Investigation of Biotoxicity and Environmental Impact of Prometryn on Fish and Algae Coexistent System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Methods
2.3. Measurement of Monitoring Indicators
2.4. Statistical Analysis
2.5. Theoretical Basis
3. Results
3.1. The Effects of Prometryn on Crucian Carp
3.2. The Effects of Prometryn on M. aeruginosa
3.3. The Environmental Effects of Prometryn
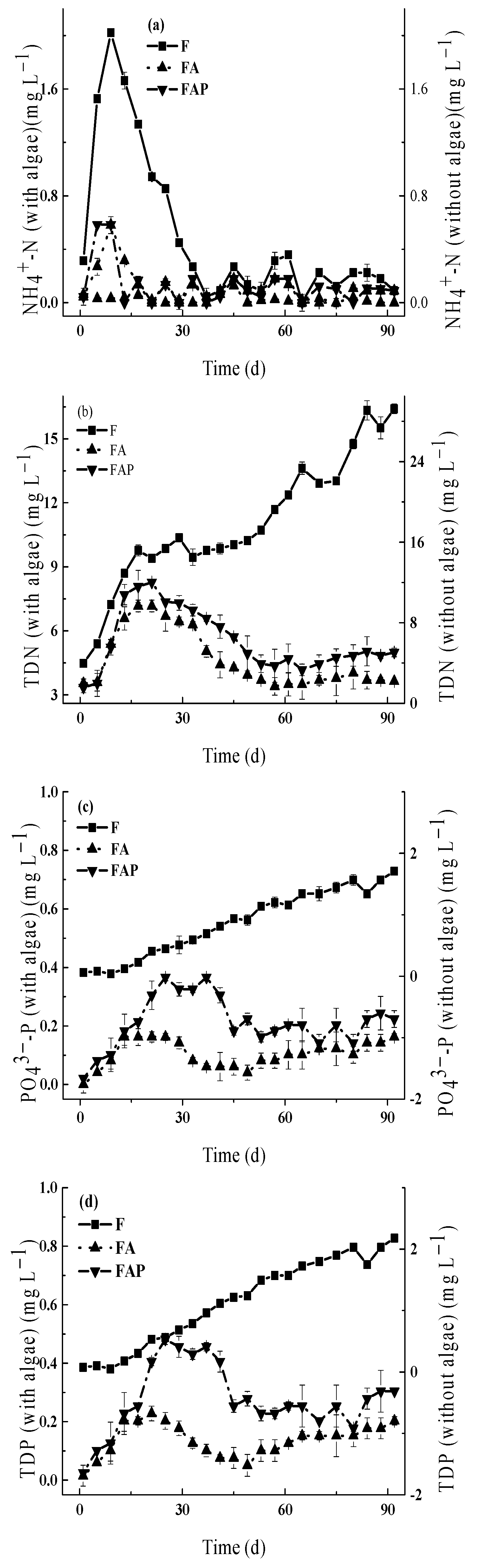
3.3.1. The Indirect Effects of Prometryn on Nutrient Concentrations
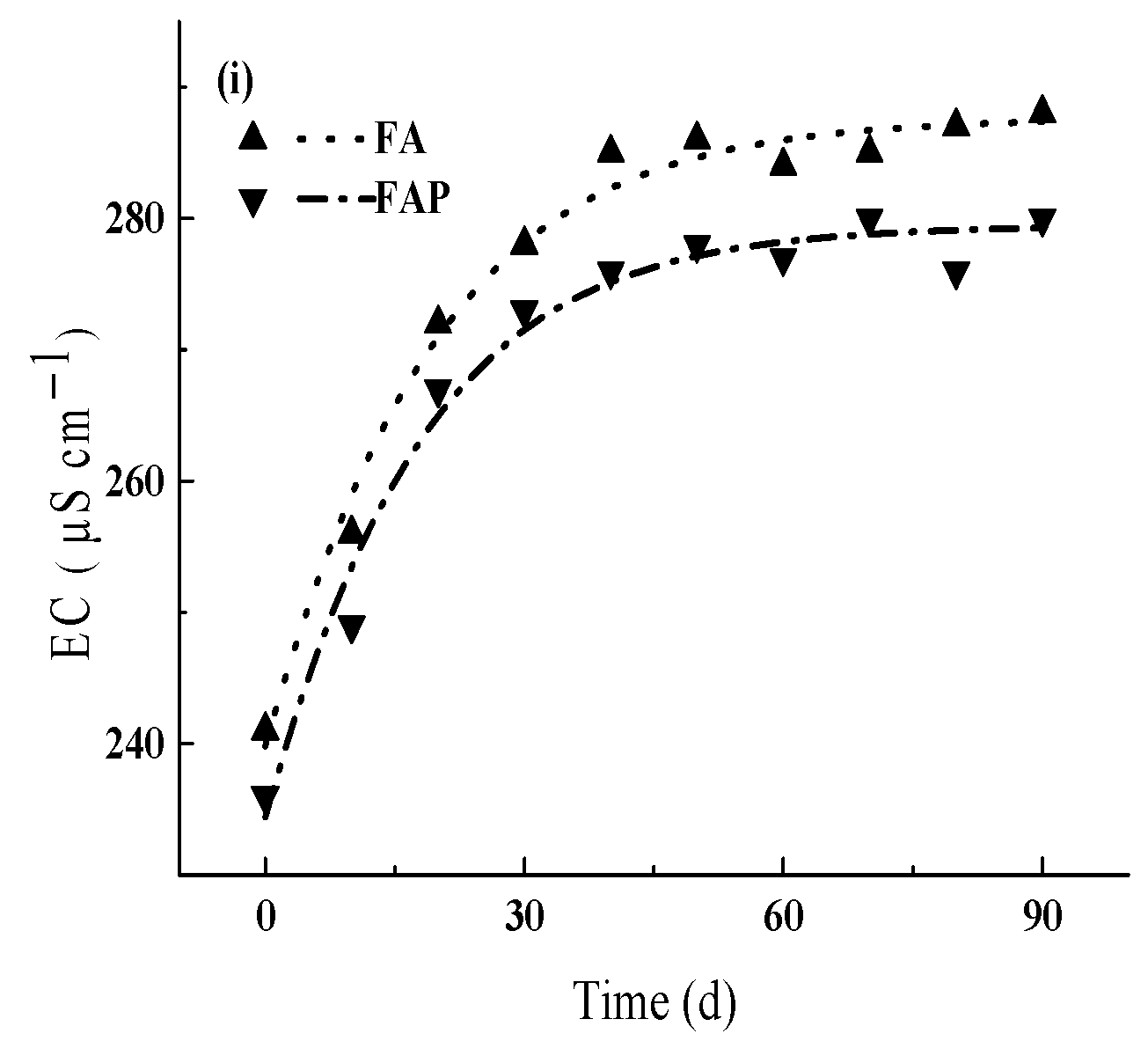
3.3.2. Indirect Effects of Prometryn on Physical Parameters of Water
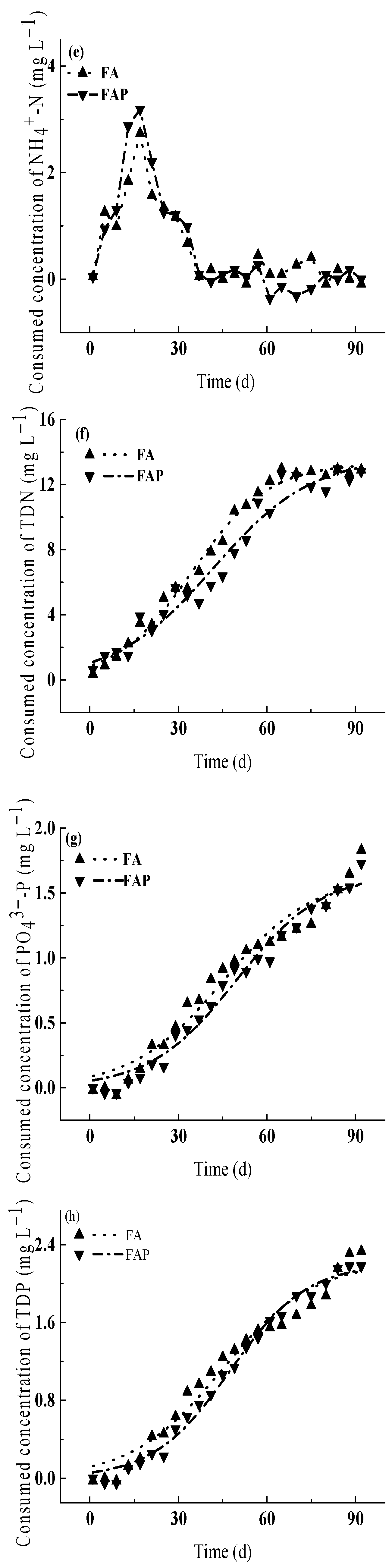
3.3.3. Indirect Effects of Prometryn on Consumed Concentrations of Nutrients
4. Discussion
4.1. Discussion of the Toxic Effects of Prometryn
4.2. Environmental Effects of Prometryn
4.3. Relationship between Inhibition Rates and Concentrations of Consumed Nutrients
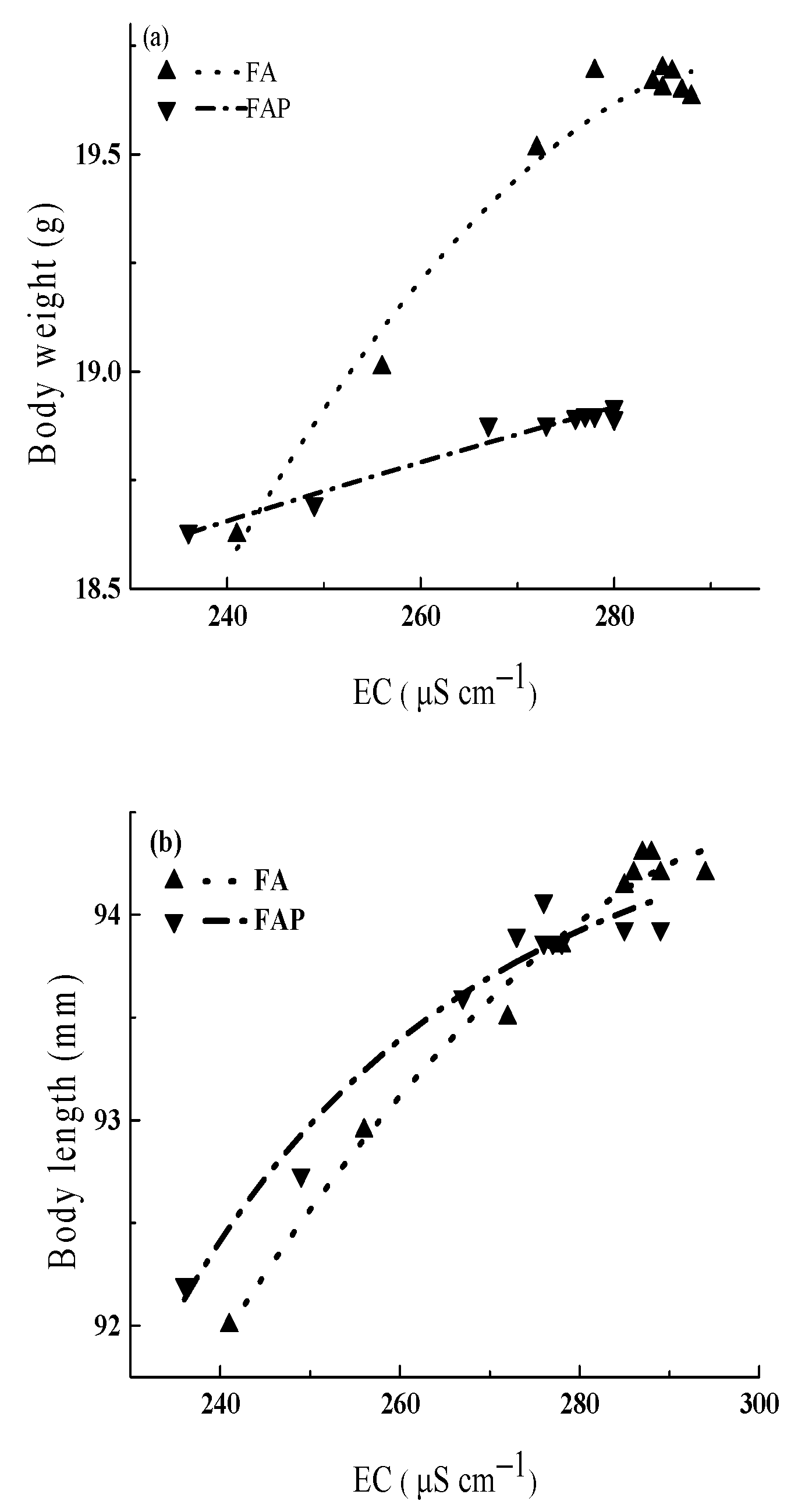
4.4. Correlations between Fish Growth and Physicochemical Parameters of Water
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Degefu, F.; Mengistu, S.; Schagerl, M. Influence of fish cage farming on water quality and plankton in fish ponds: A case study in the rift valley and north shoa reservoirs, Ethiopia. Aquaculture 2011, 316, 129–135. [Google Scholar] [CrossRef]
- Chen, L.; Yang, X.; Sun, C.; Wang, Y.; Xu, D.; Zhou, C. Feed intake prediction model for group fish using the MEA-BP neural network in intensive aquaculture. Inf. Process. Agric. 2020, 7, 261–271. [Google Scholar] [CrossRef]
- Yang, W.; Xiang, F.; Sun, H.; Chen, Y.; Minter, E.; Yang, Z. Changes in the selected hematological parameters and gill Na+/K+ ATPase activity of juvenile crucian carp Carassius auratus during elevated ammonia exposure and the post-exposure recovery. Biochem. Syst. Ecol. 2010, 38, 557–562. [Google Scholar] [CrossRef]
- Lopes, I.G.; Lalander, C.H.; Vidotti, R.M.; Vinners, B. Using Hermetia illucens larvae to process biowaste from aquaculture production. J. Clean. Prod. 2020, 251, 119753. [Google Scholar] [CrossRef]
- Rahman, M.M.; Verdegem, M.; Nagelkerke, L.; Wahab, M.D.; Milstein, A.; Verreth, J. Effects of common carp Cyprinus carpio (L.) and feed addition in rohu Labeo rohita (Hamilton) ponds on nutrient partitioning among fish, plankton and benthos. Aquacult. Res. 2008, 39, 85–95. [Google Scholar] [CrossRef]
- Anderson, D.M.; Glibert, P.M.; Burholder, J.M. Harmful algal blooms and eutrophication: Nutrient sources, composition, and consequences. Estuaries 2002, 25, 704–726. [Google Scholar] [CrossRef]
- Guo, L.; Li, Z.; Xie, P.; Ni, L. Assessment effects of cage culture on nitrogen and phosphorus dynamics in relation to fallowing in a shallow lake in China. Aquacult. Int. 2009, 17, 229–241. [Google Scholar] [CrossRef]
- Akhan, S.; Gedik, K. The nutrient releases from sea bass (Dicentrarchus labrax Linnaeus, 1758) faeces and feed in estuarine Black Sea condition. J. Food Agric. Environ. 2011, 9, 738–740. [Google Scholar]
- Huang, S.L.; Wu, M.; Zang, C.J.; Du, S.L.; Domagalski, J.; Gajewska, M.; Gao, F.; Lin, C.; Guo, Y.; Liu, B.; et al. Dynamics of algae growth and nutrients in experimental enclosures culturing bighead carp and common carp: Phosphorus dynamics. Int. J. Sediment. Res. 2016, 31, 173–180. [Google Scholar] [CrossRef]
- Tsai, K.P.; Uzun, H.; Chen, H.; Karanfil, T.; Chow, A.T. Control wildfire-induced Microcystis aeruginosa blooms by copper sulfate: Trade-offs between reducing algal organic matter and promoting disinfection byproduct formation. Water Res. 2019, 158, 227–236. [Google Scholar] [CrossRef]
- Yang, M.; Wang, X. Interactions between Microcystis aeruginosa and coexisting bisphenol A at different phosphorus levels. Sci. Total Environ. 2019, 658, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Hu, F.; Jiao, J.; Liu, M.; Li, H. Effects of bacterial-feeding nematodes and prometryne-degrading bacteria on the dissipation of prometryne in contaminated soil. J. Soil Sediment. 2012, 12, 576–585. [Google Scholar] [CrossRef]
- Starr, A.V.; Bargu, S.; Maiti, K.; Ronald, D.L. The effect of atrazine on Louisiana gulf coast estuarine phytoplankton. Arch. Environ. Con. Tox. 2017, 72, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Masia, A.; Campo, J.; Navarro-Ortega, A.; Barcelo, D.; Plco, Y. Pesticide monitoring in the basin of Llobregat River (Catalonia, Spain) and comparison with historical data. Sci. Total Environ. 2015, 503–504, 58–68. [Google Scholar] [CrossRef]
- Stara, A.; Machova, J.; Velisek, J. Effect of chronic exposure to simazine on oxidative stress and antioxidant response in common carp (Cyprinus carpio L.). Pestic. Biochem. Phys. 2013, 33, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Cao, L.; Du, J.; Gao, J.; Zhang, Y.; Sun, Y.; Li, Q.; Nie, Z.; Xu, G. Effects of prometryn exposure on hepatopancreas oxidative stress and intestinal flora in Eriocheir sinensis (Crustacea: Decapoda). Antioxidants 2023, 12, 1548. [Google Scholar] [CrossRef]
- Stara, A.; Kouba, A.; Velisek, J. Effect of chronic exposure to prometryne on oxidative stress and antioxidant response in red swamp crayfish (Procambarus clarkii). Biomed Res. Int. 2014, 2014, 680131. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhu, L. Effect of humic acid on prometryn bioaccumulation and the induction of oxidative stress in zebrafish (Danio rerio). RSC Adv. 2016, 6, 16790–16797. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, F.; Li, F.; Gu, Z.; Chen, R.; Zhang, Y. An improved logistic model illustrating Microcystis aeruginosa growth under different turbulent mixing conditions. Water 2019, 11, 669. [Google Scholar] [CrossRef]
- Aleynik, D.; Dale, A.C.; Porter, M.; Davidson, K. A high resolution hydrodynamic model system suitable for novel harmful algal bloom modelling in areas of complex coastline and topography. Harmful Algae 2016, 53, 102–117. [Google Scholar] [CrossRef]
- Hernandez-Llamas, A.; Ratkowsky, D.A. Growth of fishes, crustaceans and molluscs: Estimation of the von Bertalanffy, Logistic, Gompertz and Richards curves and a new growth model. Mar. Ecol. Prog. Ser. 2004, 282, 237–244. [Google Scholar] [CrossRef]
- Lv, Q.; Pitchford, J.W. Stochastic von Bertalanffy models, with applications to fish recruitment. J. Theor. Biol. 2007, 244, 640–655. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Huang, S.L.; Zang, C.J.; Du, S.L. Release of nutrient from fish feed and effects on Microcystis aeruginosa growth. Aquacult. Res. 2012, 43, 1460–1470. [Google Scholar] [CrossRef]
- Huang, S.L.; Kong, W.W.; Yang, Z.J.; Yu, H.; Li, F.Y. Combination of Logistic and modified Monod functions to study Microcystis aeruginosa growth stimulated by fish feed. Ecotoxicol. Environ. Saf. 2019, 167, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.J.; Huang, S.L.; Kong, W.W.; Yu, H.; Li, F.Y.; Khatoon, Z.; Ashraf, M.N.; Akram, W. Effect of different fish feeds on water quality and growth of crucian carp (Carassius carassius) in the presence and absence of prometryn. Ecotoxicol. Environ. Saf. 2021, 227, 112914. [Google Scholar] [CrossRef]
- Xie, J.; Dai, X.L.; Zang, W.L.; Wang, J.W.; Zhang, Z.H.; Ding, F.J. Study on the toxicity of prometryn to two kinds of shrimp and two kinds of aquatic plants. Hunan Agric. Sci. 2010, 23, 147–150. [Google Scholar]
- Yang, Z.J.; Huang, S.L.; Huang, X.W.; Liang, X.F.; Akram, W.; Khatoon, Z. Effects of prometryn exposure scenarios on Microcystis aeruginosa growth and water qualities in incubator experiments. Water 2021, 13, 1346. [Google Scholar] [CrossRef]
- Madilonga, R.T.; Edokpayi, J.N.; Volenzo, E.T.; Durowoju, O.S.; Odiyo, J.O. Water quality assessment and evaluation of human health risk in Mutangwi river, Limpopo Province, South Africa. Int. J. Environ. Res. Public Health 2021, 18, 6765. [Google Scholar] [CrossRef]
- Daendee, S.; Thongsong, B.; Kalandakanond-Thongsong, S. Effects of time of estrogen deprivation on anxiety-like behavior and GABAA receptor plasticity in ovariectomized rats. Behav. Brain Res. 2013, 246, 86–93. [Google Scholar] [CrossRef]
- Handeland, S.O.; Imsland, A.K.; Stefansson, S.O. The effect of temperature and fish size on growth, feed intake, food conversion efficiency and stomach evacuation rate of Atlantic salmon post-smolts. Aquaculture 2008, 283, 36–42. [Google Scholar] [CrossRef]
- Lim, S.J.; Lee, K.J. Partial replacement of fish meal by cottonseed meal and soybean meal with iron and phytase supplementation for parrot fish Oplegnathus fasciatus. Aquaculture 2009, 290, 283–289. [Google Scholar] [CrossRef]
- Hamre, K.; Yufera, M.; Ronnestad, I.; Boglione, C.; Conceicao, L.E.C.; Izquierdo, M. Fish larval nutrition and feed formulation: Knowledge gaps and bottlenecks for advances in larval rearing. Rev. Aquacult. 2013, 5, S26–S58. [Google Scholar] [CrossRef]
- Wang, C.Y.; Wang, X.L.; Su, R.G.; Liang, S.K. No detected toxic concentrations in in situ algal growth inhibition tests-A convenient approach to aquatic ecotoxicology. Ecotoxicol. Environ. Saf. 2011, 74, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.; Yu, H. Effects of stationary phase elongation and initial nitrogen and phosphorus concentrations on the growth and lipid-producing potential of Chlorella sp. HQ. J. Appl. Phycol. 2014, 26, 141–149. [Google Scholar]
- Goudar, C.T.; Joeris, K.; Konstantinov, K.B.; Piret, J.M. Logistic equations effectively model mammalian cell batch and fed-batch kinetics by logically constraining the fit. Biotechnol. Prog. 2005, 21, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, R.; Moraes, B.S.; Loro, V.L.; Pretto, A. Tissue biochemical alterations of Cyprinus carpio exposed to commercial herbicide containing clomazone under rice-field conditions. Tox. Arch. Environ. Contam. Toxicol. 2012, 62, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Liebig, M.; Schmidt, G.; Bontje, D.; Kooi, B.W.; Streck, G.; Traunspurger, W.; Knacker, T. Direct and indirect effects of pollutants on algae and algivorous ciliates in an aquatic indoor microcosm. Aquat. Toxicol. 2008, 88, 102–110. [Google Scholar] [CrossRef]
- Kong, W.W.; Huang, S.L.; Shi, F.F.; Zhou, J.G.; Feng, Y.B.; Xiao, Y. Study on Microcystis aeruginosa growth in incubator experiments by combination of Logistic and Monod functions. Algal Res. 2018, 35, 602–612. [Google Scholar] [CrossRef]
- Li, X.Q.; Xu, L.G.; Ma, J.Y. Combination toxicity of Prometryne and permeating agent on Chlorella pyrenoidosa. China Environ. Sci. 2005, 25, 432–436. [Google Scholar]
- Jin, Z.P.; Luo, K.; Zhang, S.; Zheng, Q.; Yang, H. Bioaccumulation and catabolism of prometryne in green algae. Chemosphere 2012, 87, 278–284. [Google Scholar] [CrossRef]
- Topal, A.; Atamanalp, M.; Ucar, A.; Oruc, E.; Kocaman, E.M.; Sulukan, E.; Akdemir, F.; Beydemir, S.; Kilinc, N.; Erdogan, O.; et al. Effects of glyphosate on juvenile rainbow trout (Oncorhynchus mykiss): Transcriptional and enzymatic analyses of antioxidant defence system, histopathological liver damage and swimming performance. Ecotoxicol. Environ. Saf. 2015, 111, 206–214. [Google Scholar] [CrossRef]
- Song, M.; Fan, S.; Pang, C.; Wei, H.; Yu, S. Genetic analysis of the antioxidant enzymes, methane dicarboxylic aldehyde (MDA) and chlorophyll content in leaves of the short season cotton (Gossypium hirsutum L.). Euphytica 2014, 198, 153–162. [Google Scholar] [CrossRef]
- Bhunia, A.K.; Basu, N.K.; Roy, D.; Chakrabarti, A.; Banerjee, S.K. Growth, chlorophyll a content, nitrogen-fixing ability, and certain metabolic activities of Nostoc muscorum: Effect of methylparathion and benthiocarb. B. Environ. Contam. Tox. 1991, 47, 43–50. [Google Scholar] [CrossRef]
- Velisek, J.; Stara, A.; Koutnik, D.; Machova, J. Effects of prometryne on early life stages of common carp (Cyprinus carpio L.). Pestic. Biochem. Phys. 2015, 118, 58–63. [Google Scholar] [CrossRef]
- Yang, Z.J.; Huang, S.L.; Kong, W.W.; Chen, L.; Ekaterina, P.; Khatoon, Z.; Ashraf, M.; Akram, W. Effects of fish feed addition scenarios with prometryn on Microcystis aeruginosa growth and water qualities. Ecotoxicol. Environ. Saf. 2021, 209, 111810. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, Z.; Lavoie, M.; Fan, X.; Bai, X.; Qian, H. Ammonium reduces chromium toxicity in the freshwater alga Chlorella vulgaris. Appl. Microbiol. Biot. 2015, 99, 3249–3258. [Google Scholar] [CrossRef]
- Wang, W.X.; Dei, R.C.H. Metal stoichiometry in predicting Cd and Cu toxicity to a freshwater green alga Chlamydomonas reinhardtii. Environ. Pollut. 2006, 142, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.; Guasch, H.; Admiraal, W.; Geest, H.G.V.D. Influence of phosphorus on copper sensitivity of fluvial periphyton: The role of chemical, physiological and community-related factors. Ecotoxicology 2010, 19, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, B.E. Effect of zinc on growth of the minnow Phoxinus phoxinus. Oikos 1974, 25, 370–373. [Google Scholar] [CrossRef]
- Barman, U.K.; Jana, S.N.; Garg, S.K.; Bhatnagar, A.; Arasu, A.R.T. Effect of inland water salinity on growth, feed conversion efficiency and intestinal enzyme activity in growing grey mullet, Mugil cephalus (Linn.): Field and laboratory studies. Aquacult. Int. 2005, 13, 241–256. [Google Scholar] [CrossRef]
- Shannon, L.J.; Coll, M.; Yemane, D.; Jouffre, D.; Neira, S.; Bertrand, A.; Diaz, E.; Shin, Y.J. Comparing data-based indicators across upwelling and comparable systems for communicating ecosystem states and trends. ICES J. Mar. Sci. 2010, 67, 807–832. [Google Scholar] [CrossRef]




| Parameter | F | FA | FAP |
|---|---|---|---|
| Parameters that are related to growth of crucian carp | |||
| aW | −2.79 | −2.97 | −3.06 |
| rW | 0.08 | 0.08 | 0.05 |
| BWmax | 19.74 | 19.56 | 18.92 |
| R2 | 0.971 | 0.775 | 0.751 |
| aL | −3.67 | −3.60 | −3.92 |
| rL | 0.05 | 0.02 | 0.02 |
| BLmax | 94.33 | 94.59 | 95.66 |
| R2 | 0.995 | 0.894 | 0.915 |
| FCE | 9.01 ± 0.26 a | 8.50 ± 0.32 a | 1.01 ± 0.02 b |
| PER | 29.34 ± 0.18 a | 28.35 ± 0.21 a | 3.36 ± 0.26 b |
| Parameters of equations describing growth of M. aeruginosa | |||
| a | - | 6.66 | 7.20 |
| r/d−1 | - | 0.16 | 0.15 |
| Nmax | - | 165.68 | 130.62 |
| Nave | - | 90.86 | 61.94 |
| R2 | - | 0.992 | 0.994 |
| μ′cmax | - | 6.63 | 4.90 |
| μ′cave | - | 1.81 | 1.55 |
| μcmax | - | 0.16 | 0.15 |
| μcave | - | 0.05 | 0.05 |
| Imax | - | - | 59.48 |
| Iave | - | - | 39.25 |
| Antioxidant indexes of fish in different groups | |||
| CAT (U/mgprot) | 0.74 ± 0.01 a | 0.73 ± 0.00 a | 0.67 ± 0.01 b |
| SOD (U/mgprot) | 0.67 ± 0.02 a | 0.65 ± 0.00 a | 0.55 ± 0.03 b |
| MDA (nmol/mgprot) | 0.16 ± 0.00 a | 0.16 ± 0.02 a | 0.17 ± 0.01 a |
| Antioxidant indexes of algae in different groups | |||
| CAT (U/mgprot) | - | 7.91 ± 1.02 b | 37.75 ± 0.76 a |
| SOD (U/mgprot) | - | 25.19 ± 2.42 a | 28.14 ± 1.36 a |
| MDA (nmol/mgprot) | - | 1.36 ± 0.08 b | 1.93 ± 0.01 a |
| ΔTDN | ΔPO43−-P | ΔTDP | ||||
|---|---|---|---|---|---|---|
| Parameters | FA | FAP | FA | FAP | FA | FAP |
| aΔC (-) | 2.69 | 2.49 | 2.90 | 3.39 | 2.87 | 3.64 |
| rΔC (d−1) | 0.08 | 0.06 | 0.06 | 0.07 | 0.06 | 0.08 |
| ΔCmax (mg L−1) | 13.89 | 13.02 | 1.64 | 1.51 | 2.38 | 2.21 |
| R2 | 0.989 | 0.960 | 0.952 | 0.976 | 0.963 | 0.991 |
| ΔCave (mg L−1) | 8.66 | 7.32 | 0.87 | 0.74 | 1.21 | 1.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Zhao, D.; Gu, J.; Wu, R.; Liu, B.; Yu, G.; Dong, P.; Huang, X.; Li, M.; Li, G. Investigation of Biotoxicity and Environmental Impact of Prometryn on Fish and Algae Coexistent System. Water 2024, 16, 2531. https://doi.org/10.3390/w16172531
Yang Z, Zhao D, Gu J, Wu R, Liu B, Yu G, Dong P, Huang X, Li M, Li G. Investigation of Biotoxicity and Environmental Impact of Prometryn on Fish and Algae Coexistent System. Water. 2024; 16(17):2531. https://doi.org/10.3390/w16172531
Chicago/Turabian StyleYang, Zhenjiang, Daoquan Zhao, Jinxing Gu, Ran Wu, Bianzhi Liu, Guangqing Yu, Pengsheng Dong, Xiaocheng Huang, Ming Li, and Guoxi Li. 2024. "Investigation of Biotoxicity and Environmental Impact of Prometryn on Fish and Algae Coexistent System" Water 16, no. 17: 2531. https://doi.org/10.3390/w16172531






