Characterization of Nanoparticles in Drinking Water Using Field-Flow Fractionation Coupled with Multi-Angle Light Scattering and Inductively Coupled Plasma Mass Spectrometry
Abstract
:1. Introduction
2. Theory
2.1. Field Flow Fractionation
2.2. Calcium Carbonate Nucleation
3. Materials and Methods
3.1. AF4-UV-MALS-ICP-MS Instrumentation and Software
3.2. Water Sample Analysis
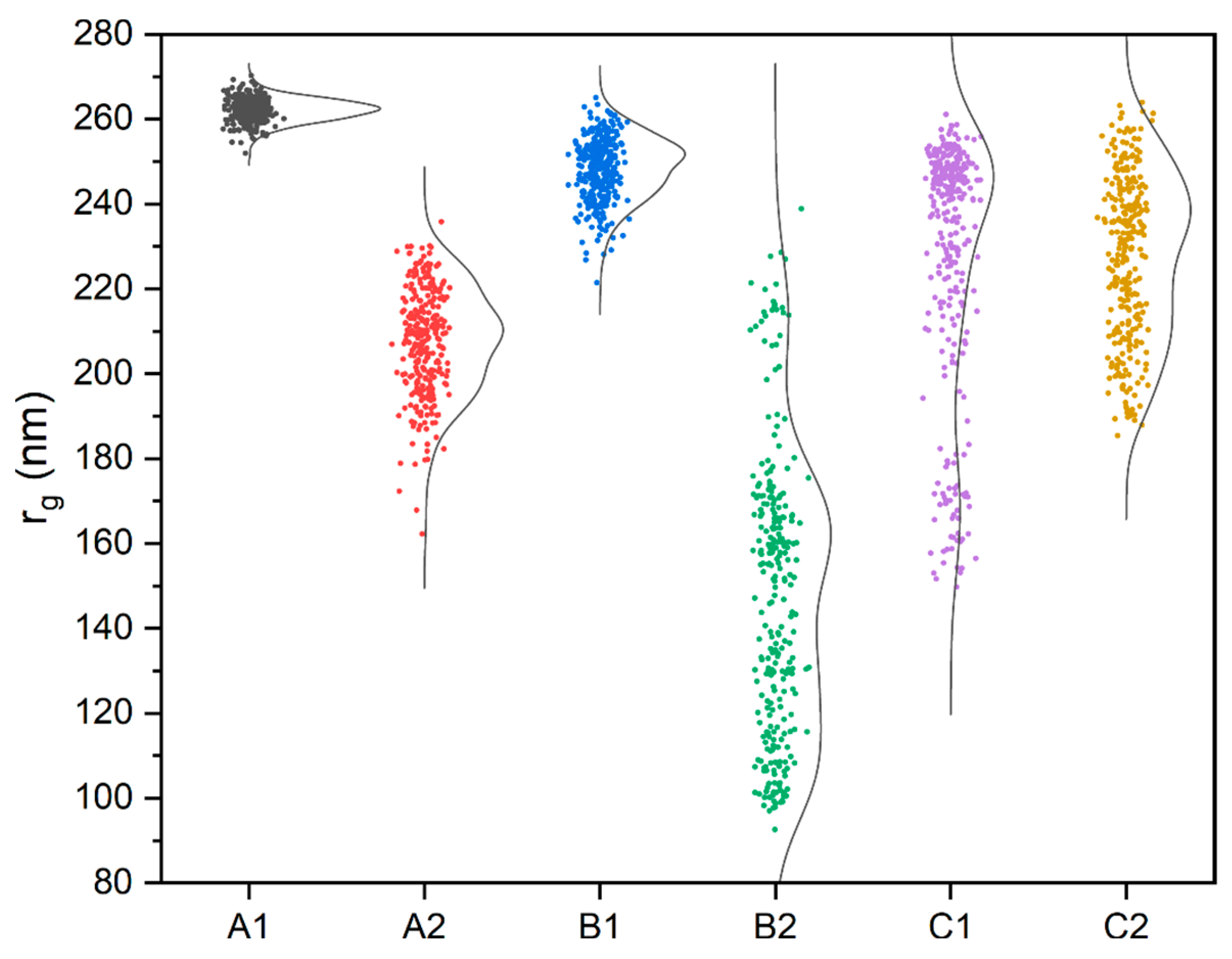
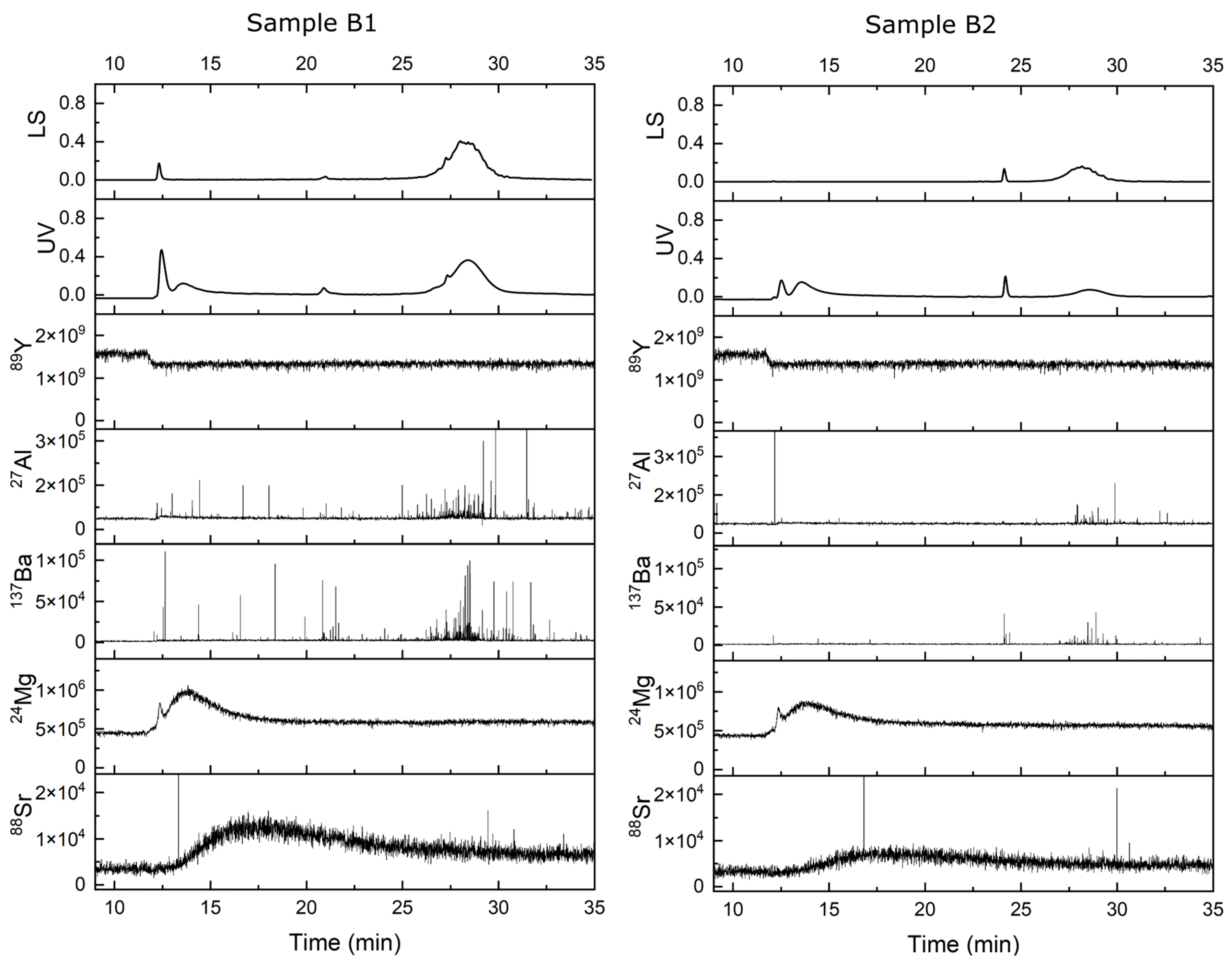
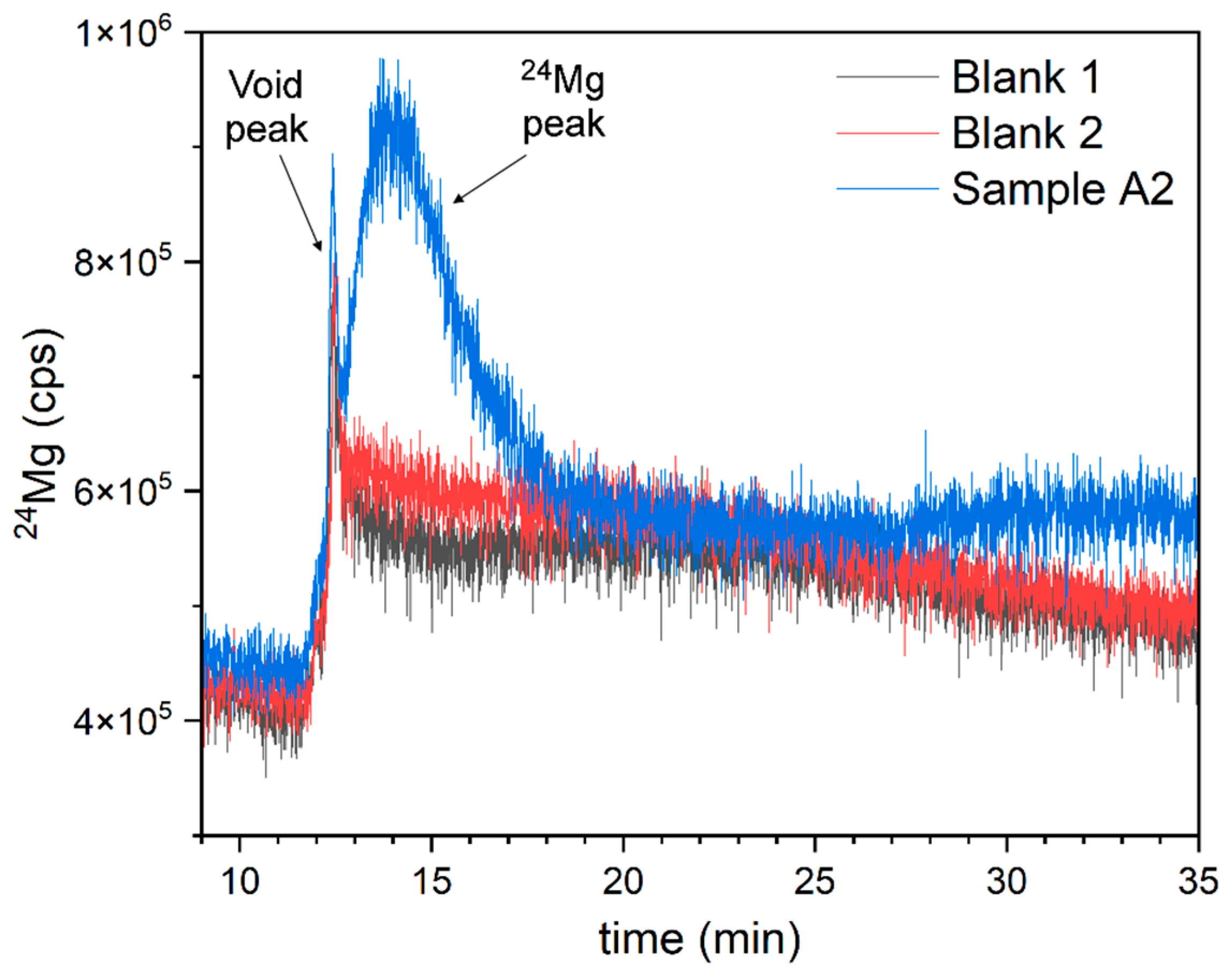
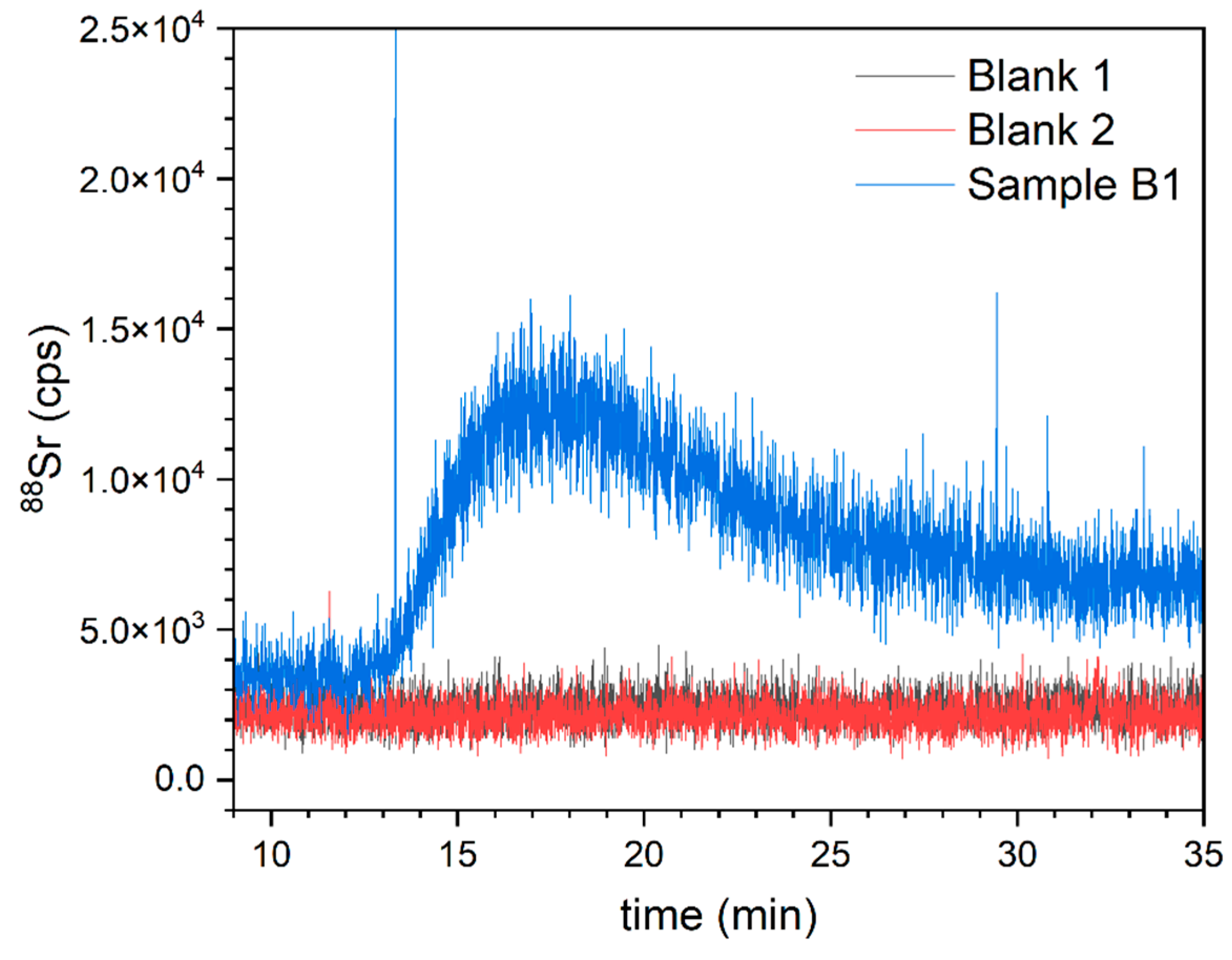
4. Results and Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pulit-Prociak, J.; Banach, M. Silver nanoparticles: A material of the future? Open Chem. 2016, 14, 76–91. [Google Scholar] [CrossRef]
- Mallia, J.d.O.; Galea, R.; Nag, R.; Cummins, E.; Gatt, R.; Valdramidis, V. Nanoparticle food applications and their toxicity: Current trends and needs in risk assessment strategies. J. Food Prot. 2022, 85, 355–372. [Google Scholar] [CrossRef] [PubMed]
- EUR-Lex. Commission Regulation (EU) 2022/63 amending Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the food additive titanium dioxide (E 171). Off. J. Eur. Union 2022, 11, OJ L11. Available online: http://data.europa.eu/eli/reg/2022/63/oj (accessed on 2 May 2023).
- Kaiser, J.-P.; Zuin, S.; Wick, P. Is nanotechnology revolutionizing the paint and lacquer industry? a critical opinion. Sci. Total. Environ. 2013, 442, 282–289. [Google Scholar] [CrossRef]
- Séby, F. Chapter eleven—Metal and metal oxide nanoparticles in cosmetics and skin care products. In Comprehensive Analytical; Milačič, R., Sčančar, J., Goenaga-Infante, H., Vidmar, J., Eds.; Part of volume ChemistryAnalysis and Characterisation of Metal-Based Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2021; Volume 93, pp. 381–427. [Google Scholar] [CrossRef]
- Rossi, A.; Zannotti, M.; Cuccioloni, M.; Minicucci, M.; Petetta, L.; Angeletti, M.; Giovannetti, R. Silver nanoparticle-based sensor for the selective detection of nickel ions. Nanomaterials 2021, 11, 1733. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Jiang, Y.; Xiao, H.; Jiang, B.; Zhang, H.; Peng, M.; Dong, G.; Yu, X.; Yang, J. Sol-gel preparation of Ag-silica nanocomposite with high electrical conductivity. Appl. Surf. Sci. 2018, 436, 732–738. [Google Scholar] [CrossRef]
- Sztandera, K.; Gorzkiewicz, M.; Klajnert-Maculewicz, B. Gold nanoparticles in cancer treatment. Mol. Pharm. 2019, 16, 1–23. [Google Scholar] [CrossRef]
- Simeonidis, K.; Mourdikoudis, S.; Kaprara, E.; Mitrakas, M.; Polavarapu, L. Inorganic engineered nanoparticles in drinking water treatment: A critical review. Environ. Sci. Water Res. Technol. 2016, 2, 43–70. [Google Scholar] [CrossRef]
- Anjum, M.; Miandad, R.; Waqas, M.; Gehany, F.; Barakat, M. Remediation of wastewater using various nano-materials. Arab. J. Chem. 2019, 12, 4897–4919. [Google Scholar] [CrossRef]
- Zhang, Y.; Leu, Y.-R.; Aitken, R.J.; Riediker, M. Inventory of engineered nanoparticle-containing consumer products available in the Singapore retail market and likelihood of release into the aquatic environment. Int. J. Environ. Res. Public Health 2015, 12, 8717–8743. [Google Scholar] [CrossRef]
- Bundschuh, M.; Filser, J.; Lüderwald, S.; McKee, M.S.; Metreveli, G.; Schaumann, G.E.; Schulz, R.; Wagner, S. Nanoparticles in the environment: Where do we come from, where do we go to? Environ. Sci. Eur. 2018, 30, 6. [Google Scholar] [CrossRef] [PubMed]
- Fent, K. Ecotoxicology of engineered nanoparticles. In Nanoparticles in the Water Cycle; Frimmel, F.H., Niessner, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 183–205. [Google Scholar] [CrossRef]
- Demichelis, R.; Raiteri, P.; Gale, J.D.; Quigley, D.; Gebauer, D. Stable prenucleation mineral clusters are liquid-like ionic polymers. Nat. Commun. 2011, 2, 590. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, D.; Völkel, A.; Cölfen, H. Stable prenucleation calcium carbonate clusters. Science 2008, 322, 1819–1822. [Google Scholar] [CrossRef]
- Pouget, E.M.; Bomans, P.H.H.; Goos, J.A.C.M.; Frederik, P.M.; de With, G.; Sommerdijk, N.A.J.M. The initial stages of template-controlled CaCO3 formation revealed by Cryo-TEM. Science 2009, 323, 1455–1458. [Google Scholar] [CrossRef] [PubMed]
- EFitzgerald, E.; Kumar, A.; Poulose, S.; Coey, J.M.D. Interaction and Stability of Nanobubbles and Prenucleation Calcium Clusters during Ultrasonic Treatment of Hard Water. ACS Omega 2024, 9, 2547–2558. [Google Scholar] [CrossRef]
- Zarei, T.; Fuchs, E.C.; Agostinho, L.L.; Gebauer, D.; Woisetschläger, J.; Offerhaus, H.L. Intrinsic CO2 nanobubbles in alkaline aqueous solutions. Colloids Surf. A Physicochem. Eng. Asp. 2024, 701, 134895. [Google Scholar] [CrossRef]
- Sammer, M.; Kamp, C.; Paulitsch-Fuchs, A.H.; Wexler, A.D.; Buisman, C.J.N.; Fuchs, E.C. Strong gradients in weak magnetic fields induce DOLLOP formation in tap water. Water 2016, 8, 79. [Google Scholar] [CrossRef]
- MBaalousha, M.; Kammer, F.V.D.; Motelica-Heino, M.; Baborowski, M.; Hofmeister, C.; Le Coustumer, P. Size-Based Speciation of Natural Colloidal Particles by Flow Field Flow Fractionation, Inductively Coupled Plasma-Mass Spectroscopy, and Transmission Electron MicroscopyX-ray Energy Dispersive Spectroscopy Colloids-Trace Element Interaction. Environ. Sci. Technol. 2006, 40, 2156–2162. [Google Scholar] [CrossRef]
- Liao, P.; Li, W.; Jiang, Y.; Wu, J.; Yuan, S.; Fortner, J.D.; Giammar, D.E. Formation, aggregation, and deposition dynamics of nom-iron colloids at anoxic–oxic interfaces. Environ. Sci. Technol. 2017, 51, 12235–12245. [Google Scholar] [CrossRef]
- Allard, S.; Gutierrez, L.; Fontaine, C.; Croué, J.-P.; Gallard, H. Organic matter interactions with natural manganese oxide and synthetic birnessite. Sci. Total. Environ. 2017, 583, 487–495. [Google Scholar] [CrossRef]
- Sharma, V.K.; Filip, J.; Zboril, R.; Varma, R.S. Natural inorganic nanoparticles—Formation, fate, and toxicity in the environment. Chem. Soc. Rev. 2015, 44, 8410–8423. [Google Scholar] [CrossRef]
- Degueldre, C.; Pfeiffer, H.-R.; Alexander, W.; Wernli, B.; Bruetsch, R. Colloid properties in granitic groundwater systems. I: Sampling and characterisation. Appl. Geochem. 1996, 11, 677–695. [Google Scholar] [CrossRef]
- Plavchak, C.L.; Smith, W.C.; Bria, C.R.; Williams, S.K.R. New advances and applications in field-flow fractionation. Annu. Rev. Anal. Chem. 2021, 14, 257–279. [Google Scholar] [CrossRef]
- Wahlund, K.G.; Giddings, J.C. Properties of an asymmetrical flow field-flow fractionation channel having one permeable wall. Anal. Chem. 1987, 59, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; Wittgren, B.; Wahlund, K.-G. Accuracy in multiangle light scattering measurements for molar mass and radius estimations. Model calculations and experiments. Anal. Chem. 2003, 75, 4279–4291. [Google Scholar] [CrossRef] [PubMed]
- De Yoreo, J.; Vekilov, P.G. Principles of Crystal Nucleation and Growth. Rev. Mineral. Geochem. 2003, 54, 57. [Google Scholar] [CrossRef]
- Niederberger, M.; Cölfen, H. Oriented Attachment and Mesocrystals: Non-classical Crystallization Mechanisms Based on Nanoparticle Assembly. Phys. Chem. Chem. Phys. 2006, 8, 3271. [Google Scholar] [CrossRef]
- Rieger, J.; Frechen, T.; Cox, G.; Heckmann, W.; Schmidt, C.; Thieme, J. Precipitation and Growth of Crystalline Matter: Mesocrystals, Composites, and Morphosynthesis. Faraday Discuss. 2007, 136, 265. [Google Scholar] [CrossRef]
- Faatz, M.; Gröhn, F.; Wegner, G. Amorphous Calcium Carbonate: Synthesis and Potential Intermediate in Biomineralization. Adv. Mater. 2004, 16, 996. [Google Scholar] [CrossRef]
- Addadi, L.; Raz, S.; Weiner, S. Taking Advantage of Disorder: Amorphous Calcium Carbonate and Its Roles in Biomineralization. Adv. Mater. 2003, 15, 959. [Google Scholar] [CrossRef]
- Xu, A.W.; Ma, Y.R.; Cölfen, H. Biomimetic mineralization. J. Mater. Chem. 2007, 17, 415. [Google Scholar] [CrossRef]
- Sinn, C.G.; Dimova, R.; Antonietti, M. Isothermal Titration Calorimetry of the Polyelectrolyte/Water Interaction and Binding of Ca2+: Effects Determining the Quality of Polymeric Scale Inhibitors. Macromolecules 2004, 37, 3444. [Google Scholar] [CrossRef]
- Quigley, D.; Rodger, P.M. Metadynamics simulations of ice nucleation and growth. J. Chem. Phys. 2008, 128, 154518. [Google Scholar] [CrossRef]
- Raiteri, P.; Gale, J.D. Water is the key to nonclassical nucleation of Amorphous Calcium Carbonate. J. Am. Chem. Soc. 2010, 132, 17623–17634. [Google Scholar] [CrossRef]
- May, T.W.; Wiedmeyer, R.H. A table of polyatomic interferences in ICP-MS. At. Spectrosc. 1998, 19, 150–155. [Google Scholar]
- Han, I.; Rhee, C.; Kim, D. Investigations on Potential Applications of CaMg(CO3)2 Nanoparticles. Molecules 2022, 28, 316. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gindele, M.B.; Vinod-Kumar, S.; Rochau, J.; Boemke, D.; Groß, E.; Redrouthu, V.S.; Gebauer, D.; Mathies, G. Colloidal pathways of amorphous calcium carbonate formation lead to distinct water environments and conductivity. Nat. Commun. 2024, 15, 80. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Amstad, E. Water: How does it influence the CaCO3 formation? Angew. Chem. Int. Ed. 2020, 59, 1798–1816. [Google Scholar] [CrossRef]
- Jin, B.; Liu, Z.M.; Tang, R.K. Recent experimental explorations of non-classical nucleation. CrystEngComm 2020, 22, 4057–4073. [Google Scholar] [CrossRef]
- Gebauer, D.; Kellermeier, M.; Gale, J.D.; Bergström, L.; Cölfen, H. Pre-nucleation clusters as solute precursors in crystallisation. Chem. Soc. Rev. 2014, 43, 2348–2371. [Google Scholar] [CrossRef] [PubMed]
- Jehannin, M.; Rao, A.; Cölfen, H. New horizons of nonclassical crystallization. J. Am. Chem. Soc. 2019, 141, 10120–10136. [Google Scholar] [CrossRef] [PubMed]
- Podzimek, S. Light Scattering, Size Exclusion Chromatography and Asymmetric Flow Field Flow Fractionation: Powerful Tools for the Characterization of Polymers, Proteins and Nanoparticles; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]


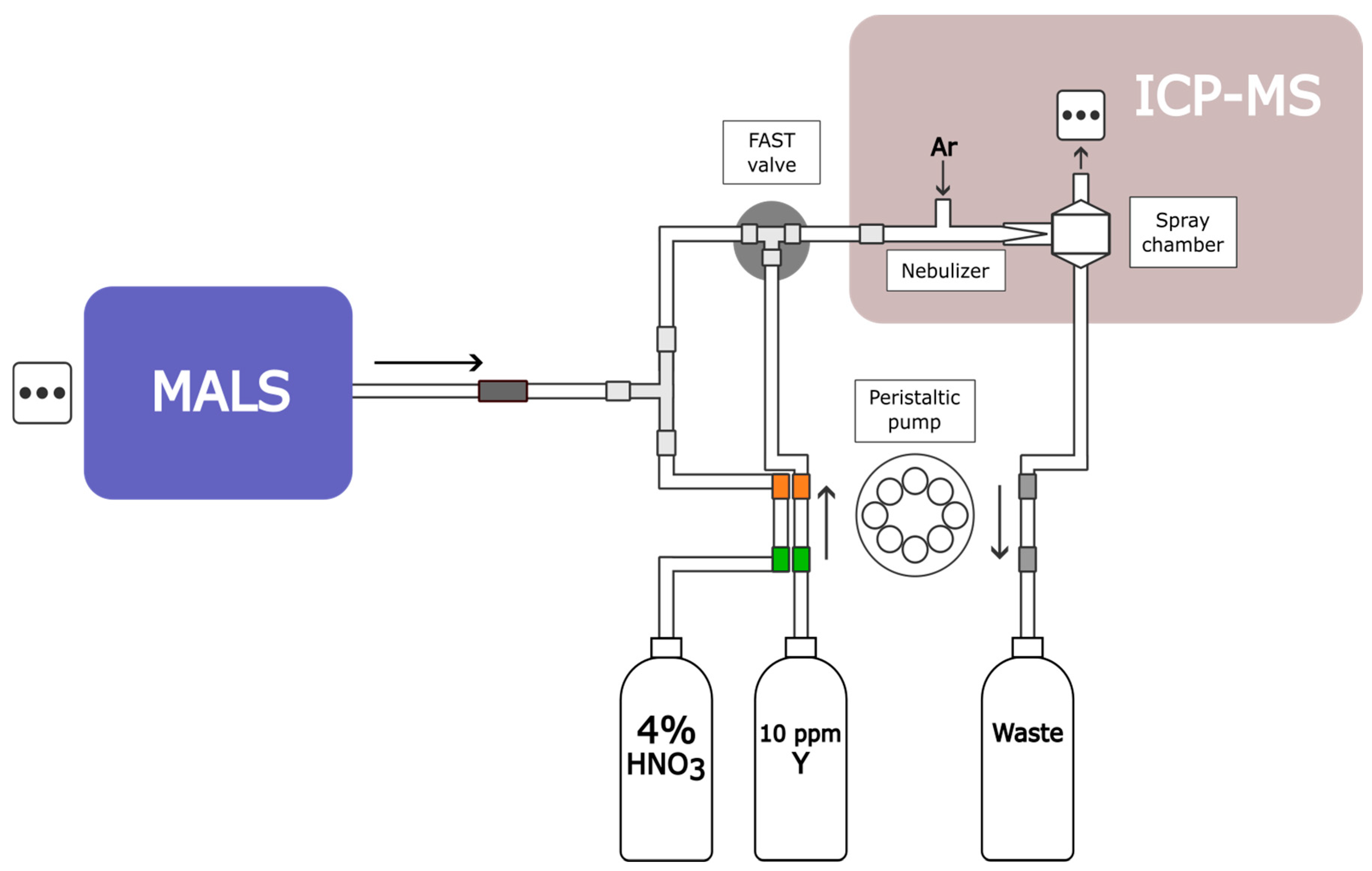





| Plasma Torch RF Power/W | Plasma Torch Nebulizer Flow/L min−1 | Plasma Torch Auxiliary Flow/L min−1 | Plasma Torch Coolant Flow/L min−1 | Quadrupole Dwell Time/s | Peri-Pump Flow Rate/mL min−1 |
|---|---|---|---|---|---|
| 1550 | 1.07 | 0.8 | 14 | 0.01 | ~0.19 |
| Company | Sample Label | Information on the Origin of the Water |
|---|---|---|
| A | A1 | Treated groundwater (Cascade aeration → Pellet softening → Multilayer filtration) |
| A2 | Treated groundwater (Limited aeration → Rapid marble filtration → Aeration → Rapid sand filtration) | |
| B | B1 | Treated groundwater (Cascade aeration → Rapid sand filtration) |
| B2 | Treated groundwater (Cascade aeration → Rapid sand filtration) | |
| C | C1 | Bottled drinking water |
| C2 | Untreated mountain spring water (source for C1) |
| Analysis | A1 | A2 | B1 | B2 | C1 | C2 |
|---|---|---|---|---|---|---|
| TOC/mg L−1 | 3.6 | 2.3 | 1.6 | 3.1 | 1.0 | 3.0 |
| IC/mg L−1 | 35.3 | 29.1 | 54.1 | 39.4 | 34.9 | 36.8 |
| Al3+/µg L−1 | 6.3 | <5.00 | 5.32 | 13.7 | <5.00 | <5.00 |
| Ba2+/µg L−1 | 14.8 | 15.3 | 32.6 | 17.4 | 56.3 | 56.7 |
| Cu2+/µg L−1 | 2.8 | 2.19 | 68.9 | 3.87 | 27.4 | 30.1 |
| Mg2+/µg L−1 | 6560 | 4360 | 9620 | 9510 | >10,000 | >10,000 |
| Sr2+/µg L−1 | 144 | 129 | 391 | 289 | 167 | 174 |
| Zn2+/µg L−1 | 2.1 | 1.93 | 2.32 | 2.02 | 5.45 | 2.77 |
| pH | 7.68 | 7.61 | 7.82 | 7.90 | 7.85 | 7.63 |
| EC/µS cm−1 | 355.7 | 402.0 | 542.3 | 514.3 | 377.0 | 394.0 |
| Turbidity/NTUs | 1 | 1 | 0 | 1 | 0 | 2 |
| Color (Hazen) | 4 | 4 | 10 | 7 | 1 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarei, T.; Colombo, M.B.A.; Fuchs, E.C.; Offerhaus, H.L.; Gebauer, D.; Agostinho, L.L.F. Characterization of Nanoparticles in Drinking Water Using Field-Flow Fractionation Coupled with Multi-Angle Light Scattering and Inductively Coupled Plasma Mass Spectrometry. Water 2024, 16, 2419. https://doi.org/10.3390/w16172419
Zarei T, Colombo MBA, Fuchs EC, Offerhaus HL, Gebauer D, Agostinho LLF. Characterization of Nanoparticles in Drinking Water Using Field-Flow Fractionation Coupled with Multi-Angle Light Scattering and Inductively Coupled Plasma Mass Spectrometry. Water. 2024; 16(17):2419. https://doi.org/10.3390/w16172419
Chicago/Turabian StyleZarei, Talie, Marcos B. A. Colombo, Elmar C. Fuchs, Herman L. Offerhaus, Denis Gebauer, and Luewton L. F. Agostinho. 2024. "Characterization of Nanoparticles in Drinking Water Using Field-Flow Fractionation Coupled with Multi-Angle Light Scattering and Inductively Coupled Plasma Mass Spectrometry" Water 16, no. 17: 2419. https://doi.org/10.3390/w16172419
APA StyleZarei, T., Colombo, M. B. A., Fuchs, E. C., Offerhaus, H. L., Gebauer, D., & Agostinho, L. L. F. (2024). Characterization of Nanoparticles in Drinking Water Using Field-Flow Fractionation Coupled with Multi-Angle Light Scattering and Inductively Coupled Plasma Mass Spectrometry. Water, 16(17), 2419. https://doi.org/10.3390/w16172419








