The Impact of Catastrophic Floods on Macroinvertebrate Communities in Low-Order Streams: A Study from the Apennines (Northwest Italy)
Abstract
:1. Introduction
2. Materials and Methods
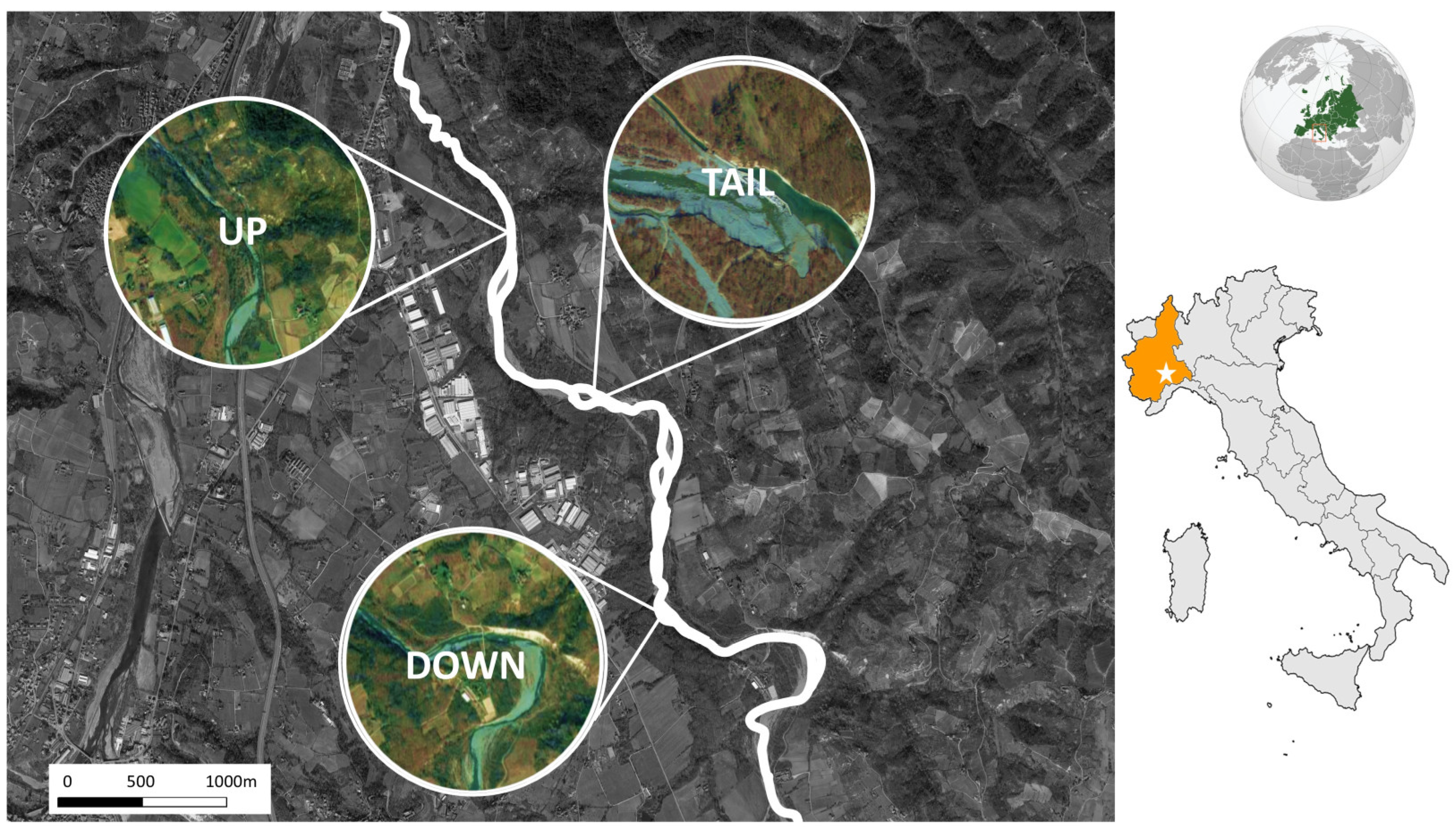
2.1. Area of Study
2.2. Macroinvertebrate Sampling and Processing
2.3. Statistical Analyses
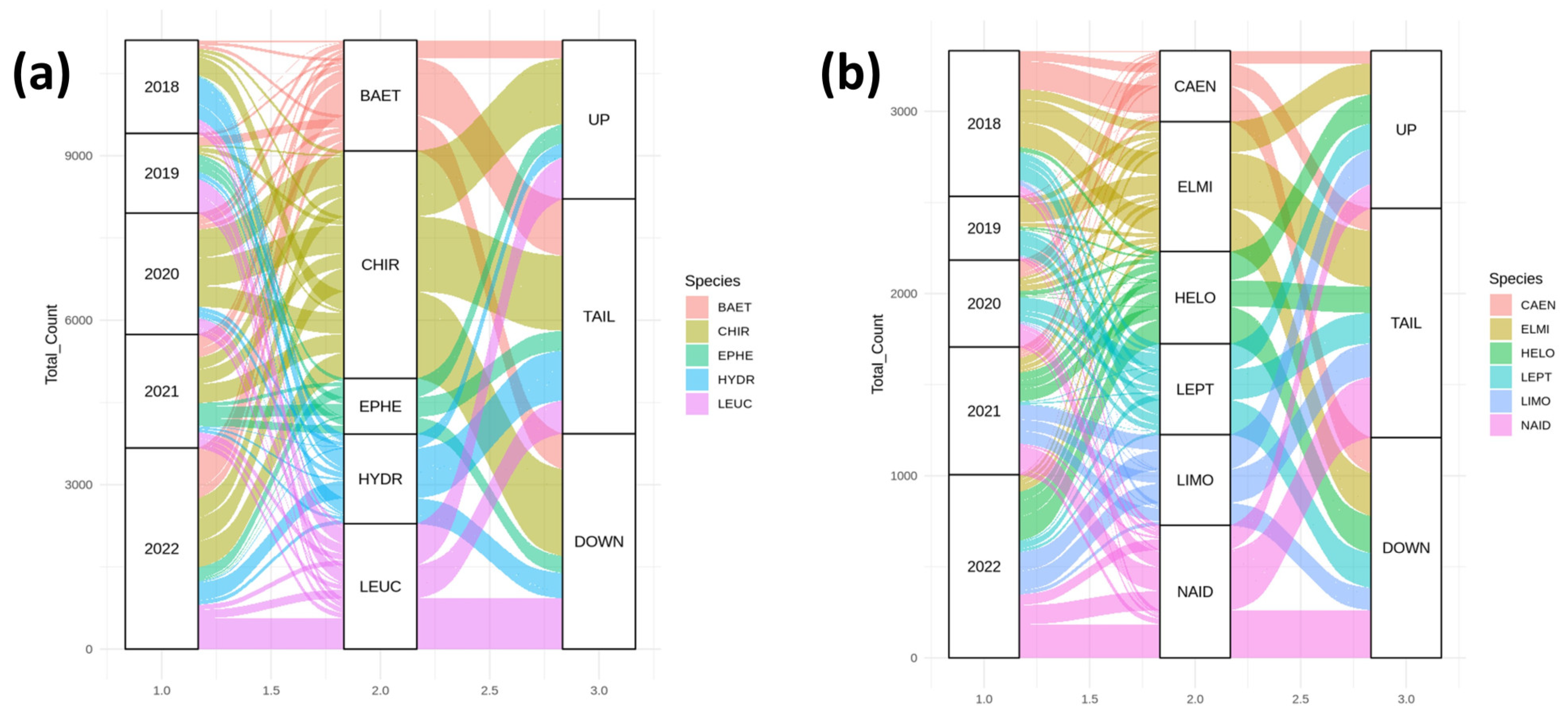
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Milner, A.M.; Picken, J.L.; Klaar, M.; Robertson, A.L.; Clitherow, L.R.; Eagle, L.J.B.; Brown, L.E. River ecosystem resilience to extreme flood events. Ecol. Evol. 2018, 8, 8354–8363. [Google Scholar] [CrossRef] [PubMed]
- Rajkhowa, S.; Sarma, J. Climate change and flood risk, global climate change. In Global Climate Change; Elsevier: Amsterdam, The Netherlands, 2021; pp. 321–339. [Google Scholar] [CrossRef]
- Aldous, A.; Fitzsimons, J.; Richter, B.D.; Bach, L. Droughts, floods and freshwater ecosystems: Evaluating climate change impacts and developing adaptation strategies. Mar. Freshwater Res. 2011, 62, 223. [Google Scholar] [CrossRef]
- Baker, V.R. Palaeoflood hydrology in a global context. Catena 2006, 66, 161–168. [Google Scholar] [CrossRef]
- Argerich, A. Effect of floods of different magnitude on the macroinvertebrate communities of Matarranya stream (Ebro river basin, NE Spain). Limnetica 2004, 23, 283–294. [Google Scholar] [CrossRef]
- Gholizadeh, M. Effects of floods on macroinvertebrate communities in the Zarin Gol River of northern Iran: Implications for water quality monitoring and biological assessment. Ecol. Process. 2021, 10, 46. [Google Scholar] [CrossRef]
- Reich, P.; Lake, P.S. Extreme hydrological events and the ecological restoration of flowing waters. Freshw. Biol. 2014, 60, 2639–2652. [Google Scholar] [CrossRef]
- Angradi, T.R. Hydrologic Context and Macroinvertebrate Community Response to Floods in an Appalachian Headwater Stream. Am. Midl. Nat. 1997, 138, 371. [Google Scholar] [CrossRef]
- Zhang, M.; Cai, Q.; Qu, X. Impacts of flood-driven water level fluctuations on macroinvertebrate assemblages in different zones of a long and narrow subtropical reservoir-bay. Quat. Int. 2017, 440, 111–118. [Google Scholar] [CrossRef]
- Lepori, F.; Hjerdt, N. Disturbance and Aquatic Biodiversity: Reconciling Contrasting Views. Bioscience 2006, 56, 809. [Google Scholar] [CrossRef]
- Wang, F.; Maberly, S.C.; Wang, B.; Liang, X. Effects of dams on riverine biogeochemical cycling and ecology. Inland Waters 2018, 8, 130–140. [Google Scholar] [CrossRef]
- Jardine, T.D.; Bond, N.; Burford, M.A.; Kennard, M.J.; Ward, D.; Bayliss, P.; Davies, P.M.; Douglas, M.M.; Hamilton, S.K.; Mélack, J.M.; et al. Does flood rhythm drive ecosystem responses in tropical riverscapes? Ecology 2015, 96, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.T.; Uehlinger, U.; Monaghan, M.T. Effects of a multi-year experimental flood regime on macroinvertebrates downstream of a reservoir. Aquat. Sci. 2003, 65, 210–222. [Google Scholar] [CrossRef]
- Chester, E.T.; Robson, B.J. Anthropogenic refuges for freshwater biodiversity: Their ecological characteristics and management. Biol. Conserv. 2013, 166, 64–75. [Google Scholar] [CrossRef]
- Mathers, K.L.; Robinson, C.T.; Weber, C. Patchiness in flow refugia use by macroinvertebrates following an artificial flood pulse. River Res. Appl. 2022, 38, 696–707. [Google Scholar] [CrossRef]
- IRSA-CNR. Notiziario dei Metodi Analitici, n° 1 Marzo; IRSA-CNR: Montelibretti, Italy, 2007; 113p. [Google Scholar]
- Campaioli, S.; Ghetti, P.F.; Minelli, A.; Ruffo, S. Manuale per il Riconoscimento dei Macroinvertebrati Delle Acque Dolci Italiane; Provincia Autonoma di Trento, Agenzia Provinciale per la Protezione dell’Ambiente: Trento, Italy, 1994; Volume I. [Google Scholar]
- Campaioli, S.; Ghetti, P.F.; Minelli, A.; Ruffo, S. Manuale per il Riconoscimento dei Macroinvertebrati Delle Acque Dolci Italiane; Provincia Autonoma di Trento, Agenzia Provinciale per la Protezione dell’Ambiente: Trento, Italy, 1999; Volume II. [Google Scholar]
- Tachet, H.; Bournaud, M.; Richoux, P.; Usseglio-Polatera, P. Invertébrés d‘eau Douce: Systématique, Biologie, Écologie; CNRS Editions: Paris, France, 2002; 588p. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O‘Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5-6. 2019. Available online: https://CRAN.R-project.org/package=vegan (accessed on 10 August 2024).
- Baselga, A.; Orme, C.D.L. betapart: An R package for the study of beta diversity. Methods Ecol. Evol. 2012, 3, 808–812. [Google Scholar] [CrossRef]
- McLeod, A.I. Kendall: Kendall Rank Correlation and Mann-Kendall Trend Test. R Package Version 2.2. 2011. Available online: https://CRAN.R-project.org/package=Kendall (accessed on 7 August 2024).
- Kroon, F.J.; Ludwig, J.A. Response and recovery of fish and invertebrate assemblages following flooding in five tributaries of a sub-tropical river. Mar. Freshwater Res. 2010, 61, 86. [Google Scholar] [CrossRef]
- Ibemenuga, K.N.; Inyang, N.M. Macroinvertebrate Fauna of a Tropical Freshwater Stream in Nigeria. Anim. Res. Int. 2008, 3, 553–561. [Google Scholar] [CrossRef]
- Abong’o, D.A.; Wandiga, S.O.; Jumba, I.O.; Brink PJ, V.D.; Naziriwo, B.; Madadi, V.O.; Wafula, G.A.; Nkedi-Kizza, P.; Kylin, H. Occurrence, abundance and distribution of benthic macroinvertebrates in the Nyando River catchment, Kenya. Afr. J. Aquat. Sci. 2015, 40, 373–392. [Google Scholar] [CrossRef]
- Bo, T.; Cucco, M.; Fenoglio, S.; Malacarne, G. Colonisation patterns and vertical movements of stream invertebrates in the interstitial zone: A case study in the Apennines, NW Italy. Hydrobiologia 2006, 568, 67–78. [Google Scholar] [CrossRef]
- Dagnino, A.; Bo, T.; Copetta, A.; Fenoglio, S.; Oliveri, C.; Bencivenga, M.; Felli, A.; Viarengo, A. Development and application of an innovative expert decision support system to manage sediments and to assess environmental risk in freshwater ecosystems. Environ. Int. 2013, 60, 171–182. [Google Scholar] [CrossRef]
- Bagalwa, M.; Mukumba, I.; Ndahama, N.; Zirirane, N.; Kalala, A.O. Assessment of River Water Quality using Macroinvertebrate Organisms as Pollution Indicators of Cirhanyobowa River, Lake Kivu, DR Congo. Int. J. Curr. Microbiol. App. Sci. 2019, 8, 2668–2680. [Google Scholar] [CrossRef]
- Papius DM, T.; William, O.; Alex, B.; Mbabazi, D.; Jimmy, O.; Kiggundu, V. Status of Kigezi minor Lakes: A limnological survey in the Lakes of Kisoro, Kabale and Rukungiri Districts. Int. J. Water Resour. Environ. Eng. 2016, 8, 60–73. [Google Scholar] [CrossRef]
- Abebe, W.B.; Tilahun, S.A.; Moges, M.M.; Wondie, A.; Derseh, M.G.; Nigatu, T.A.; Mhiret, D.A.; Steenhuis, T.S.; Camp, M.V.; Walraevens, K.; et al. Hydrological Foundation as a Basis for a Holistic Environmental Flow Assessment of Tropical Highland Rivers in Ethiopia. Water 2020, 12, 547. [Google Scholar] [CrossRef]
- Füreder, L.; Niedrist, G. Glacial Stream Ecology: Structural and Functional Assets. Water 2020, 12, 376. [Google Scholar] [CrossRef]
- Pereira, S.A.; Trindade CR, T.; Albertoni, E.F.; Palma-Silva, C. Aquatic macrophytes as indicators of water quality in subtropical shallow lakes, Southern Brazil. Acta Limnol. Bras. 2012, 24, 52–63. [Google Scholar] [CrossRef]
- Rife, G.S. Ecosystem Services Provided by Benthic Macroinvertebrate Assemblages in Marine Coastal Zones; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar] [CrossRef]
- Stephan, U.; Kainz, S.; Hengl, M.; Bickel, A.M.; Mähr, M.; Burtscher, W. Development and implementation of ecological and economical flood protection measures at an alpine river. In E3S Web Conferences; EDP Sciences: Les Ulis, France, 2018; Volume 40, p. 02030. [Google Scholar] [CrossRef]
- Chomba, I.C.; Banda, K.; Winsemius, H.; Chomba, M.; Mataa, M.; Ngwenya, V.; Sichingabula, H.M.; Nyambe, I.; Ellender, B.R. A Review of Coupled Hydrologic-Hydraulic Models for Floodplain Assessments in Africa: Opportunities and Challenges for Floodplain Wetland Management. Hydrology 2021, 8, 44. [Google Scholar] [CrossRef]
- Lawrence, J.; Blackett, P.; Cradock-Henry, N.A. Cascading climate change impacts and implications. Clim. Risk Manag. 2020, 29, 100234. [Google Scholar] [CrossRef]



| Abundance | F Value | p-Value |
|---|---|---|
| Sites | 1.937 | 0.169 |
| Site:Year | 0.493 | 0.847 |
| Richness | F value | p-value |
| Sites | 0.152 | 0.859 |
| Site:Year | 0.282 | 0.964 |
| EPT_Abundance | F value | p-value |
| Sites | 1.567 | 0.232 |
| Site:Year | 0.482 | 0.854 |
| EPT_Richness | F value | p-value |
| Sites | 0.109 | 0.896 |
| Site:Year | 0.340 | 0.940 |
| DOM-3 | F value | p-value |
| Sites | 0.006 | 0.994 |
| Site:Year | 0.327 | 0.724 |
| NCO_Richness | F value | p-value |
| Sites | 0.077 | 0.926 |
| Site:Year | 0.340 | 0.714 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marino, A.; Fenoglio, S.; Bo, T. The Impact of Catastrophic Floods on Macroinvertebrate Communities in Low-Order Streams: A Study from the Apennines (Northwest Italy). Water 2024, 16, 2646. https://doi.org/10.3390/w16182646
Marino A, Fenoglio S, Bo T. The Impact of Catastrophic Floods on Macroinvertebrate Communities in Low-Order Streams: A Study from the Apennines (Northwest Italy). Water. 2024; 16(18):2646. https://doi.org/10.3390/w16182646
Chicago/Turabian StyleMarino, Anna, Stefano Fenoglio, and Tiziano Bo. 2024. "The Impact of Catastrophic Floods on Macroinvertebrate Communities in Low-Order Streams: A Study from the Apennines (Northwest Italy)" Water 16, no. 18: 2646. https://doi.org/10.3390/w16182646
APA StyleMarino, A., Fenoglio, S., & Bo, T. (2024). The Impact of Catastrophic Floods on Macroinvertebrate Communities in Low-Order Streams: A Study from the Apennines (Northwest Italy). Water, 16(18), 2646. https://doi.org/10.3390/w16182646






