Enhancement in Sulfamethoxazole Degradation via Efficient Heterogeneous Activation of Peracetic Acid by FeS
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of FeS and PAA Solution
2.3. Experimental Procedure
2.4. Analytical Methods
2.5. Computational Methods
3. Results and Discussions
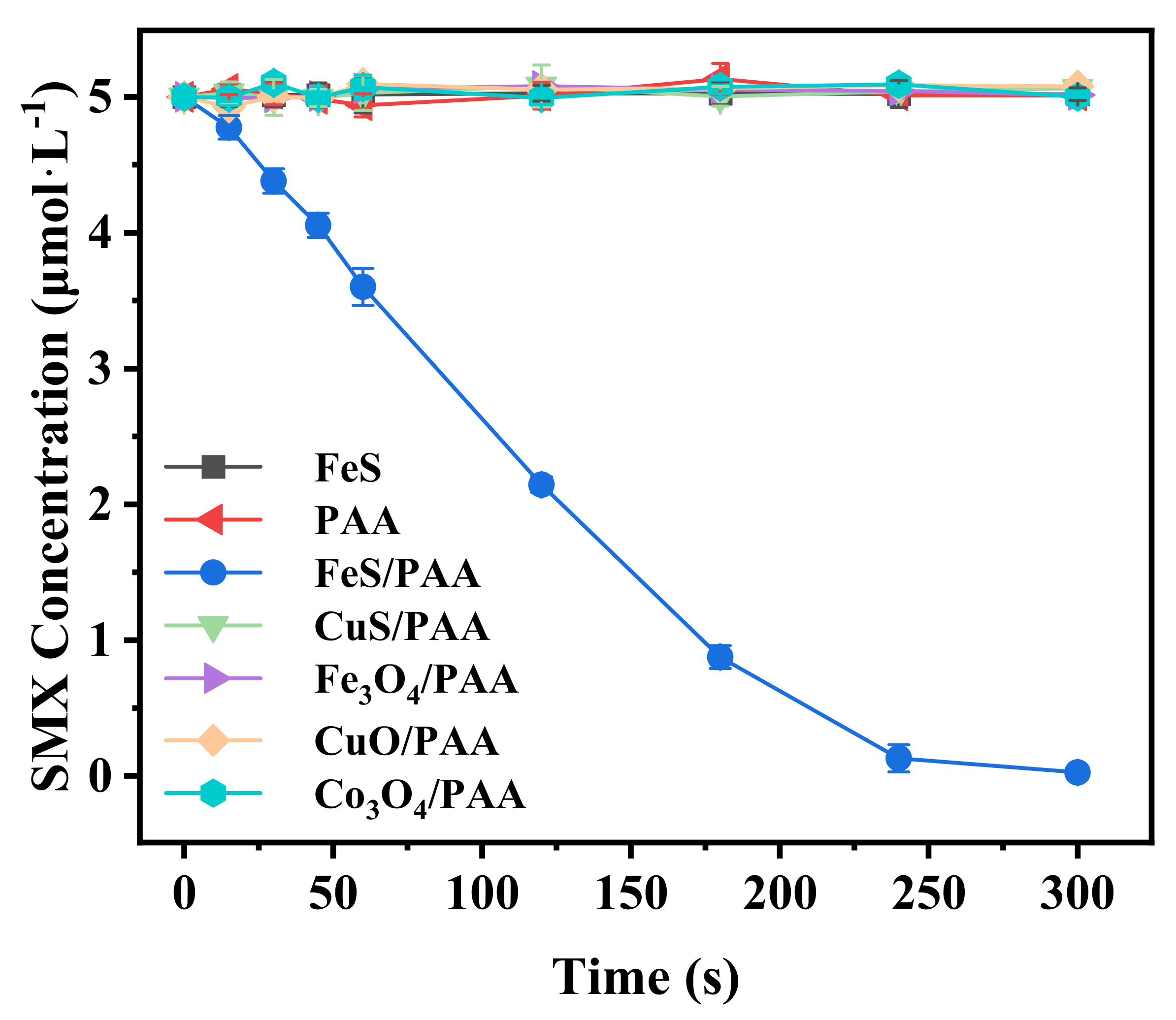
3.1. Activation of PAA by Various Activators for the Degradation of SMX
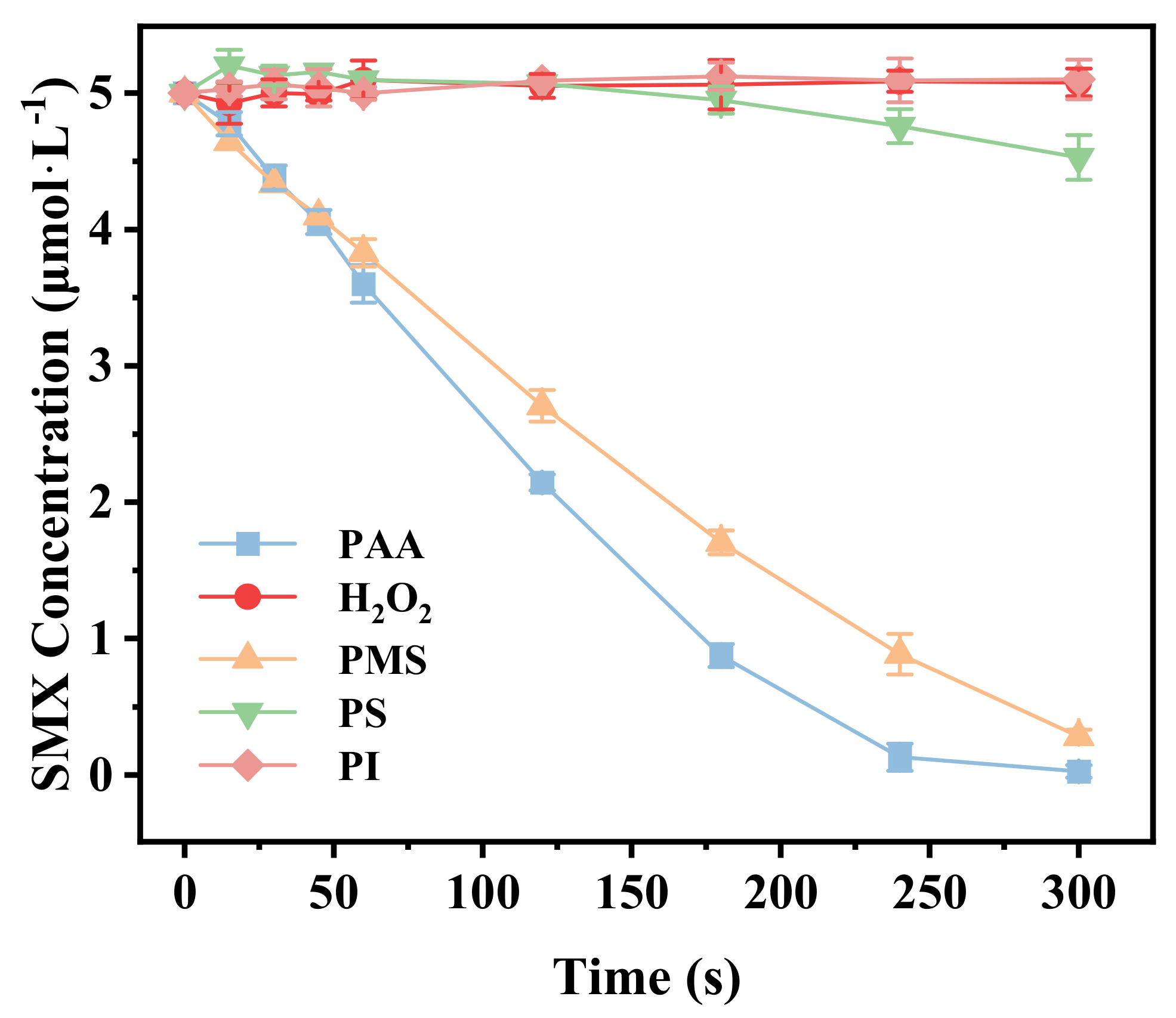
3.2. Activation of Various Oxidants by FeS for the Degradation of SMX
3.3. Effect of Reagent Dosage on SMX Degradation
3.4. Effect of Initial pH on SMX Degradation
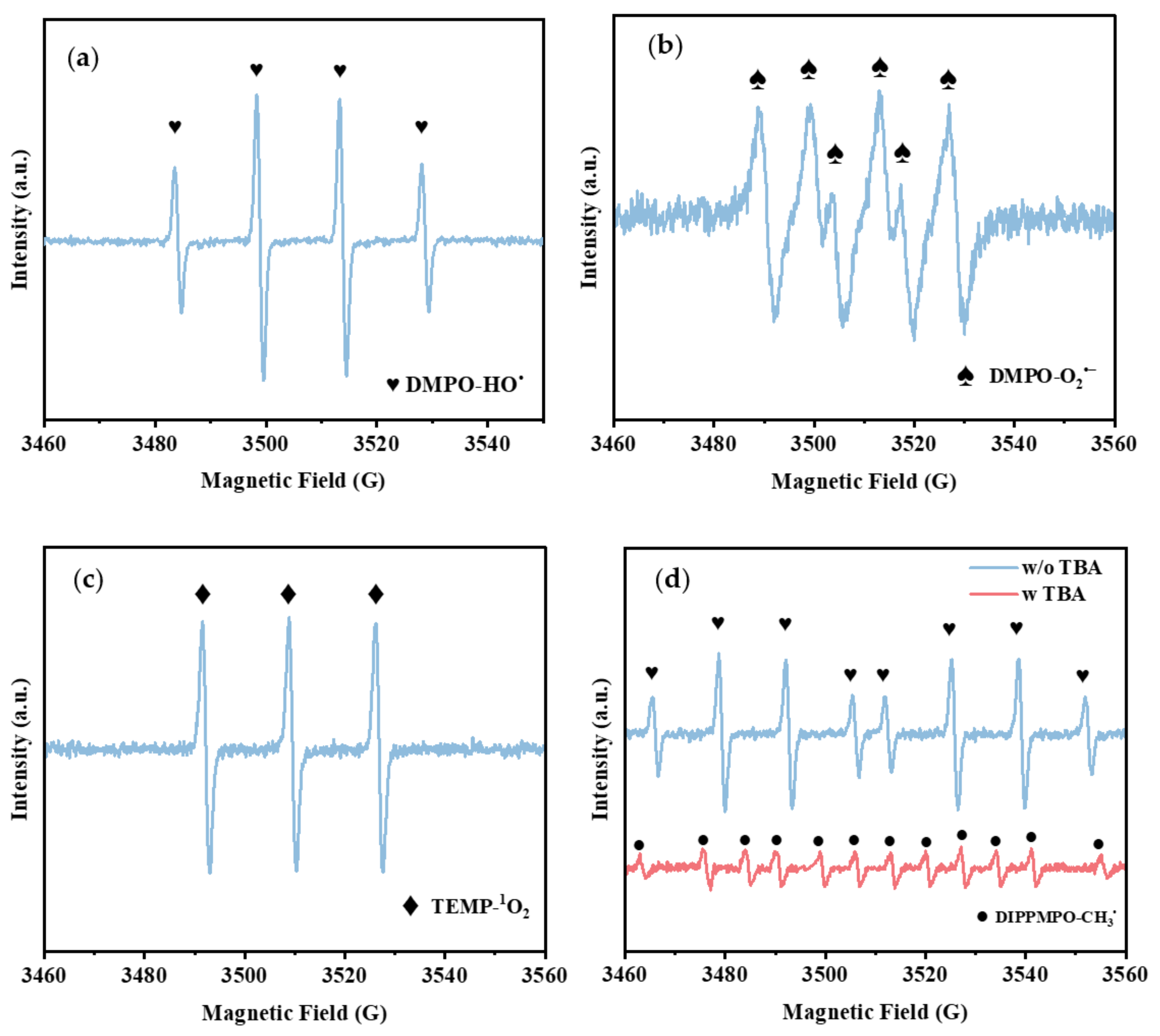
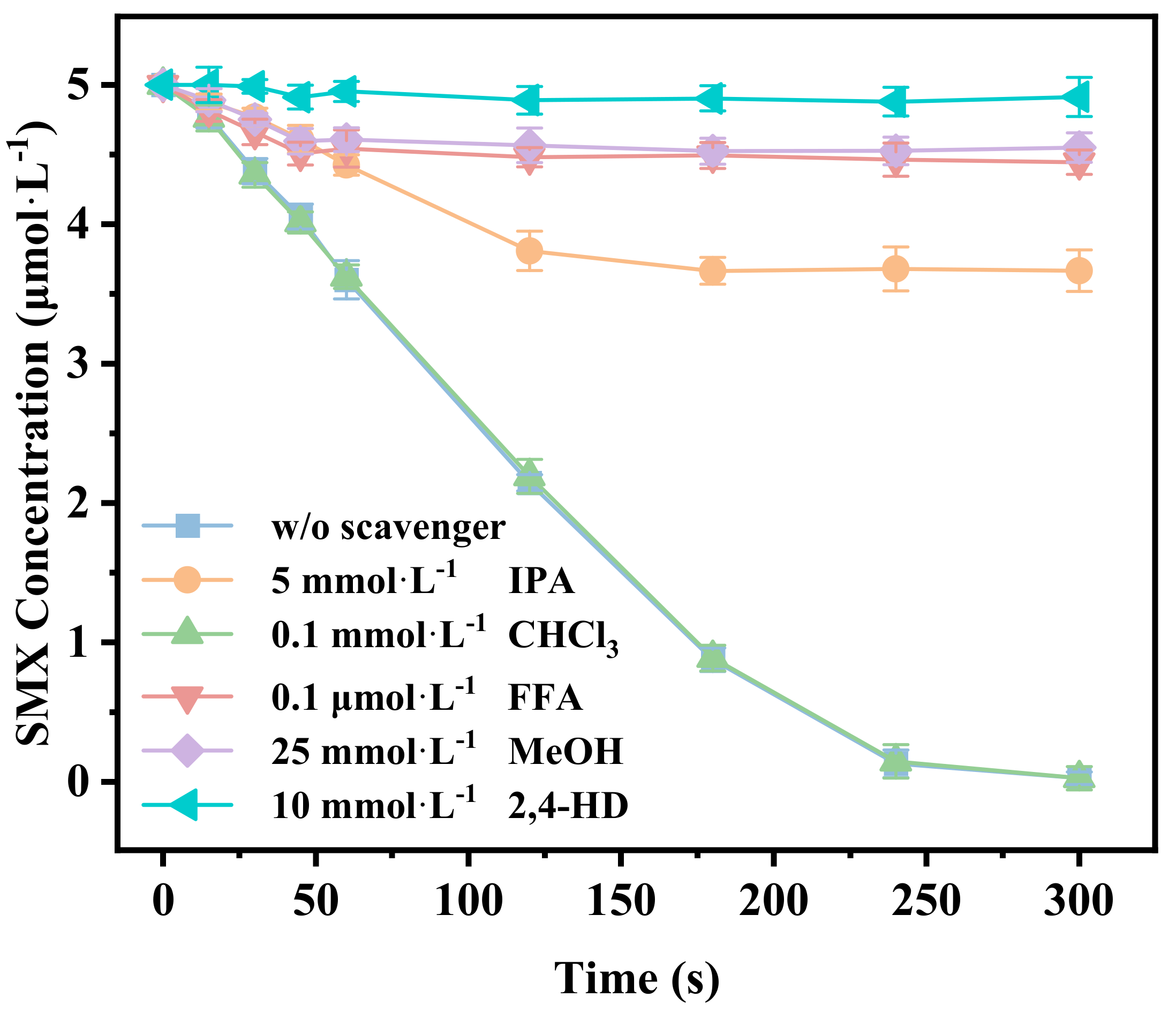
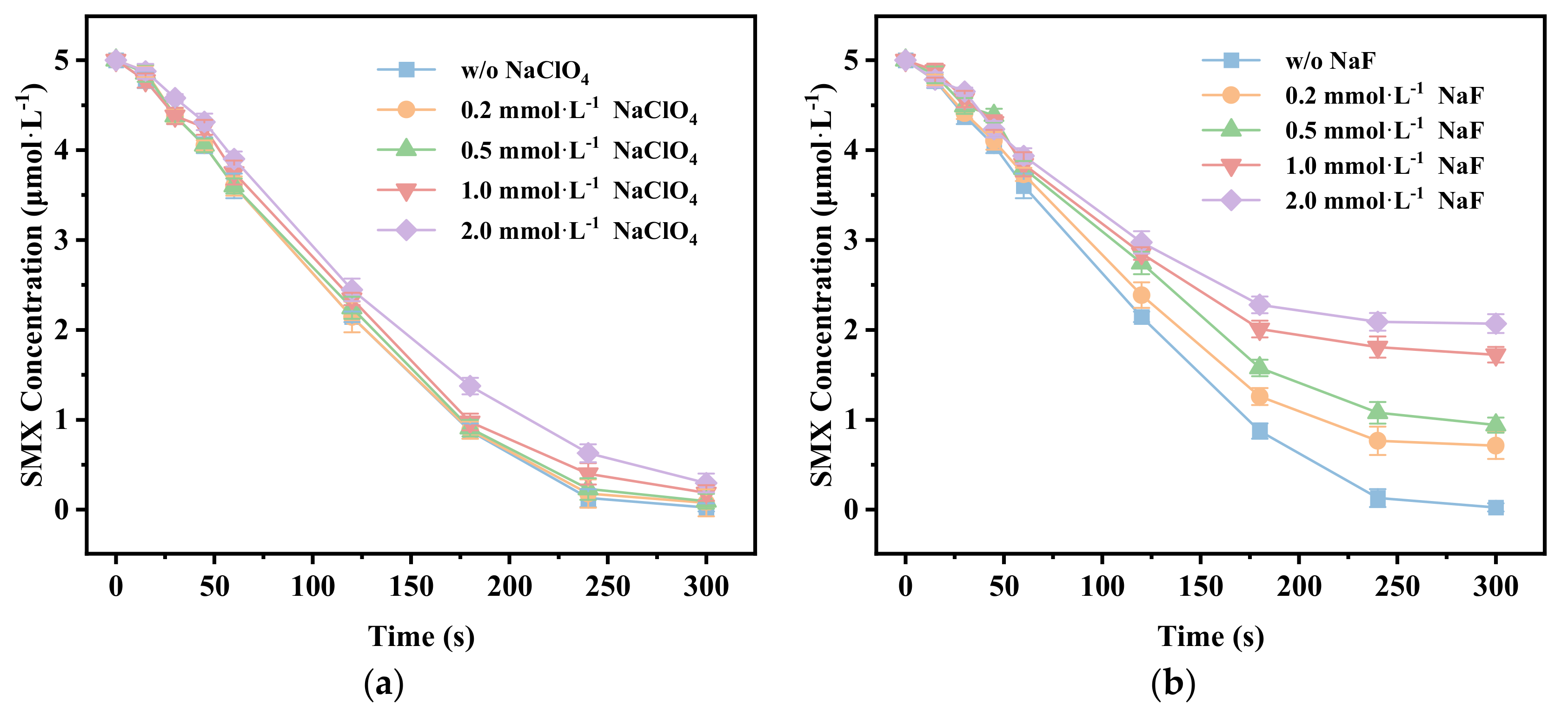
3.5. Identification of the Main Reactive Oxygen Species
3.6. Proposed Reaction Sites and Mechanism
3.7. Degradation Pathways and Toxicity Analysis of SMX
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giannakis, S.; Lin, K.-Y.A.; Ghanbari, F. A review of the recent advances on the treatment of industrial wastewaters by Sulfate Radical-based Advanced Oxidation Processes (SR-AOPs). Chem. Eng. J. 2021, 406, 127083. [Google Scholar] [CrossRef]
- Li, Y.; Dong, H.; Li, L.; Tang, L.; Tian, R.; Li, R.; Chen, J.; Xie, Q.; Jin, Z.; Xiao, J.; et al. Recent advances in waste water treatment through transition metal sulfides-based advanced oxidation processes. Water Res. 2021, 192, 116850. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.P.; Chen, Z.B.; Li, Q.B.; Wang, J.W.; Cao, L.S.; Cheng, Y.J.; Yu, S.W.; Liu, Z.Z.; Chen, Y.Q.; Yue, S.Y.; et al. Non-radical activation of peracetic acid by powdered activated carbon for the degradation of sulfamethoxazole. Environ. Sci. Technol. 2023, 57, 10478–10488. [Google Scholar] [CrossRef]
- Zhang, K.J.; Zhou, X.Y.; Du, P.H.; Zhang, T.Q.; Cai, M.Q.; Sun, P.Z.; Huang, C.H. Oxidation of β-lactam antibiotics by peracetic acid: Reaction kinetics, product and pathway evaluation. Water Res. 2017, 123, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.P.; Wang, J.W.; Xiong, B.; Bai, F.; Wang, S.L.; Wan, Y.; Zhang, L.; Xie, P.C.; Wiesner, M.R. Application of cobalt/peracetic acid to degrade sulfamethoxazole at neutral condition: Efficiency and mechanisms. Environ. Sci. Technol. 2020, 54, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Ao, X.-w.; Eloranta, J.; Huang, C.-H.; Santoro, D.; Sun, W.-j.; Lu, Z.-d.; Li, C. Peracetic acid-based advanced oxidation processes for decontamination and disinfection of water: A review. Water Res. 2021, 188, 116479. [Google Scholar] [CrossRef]
- Cao, L.; Wang, J.; Wang, Z.; Yu, S.; Cheng, Y.; Ma, J.; Xie, P. Inactivation of Microcystis Aeruginosa by peracetic acid combined with ultraviolet: Performance and characteristics. Water Res. 2022, 208, 117847. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Shao, Y.S.; Gao, N.Y.; Xu, B.; An, N.; Lu, X. Comprehensive study on the formation of brominated byproducts during heat-activated persulfate degradation. Chem. Eng. J. 2020, 381, 10. [Google Scholar] [CrossRef]
- Chen, F.; Liu, L.L.; Chen, J.J.; Li, W.W.; Chen, Y.P.; Zhang, Y.J.; Wu, J.H.; Mei, S.C.; Yang, Q.; Yu, H.Q. Efficient decontamination of organic pollutants under high salinity conditions by a nonradical peroxymonosulfate activation system. Water Res. 2021, 191, 13. [Google Scholar] [CrossRef]
- Choong, Z.Y.; Lin, K.Y.A.; Lisak, G.; Lim, T.T.; Oh, W.D. Multi-heteroatom-doped carbocatalyst as peroxymonosulfate and peroxydisulfate activator for water purification: A critical review. J. Hazard. Mater. 2022, 426, 21. [Google Scholar] [CrossRef]
- Li, R.B.; Manoli, K.; Kim, J.; Feng, M.B.; Huang, C.H.; Sharma, V.K. Peracetic acid-Ruthenium(III) oxidation process for the degradation of micropollutants in water. Environ. Sci. Technol. 2021, 55, 9150–9160. [Google Scholar] [CrossRef]
- Liu, B.; Guo, W.; Jia, W.; Wang, H.; Zheng, S.; Si, Q.; Zhao, Q.; Luo, H.; Jiang, J.; Ren, N. Insights into the oxidation of organic contaminants by Co(II) activated peracetic acid: The overlooked role of high-valent cobalt-oxo species. Water Res. 2021, 201, 117313. [Google Scholar] [CrossRef]
- Dong, J.; Xu, W.; Liu, S.; Gong, Y.; Yang, T.; Du, L.; Chen, Q.; Tan, X.; Liu, Y. Lignin-derived biochar to support CoFe2O4: Effective activation of peracetic acid for sulfamethoxazole degradation. Chem. Eng. J. 2022, 430, 132868. [Google Scholar] [CrossRef]
- Zhu, E.Y.; Yuan, D.L.; Wang, Z.B.; Zhang, Q.R.; Tang, S.F. Insight into the activation mechanism of peracetic acid by molybdenum carbide for sulfamethoxazole decomposition. Chem. Eng. J. 2023, 474, 10. [Google Scholar] [CrossRef]
- Chen, S.; Cai, M.; Liu, Y.; Zhang, L.; Feng, L. Effects of water matrices on the degradation of naproxen by reactive radicals in the UV/peracetic acid process. Water Res. 2019, 150, 153–161. [Google Scholar] [CrossRef]
- Chen, J.Y.; Pan, H.J.; Chen, Y.L.; Zhou, Z.M.; Jing, G.H.; Zhao, X.D. Efficient activation of peracetic acid for abatement of tetracycline by W-doped CuS via regulating copper redox cycling. Chem. Eng. J. 2023, 464, 16. [Google Scholar] [CrossRef]
- Kim, J.; Wang, J.Y.; Ashley, D.C.; Sharma, V.K.; Huang, C.H. Picolinic Acid-Mediated Catalysis of Mn(II) for Peracetic Acid Oxidation Processes: Formation of High-Valent Mn Species. Environ. Sci. Technol. 2023, 57, 18929–18939. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Zhang, T.Q.; Liu, W.; Du, P.H.; Dobson, J.T.; Huang, C.H. Advanced Oxidation Process with Peracetic Acid and Fe(II) for Contaminant Degradation. Environ. Sci. Technol. 2019, 53, 13312–13322. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zeng, H.; Liang, Y.H.; Cao, Y.; Xiao, Y.; Ma, J. Degradation of bisphenol AF in water by periodate activation with FeS (mackinawite) and the role of sulfur species in the generation of sulfate radicals. Chem. Eng. J. 2021, 407, 11. [Google Scholar] [CrossRef]
- Shao, S.; Li, X.S.; Gong, Z.M.; Fan, B.; Hu, J.H.; Peng, J.B.; Lu, K.; Gao, S.X. A new insight into the mechanism in Fe3O4@CuO/PMS system with low oxidant dosage. Chem. Eng. J. 2022, 438, 11. [Google Scholar] [CrossRef]
- Song, M.J.; Nguyen, Q.B.; Kim, C.; Hwang, I. Sustained activation of persulfate by slow release of Fe(II) from silica-coated nanosized zero-valent iron for in situ chemical oxidation. Water Res. 2023, 246, 11. [Google Scholar] [CrossRef]
- Gong, Y.Y.; Tang, J.C.; Zhao, D.Y. Application of iron sulfide particles for groundwater and soil remediation: A review. Water Res. 2016, 89, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, Z.L.; Mingbao, F.B.; Liu, W.; Wang, W.J.; Yang, Q.; Hu, Y.N. Degradation of 2,4-dichlorophenoxyacetic acid in water by persulfate activated with FeS (mackinawite). Chem. Eng. J. 2017, 313, 498–507. [Google Scholar] [CrossRef]
- Xu, H.D.; Sheng, Y.Q. New insights into the degradation of chloramphenicol and fluoroquinolone antibiotics by peroxymonosulfate activated with FeS: Performance and mechanism. Chem. Eng. J. 2021, 414, 9. [Google Scholar] [CrossRef]
- Fan, J.H.; Gu, L.; Wu, D.L.; Liu, Z.G. Mackinawite (FeS) activation of persulfate for the degradation of p-chloroaniline: Surface reaction mechanism and sulfur-mediated cycling of iron species. Chem. Eng. J. 2018, 333, 657–664. [Google Scholar] [CrossRef]
- Xing, D.Y.; Shao, S.J.; Yang, Y.Y.; Zhou, Z.M.; Jing, G.H.; Zhao, X.D. Mechanistic insights into the efficient activation of peracetic acid by pyrite for the tetracycline abatement. Water Res. 2022, 222, 118930. [Google Scholar] [CrossRef] [PubMed]
- Li, C.X.; Yuan, D.L.; Yang, K.; Wang, H.C.; Wang, Z.B.; Zhang, Q.R.; Tang, S.F. Reutilization of pyrite tailings in peracetic acid-based advanced oxidation process for water purification. Sep. Purif. Technol. 2024, 354, 129155. [Google Scholar] [CrossRef]
- Liu, J.L.; Wong, M.H. Pharmaceuticals and personal care products (PPCPs): A review on environmental contamination in China. Environ. Int. 2013, 59, 208–224. [Google Scholar] [CrossRef] [PubMed]
- Rout, P.R.; Zhang, T.C.; Bhunia, P.; Surampalli, R.Y. Treatment technologies for emerging contaminants in wastewater treatment plants: A review. Sci. Total Environ. 2021, 753, 17. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.B.; Guo, X.Y.; Morimoto, A.; Maetani, K.; Tanoue, R.; Tong-U-Dom, S.; Buranapratheprat, A. Transport and dilution of fluvial antibiotic in the Upper Gulf of Thailand. Environ. Pollut. 2021, 288, 10. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.; Shahzad, A.; Tahir, K.; Kim, J.; Moztahida, M.; Jang, J.; Alam, M.B.; Lee, S.H.; Jung, H.Y.; Lee, D.S. Photo-Fenton reaction for the degradation of sulfamethoxazole using a multi-walled carbon nanotube-NiFe2O4 composite. Chem. Eng. J. 2020, 382, 12. [Google Scholar] [CrossRef]
- Lian, J.J.; Wang, H.L.; He, H.P.; Huang, W.L.; Yang, M.; Zhong, Y.; Peng, P.A. The reaction of amorphous iron sulfide with Mo(VI) under different pH conditions. Chemosphere 2021, 266, 10. [Google Scholar] [CrossRef]
- Zhao, X.B.; Cheng, K.K.; Hao, J.B.; Liu, D.H. Preparation of peracetic acid from hydrogen peroxide, part II: Kinetics for spontaneous decomposition of peracetic acid in the liquid phase. J. Mol. Catal. A-Chem. 2008, 284, 58–68. [Google Scholar] [CrossRef]
- Shen, P.; Hou, K.J.; Chen, F.; Pi, Z.J.; He, L.; Chen, S.J.; Li, X.M.; Yang, Q. Ultra-rapid and long-lasting activation of peracetic acid by Cu-Co spinel oxides for eliminating organic contamination: Role of radical and non-radical catalytic oxidation. Chem. Eng. J. 2023, 463, 13. [Google Scholar] [CrossRef]
- Viollier, E.; Inglett, P.W.; Hunter, K.; Roychoudhury, A.N.; Van Cappellen, P. The ferrozine method revisited: Fe(II)/Fe(III) determination in natural waters. Appl. Geochem. 2000, 15, 785–790. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F.W. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Tian, B.R.; Wu, N.N.; Liu, M.Z.; Wang, Z.Y.; Qu, R.J. Promoting effect of silver oxide nanoparticles on the oxidation of bisphenol B by ferrate(VI). Environ. Sci. Technol. 2023, 57, 15715–15724. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, Z.L.; Yang, Z.L.; Yang, Q.; Li, B.; Bai, Z.Y. Heterogeneous fenton-like catalytic degradation of 2,4-dichlorophenoxyacetic acid in water with FeS. Chem. Eng. J. 2015, 273, 481–489. [Google Scholar] [CrossRef]
- Al-Anazi, A.; Abdelraheem, W.H.; Han, C.; Nadagouda, M.N.; Sygellou, L.; Arfanis, M.K.; Falaras, P.; Sharma, V.K.; Dionysiou, D.D. Cobalt ferrite nanoparticles with controlled composition-peroxymonosulfate mediated degradation of 2-phenylbenzimidazole-5-sulfonic acid. Appl. Catal. B-Environ. 2018, 221, 266–279. [Google Scholar] [CrossRef]
- Zhang, H.X.; Li, C.W.; Lyu, L.; Hu, C. Surface oxygen vacancy inducing peroxymonosulfate activation through electron donation of pollutants over cobalt-zinc ferrite for water purification. Appl. Catal. B-Environ. 2020, 270, 10. [Google Scholar] [CrossRef]
- Zhang, T.Q.; Huang, C.H. Modeling the kinetics of UV/peracetic acid advanced oxidation process. Environ. Sci. Technol. 2020, 54, 7579–7590. [Google Scholar] [CrossRef]
- Cai, M.Q.; Sun, P.Z.; Zhang, L.Q.; Huang, C.H. UV/peracetic acid for degradation of pharmaceuticals and reactive species evaluation. Environ. Sci. Technol. 2017, 51, 14217–14224. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Pignatello, J.J.; Ma, J.; Mitch, W.A. Comparison of halide impacts on the efficiency of contaminant degradation by sulfate and hydroxyl radical-based advanced oxidation processes (AOPs). Environ. Sci. Technol. 2014, 48, 2344–2351. [Google Scholar] [CrossRef]
- Liu, H.H.; Zhao, J.; Wang, Y.; Wu, Y.L.; Dong, W.B.; Nie, M.H.; Wang, X.N. Enhancement of peroxymonosulfate activation by sinapic acid accelerating Fe(III)/Fe(II) cycle. Chem. Eng. J. 2022, 446, 8. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, Y.; Zhang, X.; Zhou, X.; Chen, Y.D.; Duan, X.G.; Ren, N.Q. Kinetics study of chloride-activated peracetic acid for purifying bisphenol A: Role of Cl2/HClO and carbon-centered radicals. Water Res. 2023, 242, 8. [Google Scholar] [CrossRef]
- Zhang, C.Q.; Brown, P.J.B.; Hu, Z.Q. Thermodynamic properties of an emerging chemical disinfectant, peracetic acid. Sci. Total Environ. 2018, 621, 948–959. [Google Scholar] [CrossRef]
- Kim, J.; Du, P.H.; Liu, W.; Luo, C.; Zhao, H.; Huang, C.H. Cobalt/peracetic acid: Advanced oxidation of aromatic organic compounds by acetylperoxyl radicals. Environ. Sci. Technol. 2020, 54, 5268–5278. [Google Scholar] [CrossRef]
- Yang, L.W.; She, L.H.; Xie, Z.H.; He, Y.L.; Tian, X.Y.; Zhao, C.L.; Guo, Y.Q.; Hai, C.; He, C.S.; Lai, B. Boosting activation of peracetic acid by Co@mZVI for efficient degradation of sulfamethoxazole: Interesting two-phase generation of reactive oxidized species. Chem. Eng. J. 2022, 448, 9. [Google Scholar] [CrossRef]
- Fang, Z.Y.; Zhao, J.; Li, Y.; Wang, Y.; Qiu, T.; Wu, Y.L.; Dong, W.B.; Mailhot, G. Improving Fenton-like system with Catechin, an environmental-friendly polyphenol: Effects and mechanism. Chem. Eng. J. 2021, 426, 7. [Google Scholar] [CrossRef]
- Li, G.B.; Huang, S.Q.; Zhu, N.W.; Yuan, H.P.; Ge, D.D. Near-infrared responsive upconversion glass-ceramic@BiOBr heterojunction for enhanced photodegradation performances of norfloxacin. J. Hazard. Mater. 2021, 403, 10. [Google Scholar] [CrossRef]
- Finkelstein, E.; Rosen, G.M.; Rauckman, E.J. Spin trapping-Kinetics of the reaction of superoxide and hydroxyl radicals with nitrones. J. Am. Chem. Soc. 1980, 102, 4994–4999. [Google Scholar] [CrossRef]
- Chalier, F.; Tordo, P. 5-Diisopropoxyphosphoryl-5-methyl-1-pyrroline <i>N</i>-oxide, DIPPMPO, a crystalline analog of the nitrone DEPMPO: Synthesis and spin trapping properties. J. Chem. Soc.-Perkin Trans. 2002, 2, 2110–2117. [Google Scholar] [CrossRef]
- Rokhina, E.V.; Makarova, K.; Golovina, E.A.; Van As, H.; Virkutyte, J. Free Radical Reaction Pathway, Thermochemistry of Peracetic Acid Homolysis, and Its Application for Phenol Degradation: Spectroscopic Study and Quantum Chemistry Calculations. Environ. Sci. Technol. 2010, 44, 6815–6821. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, Y.N.; Dong, W.B. Trace catechin enhanced degradation of organic pollutants with activated peroxymonosulfate: Comprehensive identification of working oxidizing species. Chem. Eng. J. 2022, 429, 7. [Google Scholar] [CrossRef]
- Xu, Y.; Ai, J.; Zhang, H. The mechanism of degradation of bisphenol A using the magnetically separable CuFe2O4/peroxymonosulfate heterogeneous oxidation process. J. Hazard. Mater. 2016, 309, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Chen, J.B.; Zhang, Y.L.; Xu, Y.; Zheng, T.L.; Zhou, X.F. Highly efficient activation of peracetic acid by nano-CuO for carbamazepine degradation in wastewater: The significant role of H2O2 and evidence of acetylperoxy radical contribution. Water Res. 2022, 216, 10. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.J.; Huang, X.P.; Jia, F.L.; Ai, Z.H.; Zhao, J.C.; Zhang, L.Z. Hydroxylamine Promoted Goethite Surface Fenton Degradation of Organic Pollutants. Environ. Sci. Technol. 2017, 51, 5118–5126. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Zhao, R.X.; Cao, L.J.; Lei, Y.S.; Liu, J.; Feng, J.; Fu, W.J.; Li, X.Y.; Li, B. High-efficiency biodegradation of chloramphenicol by enriched bacterial consortia: Kinetics study and bacterial community characterization. J. Hazard. Mater. 2020, 384, 11. [Google Scholar] [CrossRef]
- An, T.C.; Gao, Y.P.; Li, G.Y.; Kamat, P.V.; Peller, J.; Joyce, M.V. Kinetics and mechanism of •OH mediated degradation of dimethyl phthalate in aqueous solution: Experimental and theoretical studies. Environ. Sci. Technol. 2014, 48, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.V.; Howard, J.A.; Ingold, K.U. The inhibition of autoxidation by 2,4,6-tri-tert-butyl substituted phenol, aniline, and thiophenol. Can. J. Chem. 1964, 42, 2847–2851. [Google Scholar] [CrossRef]
- Li, T.Y.; Ge, L.F.; Peng, X.X.; Wang, W.; Zhang, W.X. Enhanced degradation of sulfamethoxazole by a novel Fenton-like system with significantly reduced consumption of H2O2 activated by g-C3N4/MgO composite. Water Res. 2021, 190, 12. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Wu, Y.; Dong, W. Enhancement in Sulfamethoxazole Degradation via Efficient Heterogeneous Activation of Peracetic Acid by FeS. Water 2024, 16, 2405. https://doi.org/10.3390/w16172405
Li L, Wu Y, Dong W. Enhancement in Sulfamethoxazole Degradation via Efficient Heterogeneous Activation of Peracetic Acid by FeS. Water. 2024; 16(17):2405. https://doi.org/10.3390/w16172405
Chicago/Turabian StyleLi, Linyi, Yanlin Wu, and Wenbo Dong. 2024. "Enhancement in Sulfamethoxazole Degradation via Efficient Heterogeneous Activation of Peracetic Acid by FeS" Water 16, no. 17: 2405. https://doi.org/10.3390/w16172405
APA StyleLi, L., Wu, Y., & Dong, W. (2024). Enhancement in Sulfamethoxazole Degradation via Efficient Heterogeneous Activation of Peracetic Acid by FeS. Water, 16(17), 2405. https://doi.org/10.3390/w16172405





