January 2021).
- Bansiwal, A.; Pillewan, P.; Biniwale, R.B.; Rayalu, S.S. Copper oxide incorporated mesoporous alumina for defluoridation of drinking water. Microporous Mesoporous Mater. 2010, 129, 54–61. [Google Scholar] [CrossRef]
- Maliyekkal, S.M.; Shukla, S.; Philip, L.; Nambi, I.M. Enhanced fluoride removal from drinking water by magnesia-amended activated alumina granules. Chem. Eng. J. 2008, 140, 183–192. [Google Scholar] [CrossRef]
- Mondal, P.; George, S. A review on adsorbents used for defluoridation of drinking water. Rev. Environ. Sci. Biotechnol. 2015, 14, 195–210. [Google Scholar] [CrossRef]
- Rice, E.W.; Baird, R.B.; Eaton, A.D. (Eds.) Standard Methods for the Examination of Water and Wastewater, 23rd ed.; Method 4500-F- Fluoride; American Public Health Association: Washington, DC, USA; American Water Works Association: Denver, CO, USA; Water Environment Federation: Alexandria, VA, USA, 2018. [Google Scholar]
- Lagergren, S. Zur Theorie der Sogenannten Adsorption Gelöster Stoffe. K. Sven. Vetenskapsakademiens Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption on carbon from solutions. Journal of the Sanitary Engineering Division. Am. Soc. Civ. Eng. 1963, 89, 31–60. [Google Scholar]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Freundlich, H. Über die Adsorption in Lösungen. Z. Für Phys. Chem. 1907, 57, 385–470. [Google Scholar] [CrossRef]
- Dubinin, M.M.; Radushkevich, L.V. Equation of the characteristic curve of activated charcoal. Proc. Acad. Sci. USSR Phys. Chem. Sect. 1947, 55, 331–333. [Google Scholar]
- Modaresahmadi, K.; Khodadoust, A.P.; Wescott, J. Adsorption of Fluoride from Water Using Aluminum-Coated Silica Adsorbents: Comparison of Silica Sand and Microcrystalline Silica. Separations 2024, 11, 125. [Google Scholar] [CrossRef]
- Wu, K.; Zhang, N.; Liu, T.; Ma, C.; Jin, P.; Zhang, F.; Zhang, J.; Wang, X. Competitive adsorption behaviors of arsenite and fluoride onto manganese-aluminum binary adsorbents. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 185–194. [Google Scholar] [CrossRef]
- Zhu, P.; Wang, H.; Sun, B.; Deng, P.; Hou, S.; Yu, Y. Adsorption of fluoride from aqueous solution by magnesia-amended silicon dioxide granules. J. Chem. Technol. Biotechnol. 2009, 84, 1449–1455. [Google Scholar] [CrossRef]
- Guo, W.; Lin, H.; Zhu, H.; Mo, W.; Su, X.; Yang, J.; Ma, S.; Feng, J.; Lei, M. Efficient removal of fluorine by carbon fiber supported Mg-Fe binary metal oxide composite adsorbent and mechanism analysis based on DFT. Sep. Purif. Technol. 2024, 330, 125320. [Google Scholar] [CrossRef]
- Blanco-Flores, A.; Arteaga-Larios, N.; Pérez-García, V.; Martínez-Gutiérrez, J.; Ojeda-Escamilla, M.; Rodríguez-Torres, I. Efficient fluoride removal using Al-Cu oxide nanoparticles supported on steel slag industrial waste solid. Environ. Sci. Pollut. Res. 2018, 25, 6414–6428. [Google Scholar] [CrossRef]
- Gao, M.; Wang, W.; Yang, H.; Ye, B.C. Efficient removal of fluoride from aqueous solutions using 3D flower-like hierarchical zinc magnesium-aluminum ternary oxide microspheres. Chem. Eng. J. 2020, 380, 122459. [Google Scholar] [CrossRef]
- Bazrafshan, E.; Balarak, D.; Hossein Panahi, A.; Kamani, H.; Hossein Mahvi, A. Fluoride removal from aqueous solutions by cupricoxide nanoparticles. Res. Rep. Fluoride 2016, 49 Pt 1, 233–244. [Google Scholar]
- Tripathy, S.S.; Bersillon, J.L.; Gopal, K. Removal of fluoride from drinking water by adsorption onto alum-impregnated activated alumina. Sep. Purif. Technol. 2006, 50, 310–317. [Google Scholar] [CrossRef]
- Amalraj, A.; Pius, A. Removal of fluoride from drinking water using aluminum hydroxide coated activated carbon prepared from bark of Morinda tinctoria. Appl. Water Sci. 2017, 7, 2653–2665. [Google Scholar] [CrossRef]







| Adsorption Kinetics Model | Parameters | CMCS Sorbent |
|---|---|---|
| Experimental qe (mg/kg) | 270.5 | |
| Pseudo-first order kinetics model | k1 (1/min) | 0.003 |
| qe (mg/kg) | 20.1 | |
| R2 | 0.6589 | |
| Pseudo-second order kinetics model | k2 (g/mg-min) | 0.1148 |
| qe (mg/kg) | 270.3 | |
| R2 | 1 | |
| Intraparticle diffusion kinetics model | kid (mg/g-min1/2) | 3.92 |
| C | 167.6 | |
| R2 | 0.3303 |
| Adsorption Equilibrium Model | Equation | Linear Plot | Reference | |
|---|---|---|---|---|
| Langmuir | (6) | Ce/qe vs. Ce | [40] | |
| (7) | ||||
| Freundlich | (8) | log qe vs. log Ce | [41] | |
| Dubinin–Radushkevich (D-R) | (9) | lnqe vs. ε2 | [42] | |
| Ε = RT ln (1 + 1/Ce) | (10) | |||
| (11) | ||||
| Adsorbent | Langmuir | Freundlich | Dubinin–Radushkevich |
|---|---|---|---|
| CMCS Sorbent | qm = 1667 mg/kg | 1/n = 0.342 | KDR = 4.08 × 10−8 |
| KL = 1.2 L/mg | KF = 739 | E = 3.5 kJ/mol | |
| R2 = 0.9844 | R2 = 0.9404 | R2 = 0.9217 |
| Adsorbent | Langmuir Adsorption Capacity qm (mg/kg) | BET Surface Area (m2/g) | Surface-Normalized Adsorption Capacity (µg/m2) | Langmuir Adsorption Constant KL (L/mg) | Reference |
|---|---|---|---|---|---|
| Manganese–aluminum binary adsorbent | 58,070 | 41.24 | 1408 | _ | [44] |
| Ce-Zn binary adsorbent | 194,000 | 498.5 | 389.2 | 0.132 | [26] |
| Chitosan based mesoporous Ti–Al-binary-metal-oxide-supported beads | 2220 | 323.83 | 6.855 | 0.11 | [28] |
| Magnesia-amended silicon dioxide granules | 12,600 | 494.95 | 25.46 | _ | [45] |
| Fe(III)-Zr(IV) binary mixed oxide | 981 | 74.61 | 13.14 | 0.482 | [27] |
| Mg-Fe binary metal oxide composite adsorbent | 249,000 | 264.84 | 940.2 | _ | [46] |
| Magnesia/chitosan composite | 11,236 | 45.514 | 246.9 | 2.97 | [30] |
| Copper oxide incorporated mesoporous alumina | 7770 | 189.25 | 41.1 | 0.763 | [33] |
| Magnesia-amended activated alumina granules | 10,120 | 193.5 | 52.3 | 0.416 | [34] |
| Al-Cu oxide nanoparticles supported on steel slag industrial waste solid | 3990 | 2.99 | 1334 | 1.71 | [47] |
| Cu-Mg-binary-metal-oxide-coated sand | 1667 | 1.055 | 1580 | 1.2 | Present Work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modaresahmadi, K.; Khodadoust, A.P.; Wescott, J. Defluoridation of Water Using Cu-Mg-Binary-Metal-Oxide-Coated Sand. Water 2024, 16, 2178. https://doi.org/10.3390/w16152178
Modaresahmadi K, Khodadoust AP, Wescott J. Defluoridation of Water Using Cu-Mg-Binary-Metal-Oxide-Coated Sand. Water. 2024; 16(15):2178. https://doi.org/10.3390/w16152178
Chicago/Turabian StyleModaresahmadi, Kiana, Amid P. Khodadoust, and James Wescott. 2024. "Defluoridation of Water Using Cu-Mg-Binary-Metal-Oxide-Coated Sand" Water 16, no. 15: 2178. https://doi.org/10.3390/w16152178
APA StyleModaresahmadi, K., Khodadoust, A. P., & Wescott, J. (2024). Defluoridation of Water Using Cu-Mg-Binary-Metal-Oxide-Coated Sand. Water, 16(15), 2178. https://doi.org/10.3390/w16152178






