Climate Warming and Mismanagement Drive the Shift of Fish Communities in the Wadi El-Rayan Arid Lakes
Abstract
:1. Introduction
2. Materials and Methods
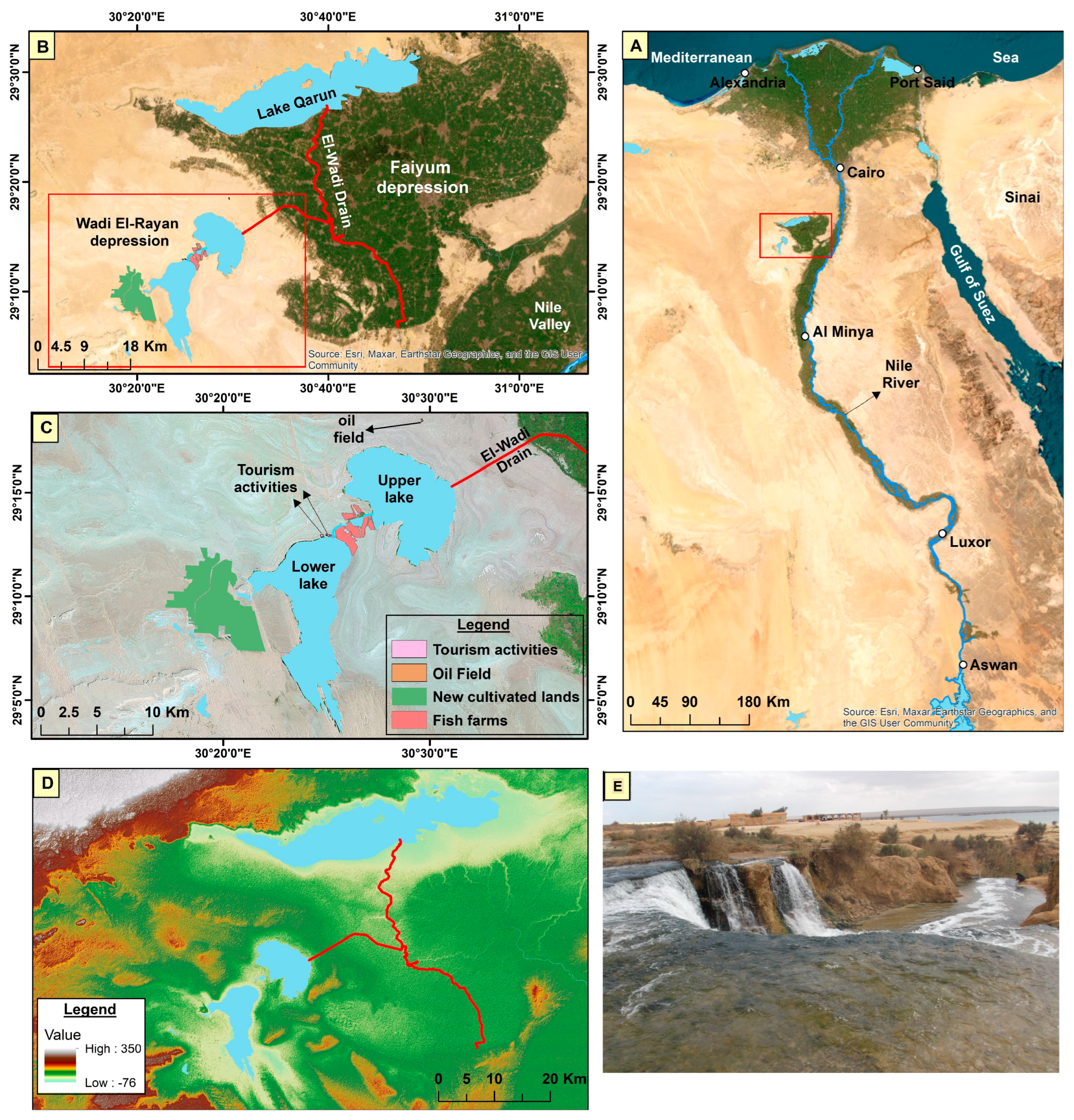
2.1. Study Area
2.2. Data Analysis
3. Results
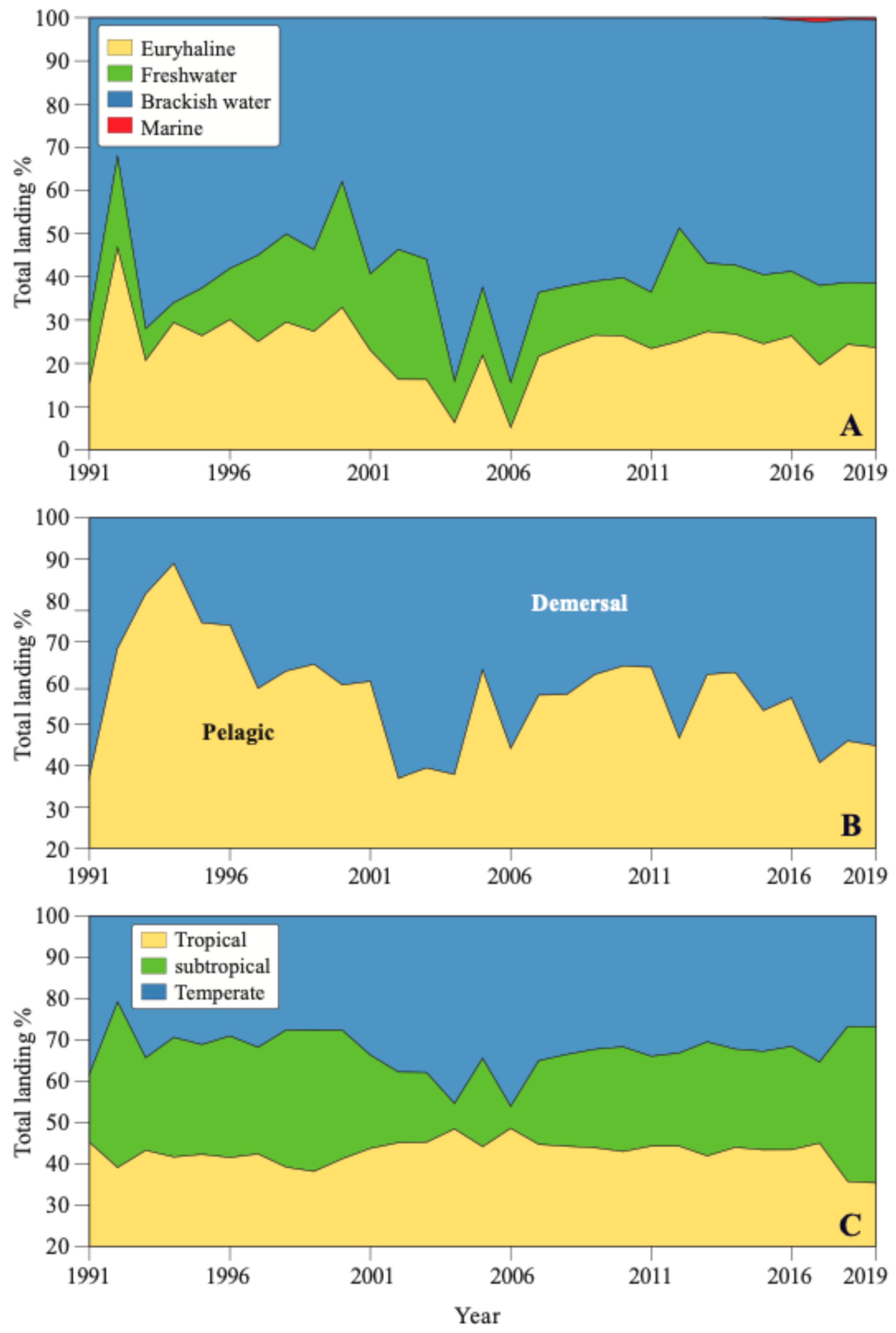
3.1. Total Fish Landing
3.2. Fish Community Composition
3.3. Environmental Impact
4. Discussion
4.1. Historical Changes and Their Drivers
4.2. Quality of the Data
4.3. Limitation and Future Prospects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC: Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Field, C.B.; Barros, V.R.; Dokken, D.J.; Mach, K.J.; Mastrandrea, M.D.; Bilir, T.E.; Chatterjee, M.; Ebi, K.L.; Estrada, Y.O.; Genova, R.C.; et al. (Eds.) Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; p. 1132. [Google Scholar]
- Collins, M.; Sutherland, M.; Bouwer, L.; Cheong, S.-M.; Frölicher, T.; Jacot Des Combes, H.; Koll Roxy, M.; Losada, I.; McInnes, K.; Ratter, B.; et al. Extremes, abrupt changes and managing risk. In IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; Pörtner, H.-O., Roberts, D.C., Masson-Delmotte, V., Zhai, P., Tignor, M., Poloczanska, E., Mintenbeck, K., Alegría, A., Nicolai, M., Okem, A., et al., Eds.; IPCC: Geneva, Switzerland, 2019. [Google Scholar] [CrossRef]
- Russo, E.; Domeisen, D.I. Increasing intensity of extreme heatwaves: The crucial role of metrics. Geophys. Res. Lett. 2023, 50, e2023GL103540. [Google Scholar] [CrossRef]
- Jeppesen, E.; Meerhoff, M.; Holmgren, K.; González-Bergonzoni, I.; Teixeira-de Mello, F.; Declerck, S.A.; De Meester, L.; Søndergaard, M.; Lauridsen, T.L.; Bjerring, R.; et al. Impacts of climate warming on lake fish community structure and potential effects on ecosystem function. Hydrobiologia 2010, 646, 73–90. [Google Scholar] [CrossRef]
- Pörtner, H.O.; Peck, M.A. Climate change effects on fishes and fisheries: Towards a cause-and-effect understanding. J. Fish Biol. 2010, 77, 1745–1779. [Google Scholar] [CrossRef]
- Seebacher, F.; Franklin, C.E. Determining environmental causes of biological effects: The need for a mechanistic physiological dimension in conservation biology. Phil. Trans. R. Soc. B 2012, 367, 1607–1614. [Google Scholar] [CrossRef]
- McKenzie, D.J.; Axelsson, M.; Chabot, D.; Claireaux, G.; Cooke, S.J.; Corner, R.A.; De Boeck, G.; Domenici, P.; Guerreiro, P.M.; Hamer, B.; et al. Conservation physiology of marine fishes: State of the art and prospects for policy. Conserv. Physiol. 2016, 4, cow046. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, D.J.; Geffroy, B.; Farrell, A.P. Effects of global warming on fishes and fisheries. J. Fish Biol. 2021, 98, 1489–1492. [Google Scholar] [CrossRef]
- Gardiner, N.M.; Munday, P.L.; Nilsson, G.E. Counter-Gradient Variation in Respiratory Performance of Coral Reef Fishes at Elevated Temperatures. PLoS ONE 2010, 5, e13299. [Google Scholar] [CrossRef] [PubMed]
- Eme, D.; Rufino, M.M.; Trenkel, V.M.; Vermard, Y.; Laffargue, P.; Petitgas, P.; Pellissier, L.; Albouy, C. Contrasted spatio-temporal changes in the demersal fish assemblages and the dominance of the environment vs fishing pressure, in the Bay of Biscay and Celtic Sea. Prog. Oceanogr. 2022, 204, 102788. [Google Scholar] [CrossRef]
- Galli, M.; Tepsich, P.; Baini, M.; Panti, C.; Rosso, M.; Vafeiadou, A.; Pantelidou, M.; Moulins, A.; Fossi, M.C. Microplastic abundance and biodiversity richness overlap: Identification of sensitive areas in the Western Ionian Sea. Mar. Pollut. Bull. 2022, 177, 113550. [Google Scholar] [CrossRef]
- Abdelhady, A.A.; Kamal, M.S.; Abdel-Raheem, K.H.M.; Ahmed, M.S.; Khalil, M.M. Historical changes in fish landings indicate a significant shift in fish catch composition and biodiversity loss in the Nile-Delta lakes. Mar. Pollut. Bull. 2023, 194, 11536. [Google Scholar] [CrossRef]
- Lappalainen, J.; Lehtonen, H.V. Temperature habitats for freshwater fishes in a warming climate. Boreal Environ. Res. 1997, 2, 69–84. Available online: https://api.semanticscholar.org/CorpusID:89365605 (accessed on 12 January 2014).
- Poloczanska, E.; Brown, C.; Sydeman, W.; Kiessling, W.; Schoeman, D.S.; Moore, P.J.; Brander, K.; Bruno, J.F.; Buckley, L.B.; Burrows, M.T.; et al. Global imprint of climate change on marine life. Nat. Clim. Change 2013, 3, 919–925. [Google Scholar] [CrossRef]
- Abdelhady, R.S.; Fahmy, H.S.; Pacini, N. Valuing of Wadi El-Rayan ecosystem through water–food–energy nexus approach. Ecohydrol. Hydrobiol. 2017, 17, 247–253. [Google Scholar] [CrossRef]
- Abudl-tawab, Y.; Said, H. An Economic Study on Fish Production and Marketing of Wadi El Rayan Lakes, Fayoum Governorate. Egypt. J. Aquat. Biol. Fish. 2023, 27, 817–836. [Google Scholar] [CrossRef]
- Allan, J.D.; Abell, R.; Hogan, Z.; Revenga, C.; Taylor, B.W.; Welcomme, R.L.; Winemiller, K. Overfishing of Inland Waters. BioScience 2005, 55, 1041–1051. [Google Scholar] [CrossRef]
- Brooks, E.G.E.; Holland, R.A.; Darwall, W.R.T.; Eigenbrod, F. Global evidence of positive impacts of freshwater biodiversity on fishery yields. Glob. Ecol. Biogeogr. 2016, 25, 553–562. [Google Scholar] [CrossRef]
- Coll, M.; Steenbeek, J.; Sole, J.; Palomera, I.; Christensen, V. Modelling the cumulative spatial–temporal effects of environmental drivers and fishing in a NW Mediterranean marine ecosystem. Ecol. Model. 2016, 331, 100–114. [Google Scholar] [CrossRef]
- Christensen, V.; Pauly, D. Changes in Models of Aquatic Ecosystems Approaching Carrying Capacity. Ecol. Appl. 1998, 8, S104–S109. [Google Scholar] [CrossRef]
- Chuenpagdee, R.; Morgan, L.E.; Maxwell, S.M.; Norse, E.A.; Pauly, D. Shifting gears: Assessing collateral impacts of fishing methods in US waters. Front. Ecol. Environ. 2003, 1, 517–524. [Google Scholar] [CrossRef]
- Welcomme, R.L.; Cowx, I.G.; Coates, D.; Béné, C.; Funge-Smith, S.; Halls, A.; Lorenzen, K. Inland capture fisheriesPhil. Trans. R. Soc. B 2010, 365, 2881–2896. [Google Scholar] [CrossRef]
- Ayyad, M.A.; Ghabbour, S.I. Hot deserts of Egypt and the Sudan. In Ecosystems of the world 12B: Hot Deserts and Arid Shrublands; Evenari, M., Noy-Meir, I., Goodall, D.W., Eds.; Elsevier: Amsterdam, The Netherlands, 1986. [Google Scholar]
- El-Sayed, M.F.; Abdel-Satar, A.M. Chemical Assessment of Wadi El-Rayan Lakes-Egypt. Am.-Eurasian J. Agric. Environ. Sci. 2009, 5, 53–62. [Google Scholar]
- Afefe, A.A. Composition and changes in the spontaneous flora of the Wadi El Rayan Ramsar site, Fayoum, Egypt, in the last 20 years. Limnol. Rev. 2020, 20, 109–121. [Google Scholar] [CrossRef]
- Abd Ellah, R.G.E. Bathymetric Study of Wadi El-Rayan Lakes, Egypt. Lakes Reserv. Ponds 2016, 10, 110–125. [Google Scholar]
- El-Shabrawy, G.M.; Dumont, H.J. The Fayum Depression and Its Lakes. In The Nile. Monographiae Biologicae; Dumont, H.J., Ed.; Springer: Dordrecht, The Netherlands, 2009; Volume 89. [Google Scholar] [CrossRef]
- Abdelhaleem, F.S.; El-Belasy, A.M.; El-Ghoreb, E.A. Managing Water and Salt Balance of Wadi El-Rayan Lakes, El-Fayoum, Egypt. Mansoura Eng. J. 2013, 38, C.35–C.53. [Google Scholar] [CrossRef]
- Abd Ellah, R.G.; Haque, M.N. The degradation scenario of man-made lakes from satellite observations: A case of Wadi El-Rayan lakes, Egypt. Egypt. J. Aquat. Res. 2022, 48, 99–106. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. (Eds.) FishBase; World Wide Web Electronic Publication: Rome, Italy, 2014; Available online: http://fishbase.sinica.edu.tw/ (accessed on 12 January 2014).
- Abdelhady, A.A.; Fürsich, F.T. Palaeobiogeography of the Bajocian–Oxfordian macrofauna of Gebel Maghara (North Sinai, Egypt): Implications for eustacy and basin topography. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2015, 417, 261–273. [Google Scholar] [CrossRef]
- Legendre, P.; Legendre, L. Developments in Environmental Modeling. In Numerical Ecology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. Available online: http://palaeo-electronica.org/2001_1/past/issue1_01.htm (accessed on 29 April 2023).
- Hereher, M.E. Assessing the dynamics of El-Rayan lakes, Egypt, using remote sensing techniques. Arab. J. Geosci. 2015, 8, 1931–1938. [Google Scholar] [CrossRef]
- Afefe, A.A.; Hatab, E.E.; Abbas, M.S.; Gaber, E.I. Assessment of threats to vegetation cover in Wadi El- -Rayan protected area, western desert, Egypt, Intern. J. Conserv. Sci. 2016, 7, 691–708. [Google Scholar]
- Bai, J.; Chen, X.; Li, J.; Yang, L.; Fang, H. Changes in the area of inland lakes in arid regions of central Asia during the past 30 years. Environ. Monit. Assess. 2011, 178, 247–256. [Google Scholar] [CrossRef]
- Goher, M.E.; Mahdy, E.S.M.; Abdo, M.H.; El Dars, F.M.; Korium, M.A.; Elsherif, A.A. Water quality status and pollution indices of Wadi El-Rayan lakes, El-Fayoum, Egypt. Sustain. Water Resour. Manag. 2019, 5, 387–400. [Google Scholar] [CrossRef]
- Hondorp, D.W.; Breitburg, D.L.; Davias, L.A. Eutrophication and fisheries: Separating the effects of nitrogen loads and hypoxia on the pelagic-to-demersal ratio and other measures of landings composition. Mar. Coast. Fish. 2011, 2, 339–361. [Google Scholar] [CrossRef]
- Pennino, M.G.; Bellido, J.M. Can a simple Pelagic-Demersal ratio explain ecosystem functioning. Biodivers. J. 2012, 3, 69–78. [Google Scholar]
- Nunes, M.; Adams, J.B.; Rishworth, G.M. Shifts in phytoplankton community structure in response to hydrological changes in the shallow St Lucia Estuary. Mar. Pollut. Bull. 2018, 128, 275–286. [Google Scholar] [CrossRef]
- De Anda, J.; Shear, H. Nutrients and Eutrophication in Lake Chapala. In The Lerma-Chapala Watershed: Evaluation and Management; Hansen, A.M., van Afferden, M., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2001; pp. 183–198. [Google Scholar] [CrossRef]
- Benoît, H.P.; Swain, D.P. Impacts of environmental change and direct and indirect harvesting effects on the dynamics of a marine fish community. Can. J. Fish. Aquat. Sci. 2008, 65, 2088–2104. [Google Scholar] [CrossRef]
- Pyšek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P.; et al. Scientists’ warning on invasive alien species. Biol. Rev. 2020, 95, 1511–1534. [Google Scholar] [CrossRef]
- Mormul, R.P.; Vieira, D.S.; Bailly, D.; Fidanza, K.; da Silva, V.F.B.; da Graça, W.J.; Pontara, V.; Bueno, M.L.; Thomaz, S.M.; Mendes, R.S. Invasive alien species records are exponentially rising across the Earth. Biol. Invasions 2022, 24, 3249–3261. [Google Scholar] [CrossRef]
- Occhipinti-Ambrogi, A. Global change and marine communities: Alien species and climate change. Mar. Pollut. Bull. 2007, 55, 342–352. [Google Scholar] [CrossRef]
- Sultana, M.; Hashim, Z.H. Invasive alien fish species in freshwater of the continents. J. Environ. Sci. Nat. Resour. 2015, 8, 63–74. [Google Scholar] [CrossRef]
- Erarto, F.; Getahun, A. Impacts of introductions of alien species with emphasis on fishes. Int. J. Fish. Aquat. Stud. 2020, 8, 207–216. [Google Scholar]
- Occhipinti-Ambrogi, A.; Galil, B. Marine alien species as an aspect of global change. Adv. Oceanogr. Limnol. 2010, 1, 199–218. [Google Scholar] [CrossRef]
- Moreira, F.; Assis, C.A.; Almeida, P.R.; Costa, J.L.; Costa, M.J. Trophic relationships in the community of the Upper Tagus Estuary (Portugal: A preliminary approach). Estuar. Coast. Shelf Sci. 1992, 34, 617–623. [Google Scholar] [CrossRef]
- Palazzi, R.; Richard, J.; Bozzato, G.; Zanella, L. Larval and juvenile rearing of common sole (Solea solea L.) in the Northern Adriatic (Italy). Aquaculture 2006, 255, 495–506. [Google Scholar] [CrossRef]
- Mohammed, H.H.; Ebrahim, M.; Youssef, M.I.; Saleem, A.S.Y.; Abdelkhalek, A. Behavior and management of carp fish: A review. Open Vet. J. 2024, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- El-Samkary, M.A.; Khallaf, M.F.; Ahmed, S.A.; Abo-Taleb, M. Studies on the utilization of Egyptian silver carp fish. Egypt. J. Aquat. Biol. Fish. 1997, 1, 71–92. [Google Scholar] [CrossRef]
- Brummett, R.E.; Beveridge, M.C.M.; Cowx, I.G. Functional aquatic ecosystems, inland fisheries and the Millennium Development Goals. Fish Fish. 2012, 14, 312–324. [Google Scholar] [CrossRef]
- Garibaldi, L. The FAO global capture production database: A six-decade effort to catch the trend. Mar. Policy 2012, 36, 760–768. [Google Scholar] [CrossRef]
- Bartley, T.J.; Braid, H.E.; McCann, K.S.; Lester, N.P.; Shuter, B.J.; Hanner, R.H. DNA barcoding increases resolution and changes structure in Canadian boreal shield lake food webs. DNA Barcodes 2015, 3, 30–43. [Google Scholar] [CrossRef]
- Pauly, D.; Christensen, V.; Dalsgaard, J.; Froese, R.; Torres, F.J.R. Fishing Down Marine Food Webs. Science 1998, 279, 860–863. [Google Scholar] [CrossRef]
- Daan, N.; Gislason, H.; Pope, J.G.; Rice, J.C. Apocalypse in world fisheries? The reports of their death are greatly exaggerated. ICES J. Mar. Sci. 2011, 68, 1375–1378. [Google Scholar] [CrossRef]
- Alverson, D.L.; Freeberg, M.H.; Pope, J.G.; Murawski, S.A. A Global Assessment of Fisheries Bycatch and Discards; FAO Fisheries Technical Paper; FAO: Rome, Italy, 1994; Volume 339, 233p. [Google Scholar]
- Caddy, J.F.; Carocci, F.; Coppola, S. Have Peak Fishery Production Levels been Passed in Continental Shelf Areas? Some Perspectives Arising from Historical Trends in Production per Shelf Area. J. Northw. Atl. Fish. Sci. 1998, 23, 191–219. [Google Scholar] [CrossRef]
- Willemse, N.E.; Pauly, D. Reconstruction and interpretation of marine fisheries catches from Namibian waters, 1950 to 2000. In Namibia’s Fisheries: Ecological, Economic and Social Aspects; Sumaila, U.R., Boyer, D., Skog, M., Steinshamm, S.I., Eds.; Eburon: Delft, The Netherlands, 2004; pp. 99–112. [Google Scholar]
- Pinnegar, J.K.; Jennings, S.; O’Brien, C.M.; Polunin, N.V.C. The effects of exploitation and environmental variability on the trophic structure of the Celtic Sea fish community. J. Appl. Ecol. 2002, 39, 377–390. [Google Scholar] [CrossRef]
- Leitão, F. Mean Size of the Landed Catch: A Fishery Community Index for Trend Assessment in Exploited Marine Ecosystems. Front. Mar. Sci. 2019, 6, 302. [Google Scholar] [CrossRef]
- Valtýsson, H.Þ.; Pauly, D. Fishing down the food web: An Icelandic case study. In Competitiveness within the Global Fisheries: Proceedings of a Conference Held in Akureyri, Iceland, 6–7 April 2003; Guðmundsson, E., Valtýsson, H.Þ., Eds.; University of Akureyri: Akureyri, Iceland, 2003; pp. 12–24. [Google Scholar]
- Mcclanahan, T.R.; Hicks, C.C. Changes in life history and ecological characteristics of coral reef fish catch composition with increasing fishery management. Fish. Manag. Ecol. 2011, 18, 50–60. [Google Scholar] [CrossRef]
- Durante, L.M.; Beentjes, M.P.; Wing, S.R. Shifting trophic architecture of marine fisheries in New Zealand: Implications for guiding effective ecosystem-based management. Fish Fish. 2020, 21, 813–830. [Google Scholar] [CrossRef]
- Sundermeyer, M.A.; Rothschild, B.J.; Robinson, A.R. Using commercial landings data to identify environmental correlates with distributions of fish stocks. Fish. Oceanogr. 2005, 14, 47–63. [Google Scholar] [CrossRef]
- Scarabotti, P.A.; Lucifora, L.O.; Espínola, L.A.; Rabuffetti, A.P.; Liotta, J.; Mantinian, J.E.; Roux, J.P.; Silva, N.; Balboni, L.; Vargas, F.; et al. Long-term trends of fishery landings and target fish populations in the lower La Plata basin. Neotrop. Ichthyol. 2021, 19, e210013. [Google Scholar] [CrossRef]
- McCluskey, S.M.; Lewison, R.L. Quantifying fishing effort: A synthesis of current methods and their applications. Fish Fish. 2008, 9, 188–200. [Google Scholar] [CrossRef]
- de Mutsert, K.; Cowan Jr, J.H.; Essington, T.E.; Hilborn, R. Reanalyses of Gulf of Mexico fisheries data: Landings can be misleading in assessments of fisheries and fisheries ecosystems. Proc. Natl. Acad. Sci. USA 2008, 105, 2740–2744. [Google Scholar] [CrossRef]
- Abdelhady, A.A.; Khalil, M.; Ismail, E.; Mohamed, R.; Ali, A.; Snousy, M.; Fan, D.; Zhang, S.; Xiao, J. Potential biodiversity threats associated with the metal pollution in the Nile-Delta ecosystem (Manzala lagoon, Egypt). Ecol. Indic. 2019, 98, 844–853. [Google Scholar] [CrossRef]
- Abdelhady, A.A. Phenotypic differentiation of the Red Sea gastropods in response to the environmental deterioration: Geometric morphometric approach. J. Afr. Earth Sci. 2016, 115, 191–202. [Google Scholar] [CrossRef]
- Khalil, M.; Abdelwahab, S.M.; Ahmed, M.; Ahmed, M.S.; Abdelhady, A.A. Mixed agricultural, industrial, and domestic drainage water discharge poses a massive strain on freshwater ecosystems: A case from the Nile River in Upper Egypt. Environ. Sci. Pollut. Res. 2023, 30, 122642–122662. [Google Scholar] [CrossRef] [PubMed]
- Abotalib, A.Z.; Abdelhady, A.A.; Heggy, E.; Salem, S.G.; Ismail, E.; Ali, A.; Khalil, M.M. Irreversible and Large-Scale Heavy Metal Pollution Arising from Increased Damming and Untreated Water Reuse in the Nile Delta. Earth’s Future 2023, 11, e2022EF002987. [Google Scholar] [CrossRef]
- Abdel-Raheem, K.H.M.; Khalil, M.; Abdelhady, A.A.; Tan, L. Anthropogenic–induced environmental and ecological changes in the Nile Delta over the past half-century. Sci. Total Environ. 2024, 926, 171941. [Google Scholar] [CrossRef] [PubMed]
- Abdelhady, A.A.; Hussain, A.M.; Samy-Kamal, M.; Ahmed, M.S.; Alexakis, D.A.; Ali, A. Morphological Variation between Life and Death Gastropod Populations in the Nile Delta: A Pollution-Induced Evolution. Water 2023, 15, 4078. [Google Scholar] [CrossRef]
- Abdelhady, A.A.; Hassan, H.; Balboul, B.A.A.; Abu Shama, A.; Abdel-Raheem, K.H.M.; Ahmed, M.S.; Hussain, A.M. Taphonomic damage of molluscan shells in the Nile Delta under natural and anthropogenic sources of environmental variability. J. Afr. Earth Sci. 2024, 210, 105155. [Google Scholar] [CrossRef]





| Temperature (°C) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Scientific Name | English Name | Family | Life Mode | Diet | Salinity | Status | Zone | Min | Max | Avg. |
| Bagrus bajad | Bagrus bajad | Bagridae | Demersal | Piscivore | Freshwater | Native | Tropical | 17 | 29 | 23 |
| Hemiramphus far | Black-barred halfbeak | Hemiramphidae | Reef ass. | Herbivore | Brackish marine | Native | Tropical | 25 | 29 | 27 |
| Clarias gariepinus | Catfish | Clariidae | Benthopelagic | Omnivore | Freshwater | Native | Subtropical | 23 | 29 | 26 |
| Solea solea | Common sole | Soleidae | Demersal | Omnivore | Brackish marine | Native | Subtropical | 8 | 24 | 16 |
| Anguilla anguilla | European eel | Anguillidae | Demersal | Carnivore | Euryhaline | Native | Temperate | 0 | 30 | 15 |
| Dicentrarchus labrax | European seabass | Moronidae | Demersal | Carnivore | Euryhaline | Native | Subtropical | 5 | 28 | 17 |
| Sparus aurata | Gilthead sea bream | Sparidae | Demersal | Carnivore | Brackish marine | Native | Subtropical | 18 | 26 | 22 |
| Ctenopharyngodon idella | Grass carp | Cyprinidae | Benthopelagic | Omnivore | Fresh–brackish | Alien | Subtropical | 20 | 30 | 25 |
| Chelon aurata | Mullet nei | Mugilidae | Pelagic | Omnivore | Euryhaline | Native | Temperate | 12 | 26 | 19 |
| Lates niloticus | Nile perch | Latidae | Demersal | Carnivore | Freshwater | Native | Tropical | 22 | 28 | 25 |
| Oreochromis niloticus | Nile tilapia | Cichlidae | Benthopelagic | Herbivore | Fresh–brackish | Native | Tropical | 28 | 34 | 31 |
| Oreochromis aureus | Blue tilapia | Cichlidae | Benthopelagic | Herbivore | Fresh–brackish | Native | Tropical | 28 | 34 | 31 |
| Tilapia zilli | Redbelly tilapia | Cichlidae | Benthopelagic | Herbivore | Fresh–brackish | Native | Tropical | 28 | 34 | 31 |
| Hypophthalmichthys molitrix | Silver carp | Xenocyprididae | Benthopelagic | Herbivore | Fresh–brackish | Alien | Subtropical | 22 | 28 | 25 |
| Pomadasys stridens | Striped piggy | Haemulidae | Demersal | Omnivore | Marine | Native | Tropical | 18 | 21 | 20 |
| 1991–2003 | 2004–2006 | 2007–2017 | 2018–2019 | |
|---|---|---|---|---|
| 1991–2003 | 0 | |||
| 2004–2006 | 0.0058 | 0 | ||
| 2007–2017 | 0.0001 | 0.0048 | 0 | |
| 2018–2019 | 0.0098 | 0.1036 | 0.0134 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelhady, A.A.; Samy-Kamal, M.; Ismail, E.; Hussain, A.M.; Gamvroula, D.E.; Ali, A.; Ahmed, M.S.; Abdel-Raheem, K.H.M.; Saibi, H.; Sami, M.; et al. Climate Warming and Mismanagement Drive the Shift of Fish Communities in the Wadi El-Rayan Arid Lakes. Water 2024, 16, 2685. https://doi.org/10.3390/w16182685
Abdelhady AA, Samy-Kamal M, Ismail E, Hussain AM, Gamvroula DE, Ali A, Ahmed MS, Abdel-Raheem KHM, Saibi H, Sami M, et al. Climate Warming and Mismanagement Drive the Shift of Fish Communities in the Wadi El-Rayan Arid Lakes. Water. 2024; 16(18):2685. https://doi.org/10.3390/w16182685
Chicago/Turabian StyleAbdelhady, Ahmed A., Mohamed Samy-Kamal, Esam Ismail, Ali M. Hussain, Dimitra E. Gamvroula, Ahmed Ali, Mohamed S. Ahmed, Khalaf H. M. Abdel-Raheem, Hakim Saibi, Mabrouk Sami, and et al. 2024. "Climate Warming and Mismanagement Drive the Shift of Fish Communities in the Wadi El-Rayan Arid Lakes" Water 16, no. 18: 2685. https://doi.org/10.3390/w16182685
APA StyleAbdelhady, A. A., Samy-Kamal, M., Ismail, E., Hussain, A. M., Gamvroula, D. E., Ali, A., Ahmed, M. S., Abdel-Raheem, K. H. M., Saibi, H., Sami, M., Alexakis, D. E., & Khalil, M. M. (2024). Climate Warming and Mismanagement Drive the Shift of Fish Communities in the Wadi El-Rayan Arid Lakes. Water, 16(18), 2685. https://doi.org/10.3390/w16182685












