Biochar Weakens the Efficiency of Nitrification Inhibitors and Urease Inhibitors in Mitigating Greenhouse Gas Emissions from Soil Irrigated with Alternative Water Resources
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Soil, Water, and Exogenous Substances
2.3. Incubation Experiment
2.4. Gas Sampling
2.5. Measurement of Soil Properties
2.6. Data Analysis
3. Results
3.1. Soil NH4+-N and NO3−-N
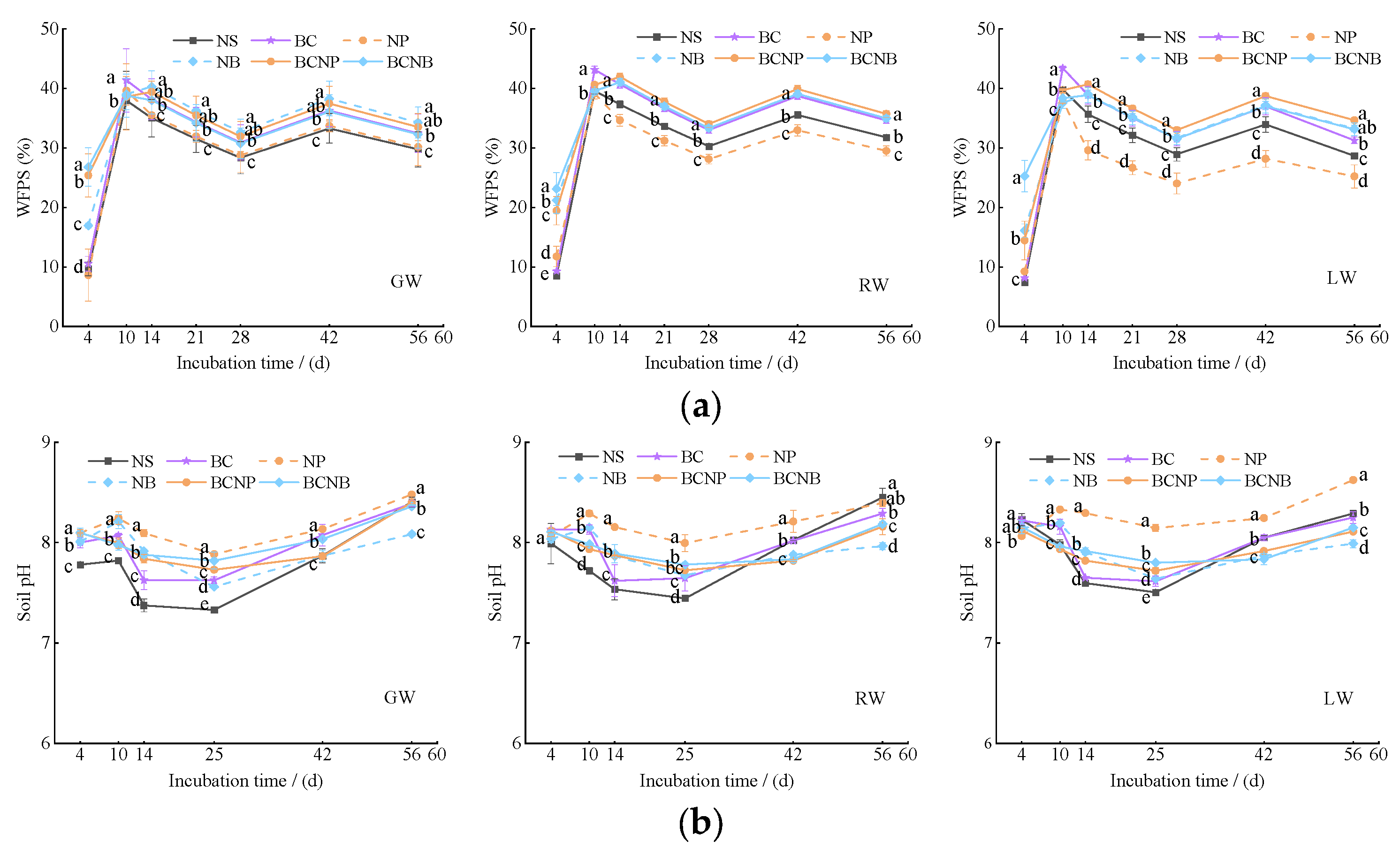
3.2. Soil Moisture and pH
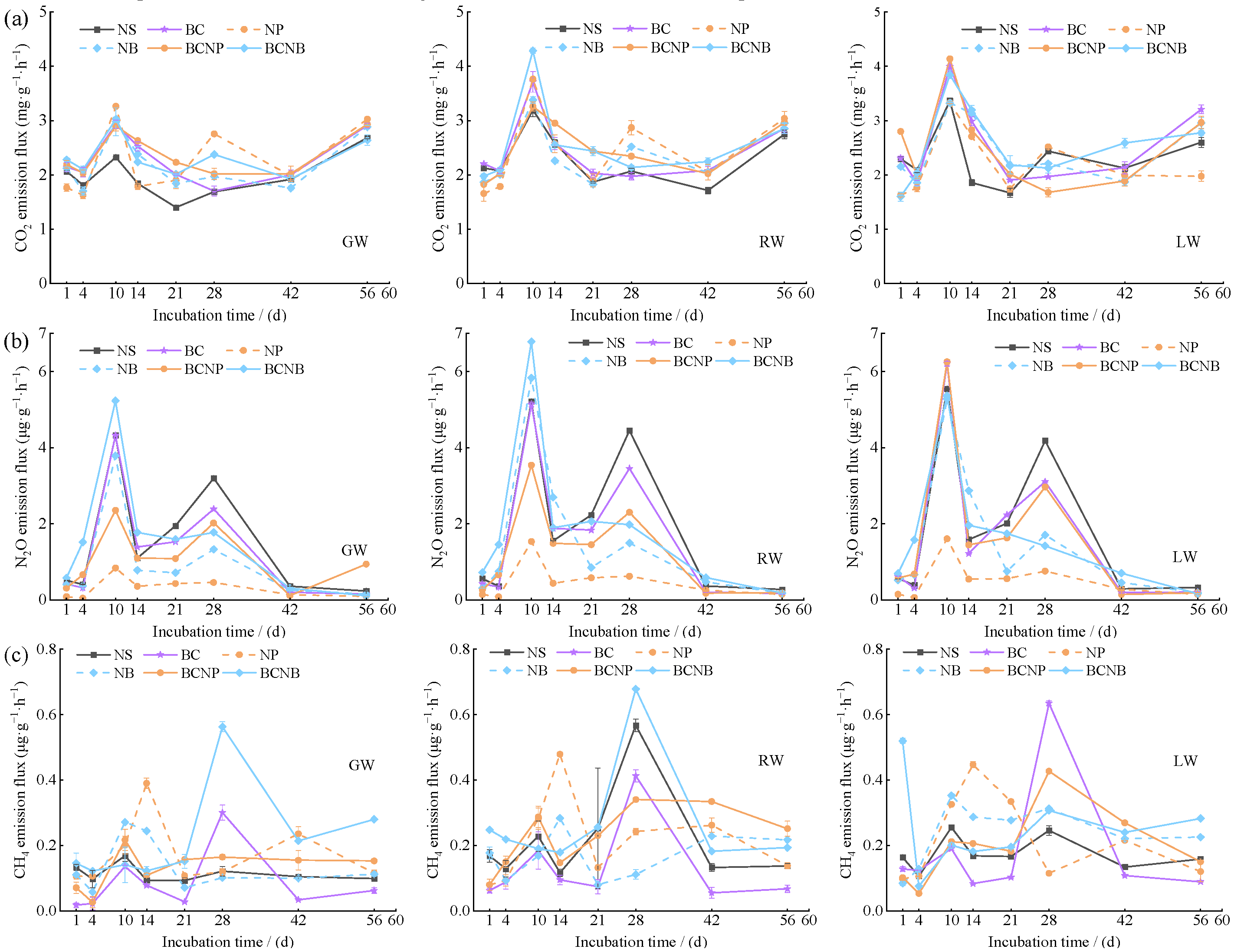
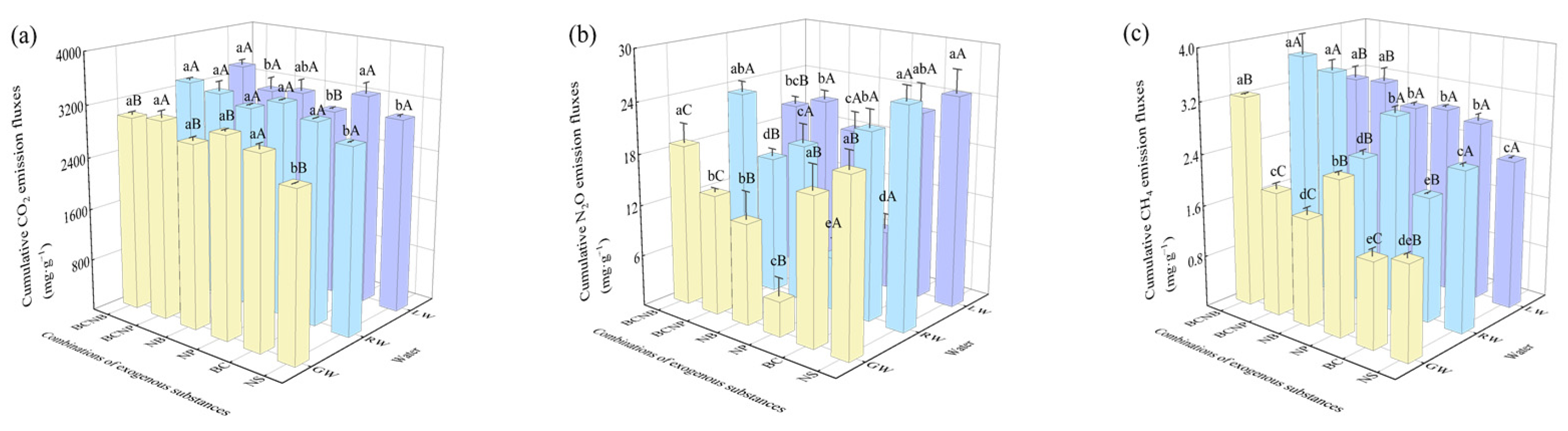
3.3. CO2, N2O, and CH4 Emissions
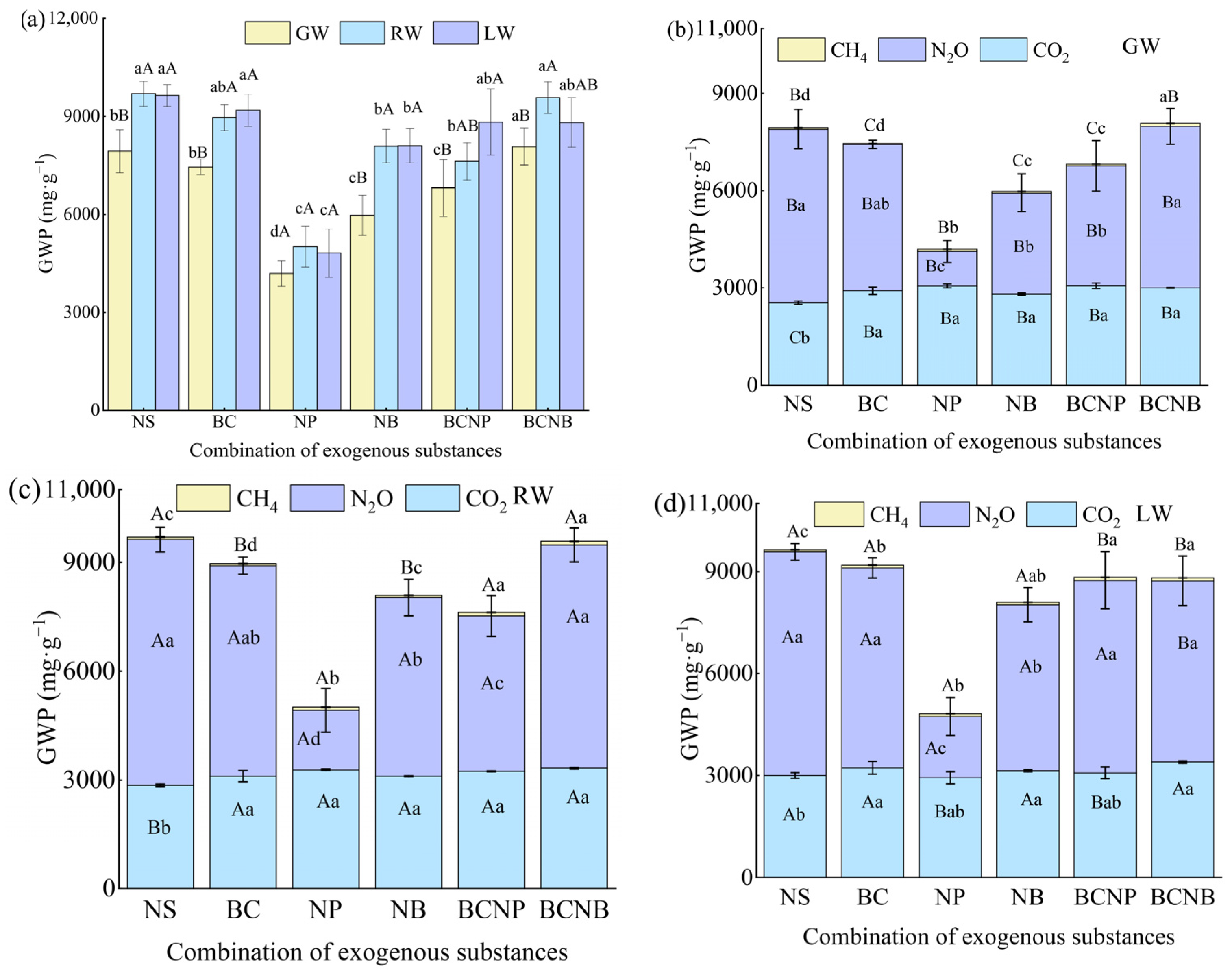
3.4. GWP
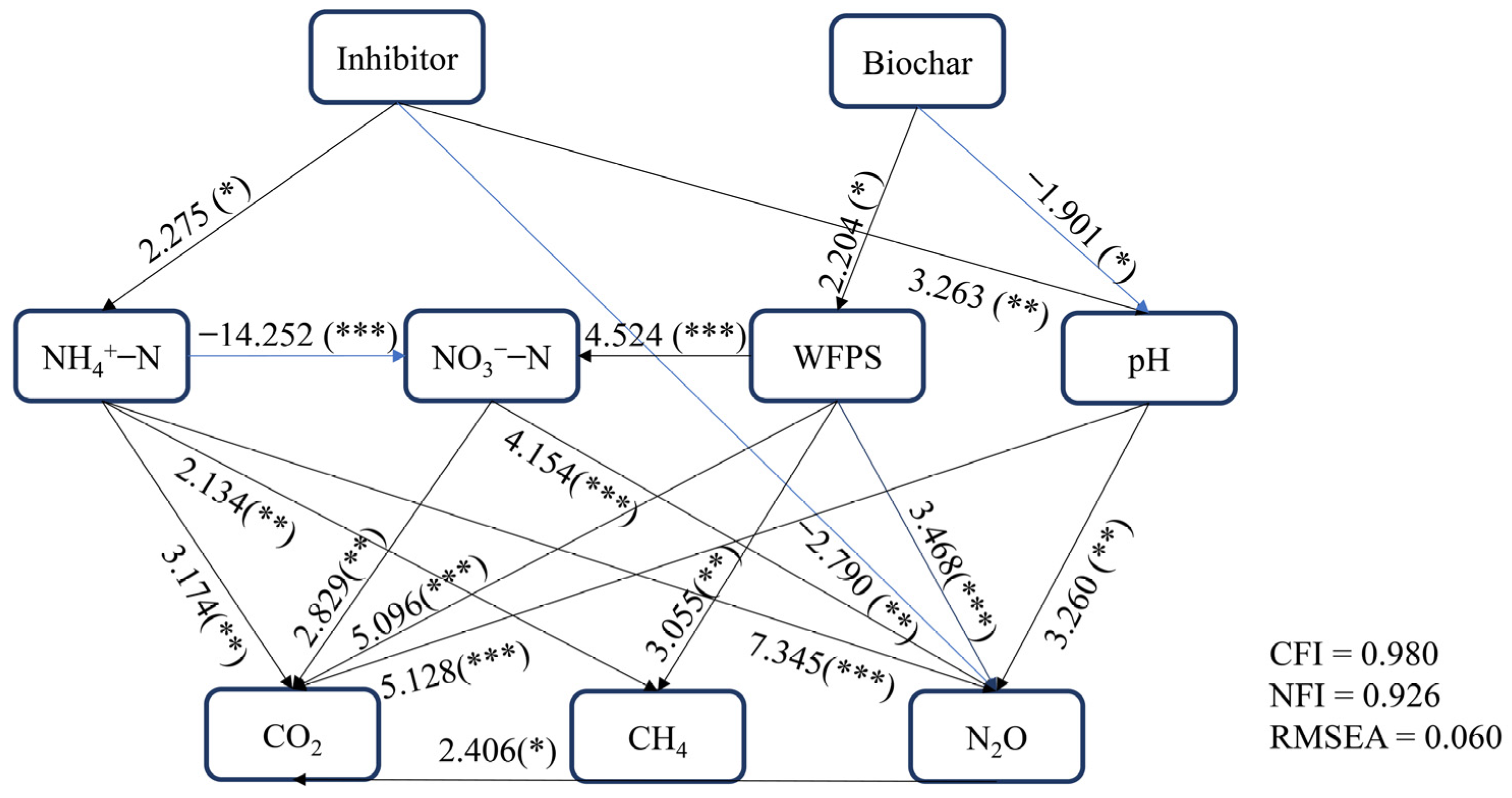
3.5. Connections between Soil Characteristics and Soil Gas Emissions
4. Discussion
4.1. Impact of Biochar Application Alone on CO2, N2O, and CH4 Emissions under Varying Water Resources
4.2. Influence of Inhibitors on CO2, N2O, and CH4 Emissions under Varying Water Resources
4.3. Effects of Co-Application of Inhibitors and Biochar on CO2, N2O, and CH4 Emissions under Varying Water Resources
4.4. Influence of Exogenous Substances and Water Resources on GWP
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2014: Synthesis Report. In Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014; p. 151. [Google Scholar]
- Sánchez, M.L.; Ozores, M.I.; López, M.J.; Colle, R.; De Torre, B.; García, M.A.; Pérez, I. Soil CO2 fluxes beneath barley on the central Spanish plateau. Agric. For. Meteorol. 2003, 118, 85–95. [Google Scholar] [CrossRef]
- Sutton, M.A.; Bleeker, A.; Howard, C.; Erisman, J.W.; Abrol, Y.P.; Bekunda, M.; Datta, A.; Davidson, E.; Vries, W.; Oenema, O.; et al. Our Nutrient World. The Challenge to Produce More Food & Energy with Less Pollution. Global Overview of Nutrient Management. 2013. Available online: https://nora.nerc.ac.uk/id/eprint/500700/1/N500700BK.pdf (accessed on 23 May 2024).
- Watanabe, T.; Kimura, M.; Asakawa, S. Dynamics of methanogenic archaeal communities based on rRNA analysis and their relation to methanogenic activity in Japanese paddy field soils. Soil Biol. Biochem. 2007, 39, 2877–2887. [Google Scholar] [CrossRef]
- Dragonetti, G.; Khadra, R.; Daccache, A.; Oubelkacem, A.; Choukr-Allah, R.; Lamaddalena, N. Development and Application of a Predictive Model for Treated Wastewater Irrigation Management in a Semiarid Area. Integr. Environ. Assess. Manag. 2020, 16, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Evanylo, G.; Ervin, E.; Zhang, X. Reclaimed Water for Turfgrass Irrigation. Water 2010, 2, 685–701. [Google Scholar] [CrossRef]
- Zhang, S.; Yao, H.; Lu, Y.; Shan, D.; Yu, X. Reclaimed Water Irrigation Effect on Agricultural Soil and Maize (Zea mays L.) in Northern China. CLEAN-Soil Air Water 2018, 46, 1800037. [Google Scholar] [CrossRef]
- Li, J.; Sun, Y.; Wang, X.; Xu, S. Changes in Microbial Community Structures under Reclaimed Water Replenishment Conditions. Int. J. Environ. Res. Public Health 2020, 17, 1174. [Google Scholar] [CrossRef]
- Li, S.; Tao, Z.; Liu, Y.; Li, S.; Kama, R.; Hu, C.; Fan, X.; Li, Z. Influence of Swine Wastewater Irrigation and Straw Return on the Accumulation of Selected Metallic Elements in Soil and Plants. Agriculture 2024, 14, 317. [Google Scholar] [CrossRef]
- Cui, B.J.; Hu, C.; Fan, X.Y.; Cui, E.P.; Li, Z.Y.; Ma, H.H.; Gao, F. Changes of endophytic bacterial community and pathogens in pepper (Capsicum annuum L.) as affected by reclaimed water irrigation. Appl. Soil Ecol. 2020, 156, 103627. [Google Scholar] [CrossRef]
- Cui, E.P.; Fan, X.Y.; Hu, C.; Neal, A.L.; Cui, B.J.; Liu, C.C.; Gao, F. Reduction effect of individual N, P, K fertilization on antibiotic resistance genes in reclaimed water irrigated soil. Ecotoxicol. Environ. Saf. 2022, 231, 113185. [Google Scholar] [CrossRef]
- Liu, J.; Yang, F.; Cai, Y.; Lu, G.; Li, Y.; Li, M.; Fan, L.; Gao, L. Unveiling the existence and ecological hazards of trace organic pollutants in wastewater treatment plant effluents across China. Eco-Environ. Health 2024, 3, 21–29. [Google Scholar] [CrossRef]
- Mukhametov, A.; Kondrashev, S.; Zvyagin, G.; Spitsov, D. Treated livestock wastewater influence on soil quality and possibilities of crop irrigation. Saudi J. Biol. Sci. 2022, 29, 2766–2771. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Neal, A.L.; Zhang, X.; Cui, E.; Gao, F.; Fan, X.; Hu, C.; Li, Z. Increasing livestock wastewater application in alternate-furrow irrigation reduces nitrification gene abundance but not nitrification rate in rhizosphere. Biol. Fertil. Soils 2019, 55, 439–455. [Google Scholar] [CrossRef]
- Xiang, M.; Li, Y.; Han, H.; Chen, M.; Liao, B.; Cui, Y. Effects of livestock wastewater irrigation on growth, yield, and water and nitrogen use efficiency of garlic. J. Irrig. Drain. 2024, 43, 16–21+54. [Google Scholar] [CrossRef]
- Guo, W.; Qi, X.; Xiao, Y.; Li, P.; Andersen, M.N.; Zhang, Y.; Zhao, Z. Effects of Reclaimed Water Irrigation on Microbial Diversity and Composition of Soil with Reducing Nitrogen Fertilization. Water 2018, 10, 365. [Google Scholar] [CrossRef]
- Kama, R.; Liu, Y.; Zhao, S.; Hamani, A.K.M.; Song, J.; Cui, B.; Aidara, M.; Liu, C.; Li, Z. Combination of intercropping maize and soybean with root exudate additions reduces metal mobility in soil-plant system under wastewater irrigation. Ecotoxicol. Environ. Saf. 2023, 266, 115549. [Google Scholar] [CrossRef]
- Tao, Z.; Liu, Y.; Li, S.; Li, B.; Fan, X.; Liu, C.; Hu, C.; Liu, H.; Li, Z. Global warming potential assessment under reclaimed water and livestock wastewater irrigation coupled with co-application of inhibitors and biochar. J. Environ. Manag. 2024, 353, 120143. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Yang, P.; Ren, S.; Ma, N.; Yang, J.; Xu, Y. Effects of fertilizer types and water quality on carbon dioxide emissions from soil in wheat-maize rotations. Sci. Total Environ. 2020, 698, 134010. [Google Scholar] [CrossRef]
- Singh, J.; Kunhikrishnan, A.; Bolan, N.S.; Saggar, S. Impact of urease inhibitor on ammonia and nitrous oxide emissions from temperate pasture soil cores receiving urea fertilizer and cattle urine. Sci. Total Environ. 2013, 465, 56–63. [Google Scholar] [CrossRef]
- Vannelli, T.; Hooper Alan, B. Oxidation of Nitrapyrin to 6-Chloropicolinic Acid by the Ammonia-Oxidizing Bacterium Nitrosomonas europaea. Appl. Environ. Microbiol. 1992, 58, 2321–2325. [Google Scholar] [CrossRef]
- Shaaban, M.; Khalid, M.S.; Hu, R.; Zhou, M. Effects of water regimes on soil N2O, CH4 and CO2 emissions following addition of dicyandiamide and N fertilizer. Environ. Res. 2022, 212, 113544. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, H.; Fan, J.; Zhang, F.; Guo, J.; Liao, Z.; Zhuang, Q. Wheat straw mulching with nitrification inhibitor application improves grain yield and economic benefit while mitigating gaseous emissions from a dryland maize field in northwest China. Field Crops Res. 2021, 265, 108125. [Google Scholar] [CrossRef]
- Xu, X.; Wu, Z.; Dong, Y.; Zhou, Z.; Xiong, Z. Effects of nitrogen and biochar amendment on soil methane concentration profiles and diffusion in a rice-wheat annual rotation system. Sci. Rep. 2016, 6, 38688. [Google Scholar] [CrossRef]
- Sial, T.A.; Shaheen, S.M.; Lan, Z.; Korai, P.K.; Ghani, M.I.; Khan, M.N.; Syed, A.-u.-A.; Hussain Asghar Ali, M.N.; Rajpar, I.; Memon, M.; et al. Addition of walnut shells biochar to alkaline arable soil caused contradictory effects on CO2 and N2O emissions, nutrients availability, and enzymes activity. Chemosphere 2022, 293, 133476. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.; Greenberg, I.; Ludwig, B.; Hippich, L.; Fischer, D.; Glaser, B.; Kaiser, M. Effect of biochar and compost on soil properties and organic matter in aggregate size fractions under field conditions. Agric. Ecosyst. Environ. 2020, 295, 106882. [Google Scholar] [CrossRef]
- Arif, M.; Jan, T.; Riaz, M.; Fahad, S.; Adnan, M.; Amanullah; Ali, K.; Mian, I.A.; Khan, B.; Rasul, F.; et al. Environment, Climate, Plant and Vegetation Growth; Fahad, S., Hasanuzzaman, M., Alam, M., Ullah, H., Saeed, M., Ali Khan, I., Adnan, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 151–171. [Google Scholar]
- Walkiewicz, A.; Kalinichenko, K.; Kubaczyński, A.; Brzezińska, M.; Bieganowski, A. Usage of biochar for mitigation of CO2 emission and enhancement of CH4 consumption in forest and orchard Haplic Luvisol (Siltic) soils. Appl. Soil Ecol. 2020, 156, 103711. [Google Scholar] [CrossRef]
- Mikhael, J.E.R.; Wang, J.J.; Dodla, S.; Scaglia, G.; Dattamudi, S. Effects of biochar and N-stabilizers on greenhouse gas emissions from a subtropical pasture field applied with organic and inorganic nitrogen fertilizers. J. Environ. Manag. 2022, 306, 114423. [Google Scholar] [CrossRef]
- Nan, Q.; Xin, L.; Qin, Y.; Waqas, M.; Wu, W. Exploring long-term effects of biochar on mitigating methane emissions from paddy soil: A review. Biochar 2021, 3, 125–134. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, Y.; Xu, Z.; Yu, Z. How does biochar amendment affect soil methane oxidation? A review. J. Soils Sediments 2021, 21, 1575–1586. [Google Scholar] [CrossRef]
- Liang, B.; Lehmann, J.; Solomon, D.; Kinyangi, J.; Grossman, J.; O’Neill, B.; Skjemstad, J.O.; Thies, J.; Luizão, F.J.; Petersen, J.; et al. Black Carbon Increases Cation Exchange Capacity in Soils. Soil Sci. Soc. Am. J. 2006, 70, 1719–1730. [Google Scholar] [CrossRef]
- He, T.; Yuan, J.; Xiang, J.; Lin, Y.; Luo, J.; Lindsey, S.; Liao, X.; Liu, D.; Ding, W. Combined biochar and double inhibitor application offsets NH3 and N2O emissions and mitigates N leaching in paddy fields. Environ. Pollut. 2022, 292, 118344. [Google Scholar] [CrossRef]
- Pokharel, P.; Chang, S.X. Biochar decreases the efficacy of the nitrification inhibitor nitrapyrin in mitigating nitrous oxide emissions at different soil moisture levels. J. Environ. Manag. 2021, 295, 113080. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Leng, Y.; Zhang, Q.; Zhao, X.; Gao, W.; Duan, P.; Li, Z.; Luo, G.; Zhang, W.; Chen, M.; et al. Synergistically mitigating nitric oxide emission by co-applications of biochar and nitrification inhibitor in a tropical agricultural soil. Environ. Res. 2022, 214, 113989. [Google Scholar] [CrossRef]
- He, T.; Liu, D.; Yuan, J.; Luo, J.; Lindsey, S.; Bolan, N.; Ding, W. Effects of application of inhibitors and biochar to fertilizer on gaseous nitrogen emissions from an intensively managed wheat field. Sci. Total Environ. 2018, 628–629, 121–130. [Google Scholar] [CrossRef]
- Deng, B.; Shen, F.; Guo, X.; Siemann, E.; Zhang, L. Urease inhibitor and biochar independently affected N2O emissions from Camellia oleifera soils. Plant Soil Environ. 2022, 68, 424–430. [Google Scholar] [CrossRef]
- He, T.; Yuan, J.; Luo, J.; Lindsey, S.; Xiang, J.; Lin, Y.; Liu, D.; Chen, Z.; Ding, W. Combined application of biochar with urease and nitrification inhibitors have synergistic effects on mitigating CH4 emissions in rice field: A three-year study. Sci. Total Environ. 2020, 743, 140500. [Google Scholar] [CrossRef]
- GB 5084-2021; Standard for Irrigation Water Quality. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China. Available online: https://english.mee.gov.cn/Resources/standards/water_environment/quality_standard/200710/t20071024_111789.shtml (accessed on 1 July 2021).
- Keiblinger, K.M.; Zehetner, F.; Mentler, A.; Zechmeister-Boltenstern, S. Biochar application increases sorption of nitrification inhibitor 3,4-dimethylpyrazole phosphate in soil. Environ. Sci. Pollut. Res. 2018, 25, 11173–11177. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.H.H.; Wang, J.; Hao, X.; Thomas, B.W. Modeling the effect of wastewater irrigation on soil salinity using a SALT-DNDC model. Land Degrad. Dev. 2022, 33, 55–67. [Google Scholar] [CrossRef]
- Lamsal, K.; Paudyal, G.N.; Saeed, M. Model for assessing impact of salinity on soil water availability and crop yield. Agric. Water Manag. 1999, 41, 57–70. [Google Scholar] [CrossRef]
- Hütsch, B.W. Methane oxidation in soils of two long-term fertilization experiments in Germany. Soil Biol. Biochem. 1996, 28, 773–782. [Google Scholar] [CrossRef]
- Razzaghi, F.; Obour, P.B.; Arthur, E. Does biochar improve soil water retention? A systematic review and meta-analysis. Geoderma 2020, 361, 114055. [Google Scholar] [CrossRef]
- Sun, F.F.; Lu, S.G. Biochars improve aggregate stability, water retention, and pore- space properties of clayey soil. J. Plant Nutr. Soil Sci. 2014, 177, 26–33. [Google Scholar] [CrossRef]
- Hussain, R.; Ravi, K.; Garg, A. Influence of biochar on the soil water retention characteristics (SWRC): Potential application in geotechnical engineering structures. Soil Tillage Res. 2020, 204, 104713. [Google Scholar] [CrossRef]
- Chen, X.; Cai, Q.; Gao, H.; Shan, S. Effects of Biochar on Water Movement in Coastal Saline-alkali Soil. J. Irrig. Drain. 2023, 42, 33–38. [Google Scholar] [CrossRef]
- Herbst, M.; Tappe, W.; Kummer, S.; Vereecken, H. The impact of sieving on heterotrophic respiration response to water content in loamy and sandy topsoils. Geoderma 2016, 272, 73–82. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, Y.; Huang, P.; Li, Z.; Zhang, X. A new concept for modelling the moisture dependence of heterotrophic soil respiration. Soil Biol. Biochem. 2023, 185, 109147. [Google Scholar] [CrossRef]
- Shen, Y.; Zhu, L.; Cheng, H.; Yue, S.; Li, S. Effects of Biochar Application on CO2 Emissions from a Cultivated Soil under Semiarid Climate Conditions in Northwest China. Sustainability 2017, 9, 1482. [Google Scholar] [CrossRef]
- Zhang, L.; Jing, Y.; Xiang, Y.; Zhang, R.; Lu, H. Responses of soil microbial community structure changes and activities to biochar addition: A meta-analysis. Sci. Total Environ. 2018, 643, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Cross, A.; Sohi, S.P. The priming potential of biochar products in relation to labile carbon contents and soil organic matter status. Soil Biol. Biochem. 2011, 43, 2127–2134. [Google Scholar] [CrossRef]
- Li, J.; Kwak, J.-H.; Chang, S.X.; Gong, X.; An, Z.; Chen, J. Greenhouse Gas Emissions from Forest Soils Reduced by Straw Biochar and Nitrapyrin Applications. Land 2021, 10, 189. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, C.; Liu, X.; Wang, X.; Zhou, D. Nonmicrobial mechanisms dominate the release of CO2 and the decomposition of organic matter during the short-term redox process in paddy soil slurry. Eco-Environ. Health 2023, 2, 227–234. [Google Scholar] [CrossRef]
- Yang, X.; Hou, R.; Fu, Q.; Li, T.; Wang, J.; Su, Z.; Shen, W.; Zhou, W.; Wang, Y. Effect of freeze-thaw cycles and biochar coupling on the soil water-soil environment, nitrogen adsorption and N2O emissions in seasonally frozen regions. Sci. Total Environ. 2023, 893, 164845. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R. N cycling in soils and emission of nitrogen gases: How well do we understand the processes and their controls (Vladimir Ivanovich Vernadsky Medal Lecture). In Proceedings of the EGU General Assembly Conference Abstracts, Vienna, Austria, 27 April–2 May 2014; p. 16536. [Google Scholar]
- Weldon, S.; Van der Veen, B.; Farkas, E.; Kocatürk-Schumacher, N.P.; Dieguez-Alonso, A.; Budai, A.; Rasse, D. A re-analysis of NH4+ sorption on biochar: Have expectations been too high? Chemosphere 2022, 301, 134662. [Google Scholar] [CrossRef] [PubMed]
- Vannelli, T.; Hooper, A.B. Reductive dehalogenation of the trichloromethyl group of nitrapyrin by the ammonia-oxidizing bacterium Nitrosomonas europaea. Appl. Environ. Microbiol. 1993, 59, 3597–3601. [Google Scholar] [CrossRef] [PubMed]
- Manunza, B.; Deiana, S.; Pintore, M.; Gessa, C. The binding mechanism of urea, hydroxamic acid and N-(N-butyl)-phosphoric triamide to the urease active site. A comparative molecular dynamics study. Soil Biol. Biochem. 1999, 31, 789–796. [Google Scholar] [CrossRef]
- Cantarella, H.; Otto, R.; Soares, J.R.; Silva, A.G.d.B. Agronomic efficiency of NBPT as a urease inhibitor: A review. J. Adv. Res. 2018, 13, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Borzouei, A.; Karimzadeh, H.; Müller, C.; Sanz-Cobena, A.; Zaman, M.; Kim, D.-G.; Ding, W. Author Correction: Relationship between nitrapyrin and varying nitrogen application rates with nitrous oxide emissions and nitrogen use efficiency in a maize field. Sci. Rep. 2023, 13, 1694. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, L.; Douglass, E.J.S.B. Metabolism of the urease inhibitor N-(n-butyl) thiophosphoric triamide (NBPT) in soils. Biochemistry 1993, 25, 1613–1618. [Google Scholar] [CrossRef]
- Reth, S.; Reichstein, M.; Falge, E. The effect of soil water content, soil temperature, soil pH-value and the root mass on soil CO2efflux—A modified model. Plant Soil 2005, 268, 21–33. [Google Scholar] [CrossRef]
- Lv, Y.; Guan, X.Y.; Ruan, B.Q.; Wang, Y.W. Multifractal characteristics of soil particle size distribution under sewage irrigation in different irrigation years. Appl. Mech. Mater. 2015, 700, 205–210. [Google Scholar] [CrossRef]
- Leuther, F.; Schlüter, S.; Wallach, R.; Vogel, H.-J. Structure and hydraulic properties in soils under long-term irrigation with treated wastewater. Geoderma 2019, 333, 90–98. [Google Scholar] [CrossRef]
- Chen, H.; Yin, C.; Fan, X.; Ye, M.; Peng, H.; Li, T.; Zhao, Y.; Wakelin, S.A.; Chu, G.; Liang, Y. Reduction of N2O emission by biochar and/or 3,4-dimethylpyrazole phosphate (DMPP) is closely linked to soil ammonia oxidizing bacteria and nosZI-N2O reducer populations. Sci. Total Environ. 2019, 694, 133658. [Google Scholar] [CrossRef]
- He, T.; Liu, D.; Yuan, J.; Ni, K.; Zaman, M.; Luo, J.; Lindsey, S.; Ding, W. A two years study on the combined effects of biochar and inhibitors on ammonia volatilization in an intensively managed rice field. Agric. Ecosyst. Environ. 2018, 264, 44–53. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, K.; Liu, J.; Chen, Y.; Han, L. Changes in soil properties and CO2 emissions after biochar addition: Role of pyrolysis temperature and aging. Sci. Total Environ. 2022, 839, 156333. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Kang, Y.; Wang, W.; Xu, L.; Liu, J.; Zhang, Z.; Wu, H. Low-density polyethylene microplastics and biochar interactively affect greenhouse gas emissions and microbial community structure and function in paddy soil. Chemosphere 2023, 340, 139860. [Google Scholar] [CrossRef] [PubMed]
- Hallam, S.J.; Girguis, P.R.; Preston, C.M.; Richardson, P.M.; De Long, E.F. Identification of methyl coenzyme M reductase A (mcrA) genes associated with methane-oxidizing archaea. Appl Env. Microbiol 2003, 69, 5483–5491. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, F.; Xu, H.; Feng, D.; Liu, J.; Han, G. Biochar promotes methane production at high acetate concentrations in anaerobic soils. Environ. Chem. Lett. 2019, 17, 1347–1352. [Google Scholar] [CrossRef]


| Water Type | COD (mg·L−1) | TN (mg·L−1) | TP (mg·L−1) | pH | NH4+-N (mg·L−1) | NO3−-N (mg·L−1) | TOC (mg·L−1) | K+ (mg·L−1) | EC (μS·cm−1) |
|---|---|---|---|---|---|---|---|---|---|
| Groundwater | - | 2.2 | 0.8 | 8.3 | 1 | 0.9 | 74.9 | 2.9 | 1570 |
| Reclaimed water | - | 15.8 | 0.8 | 8.3 | 7.2 | 6.7 | 27.1 | 10.7 | 1115 |
| Livestock wastewater (diluted) | 215.4 | 23.9 | 13.2 | 8.1 | 12.3 | 10.4 | 5.6 | 33.2 | 250 |
| Factor | Greenhouse Gas Emission Flux | ||
|---|---|---|---|
| CO2 | N2O | CH4 | |
| CO2 | 1 | ||
| N2O | 0.429 ** | 1 | |
| CH4 | 0.503 ** | −0.332 * | 1 |
| NH4+-N | 0.344 * | 0.682 ** | −0.298 |
| NO3−-N | 0.268 | 0.281 | 0.135 |
| pH | 0.161 | 0.235 | −0.251 |
| WFPS | 0.364 * | 0.423 * | 0.337 * |
| Equation | R2 | p | Equation | R2 | p | ||||
|---|---|---|---|---|---|---|---|---|---|
| NS | CO2 | y = −56.13 + 11.26 WFPS | 0.331 | <0.001 | BC | CO2 | y = −8.34 + 0.38 WFPS + 1.21 pH | 0.455 | 0.004 |
| y = −10.86 + 0.04 WFPS + 1.44 pH + 0.01 NH4+ | 0.647 | <0.001 | |||||||
| N2O | y = 0.65 − 0.08 pH | 0.201 | 0.035 | N2O | y = 0.1 + 0.04 NH4+ | 0.262 | 0.03 | ||
| y = −3.64 + 0.05 NH4+ + 0.11 WFPS | 0.608 | 0.001 | |||||||
| CH4 | y = −4.92 − 0.59 pH | 0.371 | <0.001 | CH4 | y = 2.53 − 0.29 pH + 0.06 WFPS | 0.219 | 0.029 | ||
| NP | CO2 | y = 21.86 + 0.08 WFPS | 0.385 | 0.004 | NB | CO2 | y = 21.790 + 0.632 WFPS | 0.192 | 0.039 |
| y = −190.239 + 0.707 WFPS + 26.381 pH | 0.406 | 0.008 | |||||||
| N2O | y = +0.01 + 0.01 WFPS | 0.296 | 0.011 | N2O | y = −0.002 + 0.001 NH4+ | 0.305 | <0.01 | ||
| y = −0.04 + 0.01 WFPS + 0.02 NH4+ | 0.65 | <0.001 | y = −0.145 + 0.002 NH4+ + 0.004 WFPS | 0.652 | <0.001 | ||||
| CH4 | y = 0.323 + 1.16 NH4+ | 0.277 | 0.01 | CH4 | y = −0.930 + 0.125 WFPS | 0.308 | <0.006 | ||
| y = −27.143 + 0.134 WFPS + 3.261 pH | 0.516 | 0.002 | |||||||
| BCNP | CO2 | y = 20.16 + 0.71 WFPS | 0.185 | 0.043 | BCNB | CO2 | y = 6.038 + 1.159 WFPS | 0.385 | <0.001 |
| y = −245.29 + 1.01 WFPS + 32.03 pH | 0.394 | 0.009 | |||||||
| N2O | y = 0.004 + 0.001 NH4+ | 0.715 | <0.001 | N2O | y = −0.017 + 0.009 NH4+ | 0.217 | 0.03 | ||
| CH4 | y = 35.02 + 3.22 WFPS | 0.192 | 0.039 | CH4 | y = 6.414 + 0.073 WFPS | 0.218 | 0.029 | ||
| y = 45.17 − 5.67 pH + 0.04 NO3− | 0.441 | 0.005 | y = 72.335 + 0.077 WFPS − 8.237 pH | 0.394 | 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, Z.; Liu, Y.; Li, S.; Li, B.; Fan, X.; Liu, C.; Hu, C.; Zhang, S.; Li, Z. Biochar Weakens the Efficiency of Nitrification Inhibitors and Urease Inhibitors in Mitigating Greenhouse Gas Emissions from Soil Irrigated with Alternative Water Resources. Water 2024, 16, 2671. https://doi.org/10.3390/w16182671
Tao Z, Liu Y, Li S, Li B, Fan X, Liu C, Hu C, Zhang S, Li Z. Biochar Weakens the Efficiency of Nitrification Inhibitors and Urease Inhibitors in Mitigating Greenhouse Gas Emissions from Soil Irrigated with Alternative Water Resources. Water. 2024; 16(18):2671. https://doi.org/10.3390/w16182671
Chicago/Turabian StyleTao, Zhen, Yuan Liu, Siyi Li, Baogui Li, Xiangyang Fan, Chuncheng Liu, Chao Hu, Shuiqing Zhang, and Zhongyang Li. 2024. "Biochar Weakens the Efficiency of Nitrification Inhibitors and Urease Inhibitors in Mitigating Greenhouse Gas Emissions from Soil Irrigated with Alternative Water Resources" Water 16, no. 18: 2671. https://doi.org/10.3390/w16182671










