Degradation of Bisphenol A Using Self-Excited Oscillating Jets in Synergy with Fenton and Periodate Oxidation: Experimental and Artificial Neural Network Modeling Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedure
2.3. Analysis
3. Results and Discussion
3.1. Performance of the HC + Fenton + PI Oxidation System
3.2. Review and Comparison of Similar Processes
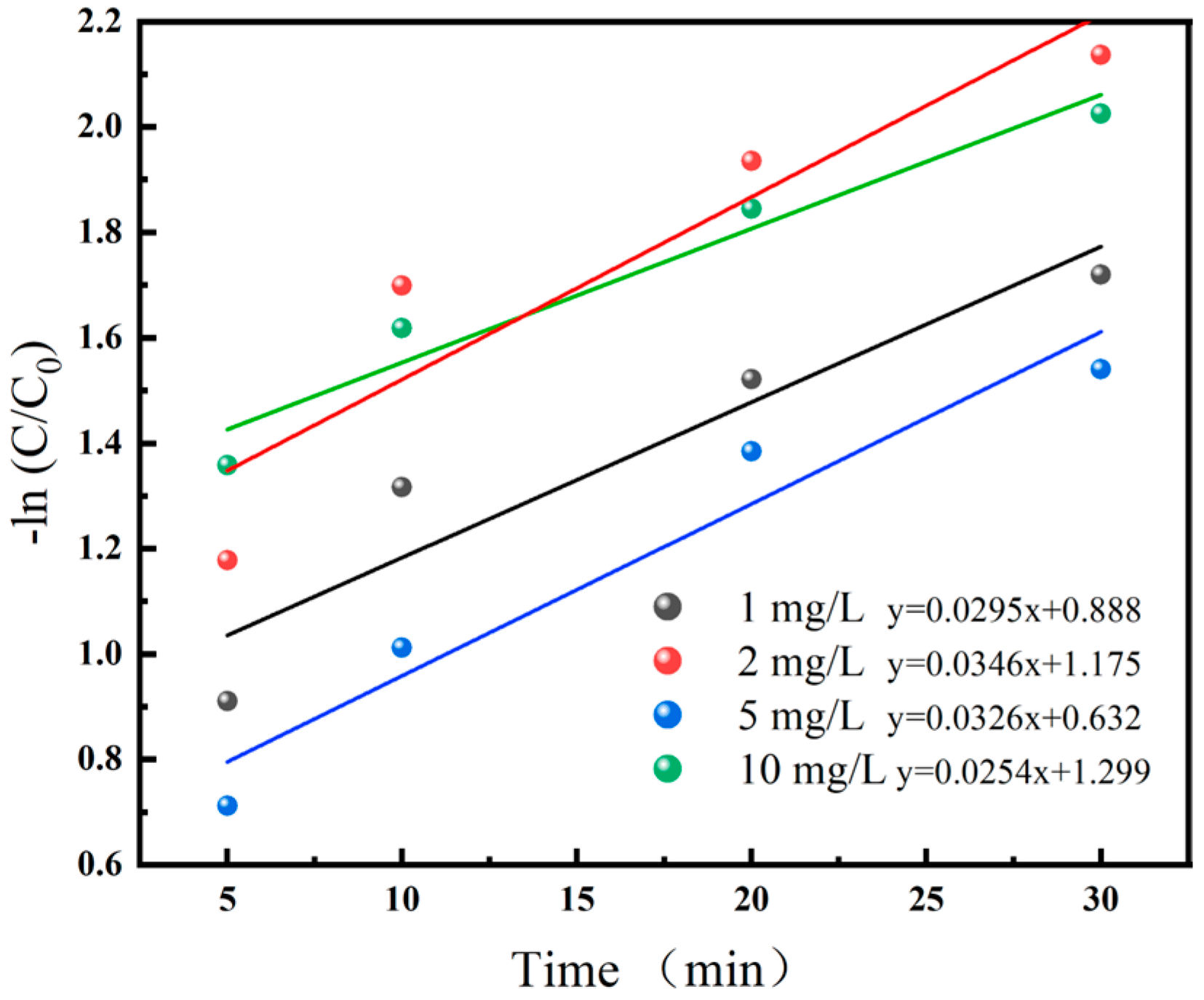
3.3. Effect of Initial Bisphenol A Concentration
3.4. Effect of Inlet Pressure
3.5. Effect of Initial Fe (II) Concentration
3.6. Effect of Initial H2O2 and PI Concentration
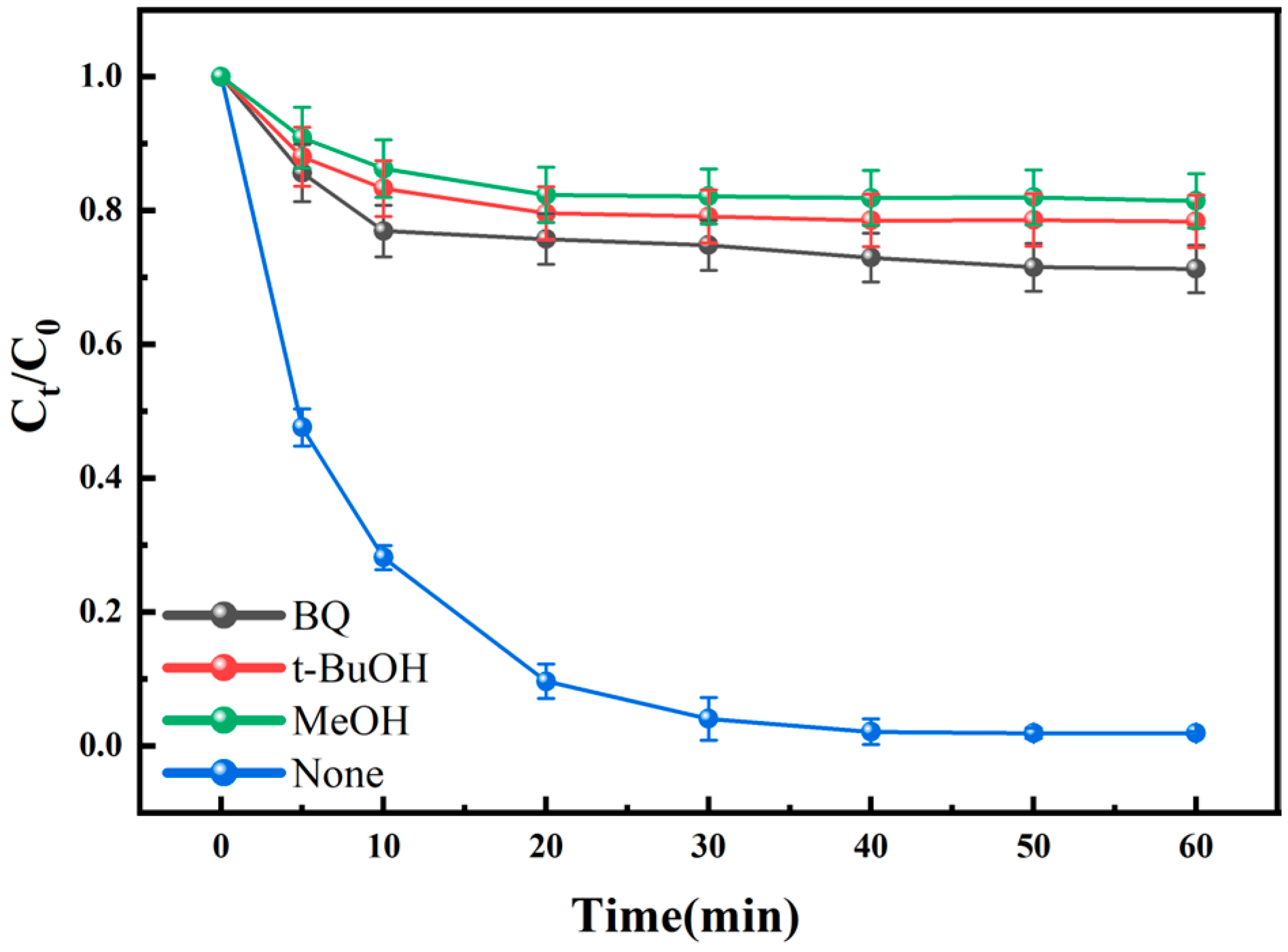
3.7. Identification of the Radicals
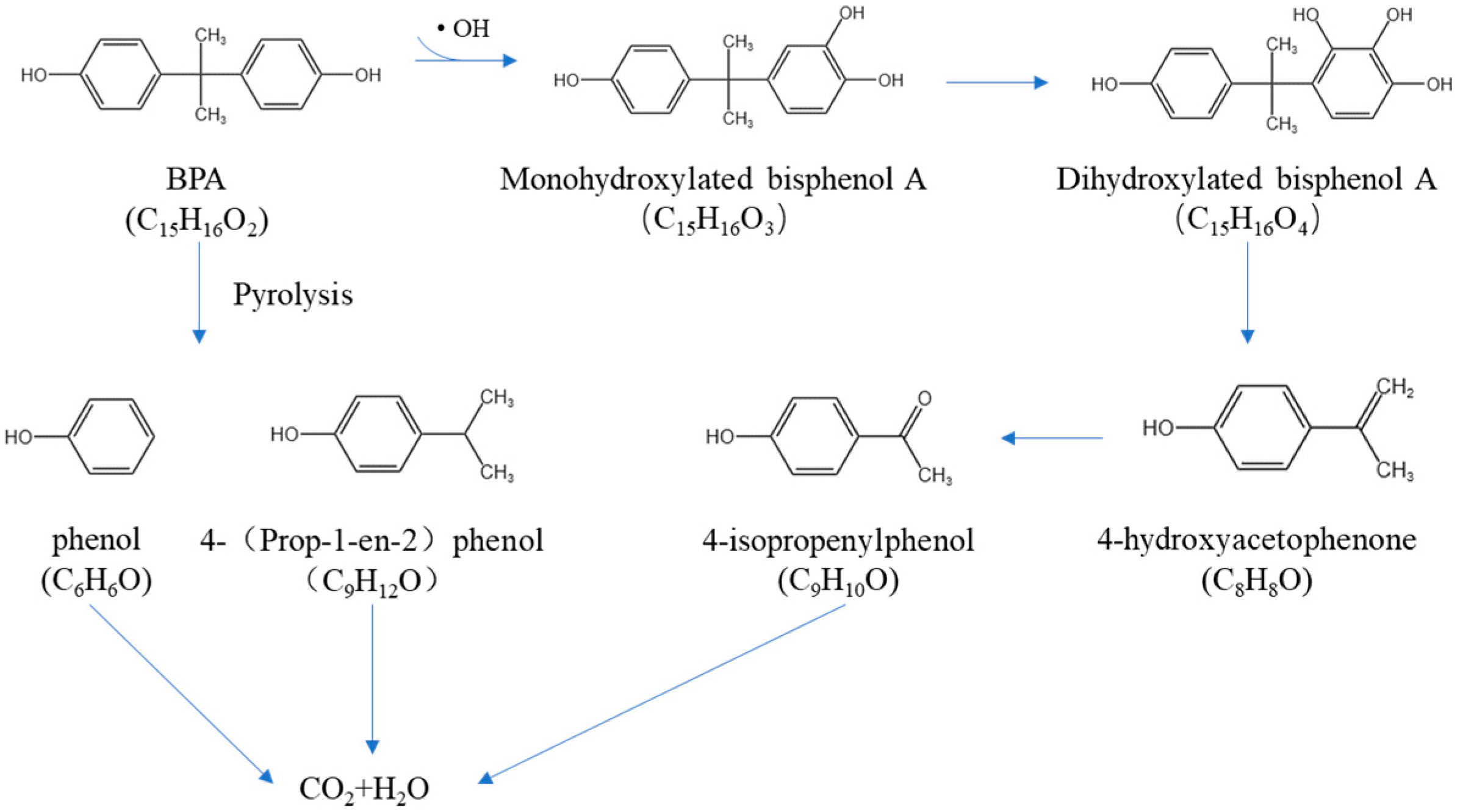
3.8. Degradation Pathway Prediction and Toxicity Assessment of BPA
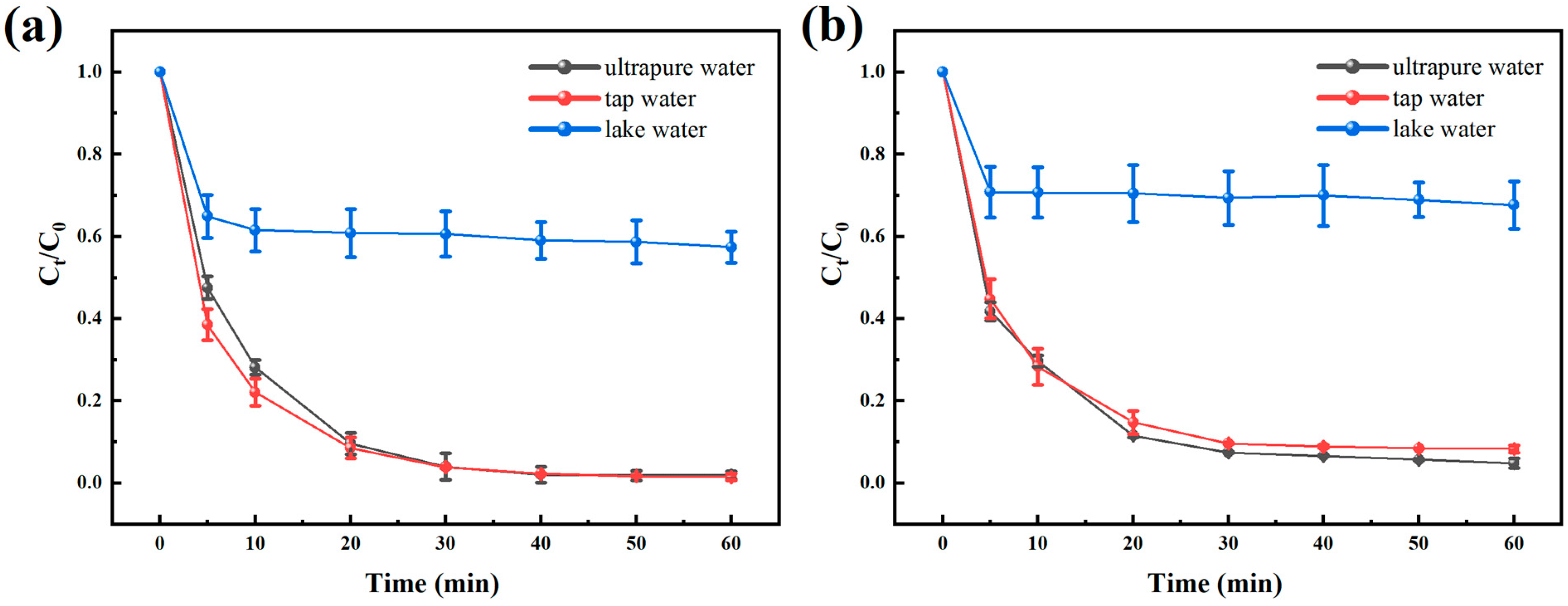
3.9. Effects of Different Water Sources
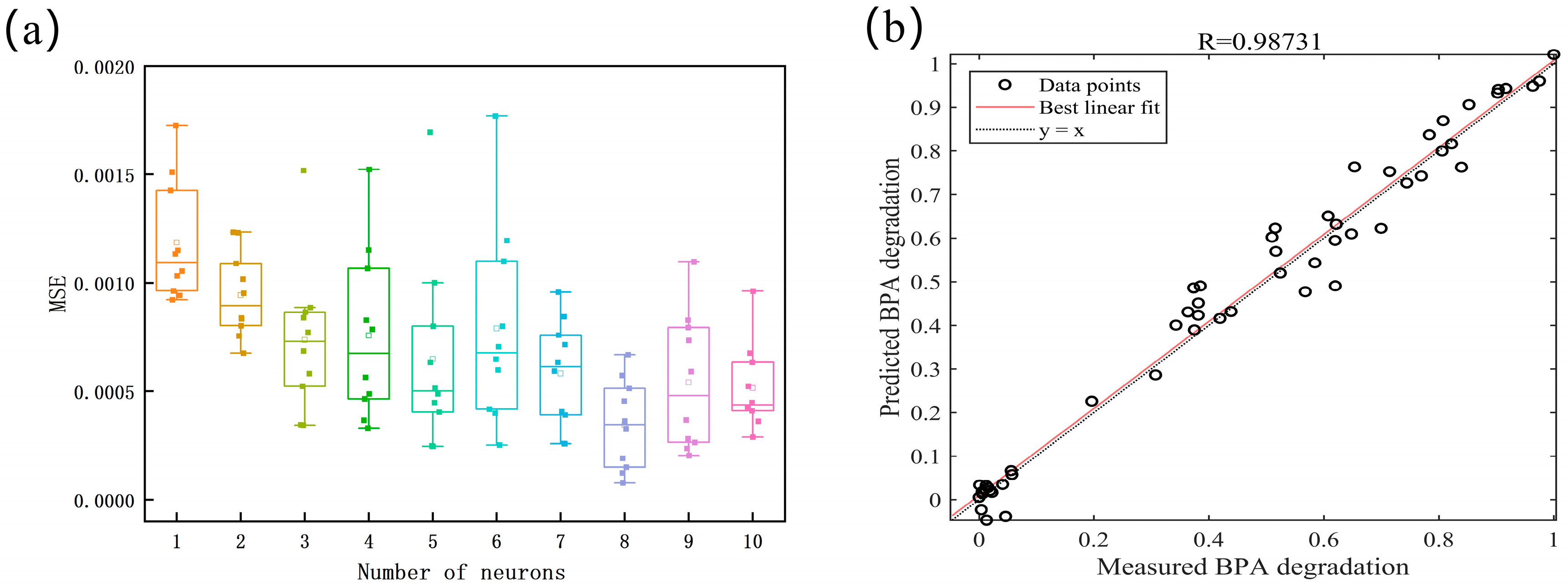
3.10. Surrogate Model and Projection
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, P.W.; Huang, Y.F.; Wang, C.H.; Fang, L.J.; Chen, M.L. Prenatal to Preschool Exposure of Nonylphenol and Bisphenol A Exposure and Neurodevelopment in Young Children. Pediatr. Neonatol. 2024, 65, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, S.; Mizutare, T.; Makishima, M.; Suzuki, N.; Fujimoto, N.; Igarashi, K.; Ohta, S. Potent Estrogenic Metabolites of Bisphenol A and Bisphenol B Formed by Rat Liver S9 Fraction: Their Structures and Estrogenic Potency. Toxicol. Sci. 2004, 78, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ying, G.G.; Zhao, J.L.; Chen, Z.F.; Lai, H.J.; Su, H.C. 4-Nonylphenol, Bisphenol-A and Triclosan Levels in Human Urine of Children and Students in China, and the Effects of Drinking These Bottled Materials on the Levels. Environ. Int. 2013, 52, 81–86. [Google Scholar] [CrossRef]
- Montévil, M.; Acevedo, N.; Schaeberle, C.M.; Bharadwaj, M.; Fenton, S.E.; Soto, A.M. A Combined Morphometric and Statistical Approach to Assess Nonmonotonicity in the Developing Mammary Gland of Rats in the Clarity-BPA Study. Environ. Health Perspect. 2020, 128, 057001. [Google Scholar] [CrossRef]
- Acevedo, N.; Davis, B.; Schaeberle, C.M.; Sonnenschein, C.; Soto, A.M. Perinatally Administered Bisphenol A as a Potential Mammary Gland Carcinogen in Rats. Environ. Health Perspect. 2013, 121, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Czarny-Krzymińska, K.; Krawczyk, B.; Szczukocki, D. Toxicity of Bisphenol A and Its Structural Congeners to Microalgae Chlorella vulgaris and Desmodesmus armatus. J. Appl. Phycol. 2022, 34, 1397–1410. [Google Scholar] [CrossRef]
- Fukazawa, H.; Hoshino, K.; Shiozawa, T.; Matsushita, H.; Terao, Y. Identification and Quantification of Chlorinated Bisphenol A in Wastewater from Wastepaper Recycling Plants. Chemosphere 2001, 44, 973–979. [Google Scholar] [CrossRef]
- Huang, Y.Q.; Wong, C.K.C.; Zheng, J.S.; Bouwman, H.; Barra, R.; Wahlström, B.; Neretin, L.; Wong, M.H. Bisphenol A (BPA) in China: A Review of Sources, Environmental Levels, and Potential Human Health Impacts. Environ. Int. 2012, 42, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.B.; Peart, T.E. Determination of Bisphenol A in Sewage Effluent and Sludge by Solid-Phase and Supercritical Fluid Extraction and Gas Chromatography/Mass Spectrometry. J. AOAC Int. 2000, 83, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Jahanshahi, S.; Badiefar, L.; Khodabandeh, M.; Heidarnia, M.A.; Yakhchali, B. Bioremediation of a Salty Petrochemical Wastewater Containing Bisphenol A by a Novel Indigenous Pseudomonas pseudoalcaligenes. RSC Adv. 2022, 13, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Takdastan, A.; Kakavandi, B.; Azizi, M.; Golshan, M. Efficient Activation of Peroxymonosulfate by Using Ferroferric Oxide Supported on Carbon/UV/US System: A New Approach into Catalytic Degradation of Bisphenol A. Chem. Eng. J. 2018, 331, 729–743. [Google Scholar] [CrossRef]
- Xu, L.; Wei, X.J.; Shang, Y.B.; Qi, Y.T.; Shi, J.; Li, K.Q.; Jin, X.; Bai, X.; Shi, X.; Jin, P.K. Ti3+ Self-Doped TiO2 Anchored with Iron Oxide Quantum Dots as an Efficient Persulfate Catalyst for the Enhanced Removal of Typical Phenolic Pollutants from Water under Visible Light. Sep. Purif. Technol. 2023, 322, 124303. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, H.; Oh, H.; Haider, Z.; Choi, J.; Shin, Y.U.; Kim, H.I.; Lee, J. Revisiting the Oxidizing Capacity of the Periodate-H Mixture: Identification of the Primary Oxidants and Their Formation Mechanisms. Environ. Sci. Technol. 2022, 56, 5763–5774. [Google Scholar] [CrossRef] [PubMed]
- Jun, B.-M.; Nam, S.-N.; Jung, B.; Choi, J.S.; Park, C.M.; Choong, C.E.; Jang, M.; Jho, E.H.; Son, A.; Yoon, Y. Photocatalytic and Electrocatalytic Degradation of Bisphenol A in the Presence of Graphene/Graphene Oxide-Based Nanocatalysts: A Review. Chemosphere 2024, 356, 141941. [Google Scholar] [CrossRef]
- Sun, S.Y.; Ren, Y.; Guo, F.S.; Zhou, Y.Y.; Cui, M.C.; Ma, J.J.; Han, Z.C.; Khim, J. Comparison of Effects of Multiple Oxidants with an Ultrasonic System under Unified System Conditions for Bisphenol A Degradation. Comput. Phys. Commun. 2023, 329, 138526. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.L.; Zhao, L.; Zhu, M.F.; Deng, C.; Liu, H.B.; Ma, J.; Li, Y. Effect of Cavitation Hydrodynamic Parameters on Bisphenol A Removal. Environ. Eng. Sci. 2019, 36, 873–882. [Google Scholar] [CrossRef]
- Sun, X.; Liu, J.; Ji, L.; Wang, G.; Zhao, S.; Yoon, J.Y.; Chen, S. A Review on Hydrodynamic Cavitation Disinfection: The Current State of Knowledge. Sci. Total Environ. 2020, 737, 139606. [Google Scholar] [CrossRef]
- Moholkar, V.S.; Senthil Kumar, P.; Pandit, A.B. Hydrodynamic Cavitation for Sonochemical Effects. Ultrason. Sonochemistry 1999, 6, 53–65. [Google Scholar] [CrossRef]
- Zampeta, C.; Bertaki, K.; Triantaphyllidou, I.-E.; Frontistis, Z.; Koutsoukos, P.G.; Vayenas, D.V. Pilot-Scale Hybrid System Combining Hydrodynamic Cavitation and Sedimentation for the Decolorization of Industrial Inks and Printing ink Wastewater. J. Environ. Manag. 2022, 302, 114108. [Google Scholar] [CrossRef]
- Agarkoti, C.; Gujar, S.K.; Gogate, P.R.; Pandit, A.B. Pilot Scale Degradation of Sulfamerazine Using Different Venturi Based Hydrodynamic Cavitation and Ultrasound Reactors in Combination with Oxidation Processes. J. Environ. Chem. Eng. 2023, 11, 109857. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. Pubchem Compound Summary for CID 6623, Bisphenol A. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Bisphenol-A (accessed on 7 August 2024).
- Choi, J.; Cui, M.; Lee, Y.; Kim, J.; Son, Y.; Khim, J. Hydrodynamic Cavitation and Activated Persulfate Oxidation for Degradation of Bisphenol A: Kinetics and Mechanism. Chem. Eng. J. 2018, 338, 323–332. [Google Scholar] [CrossRef]
- Gagol, M.; Przyjazny, A.; Boczkaj, G. Wastewater Treatment by Means of Advanced Oxidation Processes Based on Cavitation—A Review. Chem. Eng. J. 2018, 338, 599–627. [Google Scholar] [CrossRef]
- Choi, J.; Cui, M.; Lee, Y.; Ma, J.; Kim, J.; Son, Y.; Khim, J. Hybrid Reactor Based on Hydrodynamic Cavitation, Ozonation, and Persulfate Oxidation for Oxalic Acid Decomposition during Rare-Earth Extraction Processes. Ultrason. Sonochemistry 2019, 52, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Ioan, I.; Wilson, S.; Lundanes, E.; Neculai, A. Comparison of Fenton and Sono-Fenton Bisphenol A Degradation. J. Hazard. Mater. 2007, 142, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced Oxidation Processes for Organic Contaminant Destruction Based on the Fenton Reaction and Related Chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Neyens, E.; Baeyens, J. A Review of Classic Fenton’s Peroxidation as an Advanced Oxidation Technique. J. Hazard. Mater. 2003, 98, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, V.; Bingham, J.P.; Kan, E. Heterogeneous Fenton Degradation of Bisphenol A by Carbon Nanotube-Supported Fe3O4. Sep. Purif. Technol. 2014, 133, 388–395. [Google Scholar] [CrossRef]
- Chadi, N.E.; Merouani, S.; Hamdaoui, O.; Bouhelassa, M.; Ashokkumar, M. H2O2/Periodate (IO4−): A Novel Advanced Oxidation Technology for the Degradation of Refractory Organic Pollutants. Environ. Sci. Water Res. Technol. 2019, 5, 1113–1123. [Google Scholar] [CrossRef]
- Roy, K.; Moholkar, V.S. Carbamazepine Degradation Using Ternary Hybrid Advanced Oxidation Process of Hydrodynamic Cavitation + Photocatalysis (UV/ZnO/ZnFe2O4) + persulfate: Kinetic Investigations. J. Water Process Eng. 2024, 58, 104874. [Google Scholar] [CrossRef]
- Askarniya, Z.; Sadeghi, M.-T.; Baradaran, S. Decolorization of Congo Red via Hydrodynamic Cavitation in Combination with Fenton’s Reagent. Chem. Eng. Process. Process Intensif. 2020, 150, 107874. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Chen, M.-J.; Huang, C.-P.; Kuo, J.; Lo, S.-L. Efficient Sonochemical Degradation of Perfluorooctanoic Acid Using Periodate. Ultrason. Sonochemistry 2016, 31, 499–505. [Google Scholar] [CrossRef]
- Chinthala, M.; Ashwathanarayanaiah, B.K.; Kulkarni, S.; Udayakishore, Y.; Halyal, A.; Chavan, A. Intensification of Advanced Oxidation Processes (AOPs) for the Degradation of Bisphenol-A. Int. J. Chem. React. Eng. 2021, 19, 605–614. [Google Scholar] [CrossRef]
- Asadi, A.; Beigrezaee, S.; Daglioglu, N.; Guzel, E.Y.; Heydari, M.; Ravankhah, N. Combination of Hydrodynamic Cavitation with CoFe2O4 Photocatalyst for Activation of Peracetic Acid under UVC Irradiation in Synergistic Degradation of Bisphenol A. J. Water Process Eng. 2024, 64, 105608. [Google Scholar] [CrossRef]
- Gogate, P.R.; Patil, P.N. Combined Treatment Technology Based on Synergism between Hydrodynamic Cavitation and Advanced Oxidation Processes. Ultrason. Sonochemistry 2015, 25, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Grčić, I.; Vujević, D.; Koprivanac, N. Modeling the Mineralization and Discoloration in Colored Systems by (US)Fe2+/H2O2/S2O82− Processes: A Proposed Degradation Pathway. Chem. Eng. J. 2010, 157, 35–44. [Google Scholar] [CrossRef]
- Xiong, Y.H.; Zhou, T.; Bao, J.G.; Du, J.K.; Faheem, M.; Luo, L.T. Degradation Mechanism of Bisphenol S via Hydrogen Peroxide/Persulfate Activated by Sulfidated Nanoscale Zero Valent Iron. Environ. Sci. Pollut. Res. 2023, 30, 83545–83557. [Google Scholar] [CrossRef]
- Long, Y.; Feng, Y.; Li, X.; Suo, N.; Chen, H.; Wang, Z.; Yu, Y. Removal of Diclofenac by Three-Dimensional Electro-Fenton-persulfate (3D Electro-Fenton-PS). Chemosphere 2019, 219, 1024–1031. [Google Scholar] [CrossRef]
- Epold, I.; Trapido, M.; Dulova, N. Degradation of Levofloxacin in Aqueous Solutions by Fenton, Ferrous Ion-Activated Persulfate and Combined Fenton/Persulfate Systems. Chem. Eng. J. 2015, 279, 452–462. [Google Scholar] [CrossRef]
- Habib, M.; Ayaz, T.; Ali, M.; Zeeshan, M.; Sheng, X.; Fu, R.; Ullah, S.; Lyu, S. Innovative Strategy for the Effective Utilization of Coal Waste Slag in the Fenton-like Process for the Degradation of Trichloroethylene. J. Environ. Manag. 2024, 365, 121441. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.N.A.; Li, H.F.; Lin, J.M. Enhancement of Periodate-Hydrogen Peroxide Chemiluminescence by Nitrogen Doped Carbon Dots and Its Application for the Determination of Pyrogallol and Gallic Acid. Talanta 2016, 153, 23–30. [Google Scholar] [CrossRef]
- Kumagai, S.; Ono, S.; Yokoyama, S.; Kameda, T.; Yoshioka, T. Fate of Bisphenol A Pyrolysates at Low Pyrolytic Temperatures. J. Anal. Appl. Pyrolysis 2017, 125, 193–200. [Google Scholar] [CrossRef]
- Torres, R.A.; Abdelmalek, F.; Combet, E.; Pétrier, C.; Pulgarin, C. A Comparative Study of Ultrasonic Cavitation and Fenton’s Reagent for Bisphenol A Degradation in Deionised and Natural Waters. J. Hazard. Mater. 2007, 146, 546–551. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.C.C.; Teodoro, J.A.R.; Afonso, R.; Aquino, S.F.; Augusti, R. Photodegradation of Bisphenol A in Aqueous Medium: Monitoring and Identification of by-Products by Liquid Chromatography Coupled to High-Resolution Mass Spectrometry. Rapid Commun. Mass Spectrom. 2014, 28, 987–994. [Google Scholar] [CrossRef]
- Olmez-Hanci, T.; Arslan-Alaton, I.; Genc, B. Bisphenol A Treatment by the Hot Persulfate Process: Oxidation Products and Acute Toxicity. J. Hazard. Mater. 2013, 263, 283–290. [Google Scholar] [CrossRef]
- Cheng, J.; Vecitis, C.D.; Park, H.; Mader, B.T.; Hoffmann, M.R. Sonochemical Degradation of Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoate (PFOA) in Landfill Groundwater: Environmental Matrix Effects. Environ. Sci. Technol. 2008, 42, 8057–8063. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, A.; Baradaran, S.; Movahedirad, S. Synergistic Degradation of Congo Red by Hybrid Advanced Oxidation via Ultraviolet Light, Persulfate, and Hydrodynamic Cavitation. Ecotoxicol. Environ. Saf. 2024, 272, 116042. [Google Scholar] [CrossRef] [PubMed]
- Ferkous, H.; Hamdaoui, O.; Merouani, S. Sonochemical Degradation of Naphthol Blue Black in Water: Effect of Operating Parameters. Ultrason. Sonochemistry 2015, 26, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Korpe, S.; Rao, P.V. Application of Advanced Oxidation Processes and Cavitation Techniques for Treatment of Tannery Wastewater—A Review. J. Environ. Chem. Eng. 2021, 9, 105234. [Google Scholar] [CrossRef]
- Frontistis, Z.; Hapeshi, E.; Fatta-Kassinos, D.; Mantzavinos, D. Ultraviolet-Activated Persulfate Oxidation of Methyl Orange: A Comparison between Artificial Neural Networks and Factorial Design for Process Modelling. Photochem. Photobiol. Sci. 2015, 14, 528–535. [Google Scholar] [CrossRef]
- Reddy, B.S.; Maurya, A.K.; Narayana, P.L.; Pasha, S.K.K.; Reddy, M.R.; Hatshan, M.R.; Darwish, N.M.; Kori, S.A.; Cho, K.-K.; Reddy, N.S. Knowledge Extraction of Sonophotocatalytic Treatment for Acid Blue 113 Dye Removal by Artificial Neural Networks. Environ. Res. 2022, 204, 112359. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.B.; Lo, C.K.Y.; Kan, C.W. Application of Artificial Intelligence Techniques in Textile Wastewater Decolorisation Fields: A Systematic and Citation Network Analysis Review. Color. Technol. 2022, 138, 117–136. [Google Scholar] [CrossRef]
- Jiang, J.; Xiang, X.; Zhou, Q.; Zhou, L.; Bi, X.; Khanal, S.K.; Wang, Z.; Chen, G.; Guo, G. Optimization of a Novel Engineered Ecosystem Integrating Carbon, Nitrogen, Phosphorus, and Sulfur Biotransformation for Saline Wastewater Treatment Using an Interpretable Machine Learning Approach. Environ. Sci. Technol. 2024, 58, 12989–12999. [Google Scholar] [CrossRef] [PubMed]






| Type of HCR | Initial BPA Concentration | Temperature | Inlet Pressure | Reaction Rate Constant | Conversion | Bibliography |
|---|---|---|---|---|---|---|
| Venturi | 0.044 mM | 50 °C | 0.5 MPa | 1.0 × 10−3 | 15.80% | [16] |
| Orifice plate | 0.066 mM | 35 °C | 0.3 MPa | 1.8 × 10−3 | 15.50% | [22] |
| Self-excited pulsed cavitation | 0.044 mM | 25 °C | 0.5 MPa | 3.3 × 10−3 | 18.64% | This study |
| Name of Substance | Acute Toxicity (mg/L) | Bioaccumulation Factor | Developmental Toxicity | Mutagenicity |
|---|---|---|---|---|
| Bisphenol A | 3.24 | 117.27 | 0.71 | 0.09 |
| Phenol | 38.69 | 7.61 | 0.58 | 0.25 |
| 4-(Prop-1-en-2) phenol | 7.67 | 47.57 | 0.57 | 0.37 |
| Monohydroxylated Bisphenol A | 2.32 | 82.39 | 0.65 | 0.07 |
| Dihydroxylated Bisphenol A | 1.84 | 20.36 | 0.63 | 0.33 |
| 4-isopropenylphenol | 35.51 | 4.43 | 0.43 | 0.11 |
| 4-hydroxyacetophenone | 7.67 | 47.57 | 0.57 | 0.37 |
| Parameters of Lake Water | Cl− | SO42− | Ca2+ | Mg2+ | COD | TOC |
| Concentration (mg·L−1) | 38.9 ± 0.99 | 63.4 ± 1.12 | 40.8 ± 0.82 | 7.6 ± 0.15 | 37.0 ± 5.21 | 7.35 ± 1.36 |
| Parameters of Tap Water | Fe2+ | Cu2+ | TDS | pH | Residual Chlorine | |
| Concentration (mg·L−1) | 0.025 ± 0.001 | 0.039 ± 0.001 | 186 ± 3 | 7.53 ± 0.10 | 0.49 ± 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Li, B.; Xie, S.; Ji, B. Degradation of Bisphenol A Using Self-Excited Oscillating Jets in Synergy with Fenton and Periodate Oxidation: Experimental and Artificial Neural Network Modeling Study. Water 2024, 16, 2326. https://doi.org/10.3390/w16162326
Wang J, Li B, Xie S, Ji B. Degradation of Bisphenol A Using Self-Excited Oscillating Jets in Synergy with Fenton and Periodate Oxidation: Experimental and Artificial Neural Network Modeling Study. Water. 2024; 16(16):2326. https://doi.org/10.3390/w16162326
Chicago/Turabian StyleWang, Jian, Bingsheng Li, Shiwei Xie, and Bin Ji. 2024. "Degradation of Bisphenol A Using Self-Excited Oscillating Jets in Synergy with Fenton and Periodate Oxidation: Experimental and Artificial Neural Network Modeling Study" Water 16, no. 16: 2326. https://doi.org/10.3390/w16162326
APA StyleWang, J., Li, B., Xie, S., & Ji, B. (2024). Degradation of Bisphenol A Using Self-Excited Oscillating Jets in Synergy with Fenton and Periodate Oxidation: Experimental and Artificial Neural Network Modeling Study. Water, 16(16), 2326. https://doi.org/10.3390/w16162326







