Determining the Fluxes and Relative Importance of Different External Sources and Sinks of Nitrogen to the Israeli Coastal Shelf, a Potentially Vulnerable Ecosystem
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
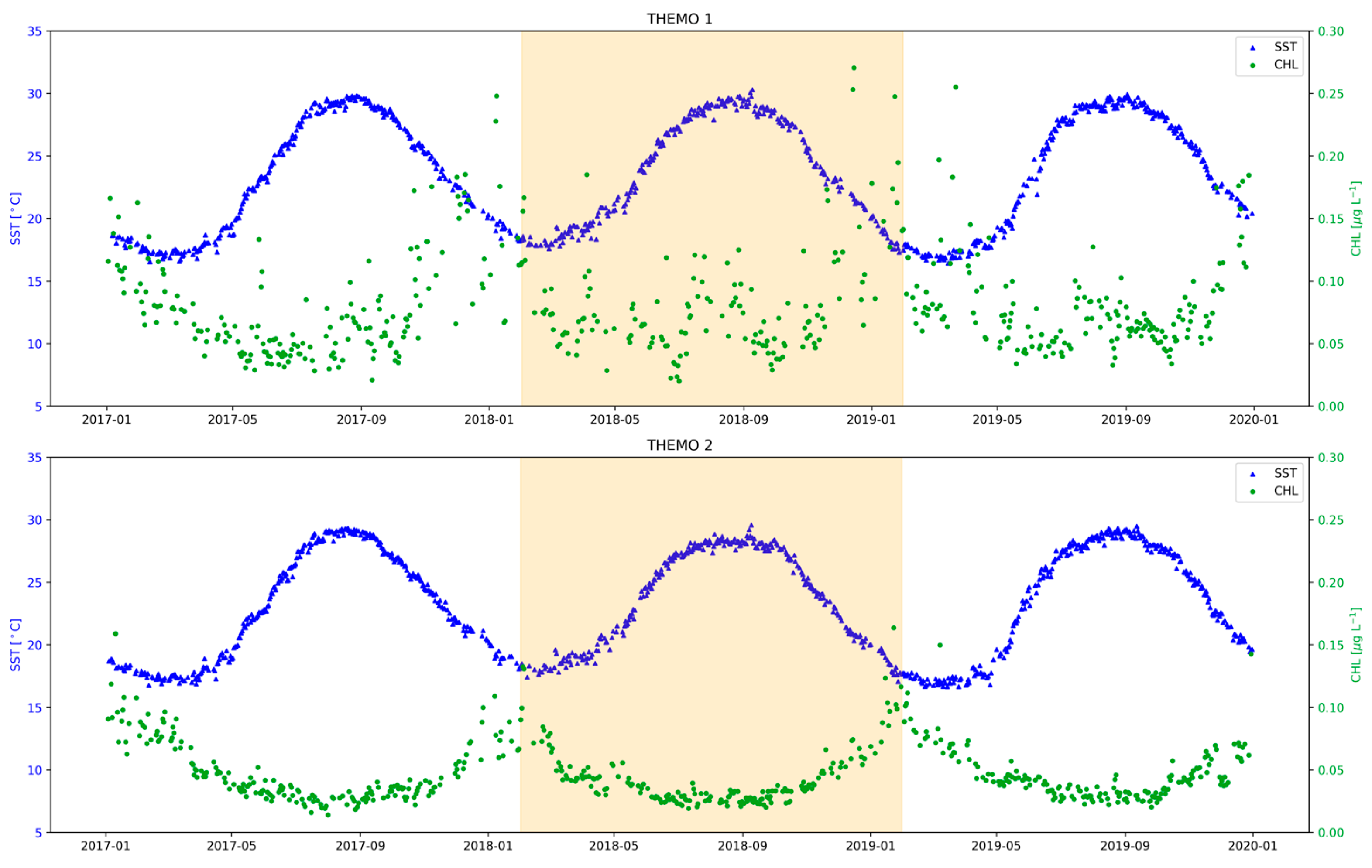
3.1. Nutrient Dynamics across the Israeli Coastal Shelf (ICS)
3.2. Seasonal Changes in Primary Productivity and Phytoplankton Biomass on the ICS
3.3. Sources and Sinks of Nutrients to the ICS
| Source of N to ICS | Average ± Range (106 mol N y−1) | Nitrate–Ammonium Ratio | Reference |
|---|---|---|---|
| Atmospheric supply | 115 | 7:5 | [7] |
| Submarine groundwater discharge | 8–38 | 19:1 | [44,45] |
| Direct wastewater discharge | 60 ± 20 | Dominantly ammonium | [46] |
| Riverine input | 123.5 | 1:1.6 | [38] |
| Total external sources | 316 | ||
| Sinks of N from ICS | |||
| Sediment | 145 ± 15 | N.A. | [16] |
| Net primary productivity | 420 | N.A. | This study |
| Fishing activity | 13 | N.A. | |
| Total sinks on the ICS | 575 | ||
| Advection = Sum of sinks− sources | 259 (45%) | Advection is dominantly nitrate and mainly in winter | This study |
3.3.1. Atmospheric Input
3.3.2. Input from Submarine Groundwater Discharge (SGD)
3.3.3. Direct Discharge of Wastewater into the ICS
3.3.4. Riverine Input
3.4. Sinks of N on the ICS
3.4.1. Flux of N across the Sediment–Water Interface
3.4.2. N Consumed by Net Primary Productivity
3.4.3. Fishing Activity on the ICS
3.4.4. Advection onto the ICS
4. Implications on Biogeochemical Processes and Seasonality across the ICS and Pelagic EMS
4.1. Implication of the Total N Budget for Biogeochemical Processes on the Shelf
4.1.1. The Relative Importance of Different Sources and Sinks of N on the Shelf
4.1.2. Why Is the Seasonality of Nutrient Dynamics on the ICS Similar to the Dynamics in the Pelagic?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Krom, M.D.; Kress, N.; Brenner, S.; Gordon, L.I. Phosphorus Limitation of Primary Productivity in the Eastern Mediterranean Sea. Limnol. Oceanogr. 1991, 36, 424–432. [Google Scholar] [CrossRef]
- Kress, N.; Herut, B. Spatial and Seasonal Evolution of Dissolved Oxygen and Nutrients in the Southern Levantine Basin (Eastern Mediterranean Sea): Chemical Characterization of the Water Masses and Inferences on the N: P Ratios. Deep Sea Res. Part I Oceanogr. Res. Pap. 2001, 48, 2347–2372. [Google Scholar] [CrossRef]
- Thingstad, T.F.; Krom, M.D.; Mantoura, R.F.C.; Flaten, C.A.F.; Groom, S.; Herut, B.; Kress, N.; Law, C.S.; Pasternak, A.; Pitta, P.; et al. Nature of Phosphorus Limitation in the Ultraoligotrophic Eastern Mediterranean. Science 2005, 309, 1068–1071. [Google Scholar] [CrossRef]
- Pujo-Pay, M.; Conan, P.; Oriol, L.; Cornet-Barthaux, V.; Falco, C.; Ghiglione, J.F.; Goyet, C.; Moutin, T.; Prieur, L. Integrated Survey of Elemental Stoichiometry (C, N, P) from the Western to Eastern Mediterranean Sea. Biogeosciences 2011, 8, 883–899. [Google Scholar] [CrossRef]
- Powley, H.R.; Krom, M.D.; Van Cappellen, P. Understanding the Unique Biogeochemistry of the Mediterranean Sea: Insights from a Coupled Phosphorus and Nitrogen Model. Glob. Biogeochem. Cycles 2017, 31, 1010–1031. [Google Scholar] [CrossRef]
- Djaoudi, K.; Van Wambeke, F.; Coppola, L.; D’Ortenzio, F.; Helias-Nunige, S.; Raimbault, P.; Taillandier, V.; Testor, P.; Wagener, T.; Pulido-Villena, E. Sensitive Determination of the Dissolved Phosphate Pool for an Improved Resolution of Its Vertical Variability in the Surface Layer: New Views in the P-Depleted Mediterranean Sea. Front. Mar. Sci. 2018, 5, 234. [Google Scholar] [CrossRef]
- Ben Ezra, T.; Krom, M.D.; Tsemel, A.; Berman-Frank, I.; Herut, B.; Lehahn, Y.; Rahav, E.; Reich, T.; Thingstad, T.F.; Sher, D. Seasonal Nutrient Dynamics in the P Depleted Eastern Mediterranean Sea. Deep. Res. Part I Oceanogr. Res. Pap. 2021, 176, 103607. [Google Scholar] [CrossRef]
- Balkis, N. Seasonal Variations of Microphytoplankton Assemblages and Environmental Variables in the Coastal Zone of Bozcaada Island in the Aegean Sea (NE Mediterranean Sea). Aquat. Ecol. 2009, 43, 249–270. [Google Scholar] [CrossRef]
- Ounissi, M.; Amira, A.B.; Dulac, F. Riverine and Wet Atmospheric Inputs of Materials to a North Africa Coastal Site (Annaba Bay, Algeria). Prog. Oceanogr. 2018, 165, 19–34. [Google Scholar] [CrossRef]
- Zaafrane, S.; Maatouk, K.; Akrout, F.; Trabelsi, I.; Drira, N. Spatio-Temporal Distribution of Physicochemical and Bacteriological Parameters in the North Area of Monastir Bay, Eastern Coast of Tunisia. Arab. J. Geosci. 2019, 12, 210. [Google Scholar] [CrossRef]
- Ignatiades, L.; Psarra, S.; Zervakis, V.; Pagou, K.; Souvermezoglou, E.; Assimakopoulou, G.; Gotsis-Skretas, O. Phytoplankton Size-Based Dynamics in the Aegean Sea (Eastern Mediterranean). J. Mar. Syst. 2002, 36, 11–28. [Google Scholar] [CrossRef]
- Psarra, S.; Tselepides, A.; Ignatiades, L. Primary Productivity in the Oligotrophic Cretan Sea (NE Mediterranean): Seasonal and Interannual Variability. Prog. Oceanogr. 2000, 46, 187–204. [Google Scholar] [CrossRef]
- Souvermezoglou, E.; Krasakopoulou, E.; Pavlidou, A. Temporal and Spatial Variability of Nutrients and Oxygen in the North Aegean Sea during the Last Thirty Years. Mediterr. Mar. Sci. 2014, 15, 805–822. [Google Scholar] [CrossRef][Green Version]
- Doğan-Sağlamtimur, N.; Tuğrul, S. Effect of Riverine Nutrients on Coastal Water Ecosystems: A Case Study from the Northeastern Mediterranean Shelf. Fresenius Environ. Bull. 2004, 13, 1288–1294. [Google Scholar]
- Townsend, D.W.; Christensen, J.P.; Berman, T.; Walline, P.; Schneller, A.; Yentsch, C.S. Near-Bottom Chlorophyll Maxima in Southeastern Mediterranean Shelf Waters: Upwelling and Sediments as Possible Nutrient Sources. Oceanol. Acta SP 1988, 235–244. [Google Scholar]
- Christensen, J.P.; Goldsmith, V.; Walline, P.; Schneller, A.; El Sayed, S.Z. Sedimentary Nutrient Regeneration on the Oligotrophic Eastern Mediterranean Continental Shelf. Oceanol. Acta Spec. Issue 1988, 1–27. [Google Scholar]
- Varkitzi, I.; Markogianni, V.; Pantazi, M.; Pagou, K.; Pavlidou, A.; Dimitriou, E. Effect of River Inputs on Environmental Status and Potentially Harmful Phytoplankton in a Coastal Area of Eastern Mediterranean (Maliakos Gulf, Greece). Mediterr. Mar. Sci. 2018, 19, 326–343. [Google Scholar] [CrossRef]
- Kress, N.; Herut, B. Hypernutrification in the Oligotrophic Eastern Mediterranean: A Study in Haifa Bay (Israel). Estuar. Coast. Shelf Sci. 1998, 46, 645–656. [Google Scholar] [CrossRef]
- Rekik, A.; Ayadi, H.; Elloumi, J. Spatial and Seasonal Variability of the Planktonic Ciliates Assemblages along the Eastern Mediterranean Coast (Sfax Coast, Tunisia). Reg. Stud. Mar. Sci. 2020, 40, 101529. [Google Scholar] [CrossRef]
- Simboura, N.; Zenetos, A.; Pancucci-Papadopoulou, M.A. Benthic Community Indicators over a Long Period of Monitoring (2000-2012) of the Saronikos Gulf, Greece, Eastern Mediterranean. Environ. Monit. Assess. 2014, 186, 3809–3821. [Google Scholar] [CrossRef]
- Kress, N.; Rahav, E.; Silverman, J.; Herut, B. Environmental Status of Israel’s Mediterranean Coastal Waters: Setting Reference Conditions and Thresholds for Nutrients, Chlorophyll-a and Suspended Particulate Matter. Mar. Pollut. Bull. 2019, 141, 612–620. [Google Scholar] [CrossRef]
- Breitburg, D.; Levin, L.A.; Oschlies, A.; Grégoire, M.; Chavez, F.P.; Conley, D.J.; Garçon, V.; Gilbert, D.; Gutiérrez, D.; Isensee, K. Declining Oxygen in the Global Ocean and Coastal Waters. Science 2018, 359, eaam7240. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Gong, G.-C.; Shiah, F.-K. Hypoxia in the East China Sea: One of the Largest Coastal Low-Oxygen Areas in the World. Mar. Environ. Res. 2007, 64, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Berner, E.K.; Berner, R.A. Global Environment: Water, Air, and Geochemical Cycles; Princeton University Press: Princeton, NJ, USA, 2012; ISBN 0691136785. [Google Scholar]
- Rahav, E.; Raveh, O.; Hazan, O.; Gordon, N.; Kress, N.; Silverman, J.; Herut, B. Impact of Nutrient Enrichment on Productivity of Coastal Water along the SE Mediterranean Shore of Israel—A Bioassay Approach. Mar. Pollut. Bull. 2018, 127, 559–567. [Google Scholar] [CrossRef]
- Rahav, E.; Raveh, O.; Yanuka-Golub, K.; Belkin, N.; Astrahan, P.; Maayani, M.; Tsumi, N.; Kiro, Y.; Herut, B.; Silverman, J. Nitrate-Enrichment Structures Phytoplankton Communities in the Shallow Eastern Mediterranean Coastal Waters. Front. Mar. Sci. 2020, 7, 611497. [Google Scholar] [CrossRef]
- Azov, Y. Seasonal Patterns of Phytoplankton Productivity and Abundance in Nearshore Oligotrophic Waters of the Levant Basin (Mediterranean). J. Plankton Res. 1986, 8, 41–53. [Google Scholar] [CrossRef]
- Berman, T.; Townsend, D.W.; Elsayed, S.Z.; Trees, C.C.; Azov, Y. Optical Transparency, Chlorophyll and Primary Productivity in the Eastern Mediterranean near the Israeli Coast. Oceanol. Acta 1984, 7, 367–372. [Google Scholar]
- Ben Ezra, T.; Reich, T.; Tsemel, A.; Berman-Frank, I.; Lehahn, Y.; Sher, D.; Suari, Y.; Krom, M.D. Nutrient Dynamics across the Israeli Coastal Shelf: An Unusual Oligotrophic Coastal System. Cont. Shelf Res. 2023, 266, 105103. [Google Scholar] [CrossRef]
- Kamennaya, N.A.; Geraki, K.; Scanlan, D.J.; Zubkov, M. V Accumulation of Ambient Phosphate into the Periplasm of Marine Bacteria Is Proton Motive Force Dependent. Nat. Commun. 2020, 11, 2642. [Google Scholar] [CrossRef]
- Ben Ezra, T.; Blachinsky, A.; Gozali, S.; Tsemel, A.; Tchernov, D.; Berman-Frank, I.; Krom, M.D. Beyond Chlorophyll: Exploring Hidden Dimensions of Nutrient Limitation in the Eastern Mediterranean Sea. Limnol. Oceanogr. 2000. [Google Scholar]
- Zubkov, M.V.; Martin, A.P.; Hartmann, M.; Grob, C.; Scanlan, D.J. Dominant Oceanic Bacteria Secure Phosphate Using a Large Extracellular Buffer. Nat. Commun. 2015, 6, 7878. [Google Scholar] [CrossRef] [PubMed]
- Berman, T.; Azov, Y.; Schneller, A.; Walline, P.; Townsend, D.W. Extent, Transparency, and Phytoplankton Distribution of the Neritic Waters Overlying the Israeli Coastal Shelf. Oceanol. Acta 1986, 9, 439–447. [Google Scholar]
- Reich, T.; Ben Ezra, T.; Belkin, N.; Tsemel, A.; Aharonovich, D.; Roth-Rosenberg, D.; Givati, S.; Bialik, M.; Herut, B.; Berman-Frank, I.; et al. A Year in the Life of the Eastern Mediterranean: Monthly Dynamics of Phytoplankton and Bacterioplankton in an Ultra-Oligotrophic Sea. Deep. Res. Part I Oceanogr. Res. Pap. 2022, 182, 103720. [Google Scholar] [CrossRef]
- Keuter, S.; Silverman, J.; Krom, M.D.; Sisma-Ventura, G.; Yu, J.; Tsemel, A.; Ben Ezra, T.; Sher, D.; Reich, T.; Koplovitz, G.; et al. Seasonal Patterns of Coccolithophores in the Ultra-Oligotrophic South-East Levantine Basin, Eastern Mediterranean Sea. Mar. Micropaleontol. 2022, 175, 102153. [Google Scholar] [CrossRef]
- Rahav, E.; Berman-Frank, I. Temporal and Vertical Dynamics of Diatoms and Dinoflagellates in the Southeastern Mediterranean Sea. J. Plankton Res. 2023, 45, 614–624. [Google Scholar] [CrossRef]
- Rahav, E.; Bar-Zeev, E. Sewage Outburst Triggers Trichodesmium Bloom and Enhance N2 Fixation Rates. Sci. Rep. 2017, 7, 4367. [Google Scholar] [CrossRef]
- Suari, Y.; Topaz, T.; Bassa, O.; Gilboa, M.; Sedaka, H.; Sade, T.; Chefetz, B.; Yahel, G. Nutrient Concentration, Loads and Retention in a Semiarid Micro-Estuary: The Relative Contribution of Baseflow and Flood Events. Sci. Total Environ. 2024, 931, 172805. [Google Scholar] [CrossRef]
- Krom, M.D.; Brenner, S.; Kress, N.; Neori, A.; Gordon, L.I. Nutrient Dynamics and New Production in a Warm-Core Eddy from the Eastern Mediterranean Sea. Deep Sea Res. Part A. Oceanogr. Res. Pap. 1992, 39, 467–480. [Google Scholar] [CrossRef]
- Tanaka, T.; Thingstad, T.F.; Christaki, U.; Colombet, J.; Cornet-Barthaux, V.; Courties, C.; Grattepanche, J.-D.D.; Lagaria, A.; Nedoma, J.; Oriol, L.; et al. Lack of P-Limitation of Phytoplankton and Heterotrophic Prokaryotes in Surface Waters of Three Anticyclonic Eddies in the Stratified Mediterranean Sea. Biogeosciences 2011, 8, 525–538. [Google Scholar] [CrossRef]
- Eppley, R.W.; Peterson, B.J. Particulate Organic Matter Flux and Planktonic New Production in the Deep Ocean. Nature 1979, 282, 677–680. [Google Scholar] [CrossRef]
- Yogev, T.; Rahav, E.; Bar-Zeev, E.; Man-Aharonovich, D.; Stambler, N.; Kress, N.; Béjà, O.; Mulholland, M.R.; Herut, B.; Berman-Frank, I. Is Dinitrogen Fixation Significant in the Levantine Basin, East Mediterranean Sea? Environ. Microbiol. 2011, 13, 854–871. [Google Scholar] [CrossRef] [PubMed]
- Krom, M.D.; Emeis, K.C.; Van Cappellen, P. Why Is the Eastern Mediterranean Phosphorus Limited? Prog. Oceanogr. 2010, 85, 236–244. [Google Scholar] [CrossRef]
- Weinstein, Y.; Yechieli, Y.; Shalem, Y.; Burnett, W.C.; Swarzenski, P.W.; Herut, B. What Is the Role of Fresh Groundwater and Recirculated Seawater in Conveying Nutrients to the Coastal Ocean? Environ. Sci. Technol. 2011, 45, 5195–5200. [Google Scholar] [CrossRef]
- Rodellas, V.; Garcia-Orellana, J.; Masqué, P.; Feldman, M.; Weinstein, Y. Submarine Groundwater Discharge as a Major Source of Nutrients to the Mediterranean Sea. Proc. Natl. Acad. Sci. USA 2015, 112, 3926–3930. [Google Scholar] [CrossRef] [PubMed]
- Powley, H.R.; Dürr, H.H.; Lima, A.T.; Krom, M.D.; Van Cappellen, P. Direct Discharges of Domestic Wastewater Are a Major Source of Phosphorus and Nitrogen to the Mediterranean Sea. Environ. Sci. Technol. 2016, 50, 8722–8730. [Google Scholar] [CrossRef]
- Herut, B.; Collier, R.; Krom, M.D. The Role of Dust in Supplying Nitrogen and Phosphorus to the Southeast Mediterranean. Limnol. Oceanogr. 2002, 47, 870–878. [Google Scholar] [CrossRef]
- Markaki, Z.; Loÿe-Pilot, M.D.; Violaki, K.; Benyahya, L.; Mihalopoulos, N. Variability of Atmospheric Deposition of Dissolved Nitrogen and Phosphorus in the Mediterranean and Possible Link to the Anomalous Seawater N/P Ratio. Mar. Chem. 2010, 120, 187–194. [Google Scholar] [CrossRef]
- Herut, B.; Krom, M. Atmospheric Input of Nutrients and Dust to the SE Mediterranean. In The Impact of Desert Dust across the Mediterranean; Springer: Berlin/Heidelberg, Germany, 1996; pp. 349–358. [Google Scholar]
- Zektser, I.S.; Everett, L.G.; Dzhamalov, R.G. Submarine Groundwater; CRC Press: Boca Raton, FL, USA, 2006; ISBN 0429124171. [Google Scholar]
- Juanico, M.; Friedler, E. Wastewater Reuse for River Recovery in Semi-Arid Israel. Water Sci. Technol. 1999, 40, 43–50. [Google Scholar] [CrossRef]
- Herut, B.; Kress, N.; Hornung, H. Nutrient Pollution at the Lower Reaches of Mediterranean Coastal Rivers in Israel. Water Sci. Technol. 2000, 42, 147–152. [Google Scholar] [CrossRef]
- Herut, B. Israel’s National Monitoring Plan Sponsored by the Ministry of Environmental Protection; The Ministry of Water and Energy and the National Infrastructure Office: Addis Ababa, Ethiopia, 2015. (In Hebrew) [Google Scholar]
- Suari, Y.; Dadon-Pilosof, A.; Sade, T.; Amit, T.; Gilboa, M.; Gafny, S.; Topaz, T.; Zedaka, H.; Boneh, S.; Yahel, G. A Long Term Physical and Biogeochemical Database of a Hyper-Eutrophicated Mediterranean Micro-Estuary. Data Br. 2019, 27, 104809. [Google Scholar] [CrossRef]
- Kanari, M.; Tibor, G.; Hall, J.K.; Ketter, T.; Lang, G.; Schattner, U. Sediment Transport Mechanisms Revealed by Quantitative Analyses of Seafloor Morphology: New Evidence from Multibeam Bathymetry of the Israel Exclusive Economic Zone. Mar. Pet. Geol. 2020, 114, 104224. [Google Scholar] [CrossRef]
- Eijsink, L.M.; Krom, M.D.; Herut, B. Speciation and Burial Flux of Phosphorus in the Surface Sediments of the Eastern Mediterranean. Am. J. Sci. 2000, 300, 483–503. [Google Scholar] [CrossRef]
- Frid, O.; Gavriel, T.; Ben-Ari, Y.; Weinberger, A.; Yancovich-Shalom, H.; Belmaker, J. Catch estimates and species composition of recreational fishing in Israel. Fishes 2023, 8, 69. [Google Scholar] [CrossRef]
- Van Cappellen, P.; Powley, H.R.; Emeis, K.-C.; Krom, M.D. A Biogeochemical Model for Phosphorus and Nitrogen Cycling in the Eastern Mediterranean Sea: Part 1. Model Development, Initialization and Sensitivity. J. Mar. Syst. 2014, 139, 460–471. [Google Scholar] [CrossRef]
- Artioli, Y.; Friedrich, J.; Gilbert, A.J.; McQuatters-Gollop, A.; Mee, L.D.; Vermaat, J.E.; Wulff, F.; Humborg, C.; Palmeri, L.; Pollehne, F. Nutrient Budgets for European Seas: A Measure of the Effectiveness of Nutrient Reduction Policies. Mar. Pollut. Bull. 2008, 56, 1609–1617. [Google Scholar] [CrossRef] [PubMed]
- Krom, M.D.; Kress, N.; Berman-Frank, I.; Rahav, E. Past, Present and Future Patterns in the Nutrient Chemistry of the Eastern Mediterranean. In The Mediterranean Sea: Its History and Present Challenges; Springer: Dordrecht, The Netherlands, 2014; pp. 49–68. ISBN 9789400767041. [Google Scholar]
- Radtke, H.; Neumann, T.; Voss, M.; Fennel, W. Modeling Pathways of Riverine Nitrogen and Phosphorus in the Baltic Sea. J. Geophys. Res. Ocean. 2012, 117, C09024. [Google Scholar] [CrossRef]
- Pätsch, J.; Kühn, W. Nitrogen and Carbon Cycling in the North Sea and Exchange with the North Atlantic—A Model Study. Part I. Nitrogen Budget and Fluxes. Cont. Shelf Res. 2008, 28, 767–787. [Google Scholar] [CrossRef]
- Herut, B.; Gertner, Y.; Segal, Y.; Sisma-Ventura, G.; Gordon, N.; Belkin, N.; Rahav, E. Long-Term (2002–2021) Trend in Nutrient-Related Pollution at Small Stratified Inland Estuaries, the Kishon SE Mediterranean Case. Water 2023, 15, 484. [Google Scholar] [CrossRef]
- Golan, T. The Fall and Rise of the Kishon River. Water 2016, 8, 283. [Google Scholar] [CrossRef]
- Ozer, T.; Gertman, I.; Gildor, H.; Herut, B. Thermohaline Temporal Variability of the SE Mediterranean Coastal Waters (Israel)–Long-Term Trends, Seasonality, and Connectivity. Front. Mar. Sci. 2022, 8, 799457. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Ezra, T.; Tsemel, A.; Suari, Y.; Berman-Frank, I.; Tchernov, D.; Krom, M.D. Determining the Fluxes and Relative Importance of Different External Sources and Sinks of Nitrogen to the Israeli Coastal Shelf, a Potentially Vulnerable Ecosystem. Water 2024, 16, 2585. https://doi.org/10.3390/w16182585
Ben Ezra T, Tsemel A, Suari Y, Berman-Frank I, Tchernov D, Krom MD. Determining the Fluxes and Relative Importance of Different External Sources and Sinks of Nitrogen to the Israeli Coastal Shelf, a Potentially Vulnerable Ecosystem. Water. 2024; 16(18):2585. https://doi.org/10.3390/w16182585
Chicago/Turabian StyleBen Ezra, Tal, Anat Tsemel, Yair Suari, Ilana Berman-Frank, Danny Tchernov, and Michael David Krom. 2024. "Determining the Fluxes and Relative Importance of Different External Sources and Sinks of Nitrogen to the Israeli Coastal Shelf, a Potentially Vulnerable Ecosystem" Water 16, no. 18: 2585. https://doi.org/10.3390/w16182585
APA StyleBen Ezra, T., Tsemel, A., Suari, Y., Berman-Frank, I., Tchernov, D., & Krom, M. D. (2024). Determining the Fluxes and Relative Importance of Different External Sources and Sinks of Nitrogen to the Israeli Coastal Shelf, a Potentially Vulnerable Ecosystem. Water, 16(18), 2585. https://doi.org/10.3390/w16182585







