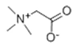Abstract
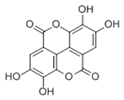
This study investigates the synthesis of iron-based nanoparticles (Fe NPs) using pomegranate leaf extracts and their application in removing indole, a persistent organic pollutant commonly found in wastewater. The physicochemical properties of the synthesized Fe NPs and the active biomolecules in the pomegranate leaf extracts were comprehensively characterized. Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM) analyses revealed that the Fe NPs exhibited quasi-spherical shapes, with sizes ranging from 75 to 105 nm. Energy-Dispersive X-ray Spectroscopy (EDS) confirmed a homogeneous distribution of elements, including C, O, Fe, and S, on the nanoparticle surfaces, with weight percentages of 43.59%, 42.95%, 12.58%, and 0.88%, respectively. Fourier-transform infrared spectroscopy (FTIR) identified key functional groups like −OH, −COOH, and −C=O, which are essential for the capping and stabilization of the nanoparticles. Biomolecules such as ellagic acid (C14H6O8) and gallic acid (C7H6O5) functioned as reducing agents, improving nanoparticle dispersion and preventing aggregation. The synthesized Fe NPs quickly achieved 45.5% removal of indole within just 20 min and maintained a stable removal efficiency of approximately 51.4% after 90 min. This performance was attributed to the synergetic interaction between the biomolecules and the nanoparticles, with the monolayer adsorption of indole molecules on the Fe NP surfaces likely setting an upper limit on the maximum achievable removal efficiency. It appears from this study that pomegranate leaf extracts can be effectively utilized to synthesize Fe NPs as a novel and eco-friendly approach, demonstrating promising potential for the rapid removal of indole from aqueous solutions.
1. Introduction
Iron-based nanoparticles (Fe NPs), characterized by their nanoscale size, extensive surface area, and the presence of zero-valent iron, have emerged as effective agents for the rapid removal of a wide range of contaminants from water [1]. Traditional synthesis methods, such as physical vapor deposition, chemical reduction, and hydrothermal synthesis, predominantly utilize liquid-phase reduction techniques. However, these approaches often encounter obstacles including nanoparticle agglomeration and environmental risks due to increased solubility in water [2,3,4]. To overcome these challenges, recent research has increasingly focused on the development of environmentally friendly, cost-effective, and biologically safer nano-iron materials.
Green synthesis, which utilizes plant extracts or microbial tissue to reduce iron salts to zero-valent iron, has garnered attention due to its avoidance of toxic chemicals, reduced energy consumption, and enhanced dispersion of nanoparticles [5,6,7,8]. Particularly, the use of plant-derived substances for nanoparticle synthesis has been extensively explored, with natural components such as polyphenols, sugars, alkaloids, and proteins acting as reducing, capping, and stabilizing agents in the biosynthesis process, thereby mitigating the oxidation and agglomeration of Fe NPs [9,10]. Species such as eucalyptus leaves and green tea have shown potential in producing nanoparticles, with active biomolecules in eucalyptus leaf extracts serving as capping and dispersive agents, preventing nanoparticle agglomeration and oxidation [11,12]. Additionally, the average size of these plant-synthesized Fe NPs is smaller than that of chemically synthesized ones, contributing to their enhanced properties [13,14]. For example, Fe NPs synthesized with green tea extracts exhibit higher mobility in soil due to the stabilizing effects of plant biomolecules [15]. Furthermore, these plant-synthesized Fe NPs have been proven to effectively catalyze the degradation of organic pollutants such as 2,4-dichlorophenol, establishing their utility as Fenton-like catalysts for water purification [16,17].
Pomegranate (Punica granatum) trees, which are abundant in central China, offer a promising alternative due to the rich natural antioxidant content of their leaves [14,18,19]. These antioxidants, including polyphenols and flavonoids, possess strong reducing abilities and serve as capping agents in nanoparticle synthesis, enhancing Fe NPs’ stability and preventing aggregation. Notably, the polyphenol content in pomegranate leaves is significantly higher than in other plants, which grants them superior antioxidant properties, surpassing even those found in green tea [20,21]. Previous studies have demonstrated that using pomegranate leaf extract for Fe NP synthesis, with its relatively high polyphenol content, can produce smaller, more uniformly dispersed nanoparticles, thereby enhancing their reactivity and improving pollutant degradation efficiency [15]. Therefore, it is reasonable to consider pomegranate leaf extract as an especially effective medium for the green synthesis of Fe NPs, with significant potential for application in environmental pollutant remediation.
Indole, a recalcitrant pollutant frequently found in various industrial wastewaters, presents significant challenges for biodegradation due to its stable structure and resistance to conventional treatment methods [22,23,24]. Although traditional methods such as activated carbon adsorption and ozone oxidation offer some efficacy, they are often limited by high costs and inefficiency. In contrast, Fe NPs have demonstrated considerable promise in breaking down organic dyes in wastewater, owing to the structural and chemical similarities between indole and these dyes [25]. This suggests that green-synthesized Fe NPs, particularly those derived from pomegranate leaves, could offer an effective, eco-friendly solution for indole degradation. However, despite the potential of plant extracts for nanoparticle synthesis, the use of pomegranate leaf extracts specifically for Fe NP synthesis targeting indole degradation has been scarcely explored.
Therefore, this study aims to explore the feasibility of synthesizing Fe NPs using pomegranate leaf extracts and to identify the key components influencing the characteristics of the synthesized nanoparticles. To achieve this, pomegranate leaf extracts were utilized to synthesize Fe NPs, which were subsequently characterized to elucidate the role of active biomolecules in their formation and stabilization. The effectiveness of these Fe NPs in removing indole was then evaluated. It is expected that this study could offer valuable insights into the green synthesis of Fe NPs and their potential application in environmental remediation, particularly in sustainable water treatment practices.
2. Materials and Methods
2.1. Materials and Reagents
Pomegranate leaves (Punica granatum L.) were sourced from Hongtong city,, Shanxi Province, China. Ferrous sulfate heptahydrate (FeSO4·7H2O, AR) was acquired from Sinopharm Chemical Reagent Co., Ltd, Shanghai, China. Indole (C8H7N, AR), anhydrous ethanol (C2H6O, AR), and solvent methylene chloride (C2H4Cl2, HPLC) were obtained from Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China. Ultrapure water used in all experiments was produced by a Millipore purification system (Milli-Q® IQ 7003, Millipore (China) Ltd., Shanghai, China) to ensure reaction purity.
2.2. Preparation of Fe NPs
Fresh pomegranate leaves, harvested in early July, were first washed and then air-dried for 48 h. The leaves were further dried in an electric thermostatic drying oven (DHG-9030, Shanghai Yiheng Technology Co., Ltd., Shanghai, China) at 333 K for over 24 h until completely desiccated. Subsequently, the dried leaves were ground using a high-speed grinder (FW-200, Tianjin Huaxin Instrument Factory, Tianjin, China) and sieved through a 200-mesh screen. The resulting leaf powder was sealed and stored in a desiccator.
A 3.00 g portion of the dried leaf powder was added to a conical flask containing 300 mL of ultrapure water and agitated in a water bath at 333 K for 2 h at a speed of 180 rpm. After cooling to room temperature, the mixture was vacuum-filtered through a 0.45 μm membrane to obtain the pomegranate leaf extracts. Under a continuous nitrogen flow at 303 K, 200 mL of the pomegranate leaf extract was placed in a three-neck flask. Subsequently, a 0.10 M solution of FeSO4·7H2O (100 mL) was gradually introduced to the leaf extract in a 1:2 volume ratio under a nitrogen atmosphere, ensuring thorough mixing at 303 K with a magnetic stirrer. This reaction led to the formation of PL-Fe NP suspension.
The resulting PL-Fe NP suspension was then centrifuged at a high speed of 10,000 rpm for 20 min, after which the supernatant was decanted. The PL-Fe NPs that adhered to the tube walls were subsequently washed with anhydrous ethanol. Following the washing step, the PL-Fe NPs were vacuum-dried for 48 h, then ground into a fine powder, and finally stored in a desiccator for future use.
2.3. Indole Degradation Experiment
The capability of the synthesized Fe NPs to degrade indole was evaluated using a 30 mg·L−1 indole solution. Specifically, 100 mL of pomegranate leaf extract and PL-Fe NP suspension were separately added to conical flasks containing 300 mL of this indole solution and thoroughly mixed. The mixtures were then reacted at 303 K with an agitation speed of 180 rpm. Samples were collected at predetermined intervals of 0, 20, 40, 60, 90, 120, 150, and 180 min for subsequent analysis. The reaction mixtures were filtered through a 0.45 μm membrane, and phenol at a concentration of 0.1 mol·L−1 was added as an internal standard according to previous study [26]. The concentration of indole was determined via Gas Chromatography-Mass Spectrometry (GC-MS) analysis (7890B GC/5977A MSD, Agilent Technologies, Inc., Santa Clara, CA, USA), following sample preparation steps including extraction, rotary evaporation, and nitrogen gas stripping according to a previous study [2]. The efficiency of indole removal was calculated using the following equation:
where C0 represents the initial concentration of indole, and Ct denotes the concentration of indole at a certain time post-reaction initiation.
To further elucidate the impact of temperature on the indole removal efficiency of PL-Fe NPs, experiments were conducted at fixed temperatures of 293 K, 303 K, and 313 K in a thermostatic water bath, each with a reaction time of 60 min. All other procedures were consistent with the methods described above. Adsorption isotherms for each temperature were subsequently determined by varying the initial indole concentration.
2.4. Characterization of Fe NPs
The particle size and morphology of PL-Fe NPs were determined using Scanning Electron Microscopy (SEM, JSM-7001F, JEOL Ltd., Tokyo, Japan) at an accelerating voltage of 10.0 kV and Transmission Electron Microscopy (TEM, JEM-1011, JEOL Ltd., Tokyo, Japan) at 100 kV. The elemental composition and distribution of the PL-Fe NPs were further analyzed using Energy-Dispersive X-ray Spectrometry (EDS, Bruker QX200, Bruker Corporation, Karlsruhe, Germany). Additionally, X-ray Photoelectron Spectroscopy (XPS, Axis Ultra DLD, Kratos Analytical Ltd., Manchester, UK) was employed to analyze the valence state distribution of carbon (C), oxygen (O), iron (Fe), and sulfur (S) within the PL-Fe NPs, measuring surface elemental composition and various forms of iron oxidation across a 700 × 300 μm region. The infrared spectra of PL-Fe NPs were obtained through Fourier-transform infrared spectroscopy using an Antaris IGS spectrometer (FTIR, Thermo-Fisher Scientific Inc., Waltham, MA, USA). During sample preparation, 1% (w/w) of 100 mg KBr powder was mixed with the PL-Fe NPs and pressed into thin pellets, with measurements conducted at a resolution of 2 cm−1, and the average was taken from eight scans.
2.5. GC-MS Analysis
Pomegranate leaf extracts were subjected to GC-MS analysis using an Agilent 7890B GC system equipped with a split/splitless injector, coupled to an Agilent 5977A MSD mass spectrometer (Agilent Technologies, Santa Clara, CA, USA). The injector’s inlet temperature was meticulously maintained at 573 K. System operations were seamlessly managed via Agilent MSD ChemStation software (MassHunter Qualitative Analysis B.07.00). Chromatographic separation was achieved on an HP-5 fused silica capillary column (30 m × 0.25 mm × 0.25 μm). Helium was utilized as the carrier gas at a pressure of 0.55 MPa, with a sample injection volume of 1 μL.
The GC thermal program commenced at an initial temperature of 313 K, was held for 10 min, followed by a ramp to 383 K over 10 min at a rate of 20 K/min, then reaching 443 K over 3 min at 10 K/min. The MS system operated in scan mode, covering a mass range of 50 to 550 amu with a 0.1 m/z scanning step. Compound identification was achieved by matching the observed mass spectra against the NIST 14.L mass spectral library.
2.6. Liquid Chromatograph-Mass Spectrometry (LC-MS) Analysis
Analyte separation was conducted on a Dionex UltiMate 3000 UHPLC system(Agilent 1260, Agilent Technologies Inc., USA), incorporating an RS Pump, RS Autosampler, RS Column Compartment, and Diode Array Detector. Chromatographic separation was facilitated using a Thermo Scientific Hypersil GOLD column (100 mm × 2.1 mm, 1.9 µm, Thermo Scientific, Waltham, MA, USA) maintained at 45 °C. A gradient elution protocol was employed, utilizing a mobile phase-A (1% formic acid in water) and mobile phase-B (1% formic acid in acetonitrile) at a flow rate of 0.3 mL/min, with UV detection at 254.0 nm. A Thermo Scientific Q Exactive mass spectrometer (Thermo Fisher Scientific, Waltham, MA), equipped with a HESI source, enabled detection of analytes across a scanning range of 100 to 1000 m/z.
3. Results and Discussion
3.1. Characterization of Fe NPs
Figure 1 illustrates the morphological characteristics of the synthesized Fe NPs. The nanoparticles exhibited a quasi-spherical shape (Figure 1a), indicating their excellent dispersibility. TEM analysis further confirmed that the Fe NPs ranged in size from 75 to 105 nm (Figure 1b). Compared to iron-based nanoparticles synthesized using sodium borohydride, these Fe NPs displayed a variety of shapes, likely due to the influence of the biomolecular constituents from the pomegranate leaf extracts [27]. The elemental analysis through EDS in Figure 1c reveals that C, O, Fe, and S accounted for 43.59%, 42.95%, 12.58%, and 0.88% of the weight, respectively. The detection of iron peaks confirmed the successful synthesis of the nanoparticles, while the presence of oxygen suggested the formation of iron oxides. The C and O signals were attributed to the organic components of the pomegranate leaves, and the S signal originated from the precursor, FeSO4·7H2O. As shown in Figure 1d, the elements C, O, Fe, and S were uniformly distributed across the sample, indicating that the synthesis process involved interactions between iron and active biomolecules in the pomegranate leaf extracts. Additionally, the relative atomic content indicated that active biomolecules contributed to over 80% of the weight of the surface and near-surface regions of the Fe NPs.
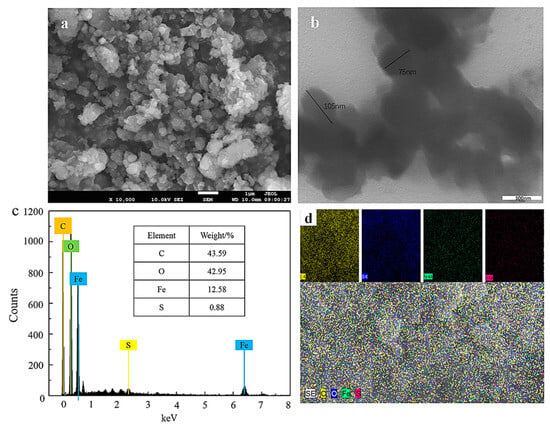
Figure 1.
Morphological and elemental characterization of Fe NPs: (a) SEM image of surface morphology; (b) TEM image; (c) EDS spectra for elemental composition; (d) EDS map for elemental distribution.
XPS was utilized to precisely identify the Fe species and their valence states. The comprehensive spectral survey displayed distinct peaks for C 1s, O 1s, and Fe 2p, as shown in Figure 2a. Specifically, Figure 2b highlights the Fe 2p spectrum, with the characteristic 2p3/2 and 2p1/2 peaks observable at 711.5 eV and 725.1 eV, respectively. These energy levels are indicative of the iron present in iron oxides (e.g., FeO, Fe2O3, Fe3O4) and iron oxyhydroxide (FeOOH) [28]. The C 1s and O 1s peaks were associated with the biomolecular capping derived from the leaf extracts on the Fe NPs surface. As illustrated in Figure 2c, the C 1s spectrum features peaks corresponding to C−C (284.7 eV), C=O (287.7 eV), and O−C−O (288.3 eV) [29], while the O 1s spectrum in Figure 2d reveals additional shoulders at 530.8 eV, 531.8 eV, and 532.6 eV. These O 1s spectral features were attributed to oxygen in the iron oxide lattice, physisorbed water, and structural hydroxyl groups, respectively. This analysis confirmed the presence of various iron oxides within the Fe NPs, with no detectable traces of metallic iron. The forms of carbon and oxygen identified were consistent with the FTIR results.
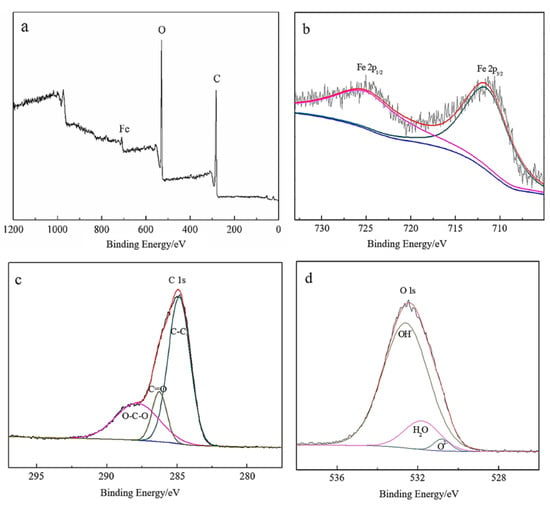
Figure 2.
Detailed XPS analysis of Fe NPs: (a) full survey spectrum; (b) high-resolution spectra of Fe 2p; (c) spectra of C 1s; (d) spectra of O 1s.
FTIR spectroscopy provided valuable insights into the functional groups essential for the formation and stabilization of Fe NPs. As depicted in Figure 3, notable changes in band intensities and spectral shifts were observed between the leaf extracts and metal ions. The prominent absorption bands at approximately 3420 cm−1 and 3393 cm−1 were attributed to O–H stretching vibrations from polyphenols, flavonoids, alkaloids, and organic acids [30], indicating their active involvement in the synthesis process. The shift from 2926 cm−1 to 2886 cm−1 highlighted the participation of saturated alkanes through symmetric and asymmetric C–H stretching vibrations in aliphatic acids during Fe NP formation. The peaks at 1707 cm−1 and 1711 cm−1 corresponded to carbonyl groups, likely contributing to carboxylic acids with aromatic rings. The slight shift in bands from 1627 cm−1 to 1624 cm−1, representing C=C aromatic skeletal vibrations, suggested their potential role in nanoparticle synthesis. Bands at 1350 cm−1 and 1335 cm−1 were associated with C–H asymmetric stretching vibrations of alkanes and aromatic C–N stretching vibrations [30,31]. The subtle shift in peaks around 1073 cm−1 to 1071 cm−1, identified as carbonyl groups, suggested the interaction of C=O groups in the leaf extract with iron, facilitating nanoparticle formation. The peaks at 765 cm−1, 762 cm−1, and 521 cm−1 denoted aromatic hydrocarbons, while additional peaks at 565 cm−1 were related to Fe–O vibrations. Broad absorption peaks were indicative of Fe2O3 and Fe3O4 stretches, aligning with the XPS results. These findings underscored the attachment of biomolecules, identified via LC-MS, as containing hydroxyl, carbonyl, and carboxyl groups, to the Fe NPs, resulting in the formation of a novel type of iron nanoparticle.
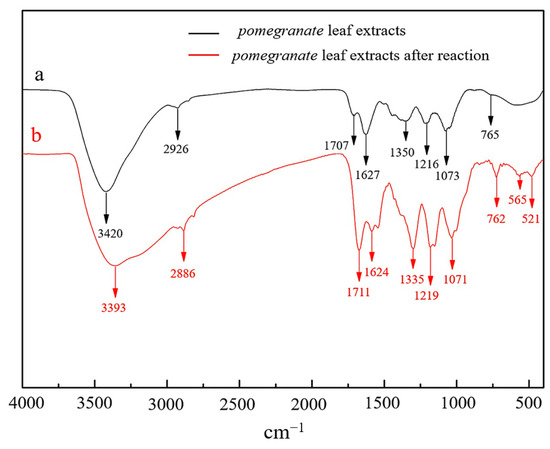
Figure 3.
FTIR spectra of pomegranate leaf extracts before and after reacting with Fe2+ solution.
3.2. Active Biomolecules in Fe NPs
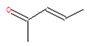
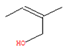
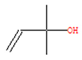
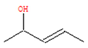
To elucidate and confirm the specific organic constituents within pomegranate leaf extracts, GC-MS analysis was conducted, and the findings are elaborated herein. As shown in Figure 4, significant variations in peak intensity were observed within the initial 20 min interval, while the subsequent 15 min period exhibited minimal changes. This pattern suggested the active involvement of low-molecular-weight volatiles in the eco-friendly synthesis of Fe NPs. Table 1 provides detailed information on six key compounds, including their nomenclature, retention times (RTs), molecular formulas, molecular weights, structural representations, and relative concentrations. Notably, the concentrations of compounds such as enols, enones, and amines decreased significantly after the synthesis process, indicating their crucial roles in the biogenic formation of Fe NPs. These findings are consistent with those of previous studies that emphasized the reactivity of phenols, amines, and alkanes in the bio-reduction, genesis, and stabilization of metallic nanoparticles [32]. During the synthesis of Fe NPs, the functional groups like –OH, =O, and –NH2 may act as reductants during oxidation, while C=C and C=N motifs likely decorate the nanoparticle surface, providing resistance against oxidative stress and enhancing the stability of Fe NPs.
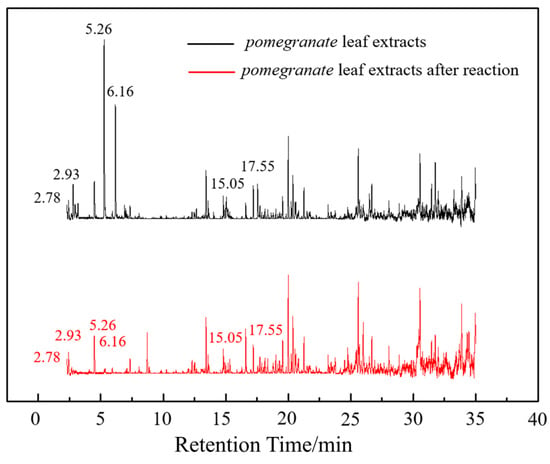
Figure 4.
GC-MS analysis of active biomolecules in Fe NPs.

Table 1.
The active biomolecules by GC-MS analysis.
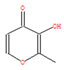
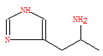
Additionally, LC-MS supplemented the GC-MS findings by identifying larger and less-volatile organic compounds such as polyphenols, alcohols, organic acids, and alkaloids. As shown in Figure 5, the chromatographic profiles (both normal and reverse-phase) of pomegranate leaf extracts were analyzed, while Table 2 details the critical biomolecules identified through LC-MS. Ellagic acid, a key component of the extracts, was found to possess half the chemical reduction potential of vitamin C in iron reduction assays [32]. Cyanidin, another significant derivative, exhibited antioxidant capacities that were 50 times greater than those of vitamin E and 20 times greater than vitamin C, respectively [33]. Flavonoids such as luteolin and apigenin were also identified, with structural features conducive to antioxidative activity. Aromatic carbonyls, by donating hydrogen atoms to free radicals, acted as hydrogen donors, facilitating the reduction of radicals [34]. Furthermore, the abundance of phenolic hydroxyl groups in these biomolecules allowed interactions with atmospheric radicals, leading to the formation of stable semiquinone radicals that inhibited radical chain reactions and provided antioxidative protection. These bioactive molecules, which surrounded the Fe NPs, ensured stable dispersion, reduced rapid oxidation, and prevented agglomeration, indicating a promising approach for nanoparticle synthesis and stabilization.
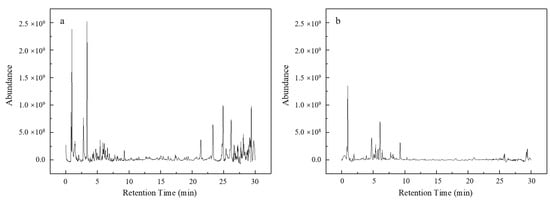
Figure 5.
LC-MS analysis of active biomolecules of Fe NPs: (a) normal-phase chromatography; (b) reverse-phase chromatography.

Table 2.
The main bioactive substances found by LC-MS analysis.
3.3. Fe NPs Synthesis Mechanisms and Indole Degradation
Figure 6 illustrates the possible mechanisms for the green synthesis of Fe NPs. The cores of Fe NPs were various forms of iron oxides such as FeO, Fe2O3, Fe3O4, and FeOOH. Parts of them were the reduction of Fe2+ to Fe0 and then further formed various forms of iron oxides with biomolecules in pomegranate leaf extracts. For the other part of them, Fe2+ reacted directly with biomolecules to form various forms of iron oxide. They were wrapped within a layer of biomolecules derived from the pomegranate leaf extracts. This layer contained active biomolecules including five carbon enols (C5H10O) and bioactive materials with large carbon numbers (C7-C14), namely ellagic acid, gallic acid (C7H6O5), and betaine (C5H11NO2). These organic substances have high antioxidant capacity, and they can act as capping, reducing, and stabilizing agents in the Fe NPs [35]. They might prevent Fe NPs from forming large aggregates and maintain high stability and reactivity. The synthesis process rendered Fe NPs with irregular morphologies due to complex biomolecular interactions, presenting a challenge in standardizing nanoparticle size. Nevertheless, the distinct physicochemical attributes of Fe NPs, enriched by bioactive molecules from pomegranate leaf extracts and iron oxide particles, were exploited for the efficient breakdown of indole [36]. Precedents in the literature have demonstrated the nanoparticles’ efficacy in degrading indole and analogous organic compounds, thus confirming the feasibility of employing Fe NPs for environmental clean-up efforts [37].
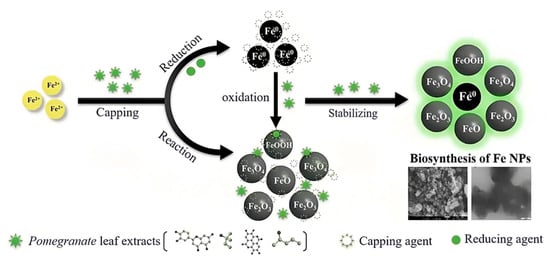
Figure 6.
Possible formation mechanisms of Fe NPs.
The results from the indole removal experiments confirmed the effectiveness of the Fe NPs in facilitating the adsorption-driven removal of indole from aqueous environments. Quantitative analysis using GC-MS with an internal standard revealed that 45.5% of the indole was removed within the first 20 min of exposure to the Fe NPs. The removal rate further increased to 51.4% after 90 min (Figure 7). The adsorption kinetics analysis indicated that the removal process followed a second-order model (Figure 8), implying that the reaction rate was dependent on the concentrations of both indole and Fe NPs. Furthermore, the adsorption isotherms showed an increase in the adsorption capacity of Fe NPs for indole with rising temperature, suggesting that higher temperatures may enhance the adsorption dynamics (Figure 9a). The linear relationship between Ce/qe and Ce at 293 K, 303 K, and 313 K aligned with the Langmuir adsorption model (Figure 9b), which confirmed the monolayer adsorption of indole molecules on the Fe NP surfaces. This monolayer adsorption likely sets an upper limit on the maximum achievable removal efficiency. Importantly, the Fe NPs exhibited superior performance in indole removal compared to pomegranate leaf extracts alone, highlighting their enhanced capacity not only for adsorption but also for reduction processes. This enhanced reactivity highlights the potential of Fe NPs not only for indole removal but also for broader applications in the detoxification of other organic pollutants, especially those with similar structural characteristics to indole, such as pyridine [38,39]. However, to further improve the removal efficiency of indole and similar compounds, more detailed studies on the removal mechanism are needed, along with the optimization of the reaction conditions or surface modification of the synthesized nanoparticles to improve their adsorption performance.
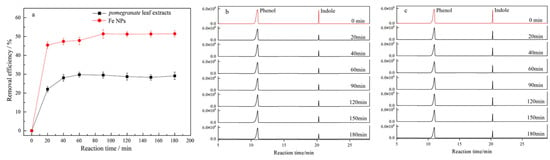
Figure 7.
(a) Indole efficiency removal by Fe NPs and pomegranate leaf extracts; GC-MS chromatogram of indole removal by Fe NPs (b) and pomegranate leaf extracts (c).
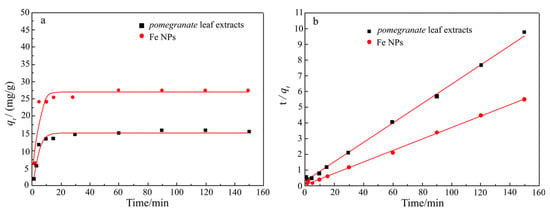
Figure 8.
Adsorption kinetics curve: (a) pseudo first-order kinetics model; (b) pseudo second-order kinetics model.
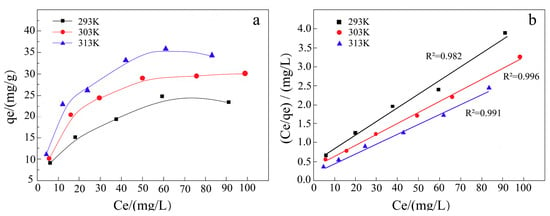
Figure 9.
(a) Adsorption isotherms at different temperatures, (b) Ce/qe–Ce relationship points and linear fitting.
4. Conclusions
This study successfully demonstrated the effectiveness of using pomegranate leaf extracts to synthesize Fe NPs and highlighted their potential for indole removal. The synthesized Fe NPs were found to be quasi-spherical, with sizes ranging from 75 to 105 nm. EDS analysis revealed a uniform distribution of elements, including C, O, Fe, and S on the nanoparticle surfaces, with respective weight percentages of 43.59%, 42.95%, 12.58%, and 0.88%. Key functional groups like −OH, −COOH, and −C=O were identified via FTIR, which played a crucial role in maintaining the stability and preventing the aggregation of Fe NPs. Further analyses through GC-MS and LC-MS revealed that small biomolecules like enols, enones, and amines were essential in the synthesis process, acting as both reductants and capping agents. Larger bioactive molecules (C7-C14), including ellagic acid, gallic acid, and betaine, were found to significantly contribute to the capping and stabilization of the nanoparticles. The synthesized Fe NPs exhibited a rapid indole removal rate, achieving 45.5% reduction within the first 20 min and stabilizing at approximately 51.4% after 90 min. This efficiency can be attributed to the synergistic interaction between the active biomolecules and the Fe NPs, which enhanced both adsorption and reduction processes. These findings highlight the potential of using pomegranate leaf extracts for the green synthesis of Fe NPs, offering a cost-effective and environmentally friendly solution for the removal of indole and possibly other similar contaminants from wastewater.
Author Contributions
H.S.: Investigation, methodology, data curation, writing—original draft, funding acquisition; Y.L.: methodology, investigation, data curation, writing—original draft; Y.Z.: methodology, investigation; Z.C.: investigation, writing—review and editing; J.L.: writing—review and editing, project administration, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (No. 22108163).
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Latif, A.; Sheng, D.; Sun, K.; Si, Y.; Azeem, M.; Abbas, A.; Bilal, M. Remediation of heavy metals polluted environment using Fe-based nanoparticles: Mechanisms, influencing factors, and environmental implications. Environ. Pollut. 2020, 264, 114728. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, Y.; Nishiwaki, K.; Suzuki, S.; Shiomi, K.; Nakanishi, I. Determination of cyanide in blood by GC-MS using a new high selectivity derivatization reagent 1,2,3,3-tetramethyl-3H-indolium iodide. Forensic Toxicol. 2022, 40, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, S.; Harini, K.; Gajula, G.P.; Sarmento, B.; Neves-Petersen, M.T.; Thiagarajan, V. Multifunctional magnetic iron oxide nanoparticles: Diverse synthetic approaches, surface modifications, cytotoxicity towards biomedical and industrial applications. BMC Mater. 2019, 1, 1–22. [Google Scholar] [CrossRef]
- Ogbezode, J.E.; Ezealigo, U.S.; Bello, A.; Anye, V.C.; Onwualu, A.P. A narrative review of the synthesis, characterization, and applications of iron oxide nanoparticles. Discov. Nano 2023, 18, 125. [Google Scholar] [CrossRef] [PubMed]
- Huston, M.; DeBella, M.; DiBella, M.; Gupta, A. Green Synthesis of Nanomaterials. Nanomaterials 2021, 11, 2130. [Google Scholar] [CrossRef]
- Singh, N.B.; Jain, P.; De, A.; Tomar, R. Green Synthesis and Applications of Nanomaterials. Curr. Pharm. Biotechnol. 2021, 22, 1705–1747. [Google Scholar] [CrossRef]
- Kumar, V.; Kaushik, N.K.; Tiwari, S.K.; Singh, D.; Singh, B. Green synthesis of iron nanoparticles: Sources and multifarious biotechnological applications. Int. J. Biol. Macromol. 2023, 127017. [Google Scholar] [CrossRef]
- Hasanzadeh, S.; Mortazavi-Derazkola, S.; Khosravi, R. Green synthesis of iron nanoparticles using Pistacia-atlantica leaf extract for enhanced removal of Cr (VI) from aqueous solution. Desalination Water Treat. 2024, 318, 100347. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Liu, Y.; Li, Y.; Zhu, Y.; Dong, Z.; Sun, D.; Lei, D. Green synthesis of iron nanoparticles using mulberry leaf extract: Characterization, identification of active biomolecules, and catalytic activity. Environ. Sci. Pollut. Res. 2024, 31, 20311–20329. [Google Scholar] [CrossRef]
- Yuan, X.; Yu, S.; Xue, N.; Li, T.; Sun, M.; Zhang, L. Activation of iron based persulfate heterogeneous nano catalyst using plant extract for removal of tetrabromobisphenol A from soil. J. Environ. Chem. Eng. 2023, 11, 109493. [Google Scholar] [CrossRef]
- Liu, Y.; Jin, X.; Chen, Z. The formation of iron nanoparticles by Eucalyptus leaf extract and used to remove Cr(VI). Sci. Total Environ. 2018, 627, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, Y.; Jin, X.; Chen, Z. Characterization of iron nanoparticles/reduced graphene oxide composites synthesized by one step eucalyptus leaf extract. Environ. Pollut. 2019, 250, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rasero, C.; Montes-Jimenez, V.; Alexandre-Franco, M.F.; Fernández-González, C.; Píriz-Tercero, J.; Cuerda-Correa, E.M. Use of Zero-Valent Iron Nanoparticles (nZVIs) from Environmentally Friendly Synthesis for the Removal of Dyes from Water-A Review. Water 2024, 16, 1607. [Google Scholar] [CrossRef]
- Patil, S.; Chandrasekaran, R. Biogenic nanoparticles: A comprehensive perspective in synthesis, characterization, application and its challenges. J. Genet. Eng. Biotechnol. 2020, 18, 67. [Google Scholar] [CrossRef]
- Haider, F.U.; Zulfiqar, U.; Ain, N.; Hussain, S.; Maqsood, M.F.; Ejaz, M.; Yong, J.W.H.; Li, Y. Harnessing plant extracts for eco-friendly synthesis of iron nanoparticle (Fe-NPs): Characterization and their potential applications for ameliorating environmental pollutants. Ecotoxicol. Environ. Saf. 2024, 281, 116620. [Google Scholar] [CrossRef]
- Huber, N.; Sirim, M.; Qian, Z.; Ferguson, C.T.J.; Wei, W.; Zhang, K.A.I. Water-Compatible Poly (methyl methacrylate) Networks for Visible Light-Driven Photocatalytic Pollutant Remediation in Aqueous Medium. ACS Appl. Polym. Mater. 2022, 4, 5728–5736. [Google Scholar] [CrossRef]
- Lv, X.; Ma, Y.; Li, Y.; Yang, Q. Heterogeneous Fenton-Like Catalytic Degradation of 2,4-Dichlorophenoxyacetic Acid by Nano-Scale Zero-Valent Iron Assembled on Magnetite Nanoparticles. Water 2020, 12, 2909. [Google Scholar] [CrossRef]
- Aly, S.H.; Eldahshan, O.A.; Al-Rashood, S.T.; Binjubair, F.A.; El Hassab, M.A.; Eldehna, W.M.; Dall’Acqua, S.; Zengin, G. Chemical Constituents, Antioxidant, and Enzyme Inhibitory Activities Supported by In-Silico Study of n-Hexane Extract and Essential Oil of Guava Leaves. Molecules 2022, 27, 8979. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, J.; Yuan, L.; Wu, F.; Xie, L.; Yan, X.; Li, H.; Li, Y.; Shi, L.; Hu, R.; et al. Neighboring Carboxylic Acid Boosts Peroxidase-Like Property of Metal-Phenolic Nano-Networks in Eradicating Streptococcus mutans Biofilms. Small 2023, 19, 2206657. [Google Scholar] [CrossRef]
- Hu, Y.; Zhou, S.; Pan, X.; Zhou, F.; Sun, Y.; Liu, M.; Zhang, D.; Zhang, L. Fe Nanoparticles Synthesized by Pomegranate Leaves for Treatment of Malachite Green. J. Wuhan Univ. Technol. Mat. Sci. Edit. 2022, 37, 350–354. [Google Scholar] [CrossRef]
- Machado, S.; Pinto, S.L.; Grosso, J.P.; Nouws, H.P.A.; Albergaria, J.T.; Delerue-Matos, C. Green production of zero-valent iron nanoparticles using tree leaf extracts. Sci. Total Environ. 2013, 445, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Duran-Alvarez, J.C.; Prado, B.; Zanella, R.; Rodriguez, M.; Diaz, S. Wastewater surveillance of pharmaceuticals during the COVID-19 pandemic in Mexico City and the Mezquital Valley: A comprehensive environmental risk assessment. Sci. Total Environ. 2023, 900, 165886. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Yang, X.; Zhu, X.; Zhao, S.; Yu, J.; Wang, D.; Yang, M. The variation of odor characteristics of wastewater sludge treated by advanced anaerobic digestion (AAD) and the contribution pattern of key odorants. Sci. Total Environ. 2022, 840, 156722. [Google Scholar] [CrossRef] [PubMed]
- Hwang, O.; Scoggin, K.; Andersen, D.; Ro, K.; Trabue, S. Swine manure dilution with lagoon effluent impact on odor reduction and manure digestion. J. Environ. Qual. 2021, 50, 336–349. [Google Scholar] [CrossRef]
- Rtimi, S.; Robyr, M.; Pulgarin, C.; Lavanchy, J.C.; Kiwi, J. A New Perspective in the Use of FeOx-TiO2 Photocatalytic Films: Indole Degradation in the Absence of Fe-Leaching. J. Catal. 2016, 342, 184–192. [Google Scholar] [CrossRef]
- Jin, G. Determination of Metacresol and Its Related Substances by Gas Chromatography. Chem. World 2016, 57, 8–11. (In Chinese) [Google Scholar]
- Kashi, R.; Bagheri-Mohagheghi, M.M.; Khorshidi, M. Synthesis and study of structural, optical, and antibacterial properties of silver, copper, and iron metallic nanoparticles prepared by green synthesis. Appl. Nanosci. 2022, 13, 4343–4360. [Google Scholar] [CrossRef]
- Krishna, D.N.G.; Anushree, C.; George, R.P.; Philip, J. Phase identification in binary mixture of nanopowders from deconvoluted valence band spectra using X-ray photoelectron spectroscopy: Case study with iron oxide and titania polymorphs. Appl. Surf. Sci. 2018, 462, 932–943. [Google Scholar] [CrossRef]
- Pham, D.M.; Miyata, Y.; Awata, T.; Nakatake, M.; Zhang, C.F.; Kanda, K.; Ogawa, S.; Ohta, S.; Yagi, S.; Katayama, A. Development of sample preparation technique to characterize chemical structure of humin by synchrotron-radiation–based X-ray photoelectron spectroscopy. Surf. Interface Anal. 2018, 51, 226–233. [Google Scholar] [CrossRef]
- Michalak, I.; Baśladyńska, S.; Mokrzycki, J.; Rutkowski, P. Biochar from A Freshwater Macroalga as A Potential Biosorbent for Wastewater Treatment. Water 2019, 11, 1390. [Google Scholar] [CrossRef]
- Gamulin, O.; Krajacic, M.; Oroz, K.; Coric, L.; Dretar, V.; Strbe, S.; Seiwerth, S.; Sikiric, P. FTIR spectroscopy reveals molecular changes in BPC 157 treated blood vessels. FASEB J. 2022, 36, R4994. [Google Scholar] [CrossRef]
- Ezeako, E.C.; Nworah, F.N.; Osuji, D.O. Phytocompounds, antioxidant potential, and inhibitory actions of ethanolic leaf fraction of Sida linifolia Linn. (Malvaceae) on enzymes linked to inflammation, diabetes, and neurological disorders. Future J. Pharm. Sci. 2023, 9, 73. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Yang, Y.; Wei, H.; Xue, L.; Zhao, M.; Cai, J. Optimization of combined microwave and hot air drying technology for purple cabbage by Response Surface Methodology (RSM). Food Sci. Nutr. 2021, 9, 4568–4577. [Google Scholar] [CrossRef] [PubMed]
- Venditto, N.J.; Liang, Y.S.; El Mokadem, R.K.; Nicewicz, D.A. Ketone-Olefin Coupling of Aliphatic and Aromatic Carbonyls Catalyzed by Excited-State Acridine Radicals. J. Am. Chem. Soc. 2022, 144, 11888–11896. [Google Scholar] [CrossRef]
- Wu, J.; Wu, P.; Weng, X.; Lin, J.; Owens, G.; Chen, Z. Mechanistic insight into the one step green synthesis of hybrid rGO/Fe NPs. Mater. Today Nano 2022, 18, 100193. [Google Scholar] [CrossRef]
- Oh, S.Y.; Seo, Y.D.; Ryu, K.S. Reductive removal of 2,4-dinitrotoluene and 2,4-dichlorophenol with zero-valent iron-included biochar. Bioresour. Technol. 2016, 216, 1014–1021. [Google Scholar] [CrossRef]
- Jin, X.; Wang, Z.; Hong, R.; Chen, Z.; Wu, B.; Ding, S.; Zhu, W.; Lin, Y.; Gu, C. Supramolecular assemblies of a newly developed indole derivative for selective adsorption and photo-destruction of perfluoroalkyl substances. Water Res. 2022, 225, 119147. [Google Scholar] [CrossRef]
- Venkateshaiah, A.; Silvestri, D.; Wacławek, S.; Ramakrishnan, R.K.; Krawczyk, K.; Saravanan, P.; Pawlyta, M.; Padil, V.V.; Černík, M.; Dionysiou, D.D. A comparative study of the degradation efficiency of chlorinated organic compounds by bimetallic zero-valent iron nanoparticles. Environ. Sci. Water Res. Technol. 2022, 8, 162–172. [Google Scholar] [CrossRef]
- Zhu, G.; Bian, Y.; Hursthouse, A.S.; Xu, S.; Xiong, N.; Wan, P. The role of magnetic MOFs nanoparticles in enhanced iron coagulation of aquatic dissolved organic matter. Chemosphere 2020, 247, 125921. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).