A Review of the Efficiency of Phosphorus Removal and Recovery from Wastewater by Physicochemical and Biological Processes: Challenges and Opportunities
Abstract
1. Introduction
2. Different Methods of P Detection and Removal
2.1. Analytical Techniques for P Detection
2.2. Removal of Different P Forms
2.2.1. Orthophosphate Removal
2.2.2. Polyphosphate Removal
2.2.3. Organic P Removal
3. Removal Efficiency of P by WWTPs
4. P Retention Process
4.1. Removal of P by Biological Treatment Technologies
4.1.1. Biological Trickling Filter and Biofilm Reactor
4.1.2. Biological Nitrification and Denitrification
4.1.3. Biologically Activated Carbon
4.1.4. Microalgae/Fungi-Based Treatment
4.1.5. Activated Sludge Process
4.1.6. Biosorption
4.1.7. Membrane Bioreactor
4.1.8. Constructed Wetland
4.2. Chemical Treatment Technologies
4.2.1. Chlorination
4.2.2. Ozonation
4.2.3. Fenton Process
4.2.4. Photolysis
4.2.5. Electro-Fenton Processes
4.2.6. Photo-Fenton Process
4.2.7. Photocatalysis
4.2.8. Solar Photocatalysis
5. Comparative Analysis and Integration of P Removal Processes
5.1. Comparative Effectiveness of P Removal Methods
5.2. Maintenance and Complexity
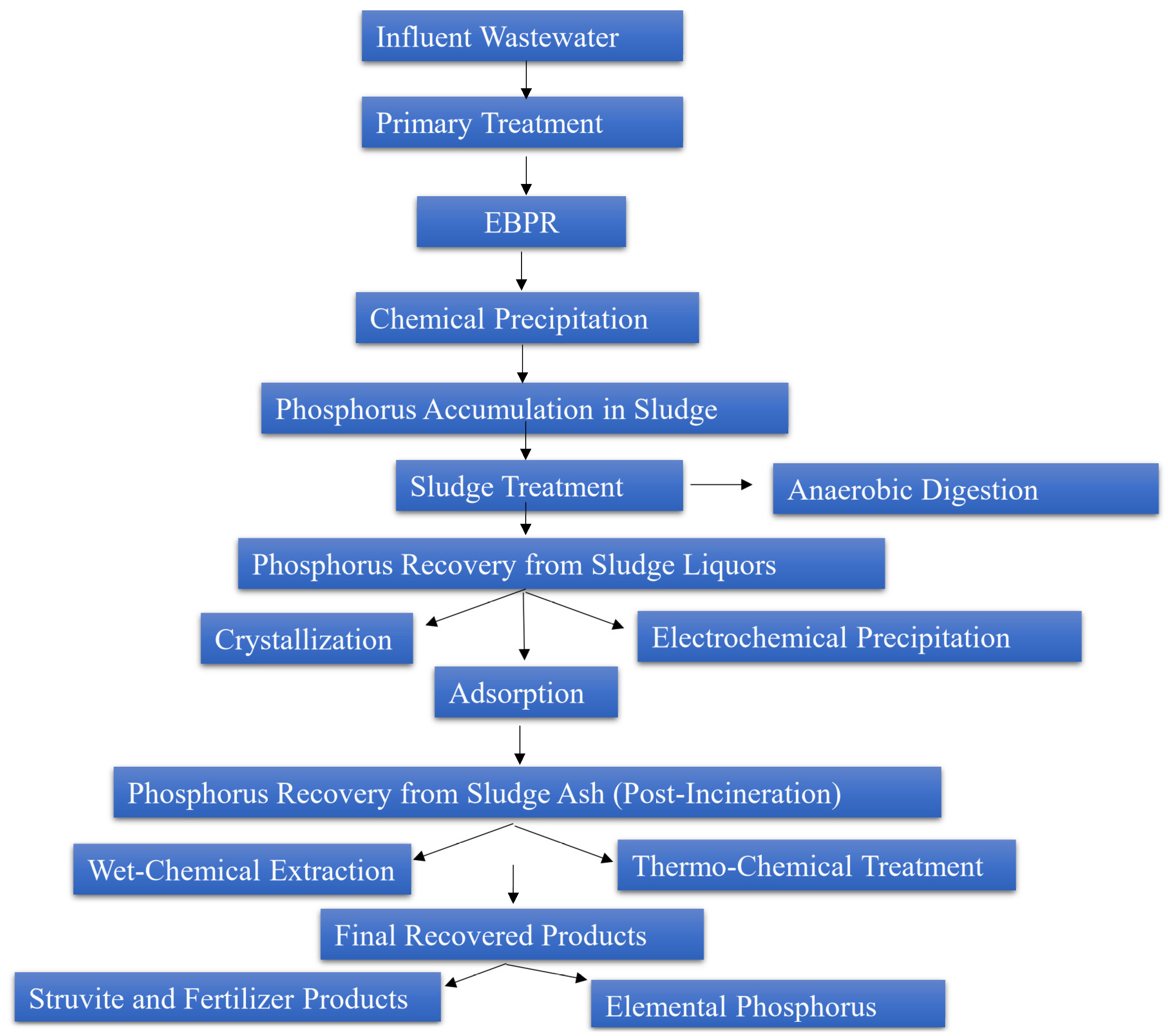
6. P Recovery from WWTP Streams
6.1. Systems for P Recovery from Liquid Streams
6.1.1. Crystallization Technologies
6.1.2. Adsorption
6.1.3. Electrochemical Precipitation
6.2. Systems for P Recovery from Raw and Digested Sewage Sludge
6.2.1. Struvite Crystallization (MagPrex® System)
6.2.2. Wet-Chemical Extraction
6.2.3. Aqua Reci® and LOPROX Systems
6.2.4. MEPHREC® Process
6.2.5. Vivianite Recovery
6.3. Systems for P Recovery from Sludge Ash
6.3.1. Wet-Chemical Leaching and Extraction
6.3.2. Thermo-Chemical Treatments
6.4. Economic Analysis of P Recovery Processes in WWTPs
7. Concluding Remarks and Perspective
7.1. Effectiveness of Biological and Chemical Methods
7.2. Operational Considerations and Redox Potential
7.3. Challenges, Opportunities, and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Boer, B.; Hirsch, P.; Johns, F.; Saul, B.; Scurrah, N. The Mekong: A Socio-Legal Approach to River Basin Development; Routledge: London, UK, 2015. [Google Scholar]
- Fones, G.R.; Bakir, A.; Gray, J.; Mattingley, L.; Measham, N.; Knight, P.; Bowes, M.J.; Greenwood, R.; Mills, G.A. Using high-frequency phosphorus monitoring for water quality management: A case study of the upper River Itchen, UK. Environ. Monit. Assess. 2020, 192, 184. [Google Scholar] [CrossRef]
- Bunce, J.T.; Ndam, E.; Ofiteru, I.D.; Moore, A.; Graham, D.W. A review of phosphorus removal technologies and their applicability to small-scale domestic wastewater treatment systems. Front. Environ. Sci. 2018, 6, 8. [Google Scholar] [CrossRef]
- Oleszkiewicz, J.; Kruk, D.; Devlin, T.; Lashkarizadeh, M.; Yuan, Q. Options for improved nutrient removal and recovery from municipal wastewater in the Canadian context. Environ. Technol. 2015, 20, 681–695. [Google Scholar]
- Li, J.W.; Xin, Z.T.; Wang, X.W.; Zheng, J.L.; Chao, F.H. Mechanisms of inactivation of hepatitis A virus by chlorine. Appl. Environ. Microbiol. 2002, 68, 4951–4955. [Google Scholar] [CrossRef]
- Xu, Z.-B.; Wang, W.-L.; Huang, N.; Wu, Q.-Y.; Lee, M.-Y.; Hu, H.-Y. 2-Phosphonobutane-1, 2, 4-tricarboxylic acid (PBTCA) degradation by ozonation: Kinetics, phosphorus transformation, anti-precipitation property changes and phosphorus removal. Water Res. 2019, 148, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Lucas, M.S.; Dias, A.A.; Sampaio, A.; Amaral, C.; Peres, J.A. Degradation of a textile reactive Azo dye by a combined chemical–biological process: Fenton’s reagent-yeast. Water Res. 2007, 41, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Cornel, P.; Schaum, C. Phosphorus recovery from wastewater: Needs, technologies and costs. Water Sci. Technol. 2009, 59, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, K.; Alliet, M.; Plana, Q.; Bernier, J.; Azimi, S.; Rocher, V.; Albasi, C. Modeling, simulation and control of biological and chemical P-removal processes for membrane bioreactors (MBRs) from lab to full-scale applications: State of the art. Sci. Total Environ. 2022, 809, 151109. [Google Scholar] [CrossRef]
- Rabbani, D.; Rashidipour, F.; Nasseri, S.; Mousavi, S.G.A.; Shaterian, M. High-efficiency removal of phosphorous from filtered activated sludge effluent using electrochemical process. J. Clean. Prod. 2020, 263, 121444. [Google Scholar] [CrossRef]
- Brix, H.; Arias, C.; Del Bubba, M. Media selection for sustainable phosphorus removal in subsurface flow constructed wetlands. Water Sci. Technol. 2001, 44, 47–54. [Google Scholar] [CrossRef]
- Yang, J.; Trela, J.; Zubrowska-Sudol, M.; Plaza, E. Intermittent aeration in one-stage partial nitritation/anammox process. Ecol. Eng. 2015, 75, 413–420. [Google Scholar] [CrossRef]
- Tian, Q.; Chen, J. Application of bioactivated carbon (BAC) Process in Water and Wastewater Treatment. Environ. Eng. 2006, 24, 84–86. [Google Scholar]
- Mohsenpour, S.F.; Hennige, S.; Willoughby, N.; Adeloye, A.; Gutierrez, T. Integrating micro-algae into wastewater treatment: A review. Sci. Total Environ. 2021, 752, 142168. [Google Scholar] [CrossRef] [PubMed]
- Vymazal, J. Subsurface horizontal-flow constructed wetlands for wastewater treatment: The Czech experience. Wetl. Ecol. Manag. 1996, 4, 199–206. [Google Scholar] [CrossRef]
- Shi, X.; Fan, J.; Zhang, J.; Shen, Y. Enhanced phosphorus removal in intermittently aerated constructed wetlands filled with various construction wastes. Environ. Sci. Pollut. Res. 2017, 24, 22524–22534. [Google Scholar] [CrossRef]
- Van Nieuwenhuijzen, A.; Mels, A. Characterisation of particulate matter in municipal wastewater. In Chemical Water and Wastewater Treatment VII; International Water Association (IWA): London, UK, 2002; pp. 203–212. [Google Scholar]
- Jarvie, H.; Neal, C.; Rowland, A.; Neal, M.; Morris, P.; Lead, J.; Lawlor, A.; Woods, C.; Vincent, C.; Guyatt, H. Role of riverine colloids in macronutrient and metal partitioning and transport, along an upland–lowland land-use continuum, under low-flow conditions. Sci. Total Environ. 2012, 434, 171–185. [Google Scholar] [CrossRef]
- Association, A.P.H. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1926; Volume 6. [Google Scholar]
- Worsfold, P.; McKelvie, I.; Monbet, P. Determination of phosphorus in natural waters: A historical review. Anal. Chim. Acta 2016, 918, 8–20. [Google Scholar] [CrossRef]
- Gache, S.A.M.; Angelini, A.A.R.; Sabeckis, M.L.; Flecha, F.L.G. Improving the stability of the malachite green method for the determination of phosphate using Pluronic F68. Anal. Biochem. 2020, 597, 113681. [Google Scholar]
- Chen, Y.-C.; Lo, K.-M.; Wang, Y.-X.; Chiu, T.-C.; Hu, C.-C. A sensitive colorimetric probe for detection of the phosphate ion. Sci. Rep. 2020, 10, 21215. [Google Scholar] [CrossRef]
- Nagul, E.A.; McKelvie, I.D.; Worsfold, P.; Kolev, S.D. The molybdenum blue reaction for the determination of orthophosphate revisited: Opening the black box. Anal. Chim. Acta 2015, 890, 60–82. [Google Scholar] [CrossRef]
- Rahutomo, S.; Kovar, J.L.; Thompson, M.L. Malachite green method for determining phosphorus concentration in diverse matrices. Commun. Soil Sci. Plant Anal. 2019, 50, 1743–1752. [Google Scholar] [CrossRef]
- Nagare, H.; Tsuno, H.; Saktaywin, W.; Soyama, T. Sludge ozonation and its application to a new advanced wastewater treatment process with sludge disintegration. Ozone: Sci. Eng. 2008, 30, 136–144. [Google Scholar] [CrossRef]
- Rott, E.; Minke, R.; Bali, U.; Steinmetz, H. Removal of phosphonates from industrial wastewater with UV/FeII, Fenton and UV/Fenton treatment. Water Res. 2017, 122, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadoon, A.A.T.; Salman, E.A.-A.; Mohammed, M. Critical review of organic pollutants in Refinery wastewater by advanced oxidation processes. J. Univ. Anbar Pure Sci. 2022, 16, 46–57. [Google Scholar] [CrossRef]
- Gilmour, D.; Blackwood, D.; Comber, S.; Thornell, A. Identifying human waste contribution of phosphorus loads to domestic wastewater. In Proceedings of the 11th International Conference on Urban Drainage, Held in Edinburgh, Scoltand, UK, 31 August–5 September 2008; pp. 1–10. [Google Scholar]
- Belivermiş, M.; Kılıç, Ö.; Çotuk, Y. Assessment of metal concentrations in indigenous and caged mussels (Mytilus galloprovincialis) on entire Turkish coastline. Chemosphere 2016, 144, 1980–1987. [Google Scholar] [CrossRef]
- Squadrone, S.; Benedetto, A.; Brizio, P.; Prearo, M.; Abete, M. Mercury and selenium in European catfish (Silurus glanis) from Northern Italian Rivers: Can molar ratio be a predictive factor for mercury toxicity in a top predator? Chemosphere 2015, 119, 24–30. [Google Scholar] [CrossRef]
- Arias, C.; Brix, H.; Johansen, N.-H. Phosphorus removal from municipal wastewater in an experimental two-stage vertical flow constructed wetland system equipped with a calcite filter. Water Sci. Technol. 2003, 48, 51–58. [Google Scholar] [CrossRef]
- Sukačová, K.; Trtílek, M.; Rataj, T. Phosphorus removal using a microalgal biofilm in a new biofilm photobioreactor for tertiary wastewater treatment. Water Res. 2015, 71, 55–63. [Google Scholar] [CrossRef]
- Kesaano, M.; Gardner, R.D.; Moll, K.; Lauchnor, E.; Gerlach, R.; Peyton, B.M.; Sims, R.C. Dissolved inorganic carbon enhanced growth, nutrient uptake, and lipid accumulation in wastewater grown microalgal biofilms. Bioresour. Technol. 2015, 180, 7–15. [Google Scholar] [CrossRef]
- Boelee, N.; Janssen, M.; Temmink, H.; Shrestha, R.; Buisman, C.; Wijffels, R. Nutrient removal and biomass production in an outdoor pilot-scale phototrophic biofilm reactor for effluent polishing. Appl. Biochem. Biotechnol. 2014, 172, 405–422. [Google Scholar] [CrossRef]
- He, S.; Xue, G. Algal-based immobilization process to treat the effluent from a secondary wastewater treatment plant (WWTP). J. Hazard. Mater. 2010, 178, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Yang, Z.-H.; Li, C.; Zeng, G.-M.; Ma, D.-H.; Zhou, L. A novel algal biofilm membrane photobioreactor for attached microalgae growth and nutrients removal from secondary effluent. Bioresour. Technol. 2015, 179, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Min, M.; Wang, L.; Li, Y.; Mohr, M.J.; Hu, B.; Zhou, W.; Chen, P.; Ruan, R. Cultivating Chlorella sp. in a pilot-scale photobioreactor using centrate wastewater for microalgae biomass production and wastewater nutrient removal. Appl. Biochem. Biotechnol. 2011, 165, 123–137. [Google Scholar] [CrossRef]
- Xu, M.; Bernards, M.; Hu, Z. Algae-facilitated chemical phosphorus removal during high-density Chlorella emersonii cultivation in a membrane bioreactor. Bioresour. Technol. 2014, 153, 383–387. [Google Scholar] [CrossRef]
- Praveen, P.; Loh, K.-C. Nitrogen and phosphorus removal from tertiary wastewater in an osmotic membrane photobioreactor. Bioresour. Technol. 2016, 206, 180–187. [Google Scholar] [CrossRef]
- Chen, L.; Gu, Y.; Cao, C.; Zhang, J.; Ng, J.-W.; Tang, C. Performance of a submerged anaerobic membrane bioreactor with forward osmosis membrane for low-strength wastewater treatment. Water Res. 2014, 50, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Hai, F.I.; Price, W.E.; Nghiem, L.D. Water extraction from mixed liquor of an aerobic bioreactor by forward osmosis: Membrane fouling and biomass characteristics assessment. Sep. Purif. Technol. 2015, 145, 56–62. [Google Scholar] [CrossRef]
- Sun, F.-y.; Wang, X.-m.; Li, X.-y. An innovative membrane bioreactor (MBR) system for simultaneous nitrogen and phosphorus removal. Process Biochem. 2013, 48, 1749–1756. [Google Scholar] [CrossRef]
- Smith, S.; Kim, G.; Doan, L.; Roh, H. Improving biological phosphorus removal in membrane bioreactors–a pilot study. J. Water Reuse Desalination 2014, 4, 25–33. [Google Scholar] [CrossRef]
- Yang, S.; Yang, F.; Fu, Z.; Wang, T.; Lei, R. Simultaneous nitrogen and phosphorus removal by a novel sequencing batch moving bed membrane bioreactor for wastewater treatment. J. Hazard. Mater. 2010, 175, 551–557. [Google Scholar] [CrossRef]
- Yin, J.; Zhang, P.; Li, F.; Li, G.; Hai, B. Simultaneous biological nitrogen and phosphorus removal with a sequencing batch reactor–biofilm system. Int. Biodeterior. Biodegrad. 2015, 103, 221–226. [Google Scholar] [CrossRef]
- Rahimi, Y.; Torabian, A.; Mehrdadi, N.; Shahmoradi, B. Simultaneous nitrification–denitrification and phosphorus removal in a fixed bed sequencing batch reactor (FBSBR). J. Hazard. Mater. 2011, 185, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xing, X.-H.; Wang, B.-Z. Characteristics of phosphorus removal from wastewater by biofilm sequencing batch reactor (SBR). Biochem. Eng. J. 2003, 16, 279–285. [Google Scholar] [CrossRef]
- Pronk, M.; De Kreuk, M.; De Bruin, B.; Kamminga, P.; Kleerebezem, R.v.; Van Loosdrecht, M. Full scale performance of the aerobic granular sludge process for sewage treatment. Water Res. 2015, 84, 207–217. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Z.; Wei, X.; Li, P.; Zhang, H.; Li, M.; Li, B.; Wang, S. Enhanced biological nitrogen and phosphorus removal using sequencing batch membrane-aerated biofilm reactor. Chem. Eng. Sci. 2015, 135, 559–565. [Google Scholar] [CrossRef]
- Díez-Montero, R.; De Florio, L.; González-Viar, M.; Herrero, M.; Tejero, I. Performance evaluation of a novel anaerobic–anoxic sludge blanket reactor for biological nutrient removal treating municipal wastewater. Bioresour. Technol. 2016, 209, 195–204. [Google Scholar] [CrossRef]
- Drizo, A.; Forget, C.; Chapuis, R.P.; Comeau, Y. Phosphorus removal by electric arc furnace steel slag and serpentinite. Water Res. 2006, 40, 1547–1554. [Google Scholar] [CrossRef]
- Gustafsson, J.P.; Renman, A.; Renman, G.; Poll, K. Phosphate removal by mineral-based sorbents used in filters for small-scale wastewater treatment. Water Res. 2008, 42, 189–197. [Google Scholar] [CrossRef]
- Renman, A.; Renman, G. Long-term phosphate removal by the calcium-silicate material Polonite in wastewater filtration systems. Chemosphere 2010, 79, 659–664. [Google Scholar] [CrossRef]
- Seo, Y.I.; Hong, K.H.; Kim, S.H.; Chang, D.; Lee, K.H.; Do Kim, Y. Phosphorus removal from wastewater by ionic exchange using a surface-modified Al alloy filter. J. Ind. Eng. Chem. 2013, 19, 744–747. [Google Scholar] [CrossRef]
- Martin, B.; Parsons, S.; Jefferson, B. Removal and recovery of phosphate from municipal wastewaters using a polymeric anion exchanger bound with hydrated ferric oxide nanoparticles. Water Sci. Technol. 2009, 60, 2637–2645. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Ngo, H.H.; Guo, W. Pilot scale study on a new membrane bioreactor hybrid system in municipal wastewater treatment. Bioresour. Technol. 2013, 141, 8–12. [Google Scholar] [CrossRef]
- Seviour, R.J.; Mino, T.; Onuki, M. The microbiology of biological phosphorus removal in activated sludge systems. FEMS Microbiol. Rev. 2003, 27, 99–127. [Google Scholar] [CrossRef]
- Brown, N.; Shilton, A. Luxury uptake of phosphorus by microalgae in waste stabilisation ponds: Current understanding and future direction. Rev. Environ. Sci. Bio/Technol. 2014, 13, 321–328. [Google Scholar] [CrossRef]
- Long, X.-Y.; Tang, R.; Wang, T.; Tao, G.-J.; Wang, J.-Y.; Zhou, H.-W.; Xue, M.; Yu, Y.-P. Characteristics of enhanced biological phosphorus removal (EBPR) process under the combined actions of intracellular and extracellular polyphosphate. Chemosphere 2021, 279, 130912. [Google Scholar] [CrossRef]
- Bond, P.L.; Keller, J.; Blackall, L.L. Anaerobic phosphate release from activated sludge with enhanced biological phosphorus removal. A possible mechanism of intracellular pH control. Biotechnol. Bioeng. 1999, 63, 507–515. [Google Scholar] [CrossRef]
- Barnard, J.L.; Dunlap, P.; Steichen, M. Rethinking the mechanisms of biological phosphorus removal: Barnard et al. Water Environ. Res. 2017, 89, 2043–2054. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Wei-jing, Z.; Liang, W.; Weixiang, W. Review on the main microorganisms and their metabolic mechanisms in enhanced biological phosphorus removal (EBPR) systems. Yingyong Shengtai Xuebao 2014, 25, 892. [Google Scholar]
- Jin, P.; Jin, X.; Wang, X.; Feng, Y.; Wang, X.C. Biological activated carbon treatment process for advanced water and wastewater treatment. Biomass Now Cultiv. Util. 2013, 7, 153–192. [Google Scholar]
- Mann, R.; Bavor, H. Phosphorus removal in constructed wetlands using gravel and industrial waste substrata. Water Sci. Technol. 1993, 27, 107–113. [Google Scholar] [CrossRef]
- Hoh, D.; Watson, S.; Kan, E. Algal biofilm reactors for integrated wastewater treatment and biofuel production: A review. Chem. Eng. J. 2016, 287, 466–473. [Google Scholar] [CrossRef]
- Iannacone, F.; Di Capua, F.; Granata, F.; Gargano, R.; Esposito, G. Shortcut nitrification-denitrification and biological phosphorus removal in acetate-and ethanol-fed moving bed biofilm reactors under microaerobic/aerobic conditions. Bioresour. Technol. 2021, 330, 124958. [Google Scholar] [CrossRef]
- Cai, W.; Zhang, B.; Jin, Y.; Lei, Z.; Feng, C.; Ding, D.; Hu, W.; Chen, N.; Suemura, T. Behavior of total phosphorus removal in an intelligent controlled sequencing batch biofilm reactor for municipal wastewater treatment. Bioresour. Technol. 2013, 132, 190–196. [Google Scholar] [CrossRef]
- Fanta, A.B.; Nair, A.M.; Saegrov, S.; Østerhus, S.W. Phosphorus removal from industrial discharge impacted municipal wastewater using sequencing batch moving bed biofilm reactor. J. Water Process Eng. 2021, 41, 102034. [Google Scholar] [CrossRef]
- Kodera, H.; Hatamoto, M.; Abe, K.; Kindaichi, T.; Ozaki, N.; Ohashi, A. Phosphate recovery as concentrated solution from treated wastewater by a PAO-enriched biofilm reactor. Water Res. 2013, 47, 2025–2032. [Google Scholar] [CrossRef] [PubMed]
- González-Martinez, S.; Wilderer, P.A. Phosphate removal in a biofilm reactor. Water Sci. Technol. 1991, 23, 1405–1415. [Google Scholar] [CrossRef]
- Metcalf; Eddy; Abu-Orf, M.; Bowden, G.; Burton, F.L.; Pfrang, W.; Stensel, H.D.; Tchobanoglous, G.; Tsuchihashi, R.; AECOM. Wastewater Engineering: Treatment and Resource Recovery; McGraw Hill Education: New York, NY, USA, 2014. [Google Scholar]
- Zhen, J.; Wang, Z.-B.; Ni, B.-J.; Ismail, S.; El-Baz, A.; Cui, Z.; Ni, S.-Q. Synergistic Integration of Anammox and Endogenous Denitrification Processes for the Simultaneous Carbon, Nitrogen, and Phosphorus Removal. Environ. Sci. Technol. 2024, 58, 10632–10643. [Google Scholar] [CrossRef]
- Ruiz, G.; Jeison, D.; Rubilar, O.; Ciudad, G.; Chamy, R. Nitrification–denitrification via nitrite accumulation for nitrogen removal from wastewaters. Bioresour. Technol. 2006, 97, 330–335. [Google Scholar] [CrossRef]
- Li, C.; Liu, S.; Ma, T.; Zheng, M.; Ni, J. Simultaneous nitrification, denitrification and phosphorus removal in a sequencing batch reactor (SBR) under low temperature. Chemosphere 2019, 229, 132–141. [Google Scholar] [CrossRef]
- Du, S.; Yu, D.; Zhao, J.; Wang, X.; Bi, C.; Zhen, J.; Yuan, M. Achieving deep-level nutrient removal via combined denitrifying phosphorus removal and simultaneous partial nitrification-endogenous denitrification process in a single-sludge sequencing batch reactor. Bioresour. Technol. 2019, 289, 121690. [Google Scholar] [CrossRef]
- Zeng, R.J.; Lemaire, R.; Yuan, Z.; Keller, J. Simultaneous nitrification, denitrification, and phosphorus removal in a lab-scale sequencing batch reactor. Biotechnol. Bioeng. 2003, 84, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Li, L.; Yang, Y.-Y.; Wang, X.-D.; Peng, Y.-Z. Denitrifying phosphorus removal and impact of nitrite accumulation on phosphorus removal in a continuous anaerobic–anoxic–aerobic (A2O) process treating domestic wastewater. Enzym. Microb. Technol. 2011, 48, 134–142. [Google Scholar] [CrossRef]
- Shen, N.; Zhou, Y. Enhanced biological phosphorus removal with different carbon sources. Appl. Microbiol. Biotechnol. 2016, 100, 4735–4745. [Google Scholar] [CrossRef]
- Gupta, V.K.; Ali, I.; Saleh, T.A.; Nayak, A.; Agarwal, S. Chemical treatment technologies for waste-water recycling—An overview. Rsc Adv. 2012, 2, 6380–6388. [Google Scholar] [CrossRef]
- Tong, J.; Chen, Y. Enhanced biological phosphorus removal driven by short-chain fatty acids produced from waste activated sludge alkaline fermentation. Environ. Sci. Technol. 2007, 41, 7126–7130. [Google Scholar] [CrossRef] [PubMed]
- Altmann, J.; Rehfeld, D.; Träder, K.; Sperlich, A.; Jekel, M. Combination of granular activated carbon adsorption and deep-bed filtration as a single advanced wastewater treatment step for organic micropollutant and phosphorus removal. Water Res. 2016, 92, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Batstone, D.J.; Keller, J. Biological phosphorus removal from abattoir wastewater at very short sludge ages mediated by novel PAO clade Comamonadaceae. Water Res. 2015, 69, 173–182. [Google Scholar] [CrossRef]
- Fan, Z.; Zeng, W.; Meng, Q.; Liu, H.; Liu, H.; Peng, Y. Achieving enhanced biological phosphorus removal utilizing waste activated sludge as sole carbon source and simultaneous sludge reduction in sequencing batch reactor. Sci. Total Environ. 2021, 799, 149291. [Google Scholar] [CrossRef]
- Tang, C.-C.; Tian, Y.; Liang, H.; Zuo, W.; Wang, Z.-W.; Zhang, J.; He, Z.-W. Enhanced nitrogen and phosphorus removal from domestic wastewater via algae-assisted sequencing batch biofilm reactor. Bioresour. Technol. 2018, 250, 185–190. [Google Scholar] [CrossRef]
- Vandamme, D.; Foubert, I.; Muylaert, K. Flocculation as a low-cost method for harvesting microalgae for bulk biomass production. Trends Biotechnol. 2013, 31, 233–239. [Google Scholar] [CrossRef]
- Dalecka, B.; Juhna, T.; Rajarao, G.K. Constructive use of filamentous fungi to remove pharmaceutical substances from wastewater. J. Water Process Eng. 2020, 33, 100992. [Google Scholar] [CrossRef]
- Mezule, L.; Berzina, I.; Strods, M. The impact of substrate–enzyme proportion for efficient hydrolysis of Hay. Energies 2019, 12, 3526. [Google Scholar] [CrossRef]
- Laezza, C.; Salbitani, G.; Carfagna, S. Fungal contamination in microalgal cultivation: Biological and biotechnological aspects of fungi-microalgae interaction. J. Fungi 2022, 8, 1099. [Google Scholar] [CrossRef]
- Boelee, N.; Temmink, H.; Janssen, M.; Buisman, C.; Wijffels, R. Nitrogen and phosphorus removal from municipal wastewater effluent using microalgal biofilms. Water Res. 2011, 45, 5925–5933. [Google Scholar] [CrossRef] [PubMed]
- De-Bashan, L.E.; Hernandez, J.-P.; Morey, T.; Bashan, Y. Microalgae growth-promoting bacteria as “helpers” for microalgae: A novel approach for removing ammonium and phosphorus from municipal wastewater. Water Res. 2004, 38, 466–474. [Google Scholar] [CrossRef]
- Ji, B.; Zhang, M.; Wang, L.; Wang, S.; Liu, Y. Removal mechanisms of phosphorus in non-aerated microalgal-bacterial granular sludge process. Bioresour. Technol. 2020, 312, 123531. [Google Scholar] [CrossRef]
- Sydney, E.d.; Da Silva, T.; Tokarski, A.; Novak, A.d.; De Carvalho, J.; Woiciecohwski, A.; Larroche, C.; Soccol, C. Screening of microalgae with potential for biodiesel production and nutrient removal from treated domestic sewage. Appl. Energy 2011, 88, 3291–3294. [Google Scholar] [CrossRef]
- Kim, T.-H.; Lee, Y.; Han, S.-H.; Hwang, S.-J. The effects of wavelength and wavelength mixing ratios on microalgae growth and nitrogen, phosphorus removal using Scenedesmus sp. for wastewater treatment. Bioresour. Technol. 2013, 130, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Barrington, S.; Lefsrud, M. Microalgae for phosphorus removal and biomass production: A six species screen for dual-purpose organisms. Gcb Bioenergy 2012, 4, 485–495. [Google Scholar] [CrossRef]
- Mielczarek, A.T.; Nguyen, H.T.T.; Nielsen, J.L.; Nielsen, P.H. Population dynamics of bacteria involved in enhanced biological phosphorus removal in Danish wastewater treatment plants. Water Res. 2013, 47, 1529–1544. [Google Scholar] [CrossRef]
- De-Bashan, L.E.; Moreno, M.; Hernandez, J.-P.; Bashan, Y. Removal of ammonium and phosphorus ions from synthetic wastewater by the microalgae Chlorella vulgaris coimmobilized in alginate beads with the microalgae growth-promoting bacterium Azospirillum brasilense. Water Res. 2002, 36, 2941–2948. [Google Scholar] [CrossRef]
- Larsdotter, K.; Jansen, J.L.C.; Dalhammar, G. Biologically mediated phosphorus precipitation in wastewater treatment with microalgae. Environ. Technol. 2007, 28, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Cydzik-Kwiatkowska, A.; Nosek, D. Biological release of phosphorus is more efficient from activated than from aerobic granular sludge. Sci. Rep. 2020, 10, 11076. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Benefield, L.; Randall, C.W. Phosphorus removal in the activated sludge process. Water Res. 1983, 17, 1193–1200. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Peng, Y.-Z.; Wang, R.-D.; Zhou, Y.-X. Understanding the granulation process of activated sludge in a biological phosphorus removal sequencing batch reactor. Chemosphere 2012, 86, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Benammar, L.; Menasria, T.; Ayachi, A.; Benounis, M. Phosphate removal using aerobic bacterial consortium and pure cultures isolated from activated sludge. Process Saf. Environ. Prot. 2015, 95, 237–246. [Google Scholar] [CrossRef]
- Kuroda, A.; Takiguchi, N.; Gotanda, T.; Nomura, K.; Kato, J.; Ikeda, T.; Ohtake, H. A simple method to release polyphosphate from activated sludge for phosphorus reuse and recycling. Biotechnol. Bioeng. 2002, 78, 333–338. [Google Scholar] [CrossRef]
- Cao, Y.; Kwok, B.H.; Van Loosdrecht, M.C.; Daigger, G.T.; Png, H.Y.; Long, W.Y.; Chye, C.S.; Ghani, Y.A. The occurrence of enhanced biological phosphorus removal in a 200,000 m3/day partial nitration and Anammox activated sludge process at the Changi water reclamation plant, Singapore. Water Sci. Technol. 2017, 75, 741–751. [Google Scholar] [CrossRef]
- Escudero-Curiel, S.; Pazos, M.; Sanromán, M.Á. Bio-Adsorbent-Based Integrated System. In Biodegradation of Toxic and Hazardous Chemicals; CRC Press: Boca Raton, FL, USA, 2024; pp. 58–79. [Google Scholar]
- Gadd, G.M. Biosorption: Critical review of scientific rationale, environmental importance and significance for pollution treatment. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2009, 84, 13–28. [Google Scholar] [CrossRef]
- Schiewer, S.; Volesky, B. Modeling of the proton-metal ion exchange in biosorption. Environ. Sci. Technol. 1995, 29, 3049–3058. [Google Scholar] [CrossRef]
- Davis, T.A.; Volesky, B.; Mucci, A. A review of the biochemistry of heavy metal biosorption by brown algae. Water Res. 2003, 37, 4311–4330. [Google Scholar] [CrossRef]
- Kuyucak, N. Feasibility of biosorbents application. Biosorption Heavy Met. 1990, 4, 372–377. [Google Scholar]
- Genz, A.; Kornmüller, A.; Jekel, M. Advanced phosphorus removal from membrane filtrates by adsorption on activated aluminium oxide and granulated ferric hydroxide. Water Res. 2004, 38, 3523–3530. [Google Scholar] [CrossRef]
- Zhu, D.; Chen, Y.; Yang, H.; Wang, S.; Wang, X.; Zhang, S.; Chen, H. Synthesis and characterization of magnesium oxide nanoparticle-containing biochar composites for efficient phosphorus removal from aqueous solution. Chemosphere 2020, 247, 125847. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Ngo, H.; Guo, W.; Zhou, J.; Wang, J.; Liang, H.; Li, G. Phosphorus elimination from aqueous solution using ‘zirconium loaded okara’as a biosorbent. Bioresour. Technol. 2014, 170, 30–37. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Huang, H.; Lv, X.; Zhao, N.; Guo, G.; Zhang, D. Removal of phosphate from aqueous solution by dolomite-modified biochar derived from urban dewatered sewage sludge. Sci. Total Environ. 2019, 687, 460–469. [Google Scholar] [CrossRef]
- Marrot, B.; Barrios-Martinez, A.; Moulin, P.; Roche, N. Industrial wastewater treatment in a membrane bioreactor: A review. Environ. Prog. 2004, 23, 59–68. [Google Scholar] [CrossRef]
- Krzeminski, P.; Leverette, L.; Malamis, S.; Katsou, E. Membrane bioreactors–a review on recent developments in energy reduction, fouling control, novel configurations, LCA and market prospects. J. Membr. Sci. 2017, 527, 207–227. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Lee, C.-C.; Lee, F.-Y.; Tseng, S.-K.; Liao, C.-J. Performance of subsurface flow constructed wetland taking pretreated swine effluent under heavy loads. Bioresour. Technol. 2004, 92, 173–179. [Google Scholar] [CrossRef]
- Qiu, G.; Law, Y.-M.; Das, S.; Ting, Y.-P. Direct and complete phosphorus recovery from municipal wastewater using a hybrid microfiltration-forward osmosis membrane bioreactor process with seawater brine as draw solution. Environ. Sci. Technol. 2015, 49, 6156–6163. [Google Scholar] [CrossRef]
- Fu, Z.; Yang, F.; An, Y.; Xue, Y. Simultaneous nitrification and denitrification coupled with phosphorus removal in an modified anoxic/oxic-membrane bioreactor (A/O-MBR). Biochem. Eng. J. 2009, 43, 191–196. [Google Scholar] [CrossRef]
- Vymazal, J. Removal of nutrients in various types of constructed wetlands. Sci. Total Environ. 2007, 380, 48–65. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Wei, D.; Zhang, J.; Hu, J.; Liu, Z.; Li, R. Natural pyrite to enhance simultaneous long-term nitrogen and phosphorus removal in constructed wetland: Three years of pilot study. Water Res. 2019, 148, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Wood, R.; McAtamney, C. Constructed wetlands for waste water treatment: The use of laterite in the bed medium in phosphorus and heavy metal removal. Hydrobiologia 1996, 340, 323–331. [Google Scholar] [CrossRef]
- Luo, P.; Liu, F.; Liu, X.; Wu, X.; Yao, R.; Chen, L.; Li, X.; Xiao, R.; Wu, J. Phosphorus removal from lagoon-pretreated swine wastewater by pilot-scale surface flow constructed wetlands planted with Myriophyllum aquaticum. Sci. Total Environ. 2017, 576, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Nandakumar, S.; Pipil, H.; Ray, S.; Haritash, A. Removal of phosphorous and nitrogen from wastewater in Brachiaria-based constructed wetland. Chemosphere 2019, 233, 216–222. [Google Scholar] [CrossRef]
- Maucieri, C.; Salvato, M.; Borin, M. Vegetation contribution on phosphorus removal in constructed wetlands. Ecol. Eng. 2020, 152, 105853. [Google Scholar] [CrossRef]
- Wu, H.; Wang, J.; Chen, J.; Wang, X.; Li, D.; Hou, J.; He, X. Advanced nitrogen and phosphorus removal by combining endogenous denitrification and denitrifying dephosphatation in constructed wetlands. J. Environ. Manag. 2021, 294, 112967. [Google Scholar] [CrossRef]
- Beutel, M.W.; Morgan, M.R.; Erlenmeyer, J.J.; Brouillard, E.S. Phosphorus Removal in a Surface-Flow Constructed Wetland Treating Agricultural Runoff. J. Environ. Qual. 2014, 43, 1071–1080. [Google Scholar] [CrossRef]
- Liikanen, A.; Puustinen, M.; Koskiaho, J.; Väisänen, T.; Martikainen, P.; Hartikainen, H. Phosphorus removal in a wetland constructed on former arable land. J. Environ. Qual. 2004, 33, 1124–1132. [Google Scholar] [CrossRef]
- Metcalf, L.; Eddy, H.P.; Tchobanoglous, G. Wastewater Engineering: Treatment, Disposal, and Reuse; McGraw-Hill New York: New York, NY, USA, 1991; Volume 4. [Google Scholar]
- Fuzawa, M.; Araud, E.; Li, J.; Shisler, J.L.; Nguyen, T.H. Free chlorine disinfection mechanisms of rotaviruses and human norovirus surrogate tulane virus attached to fresh produce surfaces. Environ. Sci. Technol. 2019, 53, 11999–12006. [Google Scholar] [CrossRef] [PubMed]
- Churn, C.; Bates, R.; Boardman, G. Mechanism of chlorine inactivation of DNA-containing parvovirus H-1. Appl. Environ. Microbiol. 1983, 46, 1394–1402. [Google Scholar] [CrossRef]
- Na, C.; Olson, T.M. Relative reactivity of amino acids with chlorine in mixtures. Environ. Sci. Technol. 2007, 41, 3220–3225. [Google Scholar] [CrossRef]
- Cho, M.; Kim, J.; Kim, J.Y.; Yoon, J.; Kim, J.-H. Mechanisms of Escherichia coli inactivation by several disinfectants. Water Res. 2010, 44, 3410–3418. [Google Scholar] [CrossRef]
- Léziart, T.; Dutheil de la Rochere, P.-M.; Cheswick, R.; Jarvis, P.; Nocker, A. Effect of turbidity on water disinfection by chlorination with the emphasis on humic acids and chalk. Environ. Technol. 2019, 40, 1734–1743. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, M.; Zhang, X.; Jiang, J.; Liu, J.; Yau, C.F.; Graham, N.J.; Li, X. Two-step chlorination: A new approach to disinfection of a primary sewage effluent. Water Res. 2017, 108, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Tang, Y.; Shih, K.; Li, B. Enhanced phosphorus availability and heavy metal removal by chlorination during sewage sludge pyrolysis. J. Hazard. Mater. 2020, 382, 121110. [Google Scholar] [CrossRef]
- Huang, H.; Yang, J.; Li, D. Recovery and removal of ammonia–nitrogen and phosphate from swine wastewater by internal recycling of struvite chlorination product. Bioresour. Technol. 2014, 172, 253–259. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, X.; Xia, T.; Chen, H.; Huang, F.; Wei, C.; Qiu, G. Effects of intensive chlorine disinfection on nitrogen and phosphorus removal in WWTPs. Sci. Total Environ. 2024, 918, 170273. [Google Scholar] [CrossRef]
- Wen, G.; Liang, Z.; Xu, X.; Cao, R.; Wan, Q.; Ji, G.; Lin, W.; Wang, J.; Yang, J.; Huang, T. Inactivation of fungal spores in water using ozone: Kinetics, influencing factors and mechanisms. Water Res. 2020, 185, 116218. [Google Scholar] [CrossRef]
- Zuma, F.; Lin, J.; Jonnalagadda, S.B. Ozone-initiated disinfection kinetics of Escherichia coli in water. J. Environ. Sci. Health Part A 2009, 44, 48–56. [Google Scholar] [CrossRef]
- Staehelin, J.; Hoigne, J. Decomposition of ozone in water: Rate of initiation by hydroxide ions and hydrogen peroxide. Environ. Sci. Technol. 1982, 16, 676–681. [Google Scholar] [CrossRef]
- Saktaywin, W.; Tsuno, H.; Nagare, H.; Soyama, T.; Weerapakkaroon, J. Advanced sewage treatment process with excess sludge reduction and phosphorus recovery. Water Res. 2005, 39, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Cosgun, S.; Semerci, N. Combined and individual applications of ozonation and microwave treatment for waste activated sludge solubilization and nutrient release. J. Environ. Manag. 2019, 241, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tian, Y.; Zhang, J. Release of phosphorus from sewage sludge during ozonation and removal by magnesium ammonium phosphate. Environ. Sci. Pollut. Res. 2017, 24, 23794–23802. [Google Scholar] [CrossRef]
- Haber, F.; Weiss, J. The catalytic decomposition of hydrogen peroxide by iron salts. Proc. R. Soc. Lond. Ser. A-Math. Phys. Sci. 1934, 147, 332–351. [Google Scholar]
- Bray, W.C.; Gorin, M. Ferryl ion, a compound of tetravalent iron. J. Am. Chem. Soc. 1932, 54, 2124–2125. [Google Scholar] [CrossRef]
- Duesterberg, C.K.; Mylon, S.E.; Waite, T.D. pH effects on iron-catalyzed oxidation using Fenton’s reagent. Environ. Sci. Technol. 2008, 42, 8522–8527. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Szpyrkowicz, L.; Juzzolino, C.; Kaul, S.N. A comparative study on oxidation of disperse dyes by electrochemical process, ozone, hypochlorite and Fenton reagent. Water Res. 2001, 35, 2129–2136. [Google Scholar] [CrossRef]
- Gong, C.; Jiang, J. Ultrasound coupled with Fenton oxidation pre-treatment of sludge to release organic carbon, nitrogen and phosphorus. Sci. Total Environ. 2015, 532, 495–500. [Google Scholar] [CrossRef]
- Yang, L.; Sheng, M.; Zhao, H.; Qian, M.; Chen, X.; Zhuo, Y.; Cao, G. Treatment of triethyl phosphate wastewater by Fenton oxidation and aerobic biodegradation. Sci. Total Environ. 2019, 678, 821–829. [Google Scholar] [CrossRef]
- Senn, A.M.; Russo, Y.M.; Litter, M.I. Treatment of wastewater from an alkaline cleaning solution by combined coagulation and photo-Fenton processes. Sep. Purif. Technol. 2014, 132, 552–560. [Google Scholar] [CrossRef]
- Zuo, G.-M.; Cheng, Z.-X.; Chen, H.; Li, G.-W.; Miao, T. Study on photocatalytic degradation of several volatile organic compounds. J. Hazard. Mater. 2006, 128, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Rasul, M.; Brown, R.; Hashib, M. Influence of parameters on the heterogeneous photocatalytic degradation of pesticides and phenolic contaminants in wastewater: A short review. J. Environ. Manag. 2011, 92, 311–330. [Google Scholar] [CrossRef] [PubMed]
- Devipriya, S.; Yesodharan, S. Photocatalytic degradation of pesticide contaminants in water. Sol. Energy Mater. Sol. Cells 2005, 86, 309–348. [Google Scholar] [CrossRef]
- Li, W. Photocatalysis of oxide semiconductors. J. Aust. Ceram. Soc. 2013, 108, 41–46. [Google Scholar]
- Dionysiou, D.D.; Khodadoust, A.P.; Kern, A.M.; Suidan, M.T.; Baudin, I.; Laîné, J.-M. Continuous-mode photocatalytic degradation of chlorinated phenols and pesticides in water using a bench-scale TiO2 rotating disk reactor. Appl. Catal. B Environ. 2000, 24, 139–155. [Google Scholar] [CrossRef]
- Vaya, D.; Surolia, P.K. Semiconductor based photocatalytic degradation of pesticides: An overview. Environ. Technol. Innov. 2020, 20, 101128. [Google Scholar] [CrossRef]
- Huang, Z.; Huang, J.; Liu, T.; Wen, Y.; Wu, H.; Yang, S.; Li, H. Full carbon upcycling of organophosphorus wastewater enabled by interface photolysis. Chem. Eng. J. 2024, 485, 149987. [Google Scholar] [CrossRef]
- Zarei, A.R.; Rezaeivahidian, H.; Soleymani, A.R. Investigation on removal of p-nitrophenol using a hybridized photo-thermal activated persulfate process: Central composite design modeling. Process Saf. Environ. Prot. 2015, 98, 109–115. [Google Scholar] [CrossRef]
- Sun, S.; Wang, S.; Ye, Y.; Pan, B. Highly efficient removal of phosphonates from water by a combined Fe (III)/UV/co-precipitation process. Water Res. 2019, 153, 21–28. [Google Scholar] [CrossRef]
- Oturan, N.; Oturan, M.A. Electro-fenton process: Background, new developments, and applications. In Electrochemical Water and Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2018; pp. 193–221. [Google Scholar]
- Atmaca, E. Treatment of landfill leachate by using electro-Fenton method. J. Hazard. Mater. 2009, 163, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Moreira, F.C.; Boaventura, R.A.; Brillas, E.; Vilar, V.J. Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewaters. Appl. Catal. B Environ. 2017, 202, 217–261. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Zhou, M.; Martínez-Huitle, C.A. Heterogeneous electro-Fenton and photoelectro-Fenton processes: A critical review of fundamental principles and application for water/wastewater treatment. Appl. Catal. B Environ. 2018, 235, 103–129. [Google Scholar] [CrossRef]
- Özcan, A.; Şahin, Y.; Oturan, M.A. Complete removal of the insecticide azinphos-methyl from water by the electro-Fenton method–A kinetic and mechanistic study. Water Res. 2013, 47, 1470–1479. [Google Scholar] [CrossRef] [PubMed]
- Mousset, E.; Wang, Z.; Lefebvre, O. Electro-Fenton for control and removal of micropollutants–process optimization and energy efficiency. Water Sci. Technol. 2016, 74, 2068–2074. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.-q.; Ren, L.-m. Organic matter removal from mother liquor of gas field wastewater by electro-Fenton process with the addition of H2O2: Effect of initial pH. R. Soc. Open Sci. 2019, 6, 191304. [Google Scholar] [CrossRef]
- Jinisha, R.; Gandhimathi, R.; Ramesh, S.; Nidheesh, P.; Velmathi, S. Removal of rhodamine B dye from aqueous solution by electro-Fenton process using iron-doped mesoporous silica as a heterogeneous catalyst. Chemosphere 2018, 200, 446–454. [Google Scholar] [CrossRef]
- Pozza, A.d.; Ferrantelli, P.; Merli, C.; Petrucci, E. Oxidation efficiency in the electro-Fenton process. J. Appl. Electrochem. 2005, 35, 391–398. [Google Scholar] [CrossRef]
- Safarzadeh-Amiri, A.; Bolton, J.R.; Cater, S.R. The use of iron in advanced oxidation processes. J. Adv. Oxid. Technol. 1996, 1, 18–26. [Google Scholar] [CrossRef]
- Lin, J.-G.; Ma, Y.-S. Oxidation of 2-chlorophenol in water by ultrasound/Fenton method. J. Environ. Eng. 2000, 126, 130–137. [Google Scholar] [CrossRef]
- Kang, S.-F.; Liao, C.-H.; Po, S.-T. Decolorization of textile wastewater by photo-Fenton oxidation technology. Chemosphere 2000, 41, 1287–1294. [Google Scholar] [CrossRef]
- Ebrahiem, E.E.; Al-Maghrabi, M.N.; Mobarki, A.R. Removal of organic pollutants from industrial wastewater by applying photo-Fenton oxidation technology. Arab. J. Chem. 2017, 10, S1674–S1679. [Google Scholar] [CrossRef]
- O’Dowd, K.; Pillai, S.C. Photo-Fenton disinfection at near neutral pH: Process, parameter optimization and recent advances. J. Environ. Chem. Eng. 2020, 8, 104063. [Google Scholar] [CrossRef]
- Chen, C.; Lu, C. Photocatalytic degradation of basic violet 4: Degradation efficiency, product distribution, and mechanisms. J. Phys. Chem. C 2007, 111, 13922–13932. [Google Scholar] [CrossRef]
- Okano, M.; Itoh, K.; Fujishima, A.; Honda, K. Photoelectrochemical polymerization of pyrrole on TiO2 and its application to conducting pattern generation. J. Electrochem. Soc. 1987, 134, 837. [Google Scholar] [CrossRef]
- Zeltner Jr, W. Supported titania for photodegradation. Chemtech 1993, 23, 21. [Google Scholar]
- Mills, A.; Davies, R.H.; Worsley, D. Water purification by semiconductor photocatalysis. Chem. Soc. Rev. 1993, 22, 417–425. [Google Scholar] [CrossRef]
- Robert, D.; Weber, J.V. Photocatalytic degradation of methylbutandioic acid (MBA) in aqueous TiO2 suspension: Influences of MBA adsorption on the solid semi-conductor. J. Clean. Prod. 1998, 6, 335–338. [Google Scholar] [CrossRef]
- Jia, H.; Zheng, Z.; Zhao, H.; Zhang, L.; Zou, Z. Nonaqueous sol–gel synthesis and growth mechanism of single crystalline TiO2 nanorods with high photocatalytic activity. Mater. Res. Bull. 2009, 44, 1312–1316. [Google Scholar] [CrossRef]
- Cai, H.; Mu, W.; Liu, W.; Zhang, X.; Deng, Y. Sol–gel synthesis highly porous titanium dioxide microspheres with cellulose nanofibrils-based aerogel templates. Inorg. Chem. Commun. 2015, 51, 71–74. [Google Scholar] [CrossRef]
- Xu, B.; Ahmed, M.B.; Zhou, J.L.; Altaee, A.; Wu, M.; Xu, G. Photocatalytic removal of perfluoroalkyl substances from water and wastewater: Mechanism, kinetics and controlling factors. Chemosphere 2017, 189, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Rabindranathan, S.; Devipriya, S.; Yesodharan, S. Photocatalytic degradation of phosphamidon on semiconductor oxides. J. Hazard. Mater. 2003, 102, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Vaiano, V.; Sacco, O.; Sannino, D.; Ciambelli, P. Process intensification in the removal of organic pollutants from wastewater using innovative photocatalysts obtained coupling Zinc Sulfide based phosphors with nitrogen doped semiconductors. J. Clean. Prod. 2015, 100, 208–211. [Google Scholar] [CrossRef]
- Zou, R.; Xu, T.; Lei, X.; Wu, Q.; Xue, S. Novel and efficient red phosphorus/hollow hydroxyapatite microsphere photocatalyst for fast removal of antibiotic pollutants. J. Phys. Chem. Solids 2020, 139, 109353. [Google Scholar] [CrossRef]
- Goswami, D. Engineering of solar photocatalytic detoxification and disinfection process. Adv. Sol. Energy 1995, 10, 165–210. [Google Scholar]
- Alfano, O.; Bahnemann, D.; Cassano, A.; Dillert, R.; Goslich, R. Photocatalysis in water environments using artificial and solar light. Catal. Today 2000, 58, 199–230. [Google Scholar] [CrossRef]
- Oyama, T.; Aoshima, A.; Horikoshi, S.; Hidaka, H.; Zhao, J.; Serpone, N. Solar photocatalysis, photodegradation of a commercial detergent in aqueous TiO2 dispersions under sunlight irradiation. Sol. Energy 2004, 77, 525–532. [Google Scholar] [CrossRef]
- Zou, L.; Li, Y.; Hu, E. Photocatalytic decolorization of lanasol blue CE dye solution using a flat-plate reactor. J. Environ. Eng. 2005, 131, 102–107. [Google Scholar] [CrossRef]
- Kositzi, M.; Poulios, I.; Malato, S.; Caceres, J.; Campos, A. Solar photocatalytic treatment of synthetic municipal wastewater. Water Res. 2004, 38, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, X.; Wang, Y.; Djellabi, R. Recovery of phosphorus from hypophosphite-laden wastewater: A single-compartment photoelectrocatalytic cell system integrating oxidation and precipitation. Environ. Sci. Technol. 2019, 54, 1204–1213. [Google Scholar] [CrossRef]
- Oliveira, M.; Rodrigues, A.; Ribeiro, D.; Brito, A.; Nogueira, R.; Machado, A. Phosphorus removal by a fixed-bed hybrid polymer nanocomposite biofilm reactor. Chem. Ecol. 2014, 30, 428–439. [Google Scholar] [CrossRef][Green Version]
- Sengupta, S.; Pandit, A. Selective removal of phosphorus from wastewater combined with its recovery as a solid-phase fertilizer. Water Res. 2011, 45, 3318–3330. [Google Scholar] [CrossRef]
- Fan, J.; Hu, H.; Zhang, Y.; Zhu, L. Biological phosphorus removal combined with ferrous chemical phosphorus removal. Adv. Mater. Res. 2014, 955, 3339–3342. [Google Scholar] [CrossRef]
- Zaletova, N.; Zaletov, S.; Bulychev, I. Potential of biological phosphorus removal. In MATEC Web of Conferences; EDP Sciences: Les Ulis, France, 2018; p. 09021. [Google Scholar]
- Coats, E.R.; Watkins, D.L.; Kranenburg, D. A comparative environmental life-cycle analysis for removing phosphorus from wastewater: Biological versus physical/chemical processes. Water Environ. Res. 2011, 83, 750–760. [Google Scholar] [CrossRef]
- Di Capua, F.; de Sario, S.; Ferraro, A.; Petrella, A.; Race, M.; Pirozzi, F.; Fratino, U.; Spasiano, D. Phosphorous removal and recovery from urban wastewater: Current practices and new directions. Sci. Total Environ. 2022, 823, 153750. [Google Scholar] [CrossRef]
- Egle, L.; Rechberger, H.; Krampe, J.; Zessner, M. Phosphorus recovery from municipal wastewater: An integrated comparative technological, environmental and economic assessment of P recovery technologies. Sci. Total Environ. 2016, 571, 522–542. [Google Scholar] [CrossRef]
- Di Capua, F.; Spasiano, D.; Giordano, A.; Adani, F.; Fratino, U.; Pirozzi, F.; Esposito, G. High-solid anaerobic digestion of sewage sludge: Challenges and opportunities. Appl. Energy 2020, 278, 115608. [Google Scholar] [CrossRef]
- Amann, A.; Zoboli, O.; Krampe, J.; Rechberger, H.; Zessner, M.; Egle, L. Environmental impacts of phosphorus recovery from municipal wastewater. Resour. Conserv. Recycl. 2018, 130, 127–139. [Google Scholar] [CrossRef]
- Chrispim, M.C.; Scholz, M.; Nolasco, M.A. Phosphorus recovery from municipal wastewater treatment: Critical review of challenges and opportunities for developing countries. J. Environ. Manag. 2019, 248, 109268. [Google Scholar] [CrossRef] [PubMed]
- Desmidt, E.; Ghyselbrecht, K.; Zhang, Y.; Pinoy, L.; Van der Bruggen, B.; Verstraete, W.; Rabaey, K.; Meesschaert, B. Global phosphorus scarcity and full-scale P-recovery techniques: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 336–384. [Google Scholar] [CrossRef]
- Ajmal, Z.; Muhmood, A.; Usman, M.; Kizito, S.; Lu, J.; Dong, R.; Wu, S. Phosphate removal from aqueous solution using iron oxides: Adsorption, desorption and regeneration characteristics. J. Colloid Interface Sci. 2018, 528, 145–155. [Google Scholar] [CrossRef]
- Goscianska, J.; Ptaszkowska-Koniarz, M.; Frankowski, M.; Franus, M.; Panek, R.; Franus, W. Removal of phosphate from water by lanthanum-modified zeolites obtained from fly ash. J. Colloid Interface Sci. 2018, 513, 72–81. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, B.; Wester, A.E.; Chen, J.; He, F.; Chen, H.; Gao, B. Reclaiming phosphorus from secondary treated municipal wastewater with engineered biochar. Chem. Eng. J. 2019, 362, 460–468. [Google Scholar] [CrossRef]
- Lei, Y.; Remmers, J.C.; Saakes, M.; van der Weijden, R.D.; Buisman, C.J. Is there a precipitation sequence in municipal wastewater induced by electrolysis? Environ. Sci. Technol. 2018, 52, 8399–8407. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Remy, C.; Kabbe, C.; Barjenbruch, M. Comparative environmental life cycle assessment of phosphorus recovery with different generations of the AirPrex® systems. Int. J. Environ. Sci. Technol. 2019, 16, 2427–2440. [Google Scholar] [CrossRef]
- Meyer, C.; Preyl, V.; Steinmetz, H.; Maier, W.; Mohn, R.-E.; Schönberger, H. The stuttgart process (Germany). In Phosphorus Recovery and Recycling; Springer: Singapore, 2019; pp. 283–295. [Google Scholar]
- Stendahl, K.; Jäfverström, S. Recycling of sludge with the Aqua Reci process. Water Sci. Technol. 2004, 49, 233–240. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Blöcher, C.; Niewersch, C.; Melin, T. Phosphorus recovery from sewage sludge with a hybrid process of low pressure wet oxidation and nanofiltration. Water Res. 2012, 46, 2009–2019. [Google Scholar] [CrossRef]
- Günther, S.; Grunert, M.; Müller, S. Overview of recent advances in phosphorus recovery for fertilizer production. Eng. Life Sci. 2018, 18, 434–439. [Google Scholar] [CrossRef]
- Wilfert, P.; Dugulan, A.I.; Goubitz, K.; Korving, L.; Witkamp, G.J.; Van Loosdrecht, M.C.M. Vivianite as the main phosphate mineral in digested sewage sludge and its role for phosphate recovery. Water Res. 2018, 144, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Di Costanzo, N.; Cesaro, A.; Di Capua, F.; Esposito, G. Exploiting the nutrient potential of anaerobically digested sewage sludge: A review. Energies 2021, 14, 8149. [Google Scholar] [CrossRef]
- Weigand, H.; Bertau, M.; Hübner, W.; Bohndick, F.; Bruckert, A. RecoPhos: Full-scale fertilizer production from sewage sludge ash. Waste Manag. 2013, 33, 540–544. [Google Scholar] [CrossRef]
- Hermann, L.; Schaaf, T. Outotec (AshDec®) process for P fertilizers from sludge ash. In Phosphorus Recovery and Recycling; Springer: Singapore, 2019; pp. 221–233. [Google Scholar]
- Jupp, A.R.; Beijer, S.; Narain, G.C.; Schipper, W.; Slootweg, J.C. Phosphorus recovery and recycling–closing the loop. Chem. Soc. Rev. 2021, 50, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Dewaele, C. NuReSys P & N recovery as struvite. In Proceedings of the 2nd European Sustainable Phosphorus Conference (ESPC2), Berlin, Germany, 5–6 March 2015; p. 21. Available online: https://phosphorusplatform.eu/activities/conference/espc2015/47-espc2-highlights/596-presentations-at-the-espc2 (accessed on 14 June 2024).
- Berg, U.; Knoll, G.; Kaschka, E.; Kreutzer, V.; Donnert, D.; Weidler, P.; Nüesch, R. P-RoC-Phosphorus recovery from wastewater by crystallisation of calcium phosphate compounds. J. Residuals Sci. Technol. 2005, 4, 121–126. [Google Scholar]
- Monballiu, A.; Ghyselbrecht, K.; Pinoy, L.; Meesschaert, B. Phosphorous recovery as calcium phosphate from UASB effluent after nitrification with or without subsequent denitrification. J. Environ. Chem. Eng. 2020, 8, 104119. [Google Scholar] [CrossRef]
- Law, K.P.; Pagilla, K.R. Reclaimed phosphorus commodity reserve from water resource recovery facilities—A strategic regional concept towards phosphorus recovery. Resour. Conserv. Recycl. 2019, 150, 104429. [Google Scholar] [CrossRef]
- Muys, M.; Phukan, R.; Brader, G.; Samad, A.; Moretti, M.; Haiden, B.; Pluchon, S.; Roest, K.; Vlaeminck, S.E.; Spiller, M. A systematic comparison of commercially produced struvite: Quantities, qualities and soil-maize phosphorus availability. Sci. Total Environ. 2021, 756, 143726. [Google Scholar] [CrossRef]
- Costa, R.H.; Villafranca, B.M.; Voltolini, C.A.; Guimarães, L.B.; Hoffmann, H.; Velho, V.F.; Mohedano, R.A. Effectiveness of phosphorus removal in an SBR using co-precipitation with ferric chloride, and its effects on microbial activity. Braz. J. Chem. Eng. 2019, 36, 785–795. [Google Scholar] [CrossRef]
- Raji, V.; Packialakshmi, S. Assessing the wastewater pollutants retaining for a soil aquifer treatment using batch column experiments. Civ. Eng. J. 2022, 8, 1482–1491. [Google Scholar] [CrossRef]
- Ajeng, A.A.; Rosli, N.S.M.; Abdullah, R.; Yaacob, J.S.; Qi, N.C.; Loke, S.P. Resource recovery from hydroponic wastewaters using microalgae-based biorefineries: A circular bioeconomy perspective. J. Biotechnol. 2022, 360, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Mielcarek, A.; Jóźwiak, T.; Rodziewicz, J.; Bryszewski, K.; Janczukowicz, W.; Kalisz, B.; Tavares, J.M.R. Recovery of phosphorus and other minerals from greenhouse wastewater generated during soilless tomato cultivation by means of alkalizing agents. Sci. Total Environ. 2023, 892, 164757. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.G.; Moreira, G.S.; Pereira, R.; Carvalho, S.M. Assessing the potential use of drainage from open soilless production systems: A case study from an agronomic and ecotoxicological perspective. Agric. Water Manag. 2022, 273, 107906. [Google Scholar] [CrossRef]
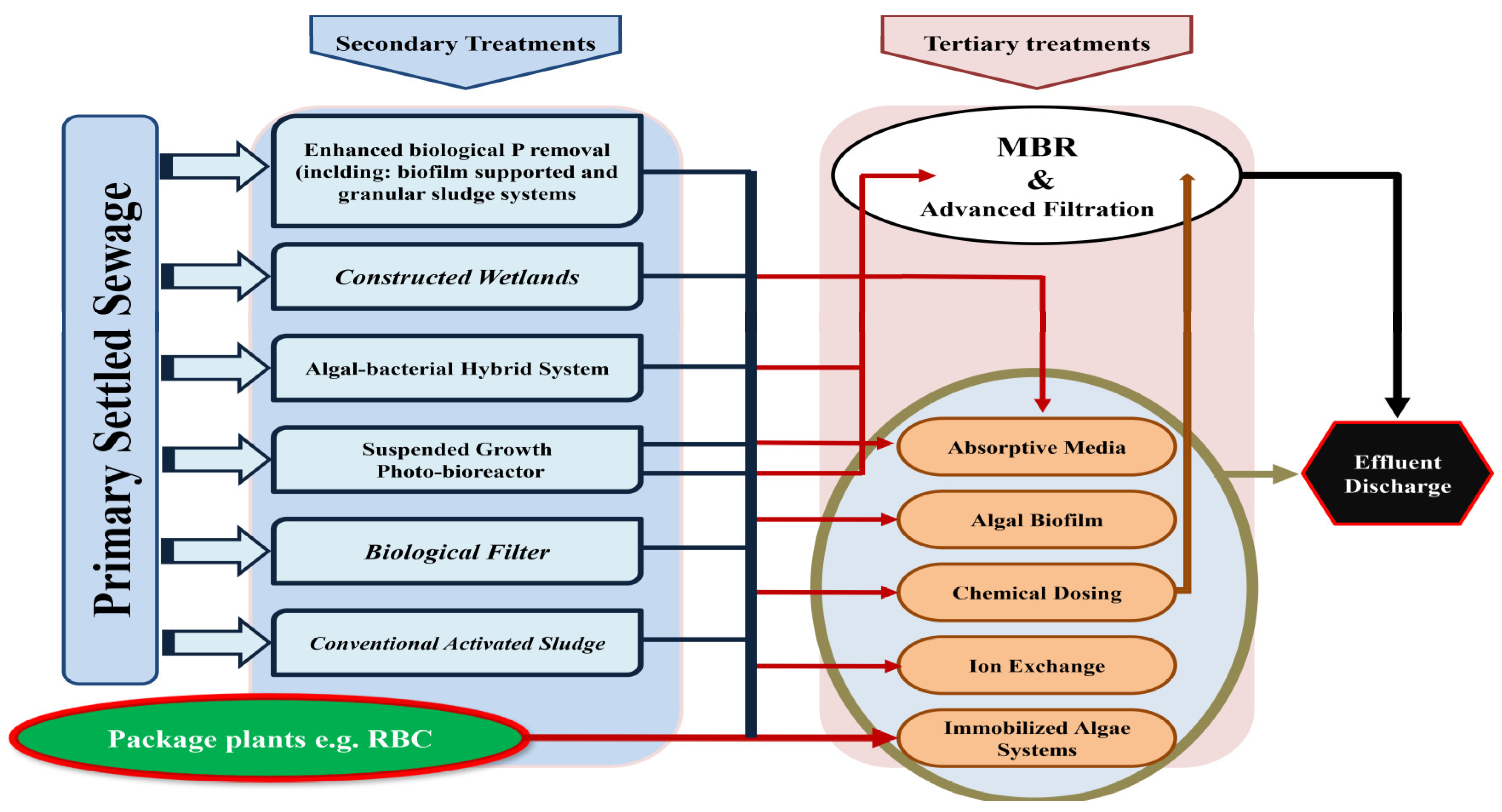



| Technology | Explanation | Rates of TP Deletion | Developmental Stage | References |
|---|---|---|---|---|
| Algal systems | ||||
| Algal biofilm reactors | Fixed-growth algal bioreactors | 41–97% | Bench-scale | [32,33] |
| Not reported | Full-scale | [34] | ||
| Algae immobilized | Algal species are into sheets or beads and immobilized | 62–90% | Bench-scale | [35,36] |
| Suspended growth photo-bioreactors | Suspended growth algal bioreactors | 61% | Pilot-scale | [37] |
| Membrane photo biofilm reactors | Membrane bioreactors with algae seed; operating promote phototrophic growth | 66–97% | Bench-scale | [38,39] |
| Osmotic MPBR | Photo-bioreactor with osmotic membrane | 90–100% | Bench-scale | [40,41] |
| EBPR systems | ||||
| MBR-UCT | Integrating a membrane bioreactor into an EBPR with continuous flow | Up to 88% | Up to 88% | [42,43] |
| Sequencing batch moving bed membrane bioreactor | Moving-bed Carriers Sequencing batch reactor with integrated membrane | 84% | Bench-scale | [44] |
| MB-SBBR | Batch biofilm reactor with moving bed sequencing | 97% | Bench-scale | [45] |
| SBBR | Sequencing batch reactor with fixed biofilm | 90% | Bench-scale | [46] |
| 70–90% | Pilot-scale | [47] | ||
| Granular sludge | SBR advanced activated sludge process | 87% | Full-scale | [48] |
| MABR-SBR hybrid | Membrane-aerated biofilm reactor operating in combination with a sequencing batch reactor | 90% | Bench-scale | [49] |
| AnoxAn | Vertical flow anaerobic-anoxic reactor | 89% | Bench-scale | [50] |
| Physico-chemical systems | ||||
| Active filter media | Materials that remove P by precipitation or absorption, whether naturally occurring or man-made. | 95% (PO4) | Bench-scale | [51,52] |
| 77–91% | Full-scale (other) | [53] | ||
| The exchange of ions | 80–90% | Bench-scale | [54,55] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdoli, S.; Asgari Lajayer, B.; Dehghanian, Z.; Bagheri, N.; Vafaei, A.H.; Chamani, M.; Rani, S.; Lin, Z.; Shu, W.; Price, G.W. A Review of the Efficiency of Phosphorus Removal and Recovery from Wastewater by Physicochemical and Biological Processes: Challenges and Opportunities. Water 2024, 16, 2507. https://doi.org/10.3390/w16172507
Abdoli S, Asgari Lajayer B, Dehghanian Z, Bagheri N, Vafaei AH, Chamani M, Rani S, Lin Z, Shu W, Price GW. A Review of the Efficiency of Phosphorus Removal and Recovery from Wastewater by Physicochemical and Biological Processes: Challenges and Opportunities. Water. 2024; 16(17):2507. https://doi.org/10.3390/w16172507
Chicago/Turabian StyleAbdoli, Sima, Behnam Asgari Lajayer, Zahra Dehghanian, Nazila Bagheri, Amir Hossein Vafaei, Masoud Chamani, Swati Rani, Zheya Lin, Weixi Shu, and G. W. Price. 2024. "A Review of the Efficiency of Phosphorus Removal and Recovery from Wastewater by Physicochemical and Biological Processes: Challenges and Opportunities" Water 16, no. 17: 2507. https://doi.org/10.3390/w16172507
APA StyleAbdoli, S., Asgari Lajayer, B., Dehghanian, Z., Bagheri, N., Vafaei, A. H., Chamani, M., Rani, S., Lin, Z., Shu, W., & Price, G. W. (2024). A Review of the Efficiency of Phosphorus Removal and Recovery from Wastewater by Physicochemical and Biological Processes: Challenges and Opportunities. Water, 16(17), 2507. https://doi.org/10.3390/w16172507








