Impact of Chemical Oxygen Demand/Total Nitrogen Ratio on Shifting Autotrophic Partial Nitrification to Heterotrophic Nitrification and Aerobic Denitrification in High-Strength Ammonium Wastewater Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Set-Up and Operation Strategy
2.2. Seeding Sludge and Synthetic Wastewater
2.3. Analytical Methods
2.4. Extracellular Polymeric Substance Extraction and Analysis
2.5. Specific Oxygen Uptake Rate
2.6. Bacterial Analysis
2.7. Statistical Analysis
3. Results
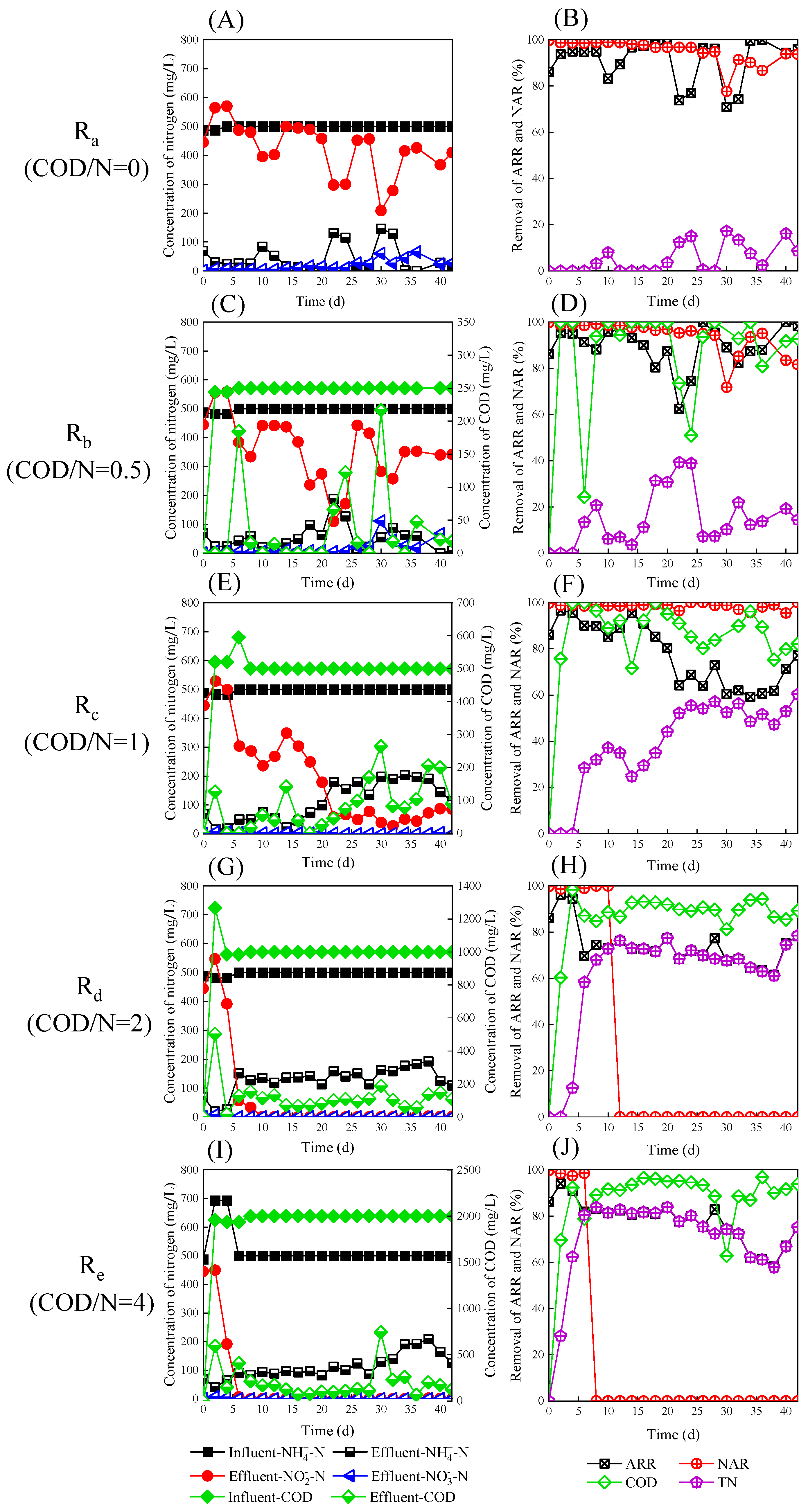
3.1. Reactor Performance
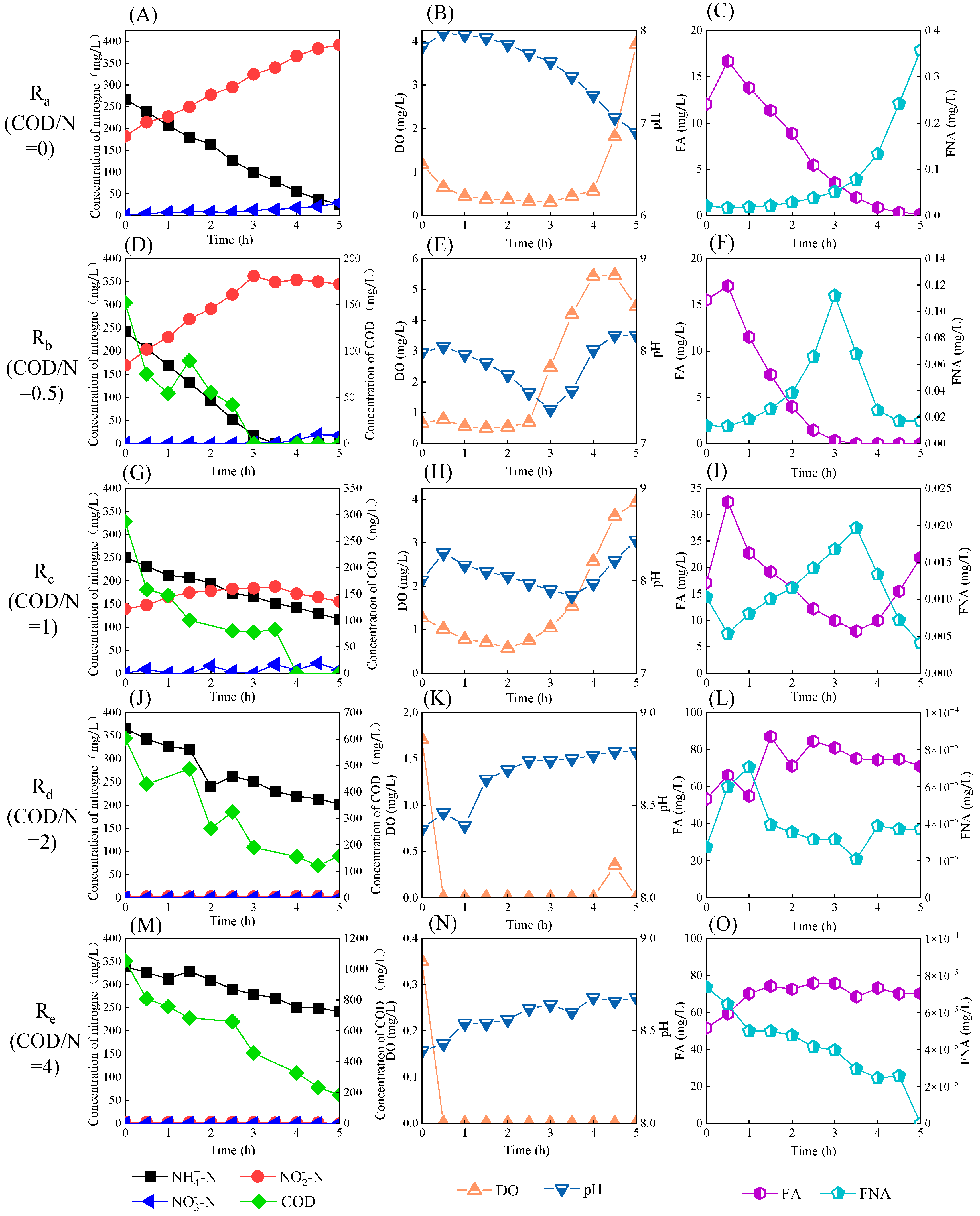
3.2. Typical Single Cycle in Reactors
3.3. EPS Analysis
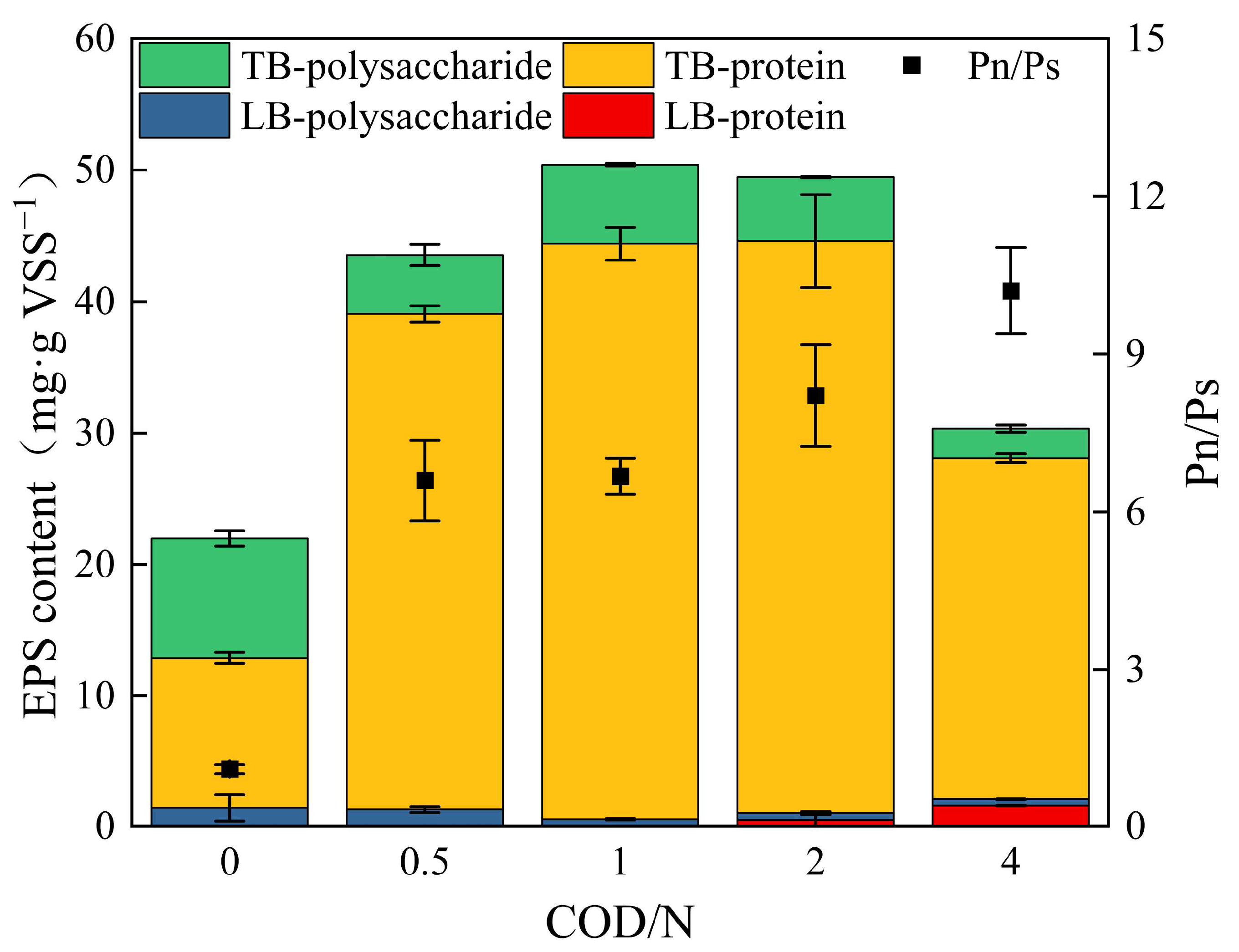
3.3.1. EPS Content
3.3.2. Characterization of EPS Components Using 3D-EEM-PARAFAC
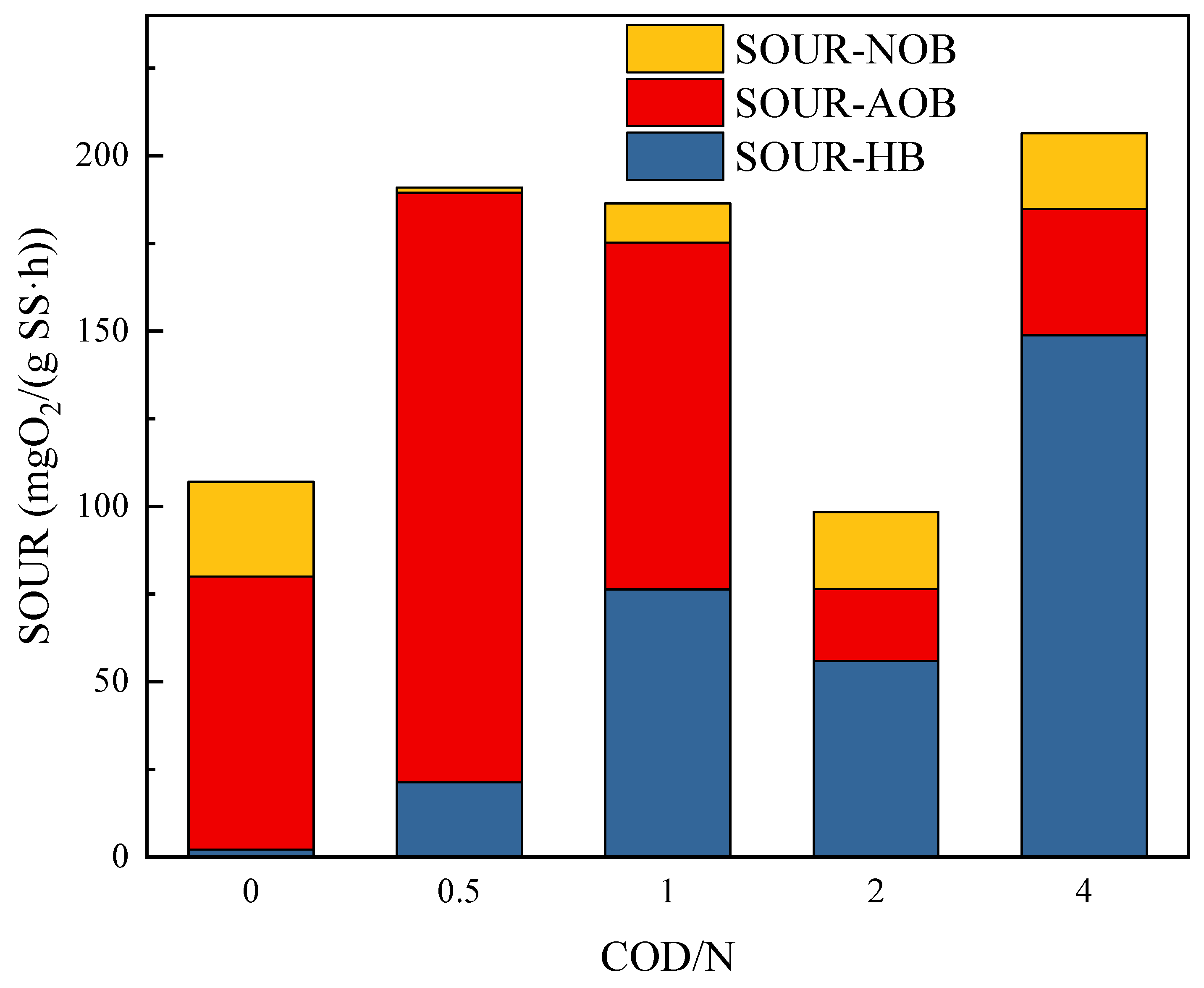
3.4. The Influence of COD/N Ratio on Specific Oxygen Uptake Rates
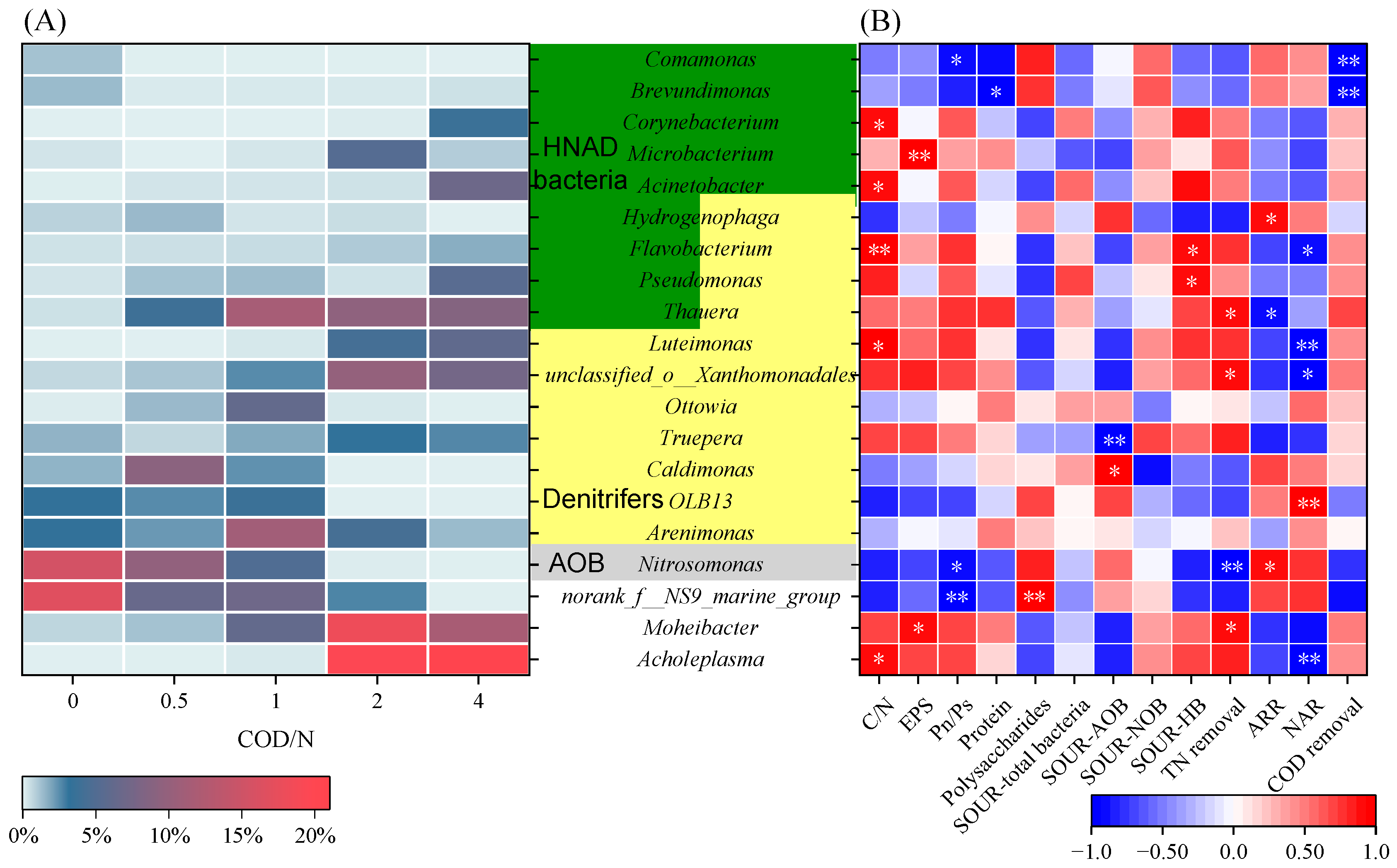
3.5. Bacterial Community
3.6. Prediction of Functional Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AOB | Ammonia-oxidizing bacteria |
| ARR | Ammonium removal rate |
| DNRA | Dissimilatory nitrate reduction to ammonium |
| DO | Dissolved oxygen |
| EEM | Excitation–emission matrix |
| EPS | Extracellular polymeric substance |
| Ex/Em | Excitation/emission |
| Fmax | Maximum fluorescence |
| FA | Free ammonia |
| FNA | Free nitrous acid |
| HB | Heterotrophic bacteria |
| HNAD | Heterotrophic nitrification and heterotrophic denitrification |
| LB-EPS | Loosely bound EPS |
| NRR | Nitrite accumulation rate |
| PARAFAC | Parallel factor analysis |
| PBS | Phosphate buffer solution |
| PICRUSt | Phylogenetic investigation of communities by reconstruction of unobserved states |
| Pn/Ps | Protein to polysaccharide ratios |
| PN | Partial nitrification |
| SBR | Sequence batch reactor |
| SOUR | Specific oxygen uptake rate |
| TB-EPS | Tightly bound EPS |
References
- Ronan, E.; Aqeel, H.; Wolfaardt, G.M.; Liss, S.N. Recent advancements in the biological treatment of high strength ammonia wastewater. World J. Microbiol. Biotechnol. 2021, 37, 158. [Google Scholar] [CrossRef] [PubMed]
- Conley, D.J.; Paerl, H.W.; Howarth, R.W.; Boesch, D.F.; Seitzinger, S.P.; Havens, K.E.; Lancelot, C.; Likens, G.E. Controlling eutrophication: Nitrogen and phosphorus. Science 2009, 323, 1014–1015. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Du, R.; Peng, Y.; Zhang, Q.; Wang, J. Characteristics of sludge granulation and EPS production in development of stable partial nitrification. Bioresour. Technol. 2020, 303, 122937. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Xu, X.; Zhou, L.; Wang, C.; Yang, F. A pilot-scale study on the start-up of partial nitrification-anammox process for anaerobic sludge digester liquor treatment. Bioresour. Technol. 2017, 241, 181–189. [Google Scholar] [CrossRef]
- Zhang, F.; Peng, Y.; Miao, L.; Wang, Z.; Wang, S.; Li, B. A novel simultaneous partial nitrification Anammox and denitrification (SNAD) with intermittent aeration for cost-effective nitrogen removal from mature landfill leachate. Chem. Eng. J. 2017, 313, 619–628. [Google Scholar] [CrossRef]
- Ge, S.; Wang, S.; Yang, X.; Qiu, S.; Li, B.; Peng, Y. Detection of nitrifiers and evaluation of partial nitrification for wastewater treatment: A review. Chemosphere 2015, 140, 85–98. [Google Scholar] [CrossRef]
- Mosquera-Corral, A.; Gonzalez, F.; Campos, J.; Méndez, R. Partial nitrification in a SHARON reactor in the presence of salts and organic carbon compounds. Process Biochem. 2005, 40, 3109–3118. [Google Scholar] [CrossRef]
- Cao, Q.; Chen, Y.; Li, X.; Li, C.; Li, X. Low C/N promotes stable partial nitrification by enhancing the cooperation of functional microorganisms in treating high-strength ammonium landfill leachate. J. Environ. Manag. 2023, 329, 116972. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Elliott, D.; Nielsen, M.; Healy, M.G.; Zhan, X. Long-term partial nitrification in an intermittently aerated sequencing batch reactor (SBR) treating ammonium-rich wastewater under controlled oxygen-limited conditions. Biochem. Eng. J. 2011, 55, 215–222. [Google Scholar] [CrossRef]
- Liang, Z.; Liu, J.-X. Control factors of partial nitritation for landfill leachate treatment. J. Environ. Sci. 2007, 19, 523–529. [Google Scholar] [CrossRef]
- Friedman, L.; Mamane, H.; Avisar, D.; Chandran, K. The role of influent organic carbon-to-nitrogen (COD/N) ratio in removal rates and shaping microbial ecology in soil aquifer treatment (SAT). Water Res. 2018, 146, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Zhang, Y.; Zheng, S. Heterotrophic nitrifying bacteria in wastewater biological nitrogen removal systems: A review. Crit. Rev. Environ. Sci. Technol. 2022, 52, 2302–2338. [Google Scholar] [CrossRef]
- Liu, X.; Dang, Y.; Sun, D.; Holmes, D.E. Identification of optimal parameters for treatment of high-strength ammonium leachate by mixed communities of heterotrophic nitrifying/aerobic denitrifying bacteria. Bioresour. Technol. 2021, 336, 125415. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, X.; Zhang, Z.; Luo, W.; Wu, H.; Zhang, L.; Zhang, X.; Zhao, T. Performance and microbial ecology of a novel moving bed biofilm reactor process inoculated with heterotrophic nitrification-aerobic denitrification bacteria for high ammonia nitrogen wastewater treatment. Bioresour. Technol. 2020, 315, 123813. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zhou, J.; He, L.; He, X.; Pan, Z.; Wang, Y.; He, Q. High-temperature biofilm system based on heterotrophic nitrification and aerobic denitrification treating high-strength ammonia wastewater: Nitrogen removal performances and temperature-regulated metabolic pathways. Bioresour. Technol. 2022, 344, 126184. [Google Scholar] [CrossRef]
- Peng, Z.; Lei, Y.; Liu, Y.; Wan, X.; Yang, B.; Pan, X. Fast start-up and reactivation of anammox process using polyurethane sponge. Biochem. Eng. J. 2022, 177, 108249. [Google Scholar] [CrossRef]
- Rice, E.W.; Bridgewater, L.; Association, A.P.H. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012; Volume 10. [Google Scholar]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence excitation−emission matrix regional integration to quantify spectra for dissolved organic matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef]
- He, W.; Hur, J. Conservative behavior of fluorescence EEM-PARAFAC components in resin fractionation processes and its applicability for characterizing dissolved organic matter. Water Res. 2015, 83, 217–226. [Google Scholar] [CrossRef]
- Surmacz-Gorska, J.; Gernaey, K.; Demuynck, C.; Vanrolleghem, P.; Verstraete, W. Nitrification monitoring in activated sludge by oxygen uptake rate (OUR) measurements. Water Res. 1996, 30, 1228–1236. [Google Scholar] [CrossRef]
- Tian, X.; Shen, Z.; Han, Z.; Zhou, Y. The effect of extracellular polymeric substances on exogenous highly toxic compounds in biological wastewater treatment: An overview. Bioresour. Technol. Rep. 2019, 5, 28–42. [Google Scholar] [CrossRef]
- Lu, H.; Chandran, K.; Stensel, D. Microbial ecology of denitrification in biological wastewater treatment. Water Res. 2014, 64, 237–254. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.; Eldyasti, A. Development of partial nitrification as a first step of nitrite shunt process in a Sequential Batch Reactor (SBR) using Ammonium Oxidizing Bacteria (AOB) controlled by mixing regime. Bioresour. Technol. 2016, 221, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, J.; Peng, Y.; Wang, S.; Zhang, L.; Yang, S.; Li, S. Insight into the impacts of organics on anammox and their potential linking to system performance of sewage partial nitrification-anammox (PN/A): A critical review. Bioresour. Technol. 2020, 300, 122655. [Google Scholar] [CrossRef]
- Zhang, Z.-J.; Chen, S.-H.; Wang, S.-M.; Luo, H.-Y. Characterization of extracellular polymeric substances from biofilm in the process of starting-up a partial nitrification process under salt stress. Appl. Microbiol. Biotechnol. 2011, 89, 1563–1571. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Meng, F.; Chen, G.-H. Spectroscopic characterization of extracellular polymeric substances from a mixed culture dominated by ammonia-oxidizing bacteria. Water Res. 2015, 68, 740–749. [Google Scholar] [CrossRef]
- Xu, J.; Li, C.; Shen, Y.; Zhu, N. Anaerobic ammonium oxidation (anammox) promoted by pyrogenic biochar: Deciphering the interaction with extracellular polymeric substances (EPS). Sci. Total Environ. 2022, 802, 149884. [Google Scholar] [CrossRef]
- Arshad, Z.; Maqbool, T.; Shin, K.H.; Kim, S.-H.; Hur, J. Using stable isotope probing and fluorescence spectroscopy to examine the roles of substrate and soluble microbial products in extracellular polymeric substance formation in activated sludge process. Sci. Total Environ. 2021, 788, 147875. [Google Scholar] [CrossRef]
- Kahane, I.; Muhlrad, A. Purification and properties of acetate kinase from Acholeplasma laidlawii. J. Bacteriol. 1979, 137, 764–772. [Google Scholar] [CrossRef]
- Du, B.; Xuan, H.; Geng, L.; Li, W.; Zhang, J.; Xiang, W.; Liu, R.; Shu, C. Microflora for improving the Auricularia auricula spent mushroom substrate for Protaetia brevitarsis production. Iscience 2022, 25, 105307. [Google Scholar] [CrossRef]
- Fu, X.; Hou, R.; Yang, P.; Qian, S.; Feng, Z.; Chen, Z.; Wang, F.; Yuan, R.; Chen, H.; Zhou, B. Application of external carbon source in heterotrophic denitrification of domestic sewage: A review. Sci. Total Environ. 2022, 817, 153061. [Google Scholar] [CrossRef]
- Winkler, M.K.; Straka, L. New directions in biological nitrogen removal and recovery from wastewater. Curr. Opin. Biotechnol. 2019, 57, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Zhang, X.; Li, J.; Wu, X.; Feng, H.; Dong, W. A review of research progress of heterotrophic nitrification and aerobic denitrification microorganisms (HNADMs). Sci. Total Environ. 2021, 801, 149319. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, Q.; Cui, Q.; Ni, S.-Q. Nitrogen recovery through fermentative dissimilatory nitrate reduction to ammonium (DNRA): Carbon source comparison and metabolic pathway. Chem. Eng. J. 2022, 441, 135938. [Google Scholar] [CrossRef]
- Berger, S.; Welte, C.; Deppenmeier, U. Acetate activation in Methanosaeta thermophila: Characterization of the key enzymes pyrophosphatase and acetyl-CoA synthetase. Archaea 2012, 2012, 315153. [Google Scholar] [CrossRef]
- Bao, P.; Wang, S.; Ma, B.; Zhang, Q.; Peng, Y. Achieving partial nitrification by inhibiting the activity of Nitrospira-like bacteria under high-DO conditions in an intermittent aeration reactor. J. Environ. Sci. 2017, 56, 71–78. [Google Scholar] [CrossRef]
- Cui, B.; Yang, Q.; Liu, X.; Huang, S.; Yang, Y.; Liu, Z. The effect of dissolved oxygen concentration on long-term stability of partial nitrification process. J. Environ. Sci. 2020, 90, 343–351. [Google Scholar] [CrossRef]
- Luan, Y.-N.; Yin, Y.; An, Y.; Zhang, F.; Wang, X.; Zhao, F.; Xiao, Y.; Liu, C. Investigation of an intermittently-aerated moving bed biofilm reactor in rural wastewater treatment under low dissolved oxygen and C/N condition. Bioresour. Technol. 2022, 358, 127405. [Google Scholar] [CrossRef]
- Liu, G.; Wang, J. Long-term low DO enriches and shifts nitrifier community in activated sludge. Environ. Sci. Technol. 2013, 47, 5109–5117. [Google Scholar] [CrossRef]
- Nogueira, R.; Melo, L.F. Competition between Nitrospira spp. and Nitrobacter spp. in nitrite-oxidizing bioreactors. Biotechnol. Bioeng. 2006, 95, 169–175. [Google Scholar] [CrossRef]
- Dytczak, M.A.; Londry, K.L.; Oleszkiewicz, J.A. Activated sludge operational regime has significant impact on the type of nitrifying community and its nitrification rates. Water Res. 2008, 42, 2320–2328. [Google Scholar] [CrossRef]
- Kim, D.-J.; Lee, D.-I.; Cha, G.-C.; Keller, J. Analysis of free ammonia inhibition of nitrite oxidizing bacteria using a dissolved oxygen respirometer. Environ. Eng. Res. 2008, 13, 125–130. [Google Scholar] [CrossRef]
- Vadivelu, V.; Keller, J.; Yuan, Z. Free ammonia and free nitrous acid inhibition on the anabolic and catabolic processes of Nitrosomonas and Nitrobacter. Water Sci. Technol. 2007, 56, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.A.; Ibrahim, S.; Aroua, M.K. Effect of carbon source on acclimatization of nitrifying bacteria to achieve high-rate partial nitrification of wastewater with high ammonium concentration. Appl. Water Sci. 2017, 7, 165–173. [Google Scholar] [CrossRef]
- Cho, K.; Bae, S.; Jung, J.; Choi, D. Effect of aerobic microbes’ competition for oxygen on nitrogen removal in mainstream nitritation-anammox systems. Chemosphere 2022, 305, 135493. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Leng, J.; Zhu, J.; Zhang, K.; Zhao, J.; Wu, P.; Xing, Q.; Tang, K.; Li, X.; Hu, B. Influence mechanism of C/N ratio on heterotrophic nitrification-aerobic denitrification process. Bioresour. Technol. 2022, 343, 126116. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, Q.; Zhu, Y.; Zhao, T. Response of wastewater treatment performance, microbial composition and functional genes to different C/N ratios and carrier types in MBBR inoculated with heterotrophic nitrification-aerobic denitrification bacteria. Bioresour. Technol. 2021, 336, 125339. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Q.; Zhu, Y.; Zhao, T. Response of rotating biological contactor started up by heterotrophic nitrification-aerobic denitrification bacteria to various C/N ratios. Chemosphere 2022, 291, 133048. [Google Scholar] [CrossRef]
- Chen, J.; Shen, L.; Li, Y.; Cao, H.; Chen, C.; Zhang, G.; Xu, Z.; Lu, Y. Insights into the nitrogen transformation mechanism of Pseudomonas sp. Y15 capable of heterotrophic nitrification and aerobic denitrification. Env. Res. 2024, 240, 117595. [Google Scholar] [CrossRef]
- Agrawal, S.; Kinh, C.T.; Schwartz, T.; Hosomi, M.; Terada, A.; Lackner, S. Determining uncertainties in PICRUSt analysis–An easy approach for autotrophic nitrogen removal. Biochem. Eng. J. 2019, 152, 107328. [Google Scholar] [CrossRef]
- Zhang, Q.-L.; Liu, Y.; Ai, G.-M.; Miao, L.-L.; Zheng, H.-Y.; Liu, Z.-P. The characteristics of a novel heterotrophic nitrification–aerobic denitrification bacterium, Bacillus methylotrophicus strain L7. Bioresour. Technol. 2012, 108, 35–44. [Google Scholar] [CrossRef]
- Zhang, M.; Li, A.; Yao, Q.; Wu, Q.; Zhu, H. Nitrogen removal characteristics of a versatile heterotrophic nitrifying-aerobic denitrifying bacterium, Pseudomonas bauzanensis DN13-1, isolated from deep-sea sediment. Bioresour. Technol. 2020, 305, 122626. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; An, Q.; He, Y.L.; Guo, J.S. N2O and N2 production during heterotrophic nitrification by Alcaligenes faecalis strain NR. Bioresour. Technol. 2012, 116, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shu, Z.; Sun, D.; Dang, Y.; Holmes, D.E. Heterotrophic nitrifiers dominate reactors treating incineration leachate with high free ammonia concentrations. ACS Sustain. Chem. Eng. 2018, 6, 15040–15049. [Google Scholar] [CrossRef]
- Li, J.; Du, Q.; Peng, H.; Zhang, Y.; Bi, Y.; Shi, Y.; Xu, Y.; Liu, T. Optimization of biochemical oxygen demand to total nitrogen ratio for treating landfill leachate in a single-stage partial nitrification-denitrification system. J. Clean. Prod. 2020, 266, 121809. [Google Scholar] [CrossRef]

| COD/N | NH4+-N (mg/L) | COD (mg/L) | NaHCO3 (mg/L) | Aeration Rate (mL/min) | pH | |
|---|---|---|---|---|---|---|
| Ra | 0 | 500 | 0 | 5500 | 600 | 7.3 ± 0.2 |
| Rb | 0.5 | 500 | 250 | 5500 | 800 | 7.3 ± 0.2 |
| Rc | 1 | 500 | 500 | 5500 | 1200 | 7.3 ± 0.2 |
| Rd | 2 | 500 | 1000 | 5500 | 1800 | 7.3 ± 0.2 |
| Re | 4 | 500 | 2000 | 5500 | 2000 | 7.3 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Z.; Lei, Y.; Zhan, Y.; Yang, B.; Pan, X. Impact of Chemical Oxygen Demand/Total Nitrogen Ratio on Shifting Autotrophic Partial Nitrification to Heterotrophic Nitrification and Aerobic Denitrification in High-Strength Ammonium Wastewater Treatment. Water 2024, 16, 2532. https://doi.org/10.3390/w16172532
Peng Z, Lei Y, Zhan Y, Yang B, Pan X. Impact of Chemical Oxygen Demand/Total Nitrogen Ratio on Shifting Autotrophic Partial Nitrification to Heterotrophic Nitrification and Aerobic Denitrification in High-Strength Ammonium Wastewater Treatment. Water. 2024; 16(17):2532. https://doi.org/10.3390/w16172532
Chicago/Turabian StylePeng, Zhenghua, Yongfei Lei, Yousheng Zhan, Benqin Yang, and Xuejun Pan. 2024. "Impact of Chemical Oxygen Demand/Total Nitrogen Ratio on Shifting Autotrophic Partial Nitrification to Heterotrophic Nitrification and Aerobic Denitrification in High-Strength Ammonium Wastewater Treatment" Water 16, no. 17: 2532. https://doi.org/10.3390/w16172532
APA StylePeng, Z., Lei, Y., Zhan, Y., Yang, B., & Pan, X. (2024). Impact of Chemical Oxygen Demand/Total Nitrogen Ratio on Shifting Autotrophic Partial Nitrification to Heterotrophic Nitrification and Aerobic Denitrification in High-Strength Ammonium Wastewater Treatment. Water, 16(17), 2532. https://doi.org/10.3390/w16172532







