Winter Season Outdoor Cultivation of an Autochthonous Chlorella-Strain in a Pilot-Scale Prototype for Urban Wastewater Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalgae
2.2. Wastewater
2.3. Experimental Design
2.4. Environmental Parameters
2.5. Growth Rate of Algae and pH of Culture Substrates
2.6. PSII Maximum Quantum Yield of Algae
2.7. Photosynthetic Pigments of Algae
2.8. Characteristics of the Cultivation Substrate
2.9. Light Microscopy of Algae Samples for Exopolysaccharides Detection (EPS)
2.10. Data Analysis
3. Results
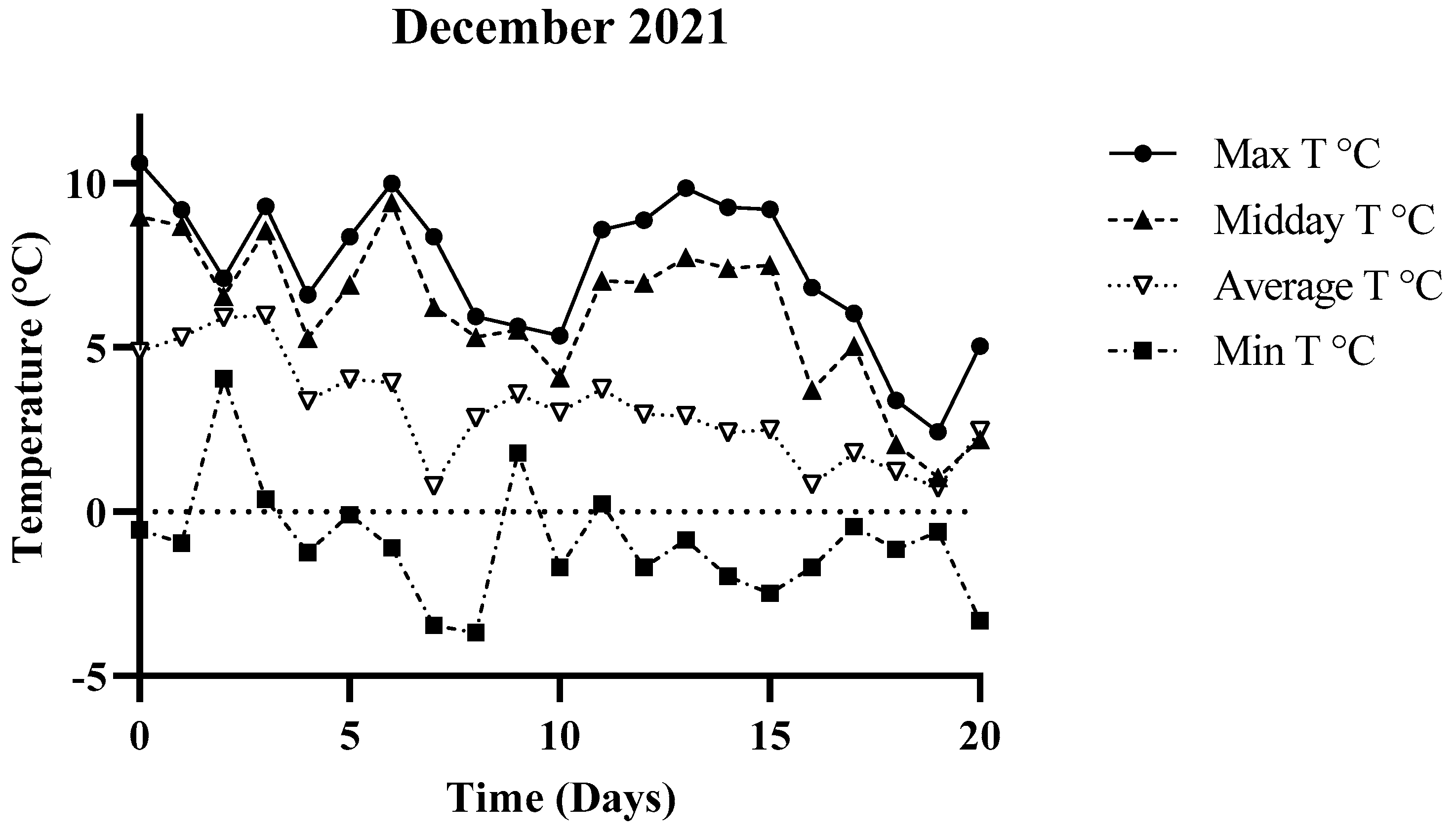
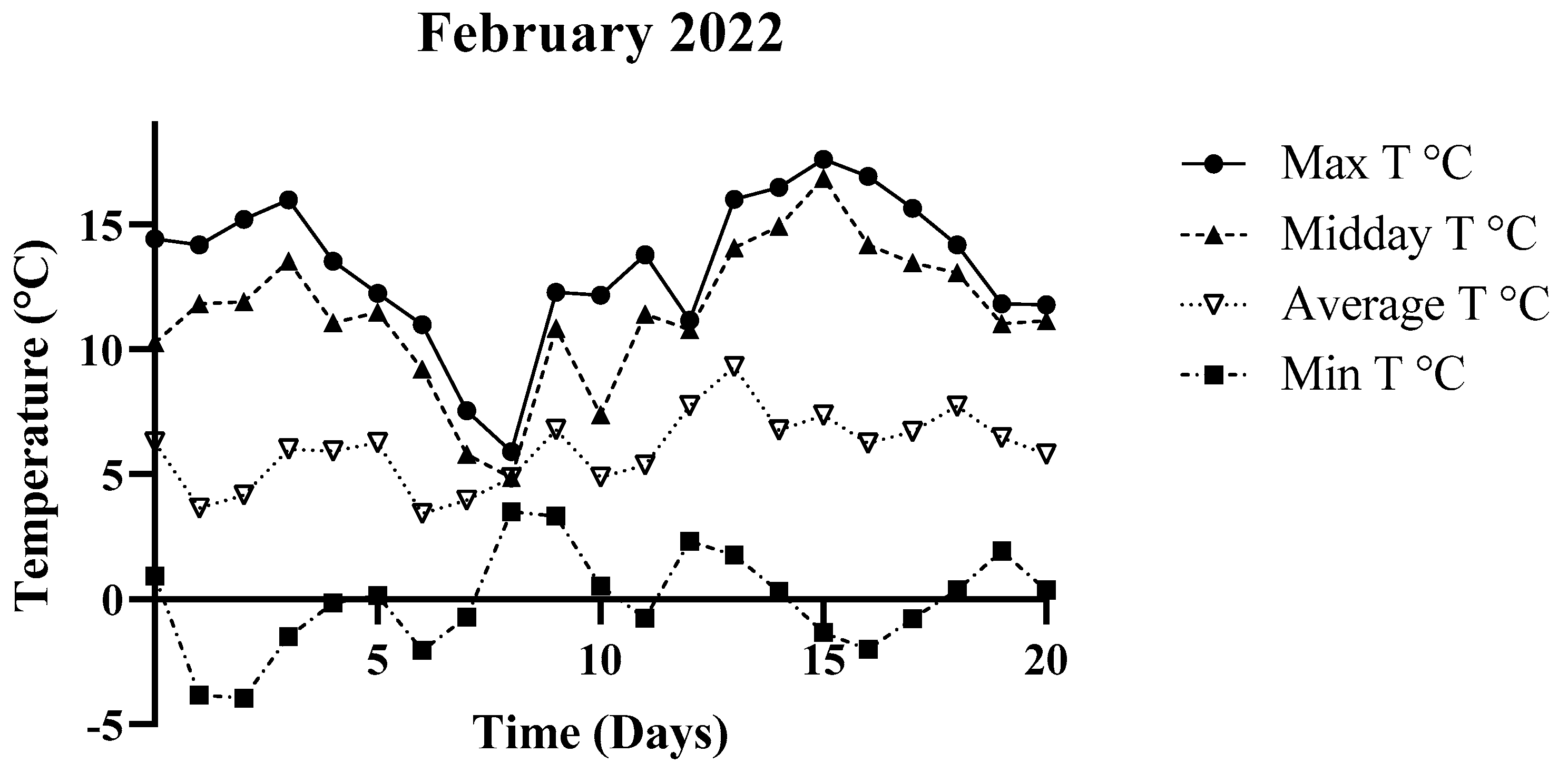
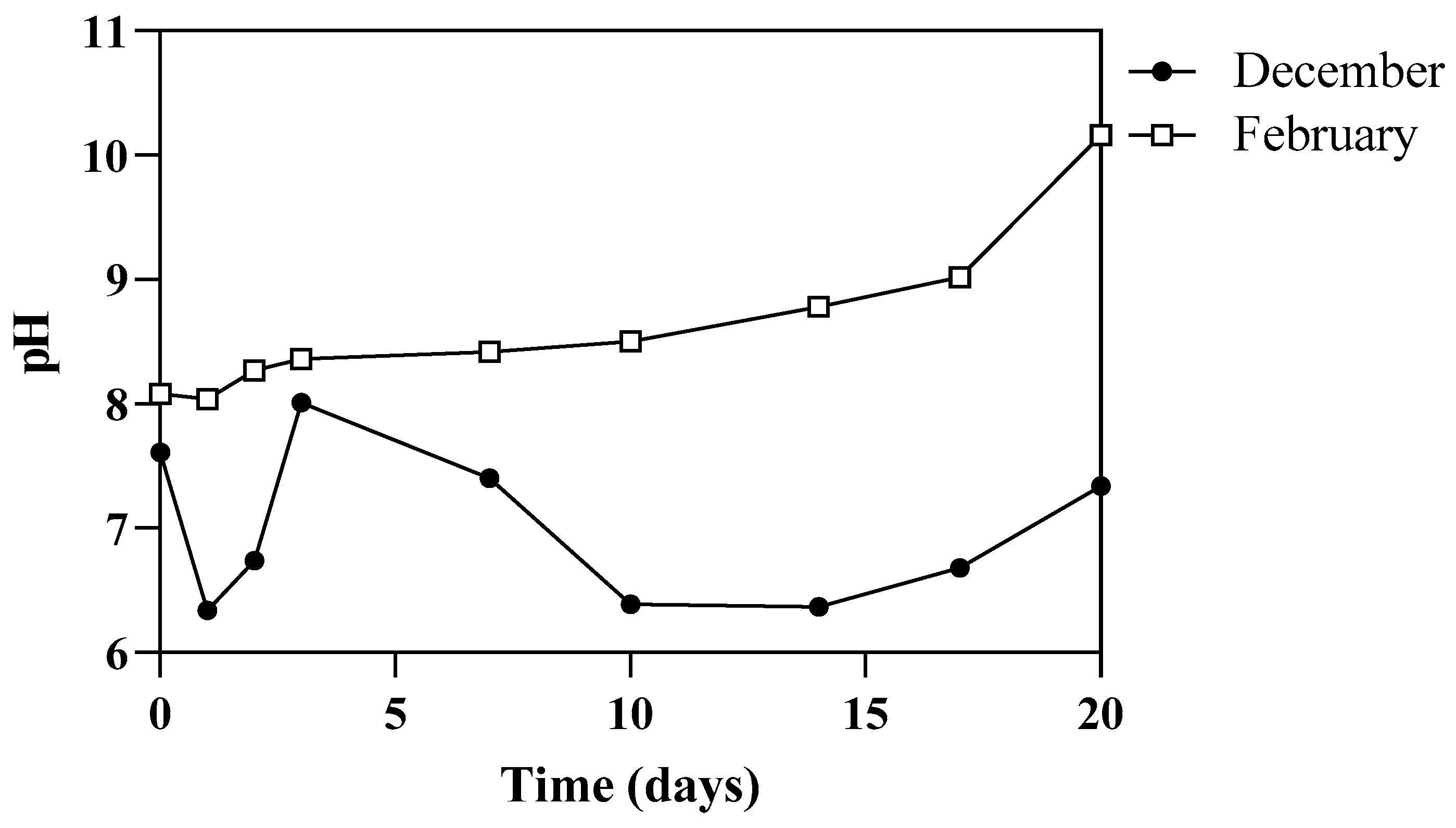
3.1. Environmental Parameters
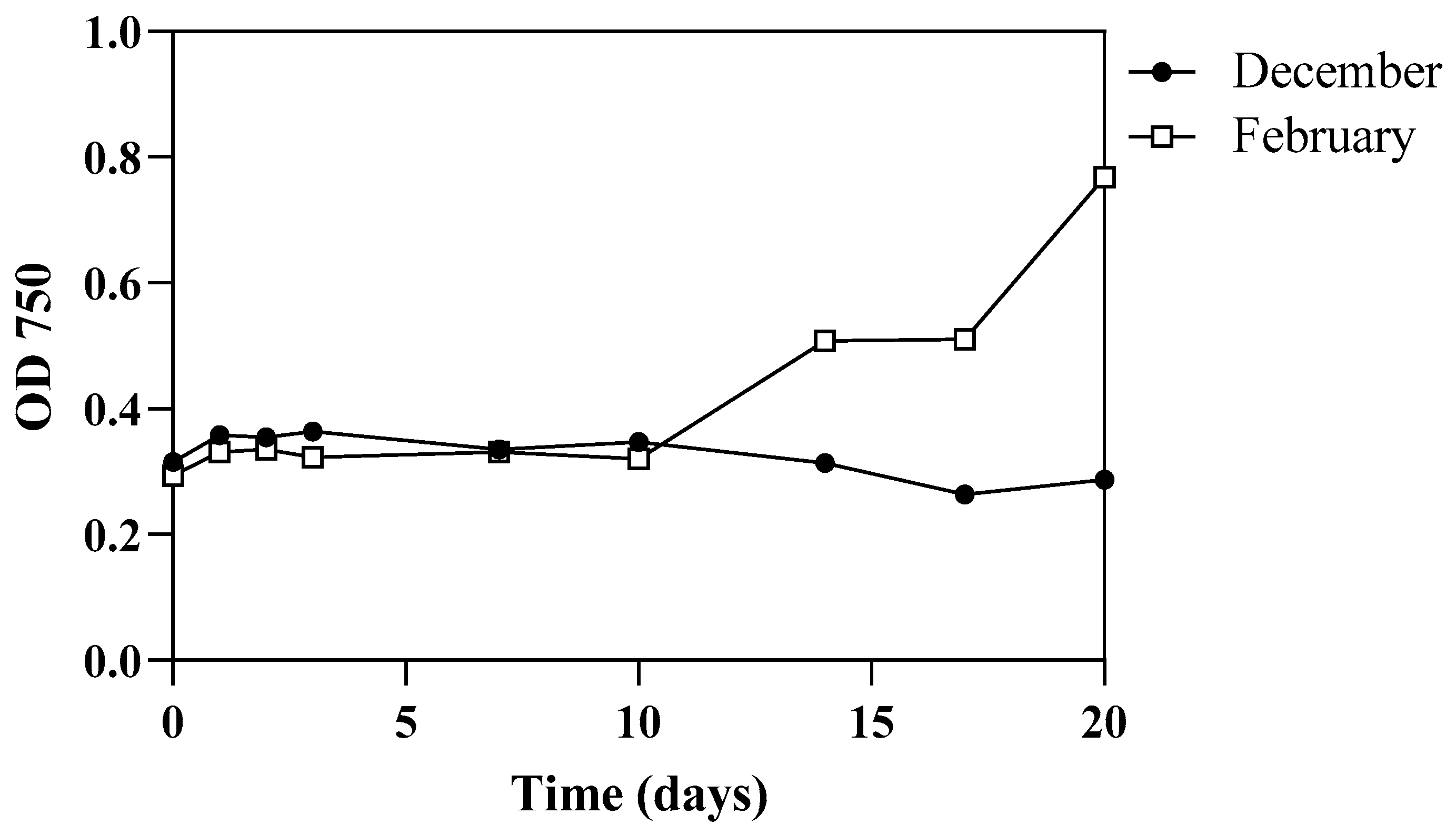
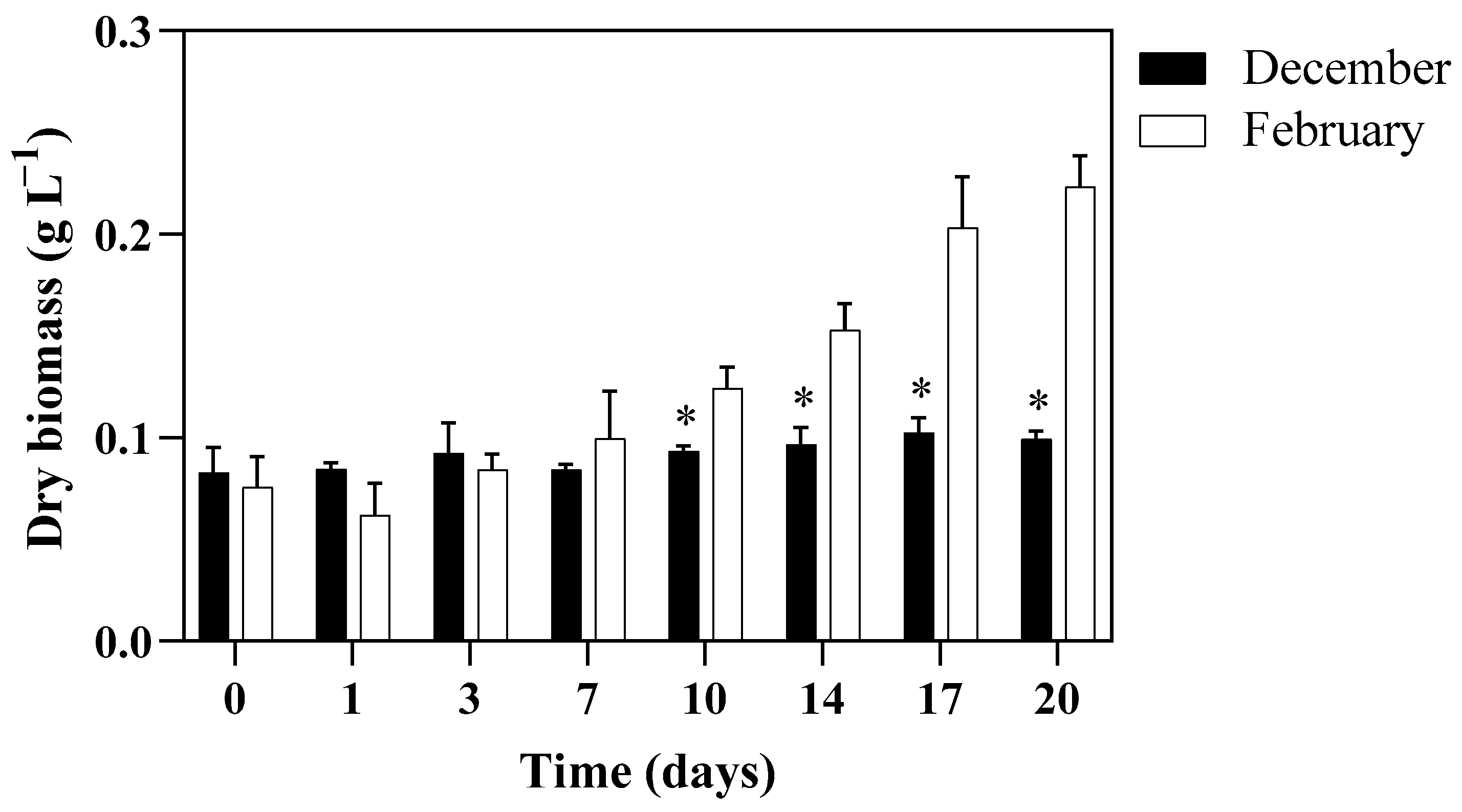
3.2. Algal Growth Aspects
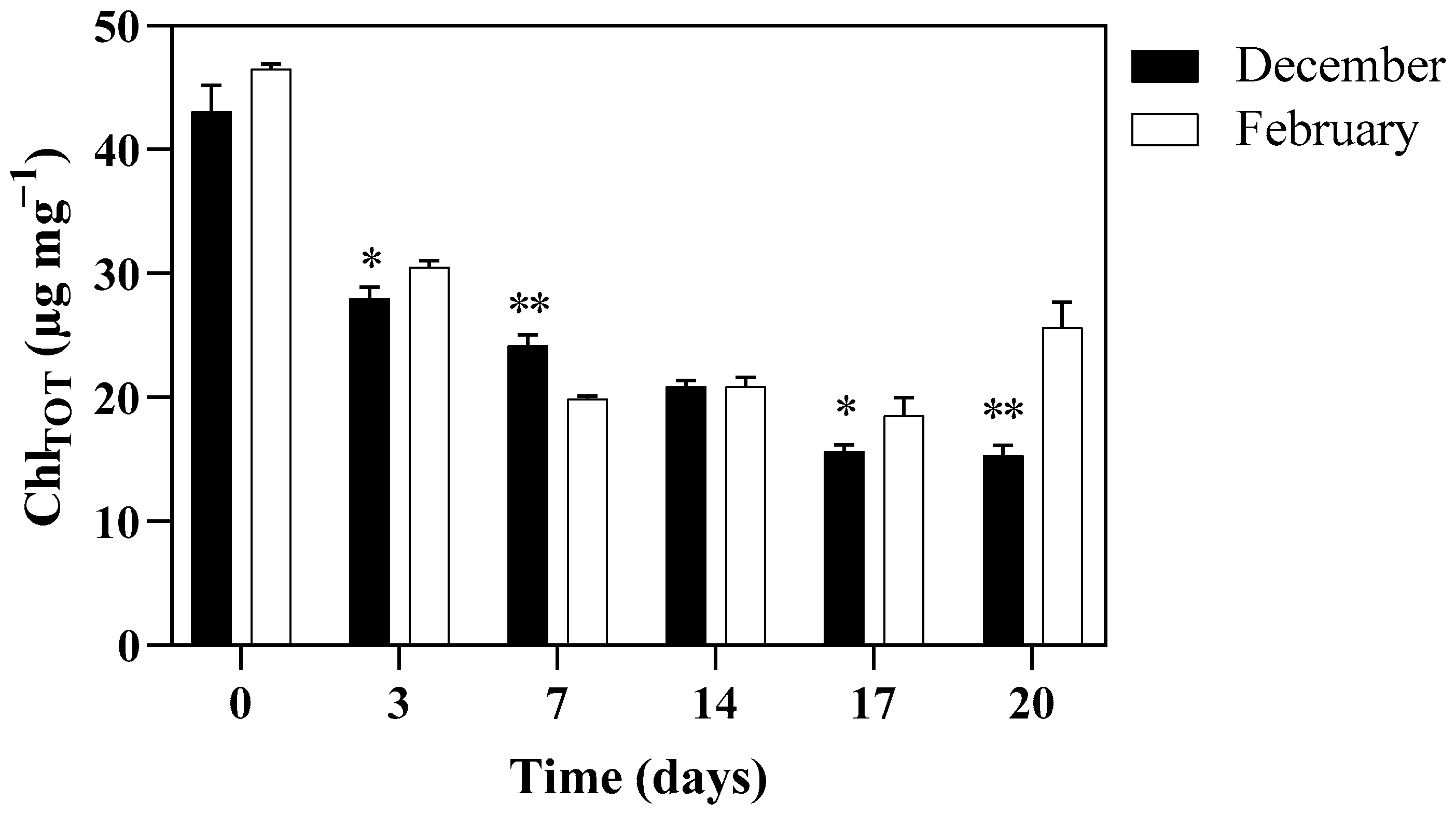
3.3. PSII Maximum Quantum Yield and Photosynthetic Pigments Content of Algae
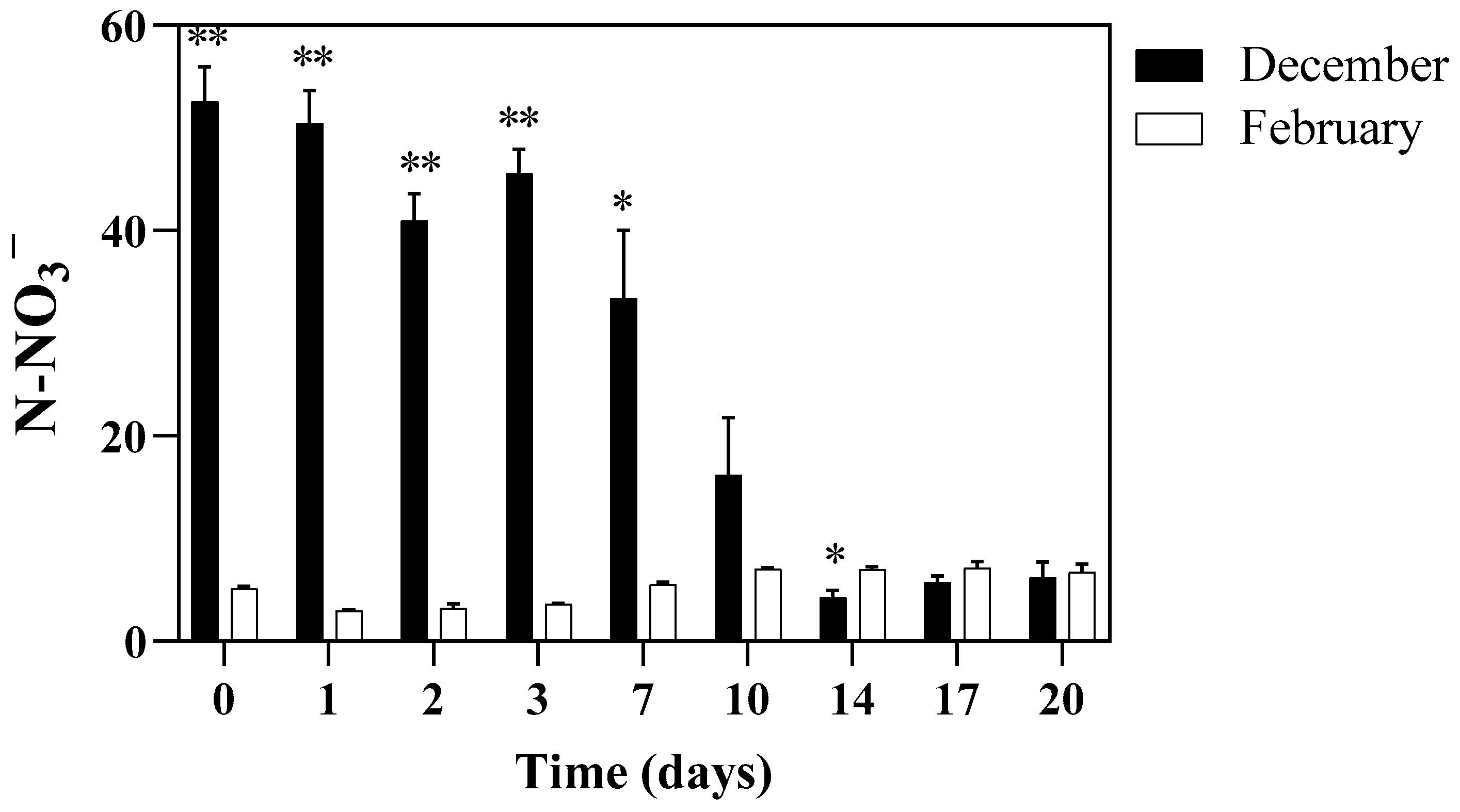
3.4. Nutrients Removal from the Cultivation Substrate and E. coli Load
3.5. Morphological Aspects of Algae
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Council directive of 21 May 1991 concerning urban waste water treatment (91/271/EEC). OJL 1991, 7, 40–52.
- Powley, H.R.; Dürr, H.H.; Lima, A.T.; Krom, M.D.; Van Cappellen, P. Direct discharges of domestic wastewater are a major source of phosphorus and nitrogen to the Mediterranean sea. Environ. Sci. Technol. 2016, 50, 8722–8730. [Google Scholar] [CrossRef]
- Jang, J.; Hur, H.-G.; Sadowsky, M.J.; Byappanahalli, M.N.; Yan, T.; Ishii, S. Environmental Escherichia coli: Ecology and public health implications-a review. J. Appl. Microbiol. 2017, 123, 570–581. [Google Scholar] [CrossRef]
- Wollmann, F.; Dietze, S.; Ackermann, J.; Bley, T.; Walther, T.; Steingroewer, J.; Krujatz, F. Microalgae wastewater treatment: Biological and technological a)pproaches. Eng. Life Sci. 2019, 19, 860–871. [Google Scholar] [CrossRef]
- Lima, S.; Villanova, V.; Grisafi, F.; Caputo, G.; Brucato, A.; Scargiali, F. Autochthonous Microalgae Grown in Municipal Wastewaters as a Tool for Effectively Removing Nitrogen and Phosphorous. J. Water Process Eng. 2020, 38, 101647. [Google Scholar] [CrossRef]
- Geremia, E.; Ripa, M.; Catone, C.M.; Ulgiati, S. A Review about Microalgae Wastewater Treatment for Bioremediation and Biomass Production—A New Challenge for Europe. Environments 2021, 8, 136. [Google Scholar] [CrossRef]
- Corpuz, M.V.A.; Borea, L.; Senatore, V.; Castrogiovanni, F.; Buonerba, A.; Oliva, G.; Ballesteros, F.; Zarra, T.; Belgiorno, V.; Choo, K.-H.; et al. Wastewater Treatment and Fouling Control in an Electro Algae-Activated Sludge Membrane Bioreactor. Sci. Total Environ. 2021, 786, 147475. [Google Scholar] [CrossRef]
- Gherghel, A.; Teodosiu, C.; De Gisi, S. A Review on Wastewater Sludge Valorisation and Its Challenges in the Context of Circular Economy. J. Clean. Prod. 2019, 228, 244–263. [Google Scholar] [CrossRef]
- Daverey, A.; Pandey, D.; Verma, P.; Verma, S.; Shah, V.; Dutta, K.; Arunachalam, K. Recent Advances in Energy Efficient Biological Treatment of Municipal Wastewater. Bioresour. Technol. Rep. 2019, 7, 100252. [Google Scholar] [CrossRef]
- La Bella, E.; Baglieri, A.; Fragalà, F.; Puglisi, I. Multipurpose Agricultural Reuse of Microalgae Biomasses Employed for the Treatment of Urban Wastewater. Agronomy 2022, 12, 234. [Google Scholar] [CrossRef]
- Di Capua, F.; de Sario, S.; Ferraro, A.; Petrella, A.; Race, M.; Pirozzi, F.; Fratino, U.; Spasiano, D. Phosphorous Removal and Recovery from Urban Wastewater: Current Practices and New Directions. Sci. Total Environ. 2022, 823, 153750. [Google Scholar] [CrossRef]
- Huang, Z.; Qie, Y.; Wang, Z.; Zhang, Y.; Zhou, W. Application of Deep-Sea Psychrotolerant Bacteria in Wastewater Treatment by Aerobic Dynamic Membrane Bioreactors at Low Temperature. J. Membr. Sci. 2015, 475, 47–56. [Google Scholar] [CrossRef]
- Zhou, H.; Li, X.; Xu, G.; Yu, H. Overview of Strategies for Enhanced Treatment of Municipal/Domestic Wastewater at Low Temperature. Sci. Total Environ. 2018, 643, 225–237. [Google Scholar] [CrossRef]
- Sala-Garrido, R.; Molinos-Senante, M.; Hernández-Sancho, F. How Does Seasonality Affect Water Reuse Possibilities? An Efficiency and Cost Analysis. Resour. Conserv. Recycl. 2012, 58, 125–131. [Google Scholar] [CrossRef]
- Singh, S.; Tiwari, S. Climate Change, Water and Wastewater Treatment: Interrelationship and Consequences. In Water Conservation, Recycling and Reuse: Issues and Challenges; Singh, R.P., Kolok, A.S., Bartelt-Hunt, S.L., Eds.; Springer: Singapore, 2019; pp. 203–214. ISBN 9789811331794. [Google Scholar]
- Li, K.; Liu, Q.; Fang, F.; Luo, R.; Lu, Q.; Zhou, W.; Huo, S.; Cheng, P.; Liu, J.; Addy, M.; et al. Microalgae-Based Wastewater Treatment for Nutrients Recovery: A Review. Bioresour. Technol. 2019, 291, 121934. [Google Scholar] [CrossRef]
- Baldisserotto, C.; Demaria, S.; Accoto, O.; Marchesini, R.; Zanella, M.; Benetti, L.; Avolio, F.; Maglie, M.; Ferroni, L.; Pancaldi, S. Removal of Nitrogen and Phosphorus from Thickening Effluent of an Urban Wastewater Treatment Plant by an Isolated Green Microalga. Plants 2020, 9, 1802. [Google Scholar] [CrossRef]
- Nirmalakhandan, N.; Selvaratnam, T.; Henkanatte-Gedera, S.M.; Tchinda, D.; Abeysiriwardana-Arachchige, I.S.A.; Delanka-Pedige, H.M.K.; Munasinghe-Arachchige, S.P.; Zhang, Y.; Holguin, F.O.; Lammers, P.J. Algal Wastewater Treatment: Photoautotrophic vs. Mixotrophic Processes. Algal Res. 2019, 41, 101569. [Google Scholar] [CrossRef]
- Nishshanka, G.K.S.H.; Thevarajah, B.; Nimarshana, P.H.V.; Prajapati, S.K.; Ariyadasa, T.U. Real-Time Integration of Microalgae-Based Bioremediation in Conventional Wastewater Treatment Plants: Current Status and Prospects. J. Water Process Eng. 2023, 56, 104248. [Google Scholar] [CrossRef]
- Wan Mahari, W.A.; Wan Razali, W.A.; Manan, H.; Hersi, M.A.; Ishak, S.D.; Cheah, W.; Chan, D.J.C.; Sonne, C.; Show, P.L.; Lam, S.S. Recent Advances on Microalgae Cultivation for Simultaneous Biomass Production and Removal of Wastewater Pollutants to Achieve Circular Economy. Bioresour. Technol. 2022, 364, 128085. [Google Scholar] [CrossRef]
- Abdelfattah, A.; Ali, S.S.; Ramadan, H.; El-Aswar, E.I.; Eltawab, R.; Ho, S.-H.; Elsamahy, T.; Li, S.; El-Sheekh, M.M.; Schagerl, M.; et al. Microalgae-Based Wastewater Treatment: Mechanisms, Challenges, Recent Advances, and Future Prospects. Environ. Sci. Ecotechnol. 2023, 13, 100205. [Google Scholar] [CrossRef]
- Wang, B.; Lan, C.Q. Biomass Production and Nitrogen and Phosphorus Removal by the Green Alga Neochloris oleoabundans in Simulated Wastewater and Secondary Municipal Wastewater Effluent. Bioresour. Technol. 2011, 102, 5639–5644. [Google Scholar] [CrossRef]
- Álvarez-Díaz, P.D.; Ruiz, J.; Arbib, Z.; Barragán, J.; Garrido-Pérez, M.C.; Perales, J.A. Freshwater Microalgae Selection for Simultaneous Wastewater Nutrient Removal and Lipid Production. Algal Res. 2017, 24, 477–485. [Google Scholar] [CrossRef]
- Jiménez-Pérez, M.V.; Sánchez-Castillo, P.; Romera, O.; Fernández-Moreno, D.; Pérez-Martínez, C. Growth and Nutrient Removal in Free and Immobilized Planktonic Green Algae Isolated from Pig Manure. Enzyme Microb. Technol. 2004, 34, 392–398. [Google Scholar] [CrossRef]
- Zhou, W.; Li, Y.; Min, M.; Hu, B.; Chen, P.; Ruan, R. Local Bioprospecting for High-Lipid Producing Microalgal Strains to Be Grown on Concentrated Municipal Wastewater for Biofuel Production. Bioresour. Technol. 2011, 102, 6909–6919. [Google Scholar] [CrossRef]
- Arbib, Z.; Ruiz, J.; Álvarez-Díaz, P.; Garrido-Pérez, C.; Perales, J.A. Capability of Different Microalgae Species for Phytoremediation Processes: Wastewater Tertiary Treatment, CO2 Bio-Fixation and Low Cost Biofuels Production. Water Res. 2014, 49, 465–474. [Google Scholar] [CrossRef]
- Chen, G.; Zhao, L.; Qi, Y. Enhancing the Productivity of Microalgae Cultivated in Wastewater toward Biofuel Production: A Critical Review. Appl. Energy 2015, 137, 282–291. [Google Scholar] [CrossRef]
- Iasimone, F.; De Felice, V.; Panico, A.; Pirozzi, F. Experimental Study for the Reduction of CO2 Emissions in Wastewater Treatment Plant Using Microalgal Cultivation. J. CO2 Util. 2017, 22, 1–8. [Google Scholar] [CrossRef]
- Singh, R.; Birru, R.; Sibi, G. Nutrient Removal Efficiencies of Chlorella vulgaris from Urban Wastewater for Reduced Eutrophication. J. Environ. Prot. 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Sisman-Aydin, G. Comparative Study on Phycoremediation Performance of Three Native Microalgae for Primary-Treated Municipal Wastewater. Environ. Technol. Innov. 2022, 28, 102932. [Google Scholar] [CrossRef]
- Qiao, S.; Hou, C.; Wang, X.; Zhou, J. Minimizing Greenhouse Gas Emission from Wastewater Treatment Process by Integrating Activated Sludge and Microalgae Processes. Sci. Total Environ. 2020, 732, 139032. [Google Scholar] [CrossRef] [PubMed]
- Henriques, J.; Catarino, J. Sustainable Value—An Energy Efficiency Indicator in Wastewater Treatment Plants. J. Clean. Prod. 2017, 142, 323–330. [Google Scholar] [CrossRef]
- Young, M.N.; Marcus, A.K.; Rittmann, B.E. A Combined Activated Sludge Anaerobic Digestion Model (CASADM) to Understand the Role of Anaerobic Sludge Recycling in Wastewater Treatment Plant Performance. Bioresour. Technol. 2013, 136, 196–204. [Google Scholar] [CrossRef]
- Pijuan, M.; Torà, J.; Rodríguez-Caballero, A.; César, E.; Carrera, J.; Pérez, J. Effect of Process Parameters and Operational Mode on Nitrous Oxide Emissions from a Nitritation Reactor Treating Reject Wastewater. Water Res. 2014, 49, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Maity, J.P.; Bundschuh, J.; Chen, C.-Y.; Bhattacharya, P. Microalgae for Third Generation Biofuel Production, Mitigation of Greenhouse Gas Emissions and Wastewater Treatment: Present and Future Perspectives—A Mini Review. Energy 2014, 78, 104–113. [Google Scholar] [CrossRef]
- Campos, J.L.; Valenzuela-Heredia, D.; Pedrouso, A.; Val del Río, A.; Belmonte, M.; Mosquera-Corral, A. Greenhouse Gases Emissions from Wastewater Treatment Plants: Minimization, Treatment, and Prevention. J. Chem. 2016, 2016, 3796352. [Google Scholar] [CrossRef]
- Bansal, A.; Shinde, O.; Sarkar, S. Industrial Wastewater Treatment Using Phycoremediation Technologies and Co-Production of Value-Added Products. J. Bioremediat. Biodegrad. 2018, 9, 428. [Google Scholar] [CrossRef]
- Song, Y.; Wang, L.; Qiang, X.; Gu, W.; Ma, Z.; Wang, G. The Promising Way to Treat Wastewater by Microalgae: Approaches, Mechanisms, Applications and Challenges. J. Water Process Eng. 2022, 49, 103012. [Google Scholar] [CrossRef]
- Khavari, F.; Saidijam, M.; Taheri, M.; Nouri, F. Microalgae: Therapeutic Potentials and Applications. Mol. Biol. Rep. 2021, 48, 4757–4765. [Google Scholar] [CrossRef]
- Hachicha, R.; Elleuch, F.; Ben Hlima, H.; Dubessay, P.; de Baynast, H.; Delattre, C.; Pierre, G.; Hachicha, R.; Abdelkafi, S.; Michaud, P.; et al. Biomolecules from Microalgae and Cyanobacteria: Applications and Market Survey. Appl. Sci. 2022, 12, 1924. [Google Scholar] [CrossRef]
- Yap, J.K.; Sankaran, R.; Chew, K.W.; Halimatul Munawaroh, H.S.; Ho, S.-H.; Rajesh Banu, J.; Show, P.L. Advancement of Green Technologies: A Comprehensive Review on the Potential Application of Microalgae Biomass. Chemosphere 2021, 281, 130886. [Google Scholar] [CrossRef]
- Sathya, A.B.; Thirunavukkarasu, A.; Nithya, R.; Nandan, A.; Sakthishobana, K.; Kola, A.K.; Sivashankar, R.; Tuan, H.A.; Deepanraj, B. Microalgal Biofuel Production: Potential Challenges and Prospective Research. Fuel 2023, 332, 126199. [Google Scholar] [CrossRef]
- Plaza, B.M.; Gómez-Serrano, C.; Acién-Fernández, F.G.; Jimenez-Becker, S. Effect of Microalgae Hydrolysate Foliar Application (Arthrospira platensis and Scenedesmus sp.) on Petunia x Hybrida Growth. J. Appl. Phycol. 2018, 30, 2359–2365. [Google Scholar] [CrossRef]
- Ronga, D.; Biazzi, E.; Parati, K.; Carminati, D.; Carminati, E.; Tava, A. Microalgal Biostimulants and Biofertilisers in Crop Productions. Agronomy 2019, 9, 192. [Google Scholar] [CrossRef]
- Media Recipes—CCAP, QA BG11. Available online: https://www.ccap.ac.uk/index.php/media-recipes/ (accessed on 16 September 2019).
- Dext3r. Available online: https://simc.arpae.it/dext3r/ (accessed on 9 October 2023).
- Giovanardi, M.; Ferroni, L.; Baldisserotto, C.; Tedeschi, P.; Maietti, A.; Pantaleoni, L.; Pancaldi, S. Morphophysiological Analyses of Neochloris oleoabundans (Chlorophyta) Grown Mixotrophically in a Carbon-Rich Waste Product. Protoplasma 2013, 250, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Popovich, C.A.; Damiani, C.; Constenla, D.; Martínez, A.M.; Freije, H.; Giovanardi, M.; Pancaldi, S.; Leonardi, P.I. Neochloris oleoabundans Grown in Enriched Natural Seawater for Biodiesel Feedstock: Evaluation of Its Growth and Biochemical Composition. Bioresour. Technol. 2012, 114, 287–293. [Google Scholar] [CrossRef]
- White, S.; Anandraj, A.; Bux, F. PAM Fluorometry as a Tool to Assess Microalgal Nutrient Stress and Monitor Cellular Neutral Lipids. Bioresour. Technol. 2011, 102, 1675–1682. [Google Scholar] [CrossRef]
- Cavieres, L.; Bazaes, J.; Marticorena, P.; Riveros, K.; Medina, P.; Sepúlveda, C.; Riquelme, C. Pilot-Scale Phycoremediation Using Muriellopsis Sp. for Wastewater Reclamation in the Atacama Desert: Microalgae Biomass Production and Pigment Recovery. Water Sci. Technol. 2020, 83, 331–343. [Google Scholar] [CrossRef]
- Ferroni, L.; Baldisserotto, C.; Giovanardi, M.; Pantaleoni, L.; Morosinotto, T.; Pancaldi, S. Revised Assignment of Room-Temperature Chlorophyll Fluorescence Emission Bands in Single Living Cells of Chlamydomonas reinhardtii. J. Bioenerg. Biomembr. 2011, 43, 163–173. [Google Scholar] [CrossRef]
- Baldisserotto, C.; Popovich, C.; Giovanardi, M.; Sabia, A.; Ferroni, L.; Constenla, D.; Leonardi, P.; Pancaldi, S. Photosynthetic Aspects and Lipid Profiles in the Mixotrophic Alga Neochloris oleoabundans as Useful Parameters for Biodiesel Production. Algal Res. 2016, 16, 255–265. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Ladle, R.J.; Goltsev, V.; Bosa, K.; Allakhverdiev, S.I.; Brestic, M.; Bussotti, F.; Calatayud, A.; Dąbrowski, P.; et al. Frequently Asked Questions about in Vivo Chlorophyll Fluorescence: Practical Issues. Photosynth. Res. 2014, 122, 121–158. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as Well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Bower, C.E.; Holm-Hansen, T. A Salicylate–Hypochlorite Method for Determining Ammonia in Seawater. Can. J. Fish. Aquat. Sci. 1980, 37, 794–798. [Google Scholar] [CrossRef]
- Available online: https://www.gruppohera.it/gruppo/attivita/ingegneria-laboratori-e-servizi-tecnici/laboratori (accessed on 12 February 2024).
- Discart, V.; Bilad, M.R.; Vankelecom, I.F.J. Critical Evaluation of the Determination Methods for Transparent Exopolymer Particles, Agents of Membrane Fouling. Crit. Rev. Environ. Sci. Technol. 2015, 45, 167–192. [Google Scholar] [CrossRef]
- Vergnes, J.B.; Gernigon, V.; Guiraud, P.; Formosa-Dague, C. Bicarbonate Concentration Induces Production of Exopolysaccharides by Arthrospira platensis That Mediate Bioflocculation and Enhance Flotation Harvesting Efficiency. ACS Sustain. Chem. Eng. 2019, 7, 13796–13804. [Google Scholar] [CrossRef]
- Tong, C.Y.; Chang, Y.S.; Ooi, B.S.; Chan, D.J.C. Physico-Chemistry and Adhesion Kinetics of Algal Biofilm on Polyethersulfone (PES) Membrane with Different Surface Wettability. J. Environ. Chem. Eng. 2021, 9, 106531. [Google Scholar] [CrossRef]
- Mowry, R.W.; Scott, J.E. Observations on the Basophilia of Amyloids. Histochemie 1967, 10, 8–32. [Google Scholar] [CrossRef] [PubMed]
- Fagan, C.; Dapson, R.W.; Horobin, R.W.; Kiernan, J.A. Revised Tests and Standards for Biological Stain Commission Certification of Alcian Blue Dyes. Biotech. Histochem. 2020, 95, 333–340. [Google Scholar] [CrossRef]
- Dayana Priyadharshini, S.; Suresh Babu, P.; Manikandan, S.; Subbaiya, R.; Govarthanan, M.; Karmegam, N. Phycoremediation of Wastewater for Pollutant Removal: A Green Approach to Environmental Protection and Long-Term Remediation. Environ. Pollut. 2021, 290, 117989. [Google Scholar] [CrossRef]
- Kaloudas, D.; Pavlova, N.; Penchovsky, R. Phycoremediation of Wastewater by Microalgae: A Review. Environ. Chem. Lett. 2021, 19, 2905–2920. [Google Scholar] [CrossRef]
- Shahid, A.; Malik, S.; Zhu, H.; Xu, J.; Nawaz, M.Z.; Nawaz, S.; Asraful Alam, M.; Mehmood, M.A. Cultivating Microalgae in Wastewater for Biomass Production, Pollutant Removal, and Atmospheric Carbon Mitigation; a Review. Sci. Total Environ. 2020, 704, 135303. [Google Scholar] [CrossRef]
- Olabi, A.G.; Shehata, N.; Sayed, E.T.; Rodriguez, C.; Anyanwu, R.C.; Russell, C.; Abdelkareem, M.A. Role of Microalgae in Achieving Sustainable Development Goals and Circular Economy. Sci. Total Environ. 2023, 854, 158689. [Google Scholar] [CrossRef]
- De Luca, M.; Pappalardo, I.; Limongi, A.R.; Viviano, E.; Radice, R.P.; Todisco, S.; Martelli, G.; Infantino, V.; Vassallo, A. Lipids from Microalgae for Cosmetic Applications. Cosmetics 2021, 8, 52. [Google Scholar] [CrossRef]
- Gonçalves, A.L. The Use of Microalgae and Cyanobacteria in the Improvement of Agricultural Practices: A Review on Their Biofertilising, Biostimulating and Biopesticide Roles. Appl. Sci. 2021, 11, 871. [Google Scholar] [CrossRef]
- Kuo, C.-M.; Sun, Y.-L.; Lin, C.-H.; Lin, C.-H.; Wu, H.-T.; Lin, C.-S. Cultivation and Biorefinery of Microalgae (Chlorella Sp.) for Producing Biofuels and Other Byproducts: A Review. Sustainability 2021, 13, 13480. [Google Scholar] [CrossRef]
- Mehariya, S.; Goswami, R.K.; Karthikeysan, O.P.; Verma, P. Microalgae for High-Value Products: A Way towards Green Nutraceutical and Pharmaceutical Compounds. Chemosphere 2021, 280, 130553. [Google Scholar] [CrossRef]
- Khan, S.; Thaher, M.; Abdulquadir, M.; Faisal, M.; Mehariya, S.; Al-Najjar, M.A.A.; Al-Jabri, H.; Das, P. Utilization of Microalgae for Urban Wastewater Treatment and Valorization of Treated Wastewater and Biomass for Biofertilizer Applications. Sustainability 2023, 15, 16019. [Google Scholar] [CrossRef]
- Guido, F.; Piero, F.; Francesca, L.; Walter, P.; Emanuela, P. Gli Indicatori del clima in Italia nel 2021—Anno XVII. Available online: https://www.isprambiente.gov.it/it/pubblicazioni/stato-dellambiente/gli-indicatori-del-clima-in-italia-nel-2021-2013-anno-xvii (accessed on 21 October 2023).
- Fraschetti, P.; Lena, F.; Perconti, W.; Piervitali, E.; Settanta, G.; Pavan, V. Il Clima in Italia nel 2022|SNPA—Sistema Nazionale Protezione Ambiente. 2023. Available online: https://www.snpambiente.it/temi/report-intertematici/cambiamenti-climatici/il-clima-in-italia-nel-2022/ (accessed on 21 October 2023).
- Persoone, G.; Baudo, R.; Cotman, M.; Blaise, C.; Thompson, K.C.; Moreira-Santos, M.; Vollat, B.; Törökne, A.; Han, T. Review on the Acute Daphnia Magna Toxicity Test—Evaluation of the Sensitivity and the Precision of Assays Performed with Organisms from Laboratory Cultures or Hatched from Dormant Eggs. Knowl. Manag. Aquat. Ecosyst. 2009, 1, 29. [Google Scholar] [CrossRef]
- Khangarot, B.S.; Ray, P.K.; Chandr, H. Daphnia magna as a Model to Assess Heavy Metal Toxicity: Comparative Assessment with Mouse System. Acta Hydrochim. Hydrobiol. 1987, 15, 427–432. [Google Scholar] [CrossRef]
- Tavares, K.P.; de Oliveira, Á.C.; Vicentini, D.S.; Melegari, S.P.; Matias, W.G.; Barbosa, S.; Kummrow, F. Acute Toxicity of Copper and Chromium Oxide Nanoparticles to Daphnia similis. Ecotoxicol. Environ. Contam. 2014, 9, 43–50. [Google Scholar] [CrossRef]
- Mennaa, F.Z.; Arbib, Z.; Perales, J.A. Urban Wastewater Treatment by Seven Species of Microalgae and an Algal Bloom: Biomass Production, N and P Removal Kinetics and Harvestability. Water Res. 2015, 83, 42–51. [Google Scholar] [CrossRef]
- Xu, K.; Zou, X.; Wen, H.; Xue, Y.; Qu, Y.; Li, Y. Effects of Multi-Temperature Regimes on Cultivation of Microalgae in Municipal Wastewater to Simultaneously Remove Nutrients and Produce Biomass. Appl. Microbiol. Biotechnol. 2019, 103, 8255–8265. [Google Scholar] [CrossRef]
- Rolton, A.; McCullough, A.; Tuckey, N.P.L.; Finnie, B.; Cooper, I.; Packer, M.A.; Vignier, J. Early Biomarker Indicators of Health in Two Commercially Produced Microalgal Species Important for Aquaculture. Aquaculture 2020, 521, 735053. [Google Scholar] [CrossRef]
- Purushanahalli Shivagangaiah, C.; Sanyal, D.; Dasgupta, S.; Banik, A. Phycoremediation and Photosynthetic Toxicity Assessment of Lead by Two Freshwater Microalgae Scenedesmus acutus and Chlorella pyrenoidosa. Physiol. Plant. 2021, 173, 246–258. [Google Scholar] [CrossRef]
- Masojídek, J.; Gómez-Serrano, C.; Ranglová, K.; Cicchi, B.; Encinas Bogeat, Á.; Câmara Manoel, J.A.; Sanches Zurano, A.; Silva Benavides, A.M.; Barceló-Villalobos, M.; Robles Carnero, V.A.; et al. Photosynthesis Monitoring in Microalgae Cultures Grown on Municipal Wastewater as a Nutrient Source in Large-Scale Outdoor Bioreactors. Biology 2022, 11, 1380. [Google Scholar] [CrossRef]
- Di Caprio, F. Methods to Quantify Biological Contaminants in Microalgae Cultures. Algal Res. 2020, 49, 101943. [Google Scholar] [CrossRef]
- Krzeminski, P.; Iglesias-Obelleiro, A.; Madebo, G.; Garrido, J.M.; van der Graaf, J.H.J.M.; van Lier, J.B. Impact of Temperature on Raw Wastewater Composition and Activated Sludge Filterability in Full-Scale MBR Systems for Municipal Sewage Treatment. J. Membr. Sci. 2012, 423–424, 348–361. [Google Scholar] [CrossRef]
- Quijano, G.; Arcila, J.S.; Buitrón, G. Microalgal-Bacterial Aggregates: Applications and Perspectives for Wastewater Treatment. Biotechnol. Adv. 2017, 35, 772–781. [Google Scholar] [CrossRef]
- Danese, P.N.; Pratt, L.A.; Kolter, R. Exopolysaccharide Production Is Required for Development of Escherichia coli K-12 Biofilm Architecture. J. Bacteriol. 2000, 182, 3593–3596. [Google Scholar] [CrossRef]
- Eboigbodin, K.E.; Biggs, C.A. Characterization of the Extracellular Polymeric Substances Produced by Escherichia coli Using Infrared Spectroscopic, Proteomic, and Aggregation Studies. Biomacromolecules 2008, 9, 686–695. [Google Scholar] [CrossRef]
- Xiao, R.; Zheng, Y. Overview of Microalgal Extracellular Polymeric Substances (EPS) and Their Applications. Biotechnol. Adv. 2016, 34, 1225–1244. [Google Scholar] [CrossRef]
- Cunha, C.; Faria, M.; Nogueira, N.; Ferreira, A.; Cordeiro, N. Marine vs Freshwater Microalgae Exopolymers as Biosolutions to Microplastics Pollution. Environ. Pollut. 2019, 249, 372–380. [Google Scholar] [CrossRef]
- Wang, X.; Hong, Y. Microalgae Biofilm and Bacteria Symbiosis in Nutrient Removal and Carbon Fixation from Wastewater: A Review. Curr. Pollut. Rep. 2022, 8, 128–146. [Google Scholar] [CrossRef]
- Myers, J.A.; Curtis, B.S.; Curtis, W.R. Improving Accuracy of Cell and Chromophore Concentration Measurements Using Optical Density. BMC Biophys. 2013, 6, 4. [Google Scholar] [CrossRef]
- Volland, S.; Lütz, C.; Michalke, B.; Lütz-Meindl, U. Intracellular Chromium Localization and Cell Physiological Response in the Unicellular Alga Micrasterias. Aquat. Toxicol. 2012, 109, 59–69. [Google Scholar] [CrossRef]
- Zou, H.; Huang, J.-C.; Zhou, C.; He, S.; Zhou, W. Mutual Effects of Selenium and Chromium on Their Removal by Chlorella vulgaris and Associated Toxicity. Sci. Total Environ. 2020, 724, 138219. [Google Scholar] [CrossRef]
- Nguyen, L.A.T.; Ward, A.J.; Lewis, D. Utilisation of Turbidity as an Indicator for Biochemical and Chemical Oxygen Demand. J. Water Process Eng. 2014, 4, 137–142. [Google Scholar] [CrossRef]
- Daliry, S.; Hallajisani, A.; Mohammadi Roshandeh, J.; Nouri, H.; Golzary, A. Investigation of Optimal Condition for Chlorella vulgaris Microalgae Growth. Glob. J. Environ. Sci. Manag. 2017, 3, 217–230. [Google Scholar] [CrossRef]
- Aloice, W. Mayo Effects of Temperature and pH on the Kinetic Growth of Unialga Chlorella vulgaris Cultures Containing Bacteria-Mayo-1997-Water Environment Research-Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/abs/10.2175/106143097X125191 (accessed on 22 October 2023).
- Zerveas, S.; Mente, M.S.; Tsakiri, D.; Kotzabasis, K. Microalgal Photosynthesis Induces Alkalization of Aquatic Environment as a Result of H+ Uptake Independently from CO2 Concentration—New Perspectives for Environmental Applications. J. Environ. Manag. 2021, 289, 112546. [Google Scholar] [CrossRef]
- Goldman, J.C.; Brewer, P.G. Effect of Nitrogen Source and Growth Rate on Phytoplankton-Mediated Changes in Alkalinity1. Limnol. Oceanogr. 1980, 25, 352–357. [Google Scholar] [CrossRef]
- Fuggi, A.; Di Martino Rigano, V.; Vona, V.; Rigano, C. Nitrate and Ammonium Assimilation in Algal Cell-Suspensions and Related pH Variations in the External Medium, Monitored by Electrodes. Plant Sci. Lett. 1981, 23, 129–138. [Google Scholar] [CrossRef]
- Cerozi, B.d.S.; Fitzsimmons, K. The Effect of pH on Phosphorus Availability and Speciation in an Aquaponics Nutrient Solution. Bioresour. Technol. 2016, 219, 778–781. [Google Scholar] [CrossRef]
- Barrow, N.J. The Effects of pH on Phosphate Uptake from the Soil. Plant Soil 2017, 410, 401–410. [Google Scholar] [CrossRef]
- Nielsen, P.H.; Mielczarek, A.T.; Kragelund, C.; Nielsen, J.L.; Saunders, A.M.; Kong, Y.; Hansen, A.A.; Vollertsen, J. A Conceptual Ecosystem Model of Microbial Communities in Enhanced Biological Phosphorus Removal Plants. Water Res. 2010, 44, 5070–5088. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiu, X.; Luo, J.; Li, H.; How, S.-W.; Wu, D.; He, J.; Cheng, Z.; Gao, Y.; Lu, H. A Review of the Phosphorus Removal of Polyphosphate-Accumulating Organisms in Natural and Engineered Systems. Sci. Total Environ. 2024, 912, 169103. [Google Scholar] [CrossRef]
- Aditya, L.; Vu, H.P.; Abu Hasan Johir, M.; Mahlia, T.M.I.; Silitonga, A.S.; Zhang, X.; Liu, Q.; Tra, V.-T.; Ngo, H.H.; Nghiem, L.D. Role of Culture Solution pH in Balancing CO2 Input and Light Intensity for Maximising Microalgae Growth Rate. Chemosphere 2023, 343, 140255. [Google Scholar] [CrossRef]
- Nguyen, B.T.; Rittmann, B.E. Predicting Dissolved Inorganic Carbon in Photoautotrophic Microalgae Culture via the Nitrogen Source. Environ. Sci. Technol. 2015, 49, 9826–9831. [Google Scholar] [CrossRef]
- Kholssi, R.; Ramos, P.V.; Marks, E.A.N.; Montero, O.; Rad, C. 2Biotechnological Uses of Microalgae: A Review on the State of the Art and Challenges for the Circular Economy. Biocatal. Agric. Biotechnol. 2021, 36, 102114. [Google Scholar] [CrossRef]
- Sun, H.; Wang, Y.; He, Y.; Liu, B.; Mou, H.; Chen, F.; Yang, S. Microalgae-Derived Pigments for the Food Industry. Mar. Drugs 2023, 21, 82. [Google Scholar] [CrossRef]
- Kula, M.; Kalaji, H.M.; Skoczowski, A. Culture Density Influence on the Photosynthetic Efficiency of Microalgae Growing under Different Spectral Compositions of Light. J. Photochem. Photobiol. B 2017, 167, 290–298. [Google Scholar] [CrossRef]
- McGee, D.; Archer, L.; Fleming, G.T.A.; Gillespie, E.; Touzet, N. Influence of Spectral Intensity and Quality of LED Lighting on Photoacclimation, Carbon Allocation and High-Value Pigments in Microalgae. Photosynth. Res. 2020, 143, 67–80. [Google Scholar] [CrossRef]
- Del Campo, J.A.; Moreno, J.; Rodríguez, H.; Angeles Vargas, M.; Rivas, J.; Guerrero, M.G. Carotenoid Content of Chlorophycean Microalgae: Factors Determining Lutein Accumulation in Muriellopsis Sp. (Chlorophyta). J. Biotechnol. 2000, 76, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Guedes, A.C.; Amaro, H.M.; Pereira, R.D.; Malcata, F.X. Effects of Temperature and pH on Growth and Antioxidant Content of the Microalga Scenedesmus obliquus. Biotechnol. Prog. 2011, 27, 1218–1224. [Google Scholar] [CrossRef] [PubMed]
- Faraloni, C.; Di Lorenzo, T.; Bonetti, A. Impact of Light Stress on the Synthesis of Both Antioxidants Polyphenols and Carotenoids, as Fast Photoprotective Response in Chlamydomonas reinhardtii: New Prospective for Biotechnological Potential of This Microalga. Symmetry 2021, 13, 2220. [Google Scholar] [CrossRef]
- Cichoński, J.; Chrzanowski, G. Microalgae as a Source of Valuable Phenolic Compounds and Carotenoids. Molecules 2022, 27, 8852. [Google Scholar] [CrossRef]
- Ras, M.; Steyer, J.-P.; Bernard, O. Temperature Effect on Microalgae: A Crucial Factor for Outdoor Production. Rev. Environ. Sci. Biotechnol. 2013, 12, 153–164. [Google Scholar] [CrossRef]
- Su, Y. Revisiting Carbon, Nitrogen, and Phosphorus Metabolisms in Microalgae for Wastewater Treatment. Sci. Total Environ. 2021, 762, 144590. [Google Scholar] [CrossRef]
- Rossi, S.; Casagli, F.; Mantovani, M.; Mezzanotte, V.; Ficara, E. Selection of Photosynthesis and Respiration Models to Assess the Effect of Environmental Conditions on Mixed Microalgae Consortia Grown on Wastewater. Bioresour. Technol. 2020, 305, 122995. [Google Scholar] [CrossRef]
- Sutherland, D.L.; Heubeck, S.; Park, J.; Turnbull, M.H.; Craggs, R.J. Seasonal Performance of a Full-Scale Wastewater Treatment Enhanced Pond System. Water Res. 2018, 136, 150–159. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, B.; Ning, D.; Zhang, Y.; Dai, T.; Wu, L.; Li, T.; Liu, W.; Zhou, J.; Wen, X. Seasonal Dynamics of the Microbial Community in Two Full-Scale Wastewater Treatment Plants: Diversity, Composition, Phylogenetic Group Based Assembly and Co-Occurrence Pattern. Water Res. 2021, 200, 117295. [Google Scholar] [CrossRef]
- Zhang, B.; Ning, D.; Yang, Y.; Van Nostrand, J.D.; Zhou, J.; Wen, X. Biodegradability of Wastewater Determines Microbial Assembly Mechanisms in Full-Scale Wastewater Treatment Plants. Water Res. 2020, 169, 115276. [Google Scholar] [CrossRef]
- Mohsenpour, S.F.; Hennige, S.; Willoughby, N.; Adeloye, A.; Gutierrez, T. Integrating Micro-Algae into Wastewater Treatment: A Review. Sci. Total Environ. 2021, 752, 142168. [Google Scholar] [CrossRef] [PubMed]
- Falkowski, P.G.; Raven, J.A. Aquatic Photosynthesis, 2nd ed.; STU-Student edition; Princeton University Press: Princeton, NJ, USA, 2007; ISBN 978-0-691-11551-1. [Google Scholar]
- Khan, F.M.; Gupta, R. Escherichia coli (E. Coli) as an Indicator of Fecal Contamination in Groundwater: A Review. In Proceedings of the Sustainable Development of Water and Environment; Jeon, H.-Y., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 225–235. [Google Scholar]
- Arcila, J.S.; Buitrón, G. Influence of Solar Irradiance Levels on the Formation of Microalgae-Bacteria Aggregates for Municipal Wastewater Treatment. Algal Res. 2017, 27, 190–197. [Google Scholar] [CrossRef]
- Tang, C.-C.; Wang, R.; Wang, T.-Y.; He, Z.-W.; Tian, Y.; Wang, X.C. Characteristic Identification of Extracellular Polymeric Substances and Sludge Flocs Affected by Microalgae in Microalgal-Bacteria Aggregates Treating Wastewater. J. Water Process Eng. 2021, 44, 102418. [Google Scholar] [CrossRef]
- Dantas, D.M.d.M.; de Oliveira, C.Y.B.; Costa, R.M.P.B.; Carneiro-da-Cunha, M.d.G.; Gálvez, A.O.; Bezerra, R.d.S. Evaluation of Antioxidant and Antibacterial Capacity of Green Microalgae Scenedesmus subspicatus. Food Sci. Technol. Int. 2019, 25, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Ilieva, Y.; Zaharieva, M.M.; Kroumov, A.D.; Najdenski, H. Antimicrobial and Ecological Potential of Chlorellaceae and Scenedesmaceae with a Focus on Wastewater Treatment and Industry. Fermentation 2024, 10, 341. [Google Scholar] [CrossRef]
- Abdel-Raouf, N.; Al-Homaidan, A.A.; Ibraheem, I.B.M. Microalgae and Wastewater Treatment. Saudi J. Biol. Sci. 2012, 19, 257–275. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, K.; Sahoo, R.K.; Gaur, M.; Subudhi, E. Algal-Bacterial System: A Novel Low-Cost Biotechnological Initiative in Wastewater Treatment. In The Role of Microalgae in Wastewater Treatment; Sukla, L.B., Subudhi, E., Pradhan, D., Eds.; Springer: Singapore, 2019; pp. 115–127. ISBN 9789811315862. [Google Scholar]
- Amaro, H.M.; Salgado, E.M.; Nunes, O.C.; Pires, J.C.M.; Esteves, A.F. Microalgae Systems—Environmental Agents for Wastewater Treatment and Further Potential Biomass Valorisation. J. Environ. Manag. 2023, 337, 117678. [Google Scholar] [CrossRef]
- Shayesteh, H.; Vadiveloo, A.; Bahri, P.A.; Moheimani, N.R. Long Term Outdoor Microalgal Phycoremediation of Anaerobically Digested Abattoir Effluent. J. Environ. Manag. 2022, 323, 116322. [Google Scholar] [CrossRef]
- Arbib, Z.; Ruiz, J.; Álvarez-Díaz, P.; Garrido-Pérez, C.; Barragan, J.; Perales, J.A. Long Term Outdoor Operation of a Tubular Airlift Pilot Photobioreactor and a High Rate Algal Pond as Tertiary Treatment of Urban Wastewater. Ecol. Eng. 2013, 52, 143–153. [Google Scholar] [CrossRef]
- Díez-Montero, R.; Belohlav, V.; Ortiz, A.; Uggetti, E.; García-Galán, M.J.; García, J. Evaluation of Daily and Seasonal Variations in a Semi-Closed Photobioreactor for Microalgae-Based Bioremediation of Agricultural Runoff at Full-Scale. Algal Res. 2020, 47, 101859. [Google Scholar] [CrossRef]
- Fahim, R.; Lu, X.; Jilani, G.A.; Mahdi, H.; Aslam, M. Synergistic Long-Term Temperate Climate Nitrogen Removal Performance in Open Raceway Pond and Horizontal Subsurface Flow Constructed Wetland Operated Under Different Regimes. Water. Air. Soil Pollut. 2020, 231, 255. [Google Scholar] [CrossRef]
- Tan, X.-B.; Yang, L.-B.; Zhang, Y.-L.; Zhao, F.-C.; Chu, H.-Q.; Guo, J. Chlorella pyrenoidosa Cultivation in Outdoors Using the Diluted Anaerobically Digested Activated Sludge. Bioresour. Technol. 2015, 198, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Posadas, E.; Morales, M.d.M.; Gomez, C.; Acién, F.G.; Muñoz, R. Influence of pH and CO2 Source on the Performance of Microalgae-Based Secondary Domestic Wastewater Treatment in Outdoors Pilot Raceways. Chem. Eng. J. 2015, 265, 239–248. [Google Scholar] [CrossRef]
- Nwoba, E.G.; Ayre, J.M.; Moheimani, N.R.; Ubi, B.E.; Ogbonna, J.C. Growth Comparison of Microalgae in Tubular Photobioreactor and Open Pond for Treating Anaerobic Digestion Piggery Effluent. Algal Res. 2016, 17, 268–276. [Google Scholar] [CrossRef]
- Ayre, J.M.; Moheimani, N.R.; Borowitzka, M.A. Growth of Microalgae on Undiluted Anaerobic Digestate of Piggery Effluent with High Ammonium Concentrations. Algal Res. 2017, 24, 218–226. [Google Scholar] [CrossRef]





| Parameter | Unit | December | February |
|---|---|---|---|
| Chemical oxygen demand (COD) | mg L−1 O2 | 80 | 26 |
| Biochemical oxygen demand (BOD5) | mg L−1 O2 | 19 | 12 |
| Total suspended solids | mg L−1 | 48 | 10 |
| Escherichia coli | UFC/100 mL | 61,000 | 1700 |
| Total nitrogen (TN) | mg L−1 | 25.2 | 6.1 |
| Nitric nitrogen (N-NO3−) | mg L−1 | 24.4 | 4.1 |
| Nitrous nitrogen (N-NO2−) Ammonia nitrogen (N-NH4+) | mg L−1 | 0.16 | 0.30 |
| Total phosphorous (TP) | mg L−1 | 1.4 | 1.7 |
| Total chromium | mg L−1 | 22.1 | 5.3 |
| Chromium VI | mg L−1 | <0.02 | <0.02 |
| Lead | mg L−1 | <0.02 | <0.02 |
| Zinc | mg L−1 | <0.005 | <0.005 |
| Selenium | mg L−1 | 0.06 | 0.12 |
| Mercury | mg L−1 | <0.01 | <0.01 |
| Nichel | mg L−1 | <0.001 | <0.001 |
| Copper | mg L−1 | <0.01 | <0.01 |
| Cadmium | mg L−1 | 0.021 | <0.005 |
| Aluminium | mg L−1 | <0.005 | <0.005 |
| Daphnia magna acute toxicity assay | % mortality | 1.33 | 0.15 |
| Chlorella-like | UWW | |
|---|---|---|
| December 2021 | 85 L | 450 L |
| February 2022 | 55 L | 540 L |
| Min T° | Max T° | Average T° | |
|---|---|---|---|
| December 2021 | −0.56 °C | 10.61 °C | 4.86 °C |
| February 2022 | 0.93 °C | 14.44 °C | 6.28 °C |
| Day of Experimentation | December 2021 | February 2022 |
|---|---|---|
| 0 | 36,000 | 210 |
| 3 | 2600 | 220 |
| 21 | 72 | 2 |
| Final RE, % | 99.8 | 99.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benà, E.; Giacò, P.; Demaria, S.; Marchesini, R.; Melis, M.; Zanotti, G.; Baldisserotto, C.; Pancaldi, S. Winter Season Outdoor Cultivation of an Autochthonous Chlorella-Strain in a Pilot-Scale Prototype for Urban Wastewater Treatment. Water 2024, 16, 2635. https://doi.org/10.3390/w16182635
Benà E, Giacò P, Demaria S, Marchesini R, Melis M, Zanotti G, Baldisserotto C, Pancaldi S. Winter Season Outdoor Cultivation of an Autochthonous Chlorella-Strain in a Pilot-Scale Prototype for Urban Wastewater Treatment. Water. 2024; 16(18):2635. https://doi.org/10.3390/w16182635
Chicago/Turabian StyleBenà, Elisa, Pierluigi Giacò, Sara Demaria, Roberta Marchesini, Michele Melis, Giulia Zanotti, Costanza Baldisserotto, and Simonetta Pancaldi. 2024. "Winter Season Outdoor Cultivation of an Autochthonous Chlorella-Strain in a Pilot-Scale Prototype for Urban Wastewater Treatment" Water 16, no. 18: 2635. https://doi.org/10.3390/w16182635







