The Sorption of Antidepressant Pharmaceuticals on Virgin and Aged Microplastics Is Lower than Bioconcentration in Protozoa
Abstract
:1. Introduction
2. Materials and Methods
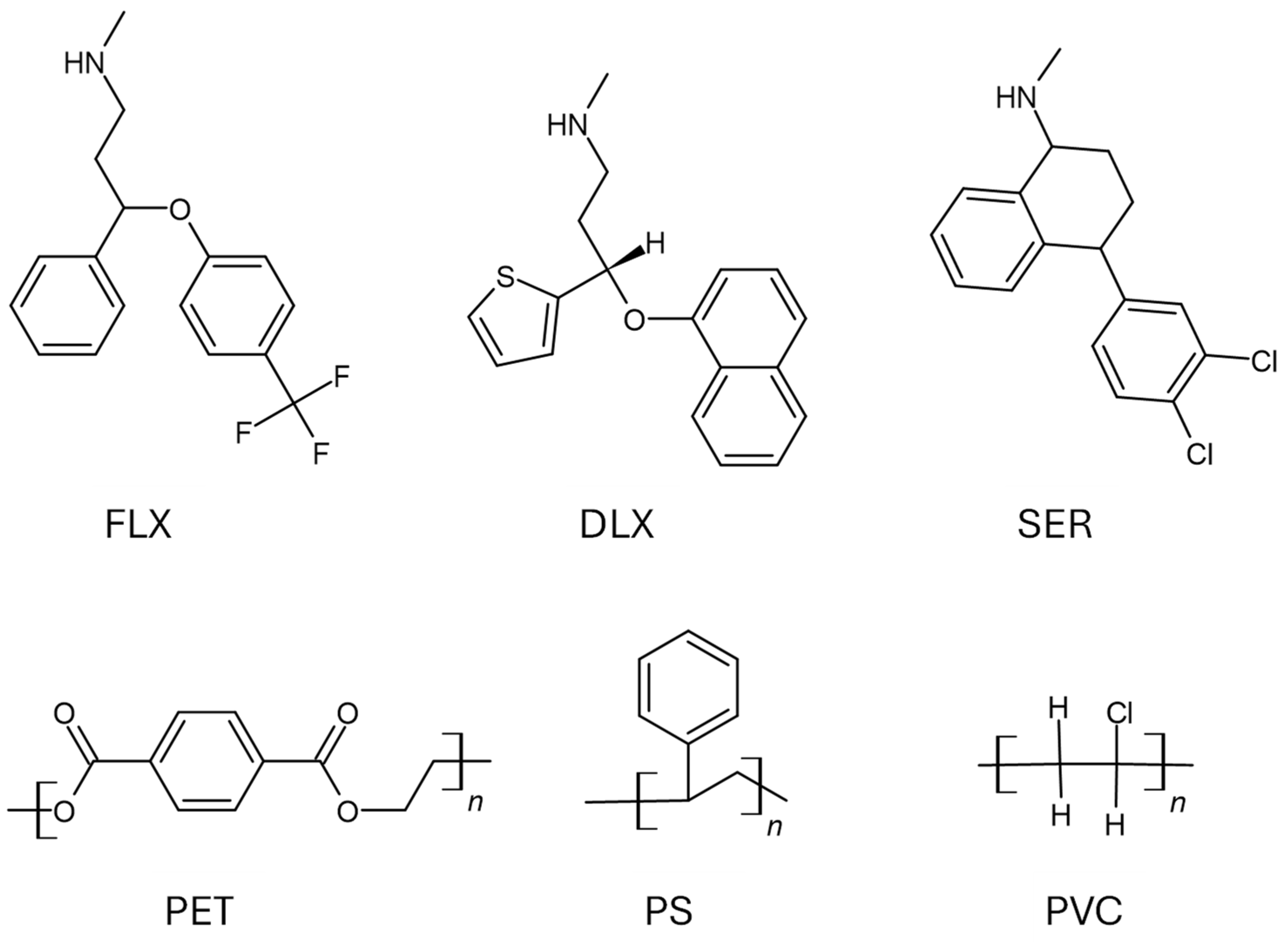
2.1. Reagents
2.2. Aging of Microplastics
2.3. Accumulation Tests
2.4. Determination of Antidepressant Concentrations in MPs and S. ambiguum with LC-MS
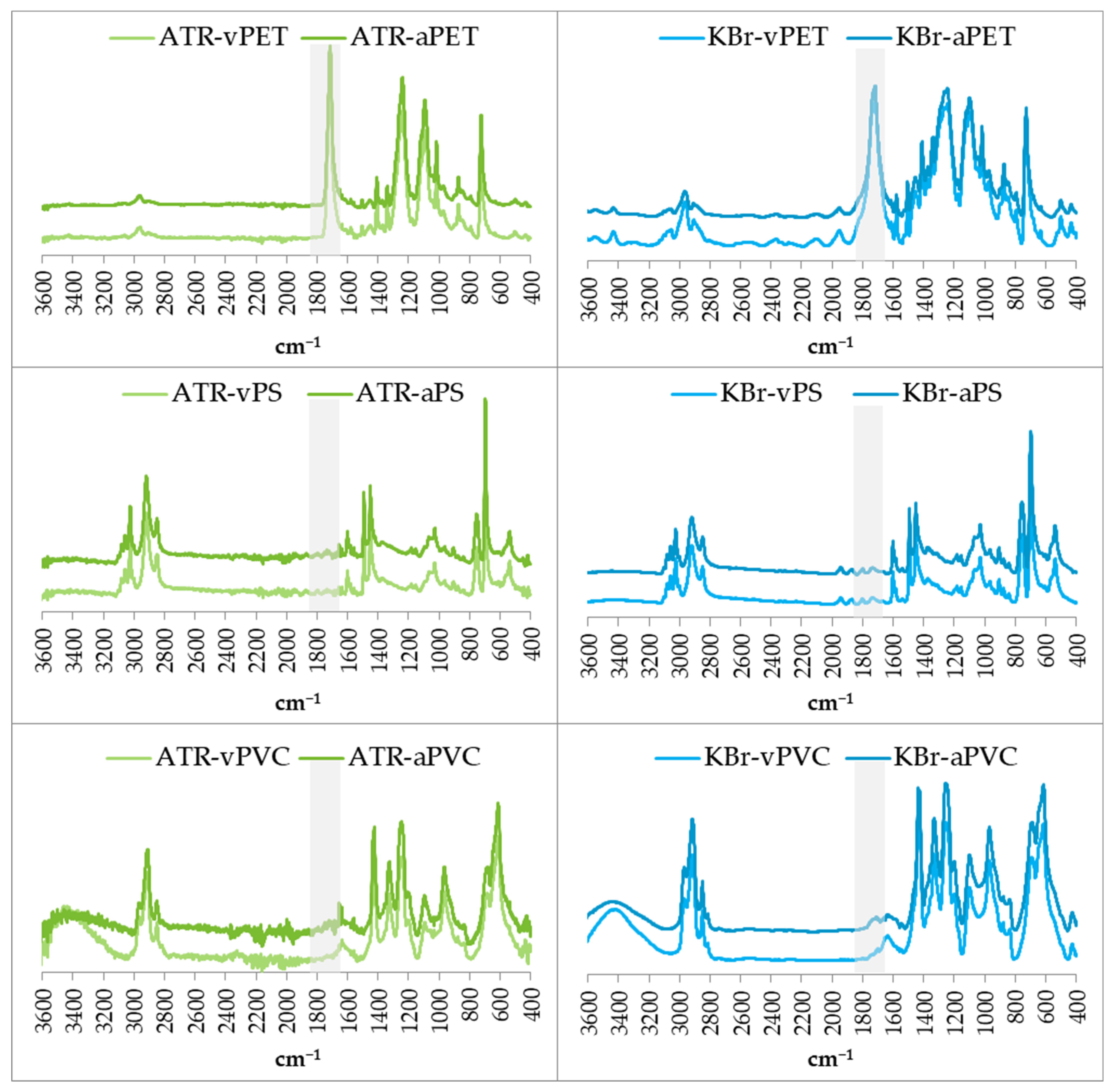
2.5. Assessment of Physicochemical Properties of Virgin and Aged MPs
2.6. Statistical Analysis
3. Results
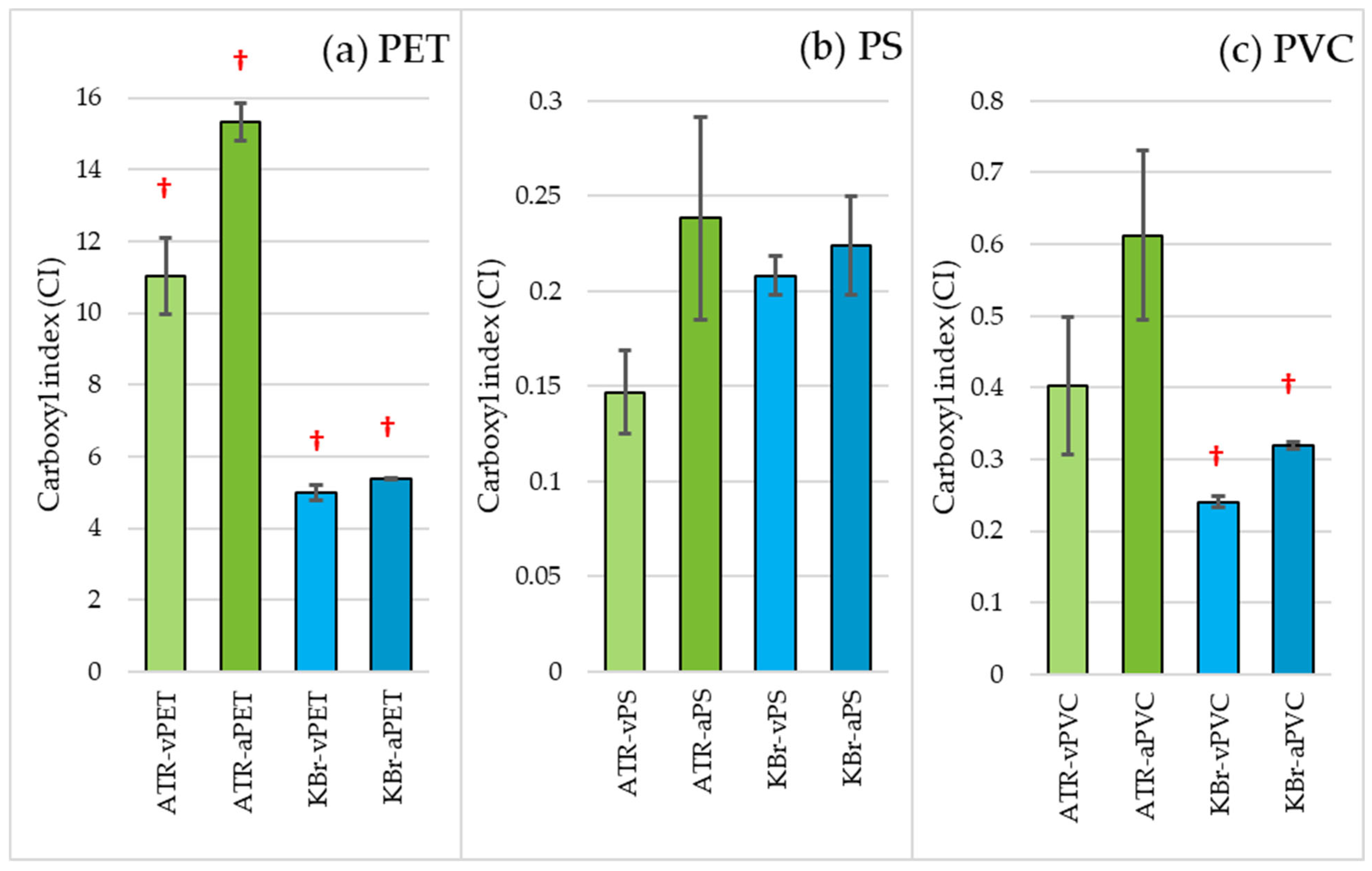
3.1. Physicochemical Properties of Virgin and Aged Microplastics
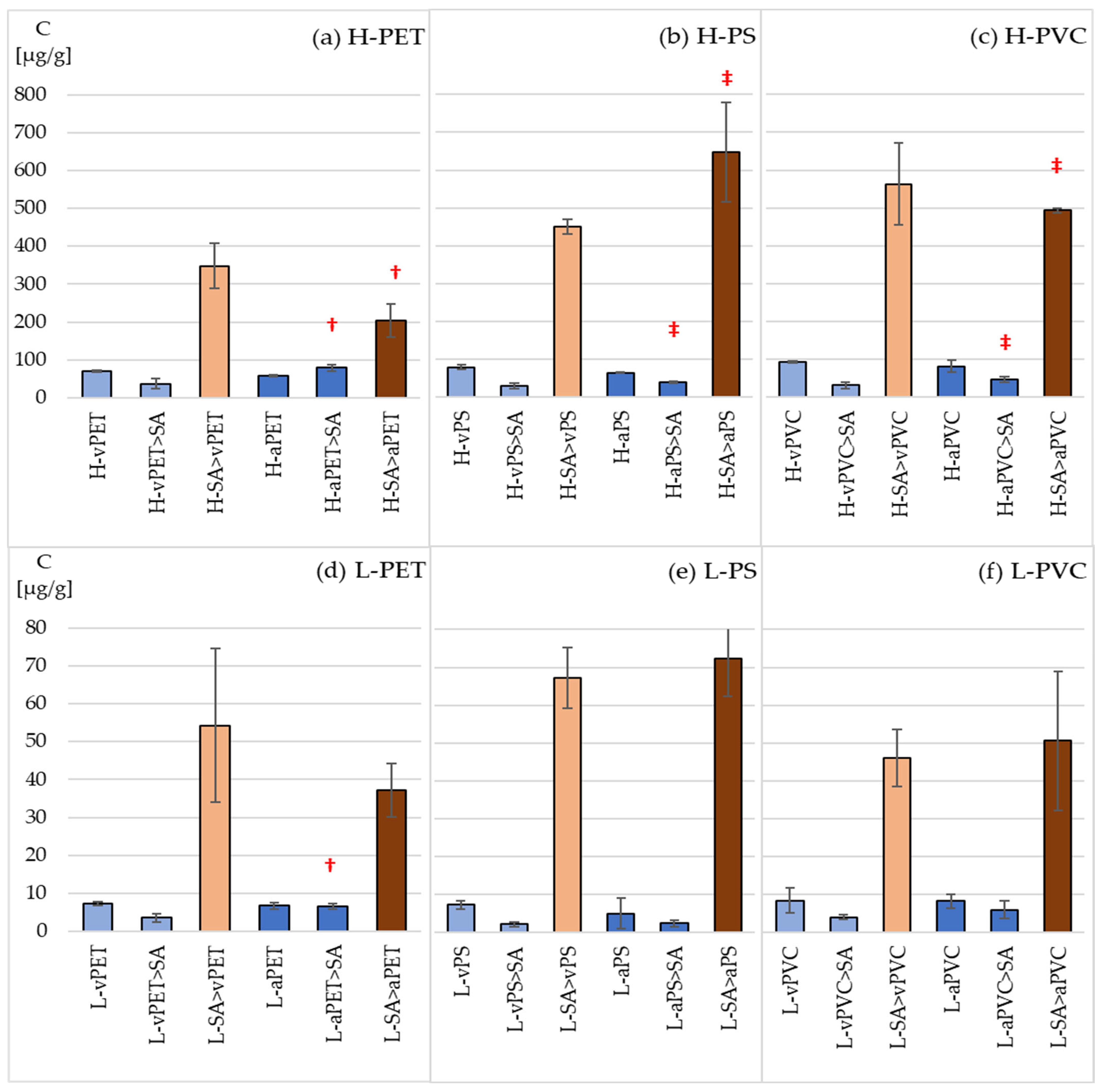
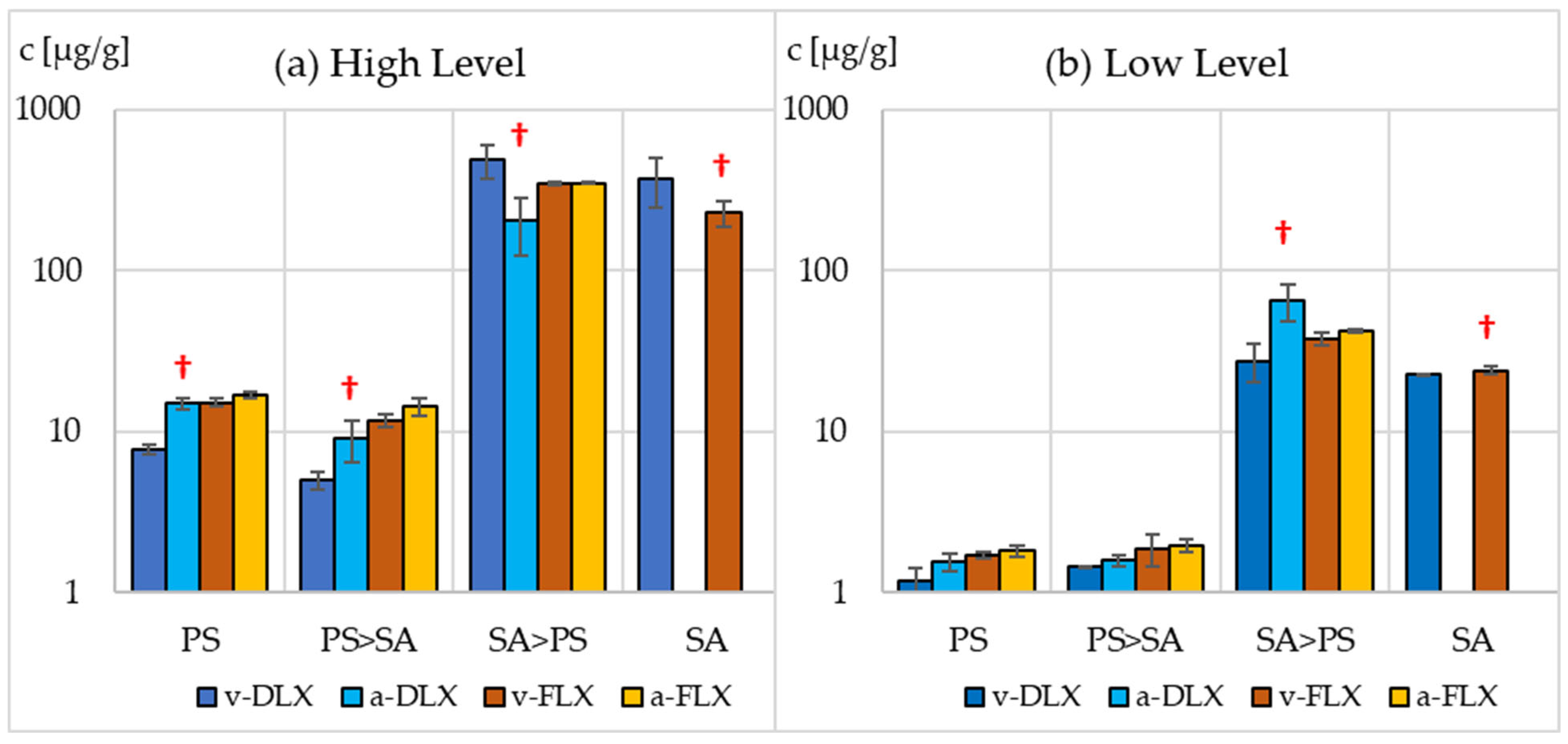
3.2. Sorption and Bioaccumulation of Sertraline
3.3. Sorption and Bioaccumulation of Fluoxetine and Duloxetine
4. Discussion
4.1. Physicochemical Properties of Virgin and Aged Microplastics
4.2. Sorption and Bioaccumulation of Pharmaceuticals
4.3. Ecological Consequences
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khan, S.A.; Abbasi, N.; Hussain, D.; Khan, T.A. Sustainable mitigation of paracetamol with a novel dual-functionalised pullulan/kaolin hydrogel nanocomposite from simulated wastewater. Langmuir 2022, 38, 8280–8295. [Google Scholar] [CrossRef] [PubMed]
- Boyd, G.R.; Reemtsma, H.; Grimm, D.A.; Mitra, S. Pharmaceuticals and personal care products (PPCPs) in surface and treated waters of Louisiana, USA and Ontario, Canada. Sci. Total Environ. 2003, 311, 135–149. [Google Scholar] [CrossRef]
- Mole, R.A.; Brooks, B.W. Global scanning of selective serotonin reuptake inhibitors: Occurrence, wastewater treatment and hazards in aquatic systems. Environ. Pollut. 2019, 250, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.H.M.L.M.; Araújo, A.N.; Fachini, A.; Pena, A.; Delerue-Matos, C.; Montenegro, M.C.B.S.M. Ecotoxicological aspects related to the presence of pharmaceuticals in the aquatic environment. J. Hazard. Mat. 2010, 175, 45–95. [Google Scholar] [CrossRef] [PubMed]
- ClinCalc DrugStats Database. Available online: https://clincalc.com (accessed on 1 June 2024).
- Arnnok, P.; Singh, R.R.; Burakham, R.; Perez-Fuentetaja, A.; Aga, D.S. Selective uptake and bioaccumulation of antidepressants in fish from effluent-impacted Niagara River. Environ. Sci. Technol. 2017, 51, 10652–10662. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.W.; Turner, P.K.; Stanley, J.K.; Weston, J.J.; Glidewell, E.A.; Foran, C.M.; Slattery, M.; La Point, T.W.; Huggett, D.B. Waterborne and sediment toxicity of fluoxetine to select organisms. Chemosphere 2003, 52, 135–142. [Google Scholar] [CrossRef]
- Giebułtowicz, J.; Nałęcz-Jawecki, G. Occurrence of antidepressant residues in the sewage-impacted Vistula and Utrata rivers and in tap water in Warsaw (Poland). Ecotoxicol. Environ. Saf. 2014, 104, 103–109. [Google Scholar] [CrossRef]
- Bojanowska-Czajka, A.; Pyszyńska, M.; Majkowska-Pilip, A.; Wawrowicz, K. Degradation of selected antidepressants sertraline and citalopram in ultrapure water and surface water using gamma radiation. Processes 2022, 10, 63. [Google Scholar] [CrossRef]
- Figuiere, R.; Waara, S.; Ahrens, L.; Golovko, O. Risk-based screening for prioritisation of organic micropollutants in Swedish freshwater. J. Hazard. Mat. 2022, 429, 128302. [Google Scholar] [CrossRef]
- Chojnacka, J.; Drobniewska, A.; Lenga, W.; Misztal, J.; Wawryniuk, M.; Nałęcz-Jawecki, G. The mutual effect of microparticles and antidepressants on the protozoan Spirostomum ambiguum (Müller, 1786) Ehrenberg, 1835. Water 2023, 15, 552. [Google Scholar] [CrossRef]
- Boström, M.L.; Ugge, G.; Jonsson, J.A.; Berglund, O. Bioaccumulation and trophodynamics of the antidepressants sertraline and fluoxetine in laboratory-constructed, 3-level aquatic food chains. Environ. Toxicol. Chem. 2017, 36, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Nałęcz-Jawecki, G.; Wawryniuk, M.; Giebułtowicz, J.; Olkowski, A.; Drobniewska, A. Influence of selected antidepressants on the ciliated protozoan Spirostomum ambiguum: Toxicity, bioaccumulation, and biotransformation products. Molecules 2020, 25, 1476. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.R.; Kasprzyk-Hordern, B. Multi-residue determination of the sorption of illicit drugs and pharmaceuticals to wastewater suspended particulate matter using pressurised liquid extraction, solid phase extraction and liquid chromatography coupled with tandem mass spectrometry. J. Chromatogr. A 2011, 1218, 7901–7913. [Google Scholar] [CrossRef] [PubMed]
- Burzio, C.; Mohammadi, A.S.; Smith, S.; Abadikhah, M.; Svahn, O.; Modin, O.; Persson, F.; Wilen, B.-M. Sorption of pharmaceuticals to foam and aerobic granular sludge with different morphologies. Resour. Environ. Sustain. 2024, 15, 100149. [Google Scholar] [CrossRef]
- Costigan, E.; Collins, A.; Hatinoglu, M.D.; Bhagat, K.; MacRae, J.; Perreault, F.; Apul, O. Adsorption of organic pollutants by microplastics: Overview of a dissonant literature. J. Hazard. Mat. Adv. 2022, 6, 100091. [Google Scholar] [CrossRef]
- Guo, X.; Wang, X.; Zhou, X.; Kong, X.; Tao, S.; Xing, B. Sorption of four hydrophobic organic compounds by three chemically distinct polymers: Role of chemical and physical composition. Environ. Sci. Technol. 2012, 46, 7252–7259. [Google Scholar] [CrossRef]
- Wagstaff, A.; Petrie, B. Enhanced desorption of fluoxetine from polyethylene terephthalate microplastics in gastric fluid and sea water. Environ. Chem. Lett. 2022, 20, 975–982. [Google Scholar] [CrossRef]
- Athey, S.N.; Albotra, A.D.; Gordon, C.A.; Monteleone, B.; Seaton, P.; Andrady, A.L.; Taylor, A.R.; Brander, S.M. Trophic transfer of microplastics in an estuarine food chain and the effects of a sorbed legacy pollutant. Limnol. Oceanol. Lett. 2020, 5, 154–162. [Google Scholar] [CrossRef]
- Atugoda, T.; Vithanage, M.; Wijesekara, H.; Bolan, N.; Sarmah, A.K.; Bank, M.S.; You, S.; Ok, Y.S. Interactions between microplastics, pharmaceuticals and personal care products: Implications for vector transport. Environ. Int. 2021, 149, 106367. [Google Scholar] [CrossRef]
- Li, J.; Zhang, K.; Zhang, H. Adsorption of antibiotics on microplastics. Environ. Pollut. 2018, 237, 460–467. [Google Scholar] [CrossRef]
- Elizalde- Velázquez, A.; Subbiah, S.; Anderson, T.A.; Green, M.J.; Zhao, X.; Cañas-Carrell, J.E. Sorption of three common nonsteroidal anti-inflammatory drugs (NSAIDs) to microplastics. Sci. Total Environ. 2020, 715, 136974. [Google Scholar] [CrossRef] [PubMed]
- Hüffer, T.; Weniger, A.-K.; Hofmann, T. Sorption of organic compounds by aged polystyrene microplastic particles. Environ. Pollut. 2018, 236, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Tourinho, P.S.; Koci, V.; Loureiro, S.; van Gestel, C.A.M. Partitioning of chemical contaminants to microplastics: Sorption mechanisms, environmental distribution and effects on toxicity and bioaccumulation. Environ. Pollut. 2019, 252, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Almond, J.; Sugumaar, P.; Wenzel, M.N.; Hill, G.; Wallis, C. Determination of the carbonyl index of polyethylene and polypropylene using specified area under band methodology with ATR-FTIR spectroscopy. e-Polymers 2020, 20, 369–381. [Google Scholar] [CrossRef]
- Zvekic, M.; Richards, L.C.; Tong, C.C.; Krogh, E.T. Characterizing photochemical ageing processes of microplastic materials using multivariate analysis of infrared spectra. Environ. Sci. Process. Impacts 2022, 24, 52. [Google Scholar] [CrossRef]
- Zhu, Z.; Cao, X.; Wang, K.; Guan, Y.; Ma, Y.; Li, Z.; Guan, J. The environmental effects of microplastics and microplastic derived dissolved organic matter in aquatic environments: A review. Sci. Total Environ. 2024, 933, 173163. [Google Scholar] [CrossRef]
- Bhagat, K.; Barrios, A.C.; Rajwade, K.; Kumar, A.; Oswald, J.; Apul, O.; Perreault, F. Aging of microplastics increases their adsorption affinity towards organic contaminants. Chemosphere 2022, 298, 134238. [Google Scholar] [CrossRef]
- Nałęcz-Jawecki, G. Spirotox test—Spirostomum ambiguum acute toxicity test. In Small-Scale Freshwater Toxicity Investigations; Blaise, C., Férard, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 1, pp. 299–322. [Google Scholar]
- Veerasingam, S.; Ranjani, M.; Venkatachalapathy, R.; Bagaev, A.; Mukhanov, V.; Litvinyuk, D.; Mugilarasan, M.; Gurumoorthi, K.; Guganathan, L.; Aboobacker, V.M.; et al. Contributions of Fourier transform infrared spectroscopy in microplastic pollution research: A review. Crit. Rev. Environ. Sci. Technol. 2021, 51, 2681–2743. [Google Scholar] [CrossRef]
- Moura, D.S.; Pestana, C.J.; Moffat, C.F.; Gkoulemani, N.; Hui, J.; Irvine, J.T.S.; Lawton, L.A. Aging microplastics enhances the adsorption of pharmaceuticals in freshwater. Sci. Tot. Environ. 2024, 912, 169467. [Google Scholar] [CrossRef]
- Korolkov, I.; Mashentseva, A.; Güven, O.; Niyazova, D.; Barsbay, M.; Zdorovets, M. The effect of oxidising agents/systems on the properties of track-etched PET membranes. Polym. Degrad. Stab. 2014, 107, 150–157. [Google Scholar] [CrossRef]
- Shi, W.; Zhang, J.; Shi, X.; Jiang, G. Different photodegradation processes of PVC with different average degrees of polymerisation. J. Appl. Polym. Sci. 2006, 107, 528–540. [Google Scholar] [CrossRef]
- Lucas, P.C.; Porter, R.S. Carbonyl functional groups in photo-oxidised polystyrene. Polym. Degrad. Stab. 1985, 13, 287–295. [Google Scholar] [CrossRef]
- Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals, Establishing a European Chemicals Agency. Available online: http://data.europa.eu/eli/reg/2006/1907/oj (accessed on 9 July 2024).
- Vieira, Y.; Lima, E.C.; Foletto, E.L.; Dotto, G.L. Microplastics physicochemical properties, specific adsorption modeling and their interaction with pharmaceuticals and other emerging contaminants. Sci. Total Environ. 2021, 753, 141981. [Google Scholar] [CrossRef]
- Liu, G.; Zhu, Z.; Yang, Y.; Sun, Y.; Yu, F.; Ma, J. Sorption behavior and mechanism of hydrophilic organic chemicals to virgin and aged microplastics in freshwater and seawater. Environ. Pollut. 2019, 246, 26–33. [Google Scholar] [CrossRef]
- Razanajatovo, R.M.; Ding, J.N.; Zhang, S.S.; Jiang, H.; Zou, H. Sorption and desorption of selected pharmaceuticals by polyethylene microplastics. Mar. Pollut. Bull. 2018, 136, 516–523. [Google Scholar] [CrossRef]
- Parmanbek, N.; Sütekin, D.S.; Barsbay, M.; Mashentseva, A.A.; Zheltov, D.A.; Aimanova, N.A.; Jakupova, Z.J.; Zdorovets, M.V. Hybrid PET track-etched membranes grafted by well-defined poly(2-(dimethylamino)ethyl methacrylate) brushes and loaded with silver nanoparticles for the removal of As(III). Polymers 2022, 13, 4026. [Google Scholar] [CrossRef] [PubMed]
- Leiva, E.; Tapia, C.; Rodriguez, C. Highly efficient removal of Cu(II) ions from acidic aqueous solution using ZnO nanoparticles as nano-adsorbents. Water 2021, 13, 2960. [Google Scholar] [CrossRef]
- Seidensticker, S.; Grathwohl, P.; Lamprecht, J.; Zarfl, C. A combined experimental and modeling study to evaluate pH-dependent sorption of polar and non-polar compounds to polyethylene and polystyrene microplastics. Environ. Sci. Eur. 2018, 30, 30. [Google Scholar] [CrossRef] [PubMed]
- Bundchuh, M.; Weyers, A.; Ebeling, M.; Elsaesser, D.; Schulz, R. Narrow pH range of surface water bodies receiving pesticide input in Europe. Bull. Environ. Contam. Toxicol. 2016, 96, 3–8. [Google Scholar] [CrossRef]
- Rybacka, A.; Andersson, P.L. Considering ionic state in modeling sorption of pharmaceuticals to sewage sludge. Chemosphere 2016, 165, 284–293. [Google Scholar] [CrossRef]
- Guo, X.; Pang, J.; Chen, S.; Jia, H. Sorption properties of tylosin on four different microplastics. Chemosphere 2018, 209, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Grabicova, K.; Grabic, R.; Fedorova, G.; Fick, J.; Cerveny, D.; Kolarova, J.; Turek, J.; Zlabek, V.; Randak, T. Bioaccumulation of psychoactive pharmaceuticals in fish in an effluent dominated streams. Water Res. 2017, 124, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Grabicova, K.; Grabic, R.; Blaha, L.; Kumar, V.; Cerveny, D.; Fedorova, G.; Randak, T. Presence of pharmaceuticals in benthic fauna living in a small stream affected by effluent from a municipal sewage treatment plant. Water Res. 2015, 72, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Anbumani, S.; Kakkar, P. Ecotoxicological effects of microplastics on biota: A review. Environ. Sci. Pollut. Res. 2018, 25, 14373–14396. [Google Scholar] [CrossRef]
- Grabowska, M.; Mazur-Marzec, H. The influence of hydrological conditions on phytoplankton community structure and cyanopeptide concentration in dammed lowland river. Environ. Monit. Assess. 2016, 188, 488. [Google Scholar] [CrossRef]
- Izydorczyk, K.; Jurczak, T.; Wojtal-Frankiewicz, A.; Skowron, A.; Mankiewicz-Boczek, J.; Tarczyńska, M. Influence of abiotic and biotic factors on microcystin content in Microcystis aeruginosa cells in a eutrophic temperate reservoir. J. Plankton Res. 2008, 30, 393–400. [Google Scholar] [CrossRef]
| Sample | The Concentration of Antidepressants in: | |
|---|---|---|
| S. ambiguum (SA) | Microplastic | |
| Low level (10 µg L−1) Virgin microplastic (vMP) | - | L–vMP |
| Low level (10 µg L−1) Virgin microplastic and protozoa | L–SA>vMP | L–vMP>SA |
| Low level (10 µg L−1) Aged microplastic (aMP) | - | L–aMP |
| Low level (10 µg L−1) Aged microplastic and protozoa | L–SA>aMP | L–aMP>SA |
| Low level (10 µg L−1) Protozoa | SA | - |
| High level (100 µg L−1) Virgin microplastic (vMP) | - | H–vMP |
| High level (100 µg L−1) Virgin microplastic and protozoa | H–SA>vMP | H–vMP>SA |
| High level (100 µg L−1) Aged microplastic (aMP) | - | H–aMP |
| High level (100 µg L−1) Aged microplastic and protozoa | H–SA>aMP | H–aMP>SA |
| High level (100 µg L−1) Protozoa | SA | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nałęcz-Jawecki, G.; Giebułtowicz, J.; Chojnacka, J.; Pajchel, Ł.; Drobniewska, A. The Sorption of Antidepressant Pharmaceuticals on Virgin and Aged Microplastics Is Lower than Bioconcentration in Protozoa. Water 2024, 16, 2514. https://doi.org/10.3390/w16172514
Nałęcz-Jawecki G, Giebułtowicz J, Chojnacka J, Pajchel Ł, Drobniewska A. The Sorption of Antidepressant Pharmaceuticals on Virgin and Aged Microplastics Is Lower than Bioconcentration in Protozoa. Water. 2024; 16(17):2514. https://doi.org/10.3390/w16172514
Chicago/Turabian StyleNałęcz-Jawecki, Grzegorz, Joanna Giebułtowicz, Justyna Chojnacka, Łukasz Pajchel, and Agata Drobniewska. 2024. "The Sorption of Antidepressant Pharmaceuticals on Virgin and Aged Microplastics Is Lower than Bioconcentration in Protozoa" Water 16, no. 17: 2514. https://doi.org/10.3390/w16172514





