Effect of Stocking Density on Growth Performance of Juvenile Gibel Carp (Carassius gibelio) and Economic Profit of Land-Based Recirculating Aquaculture System
Abstract
:1. Introduction
2. Materials and Methods
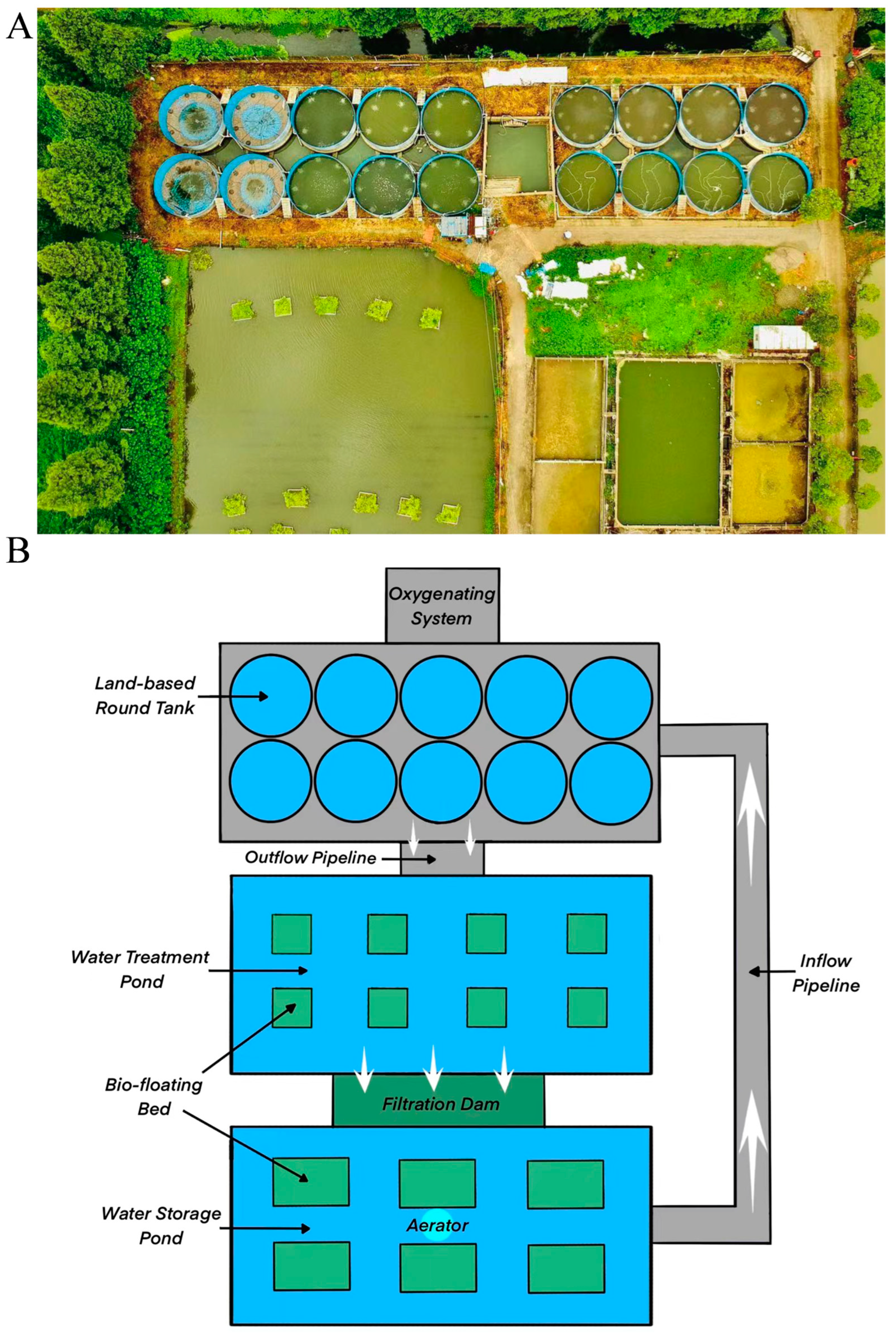
2.1. Aquaculture System
2.2. Experimental Design and Feeding Management
2.3. Sample Collection
2.4. Measurement Indicators and Methods
2.4.1. Growth Performance
2.4.2. Water Quality Parameters
2.4.3. Total RNA Extraction and Reverse Transcription
2.4.4. Real-Time Quantitative PCR (qPCR)
2.4.5. Microscopic Examination of Dorsal White Muscle Sections
2.5. Statistical Methods
3. Results
3.1. Growth Indicators
3.2. Water Quality Indicators
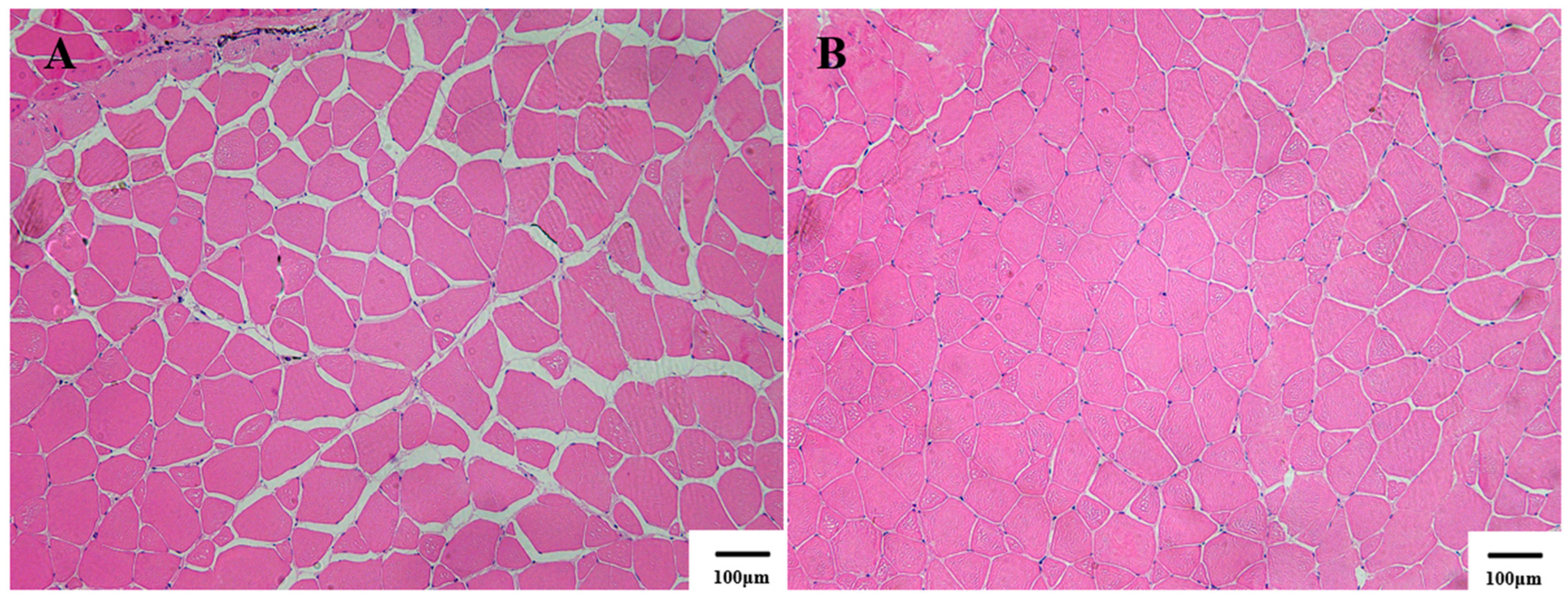
3.3. Muscle Tissue Histological Observations
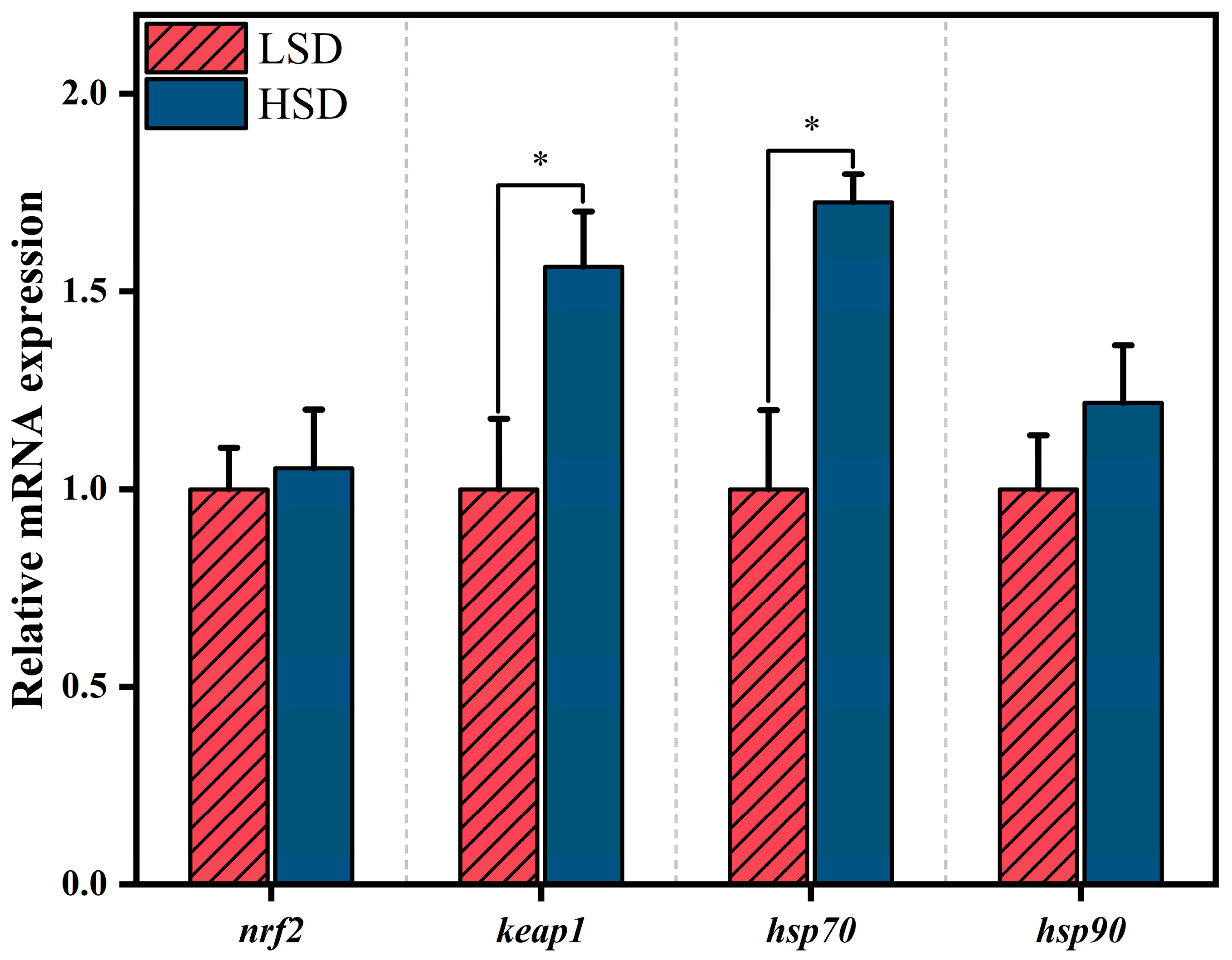
3.4. Expression of Muscle nrf2, keap1, and hsp
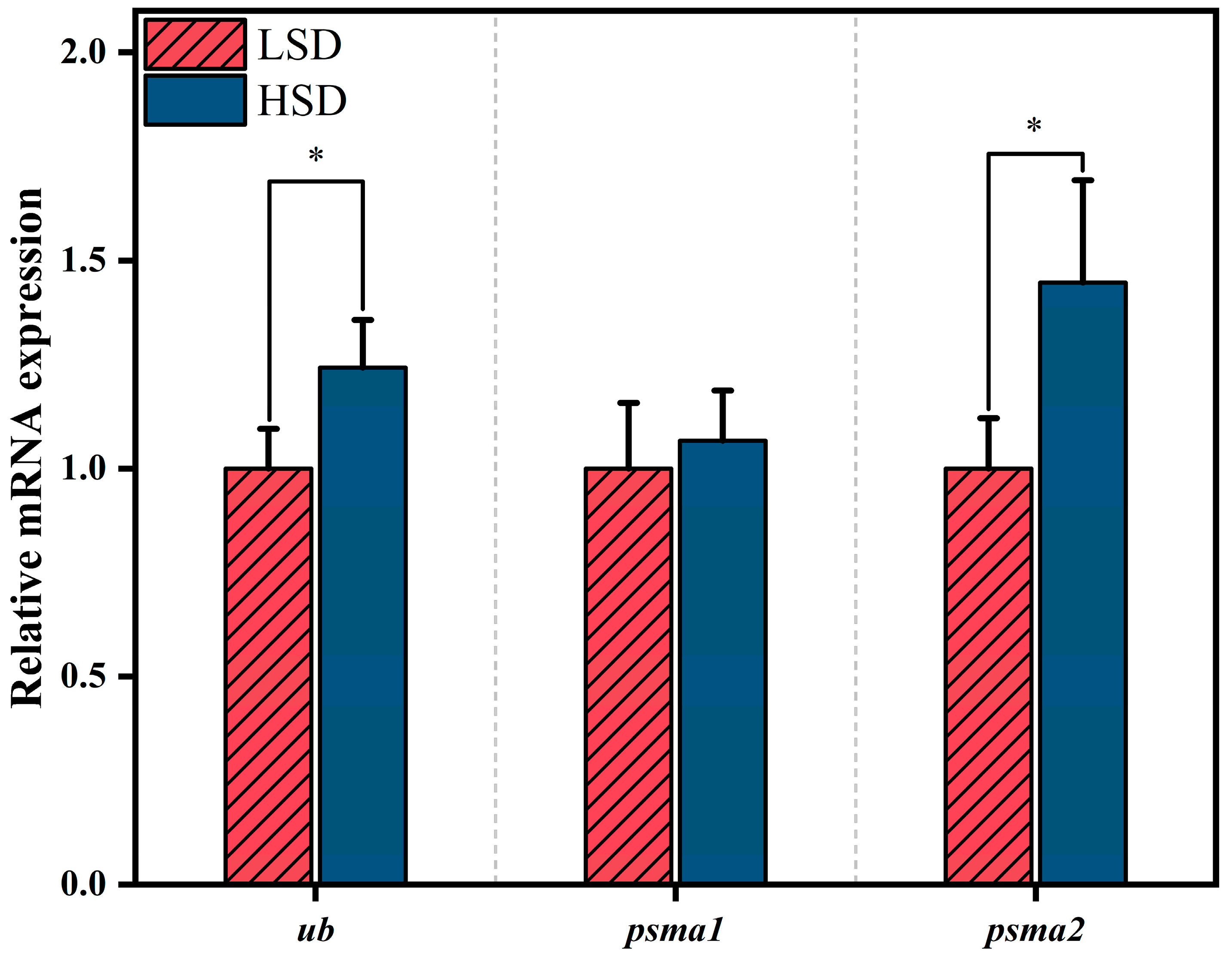
3.5. Ubiquitin–Proteasome System Activity
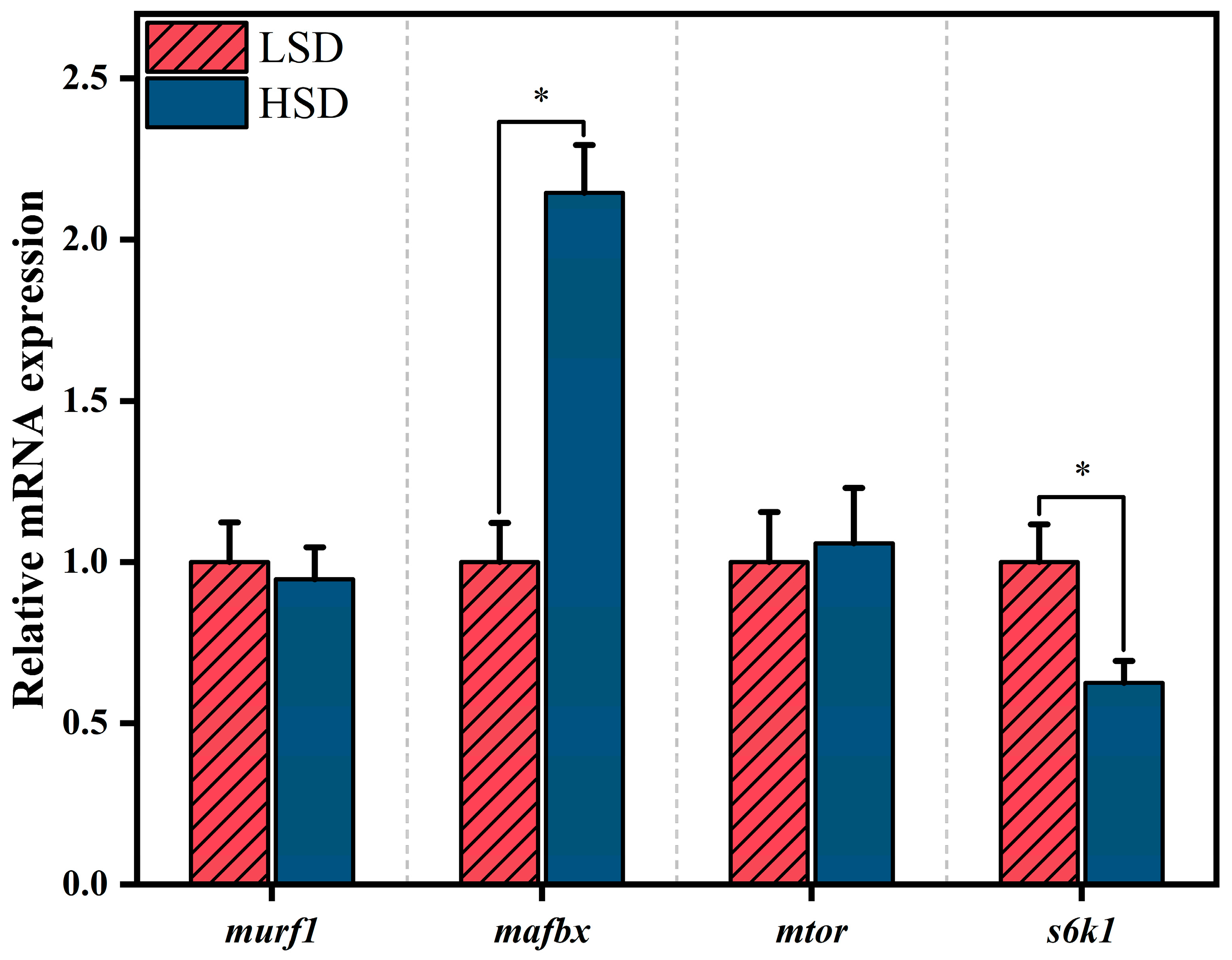
3.6. Expression of Muscle Protein Metabolism-Related Genes
3.7. Economic Profit
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. The State of World Fisheries and Aquaculture 2020: Sustainability in Action; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020. [Google Scholar]
- Béné, C.; Barange, M.; Subasinghe, R.; Pinstrup-Andersen, P.; Merino, G.; Hemre, G.-I.; Williams, M. Feeding 9 billion by 2050—Putting fish back on the menu. Food Secur. 2015, 7, 261–274. [Google Scholar] [CrossRef]
- Naylor, R.L.; Goldburg, R.J.; Primavera, J.H.; Kautsky, N.; Beveridge, M.C.; Clay, J.; Folke, C.; Lubchenco, J.; Mooney, H.; Troell, M. Effect of aquaculture on world fish supplies. Nature 2000, 405, 1017–1024. [Google Scholar] [CrossRef]
- Waite, R.; Beveridge, M.; Brummett, R.; Castine, S.; Chaiyawannakarn, N.; Kaushik, S.; Mungkung, R.; Nawapakpilai, S.; Phillips, M. Improving Productivity and Environmental Performance of Aquaculture; WorldFish: Penang, Malaysia, 2014. [Google Scholar]
- Boyd, C.E.; D’Abramo, L.R.; Glencross, B.D.; Huyben, D.C.; Juarez, L.M.; Lockwood, G.S.; McNevin, A.A.; Tacon, A.G.; Teletchea, F.; Tomasso, J.R., Jr. Achieving sustainable aquaculture: Historical and current perspectives and future needs and challenges. J. World Aquacult. Soc. 2020, 51, 578–633. [Google Scholar] [CrossRef]
- Dong, S.-L.; Dong, Y.-W. Sustainability of Aquaculture Production Systems. In Aquaculture Ecology; Springer: Berlin/Heidelberg, Germany, 2023; pp. 491–530. [Google Scholar]
- Hoseini, S.M.; Yousefi, M.; Hoseinifar, S.H.; Van Doan, H. Effects of dietary arginine supplementation on growth, biochemical, and immunological responses of common carp (Cyprinus carpio L.), stressed by stocking density. Aquaculture 2019, 503, 452–459. [Google Scholar] [CrossRef]
- Iguchi, K.i.; Ogawa, K.; Nagae, M.; Ito, F. The influence of rearing density on stress response and disease susceptibility of ayu (Plecoglossus altivelis). Aquaculture 2003, 220, 515–523. [Google Scholar] [CrossRef]
- Jiwyam, W. The effect of stocking density on yield, growth, and survival of Asian river catfish (Pangasius bocourti Sauvage, 1880) cultured in cages. Aquac. Int. 2011, 19, 987–997. [Google Scholar] [CrossRef]
- Liu, Q.; Hou, Z.; Wen, H.; Li, J.; He, F.; Wang, J.; Guan, B.; Wang, Q. Effect of stocking density on water quality and (growth, body composition and plasma cortisol content) performance of pen-reared rainbow trout (Oncorhynchus mykiss). J. Ocean Univ. China 2016, 15, 667–675. [Google Scholar] [CrossRef]
- Ashley, P.J. Fish welfare: Current issues in aquaculture. Appl. Anim. Behav. Sci. 2007, 104, 199–235. [Google Scholar] [CrossRef]
- Angel, D.; Jokumsen, A.; Lembo, G. Aquaculture production systems and environmental interactions. In Organic Aquaculture: Impacts and Future Developments; Springer: Berlin/Heidelberg, Germany, 2019; pp. 103–118. [Google Scholar]
- Domínguez-Godino, J.A.; González-Wangüemert, M. Does space matter? Optimizing stocking density of Holothuria arguinensis and Holothuria mammata. Aquac. Res. 2018, 49, 3107–3115. [Google Scholar] [CrossRef]
- Köprücü, K.; Yonar, S.M.; Köprücü, S.; Yonar, M. Effect of different stocking densities on growth, oxidative stress, antioxidant enzymes, and hematological and immunological values of European catfish (Silurus glanis L., 1758). Aquac. Int. 2024, 32, 2957–2973. [Google Scholar] [CrossRef]
- Jia, R.; Wang, L.; Hou, Y.; Feng, W.; Li, B.; Zhu, J. Effects of stocking density on the growth performance, physiological parameters, redox status and lipid metabolism of Micropterus salmoides in integrated rice–fish farming systems. Antioxidants 2022, 11, 1215. [Google Scholar] [CrossRef] [PubMed]
- Mugwanya, M.; Dawood, M.A.; Kimera, F.; Sewilam, H. A review on recirculating aquaculture system: Influence of stocking density on fish and crustacean behavior, growth performance, and immunity. Ann. Anim. Sci. 2022, 22, 873–884. [Google Scholar] [CrossRef]
- Saraiva, J.L.; Rachinas-Lopes, P.; Arechavala-Lopez, P. Finding the “golden stocking density”: A balance between fish welfare and farmers’ perspectives. Front. Vet. Sci. 2022, 9, 930221. [Google Scholar] [CrossRef]
- Ahmed, N.; Turchini, G.M. Recirculating aquaculture systems (RAS): Environmental solution and climate change adaptation. J. Clean. Prod. 2021, 297, 126604. [Google Scholar] [CrossRef]
- Yanong, R.P. Biosecurity in Aquaculture, Part 2: Recirculating Aquaculture Systems; Southern Regional Aquaculture Center: Stoneville, MS, USA, 2012. [Google Scholar]
- Pandey, A.; Pathan, M.A.; Ananthan, P.S.; Sudhagar, A.; Krishnani, K.K.; Sreedharan, K.; Kumar, P.; Thirunavukkarasar, R.; Harikrishna, V. Stocking for sustainable aqua-venture: Optimal growth, yield and economic analysis of Penaeus vannamei culture in inland saline water (ISW) of India. Environ. Dev. Sustain. 2024, 26, 6913–6942. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Nader, M.M.; Salem, H.M.; El-Tahan, A.M.; Soliman, S.M.; Khafaga, A.F. Effect of environmental factors on growth performance of Nile tilapia (Oreochromis niloticus). Int. J. Biometeorol. 2022, 66, 2183–2194. [Google Scholar] [CrossRef] [PubMed]
- Arame, H.; Adıte, A.; Adjıbade, N.; Imorou, R.S.; Sonon, P. Length-weight relationships and condition factors of Mochokidae (Pisces: Teleostei: Siluriformes) from Niger River, Northern Benin. Aquat. Res. 2020, 3, 72–84. [Google Scholar] [CrossRef]
- Rowlerson, A. Cellular mechanisms of post-embryonic muscle growth in aquaculture species. Muscle Growth and Development; Academic Press: Cambridge, MA, USA, 2001. [Google Scholar]
- Kawamura, G.; Bagarinao, T.; Yong, A.S.K.; Chen, C.Y.; Noor, S.N.M.; Lim, L.S. Low pH affects survival, growth, size distribution, and carapace quality of the postlarvae and early juveniles of the freshwater prawn Macrobrachium rosenbergii de Man. Ocean Sci. J. 2015, 50, 371–379. [Google Scholar] [CrossRef]
- Wu, X.; Li, D.; Lu, J.; Liu, L.; Yang, Q.; Tang, R.; Zhang, X.; Li, L. Adaptation strategies of juvenile grass carp (Ctenopharyngodon idella) facing different dissolved oxygen concentrations in a recirculating aquaculture system. Water Biol. Secur. 2023, 2, 100202. [Google Scholar] [CrossRef]
- Takeuchi, T. Overview of Land-Based Recirculating Aquaculture. In Application of Recirculating Aquaculture Systems in Japan; Springer: Berlin/Heidelberg, Germany, 2017; pp. 3–18. [Google Scholar]
- Lu, J.; Li, S.; He, X.; Tang, R.; Li, D. An in-pond tank culture system for high-intensive fish production: Effect of stocking density on growth of grass carp (Ctenopharyngodon idella Valenciennes, 1844) and blunt snout bream (Megalobrama amblycephala Yih, 1955). Aquaculture 2022, 549, 737808. [Google Scholar] [CrossRef]
- Basu, N.; Todgham, A.; Ackerman, P.; Bibeau, M.; Nakano, K.; Schulte, P.; Iwama, G.K. Heat shock protein genes and their functional significance in fish. Gene 2002, 295, 173–183. [Google Scholar] [CrossRef]
- Iwama, G.K.; Afonso, L.O.; Todgham, A.; Ackerman, P.; Nakano, K. Are hsps suitable for indicating stressed states in fish? J. Exp. Biol. 2004, 207, 15–19. [Google Scholar] [CrossRef]
- Liu, B.; Fei, F.; Li, X.; Wang, X.; Huang, B. Effects of stocking density on stress response, innate immune parameters, and welfare of turbot (Scophthalmus maximus). Aquac. Int. 2019, 27, 1599–1612. [Google Scholar] [CrossRef]
- Yarahmadi, P.; Miandare, H.K.; Fayaz, S.; Caipang, C.M.A. Increased stocking density causes changes in expression of selected stress-and immune-related genes, humoral innate immune parameters and stress responses of rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2016, 48, 43–53. [Google Scholar] [CrossRef]
- Roberts, R.; Agius, C.; Saliba, C.; Bossier, P.; Sung, Y. Heat shock proteins (chaperones) in fish and shellfish and their potential role in relation to fish health: A review. J. Fish Dis. 2010, 33, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; McMahon, M.; Chowdhry, S.; Dinkova-Kostova, A.T. Cancer chemoprevention mechanisms mediated through the Keap1–Nrf2 pathway. Antioxid. Redox Signal. 2010, 13, 1713–1748. [Google Scholar] [CrossRef]
- Zeng, L.; Ai, C.-X.; Wang, Y.-H.; Zhang, J.-S.; Wu, C.-W. Abrupt salinity stress induces oxidative stress via the Nrf2-Keap1 signaling pathway in large yellow croaker Pseudosciaena crocea. Fish Physiol. Biochem. 2017, 43, 955–964. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, Z.; Zhang, L.; Liu, W.; Li, H.; Li, X. Phosphatidylserine Counteracts the High Stocking Density-Induced Stress Response, Redox Imbalance and Immunosuppression in Fish Megalobrama ambylsephala. Antioxidants 2024, 13, 644. [Google Scholar] [CrossRef]
- Fanzani, A.; Conraads, V.M.; Penna, F.; Martinet, W. Molecular and cellular mechanisms of skeletal muscle atrophy: An update. J. Cachexia Sarcopenia Muscle 2012, 3, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Bonura, A.; Giacomarra, M.; Montana, G. The Keap1 signaling in the regulation of HSP90 pathway. Cell Stress Chaperones 2022, 27, 197–204. [Google Scholar] [CrossRef]
- Lee, S.; Hu, L. Nrf2 activation through the inhibition of Keap1–Nrf2 protein–protein interaction. Med. Chem. Res. 2020, 29, 846–867. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wei, S.; Ni, S.; Huang, Y.; Qin, Q. Ubiquitin–proteasome system is required for efficient replication of singapore grouper iridovirus. Front. Microbiol. 2018, 9, 2798. [Google Scholar] [CrossRef] [PubMed]
- Todgham, A.E.; Crombie, T.A.; Hofmann, G.E. The effect of temperature adaptation on the ubiquitin–proteasome pathway in notothenioid fishes. J. Exp. Biol. 2017, 220, 369–378. [Google Scholar] [CrossRef]
- Sandri, M.; Sandri, C.; Gilbert, A.; Skurk, C.; Calabria, E.; Picard, A.; Walsh, K.; Schiaffino, S.; Lecker, S.H.; Goldberg, A.L. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 2004, 117, 399–412. [Google Scholar] [CrossRef]
- Edinger, A.L.; Thompson, C.B. Akt maintains cell size and survival by increasing mTOR-dependent nutrient uptake. Mol. Biol. Cell 2002, 13, 2276–2288. [Google Scholar] [CrossRef]
- Gulati, P.; Gaspers, L.D.; Dann, S.G.; Joaquin, M.; Nobukuni, T.; Natt, F.; Kozma, S.C.; Thomas, A.P.; Thomas, G. Amino acids activate mTOR complex 1 via Ca2+/CaM signaling to hVps34. Cell Metab. 2008, 7, 456–465. [Google Scholar] [CrossRef]
- Pradeepkiran, J.A. Aquaculture role in global food security with nutritional value: A review. Transl. Anim. Sci. 2019, 3, 903–910. [Google Scholar] [CrossRef]


| Gene Name | Primer Sequences | Product Size |
|---|---|---|
| hsp70 | F:GTCCATCCTGACCATTGAA R:CCTTCTTGTGCTTCCTCTT | 195 bp |
| hsp90 | F:CAACGATGACGAGCAGTA R:GGTGAAGAATGACTCTGGTT | 138 bp |
| nrf2 | F:GTCCACAGTAAATGGGATCTGC R:ACCACCCTTCACCAAAGACA | 100 bp |
| keap1 | F:ACGCCCAGAGCAAAGACTAC R:CAGAGACTGCCGGAAGTATCC | 137 bp |
| ub | F:GCCAAGCGACACCATTGAG R:GGATGTTGTAGTCGGACAG | 156 bp |
| psma2 | F:CAGGCCAGCTTGTTCAGAGA R:TCCCAGCCAGCAATCAGAAG | 100 bp |
| psmc1 | F:CGGCTGCTCTGTGTTACTGA R:ACTCTGGATGTGTGAGGGGA | 191 bp |
| mafbx | F:GGACGAGATCTGGTTAGCC R:CTTGCGGATCTGTCTAGCT | 135 bp |
| murf1 | F:TGTCTATGGACTACAGAGGAA R:GGATTTCAAAGGAGGTTCAAG | 103 bp |
| mtor | F:CGTTCTACTGCTGGGTGTGGAT R:TGCTGGTCTTCTGCTGCGATA | 125 bp |
| s6k1 | F:GCGAAATCCGAGCCCTTAC R:GCAATAGTGAACAGCGTTATGT | 162 bp |
| β-actin | F:CATTGACTCAGGATGCGGAAACT R:CTGTGAGGGCAGAGTGGTAGACG | 144 bp |
| Parameter | LSD | HSD |
|---|---|---|
| IW (g) | 1.15 ± 0.06 | 1.15 ± 0.01 |
| IL (cm) | 3.51 ± 0.04 | 3.51 ± 0.01 |
| FW (g) | 29.68 ± 0.88 * | 15.51 ± 0.62 |
| FL (cm) | 9.63 ± 0.08 * | 7.86 ± 0.09 |
| WGR (%) | 2496.34 ± 9.19 * | 1249.9 ± 6.97 |
| SGR (%/d) | 2.03 ± 0.04 * | 1.63 ± 0.03 |
| FCR | 1.13 ± 0.02 * | 1.73 ± 0.07 |
| CF (%) | 3.32 ± 0.04 * | 3.19 ± 0.02 |
| SR (%) | 96.02 ± 0.62 | 93.34 ± 1.1 |
| VSI (%) | 14.96 ± 0.39 | 15.41 ± 0.28 |
| HSI (%) | 8.6 ± 0.33 | 8.24 ± 0.14 |
| Parameters | LSD | HSD |
|---|---|---|
| Mean size of fry (g) | 1.15 ± 0.06 | 1.15 ± 0.01 |
| Total fry weight (kg) | 8.4 ± 0.3 * | 14 ± 0.28 |
| Mean harvest weight (g) | 29.68 ± 0.88 * | 15.51 ± 0.62 |
| Total harvest weight (kg) | 156.25 ± 2.51 * | 163.2 ± 2.77 |
| Survival rate (%) | 96.02 ± 0.62 | 93.34 ± 1.1 |
| Weight gain ratio (%) | 2496.34 ± 9.19 * | 1249.9 ± 6.97 |
| Fish seed cost (USD) | 92.33 ± 3.3 * | 154.55 ± 6.62 |
| Expenses on feed (USD) | 104.16 * | 128.77 |
| Veterinary cost (USD) | 35.00 * | 58.33 |
| Electricity cost (USD) | 151.20 | 151.20 |
| Labor cost (USD) | 373.33 | 373.33 |
| Total investment (USD) | 756.02 ± 1.77 * | 866.18 ± 6.62 |
| Gross income (USD) | 875.00 ± 9.93 * | 913.92 ± 18.29 |
| Net income (USD) | 120.85 ± 6.70 * | 49.79 ± 2.39 |
| Return on investment (%) | 15.98 ± 0.82 * | 5.75 ± 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Liu, J.; Gu, X.; Li, L.; Yu, L.; Tang, R.; Wang, C.; Li, L.; Li, D. Effect of Stocking Density on Growth Performance of Juvenile Gibel Carp (Carassius gibelio) and Economic Profit of Land-Based Recirculating Aquaculture System. Water 2024, 16, 2367. https://doi.org/10.3390/w16172367
Li H, Liu J, Gu X, Li L, Yu L, Tang R, Wang C, Li L, Li D. Effect of Stocking Density on Growth Performance of Juvenile Gibel Carp (Carassius gibelio) and Economic Profit of Land-Based Recirculating Aquaculture System. Water. 2024; 16(17):2367. https://doi.org/10.3390/w16172367
Chicago/Turabian StyleLi, Huacheng, Jieya Liu, Xiao Gu, Luyi Li, Liqin Yu, Rong Tang, Chunfang Wang, Li Li, and Dapeng Li. 2024. "Effect of Stocking Density on Growth Performance of Juvenile Gibel Carp (Carassius gibelio) and Economic Profit of Land-Based Recirculating Aquaculture System" Water 16, no. 17: 2367. https://doi.org/10.3390/w16172367





