Magnetized Saline Water Drip Irrigation Alters Soil Water-Salt Infiltration and Redistribution Characteristics
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Supplies
2.1.1. Soil Sample
2.1.2. Saline Water
2.2. Experimental Design and Implementation
2.2.1. Control Variables and Laboratory Treatments
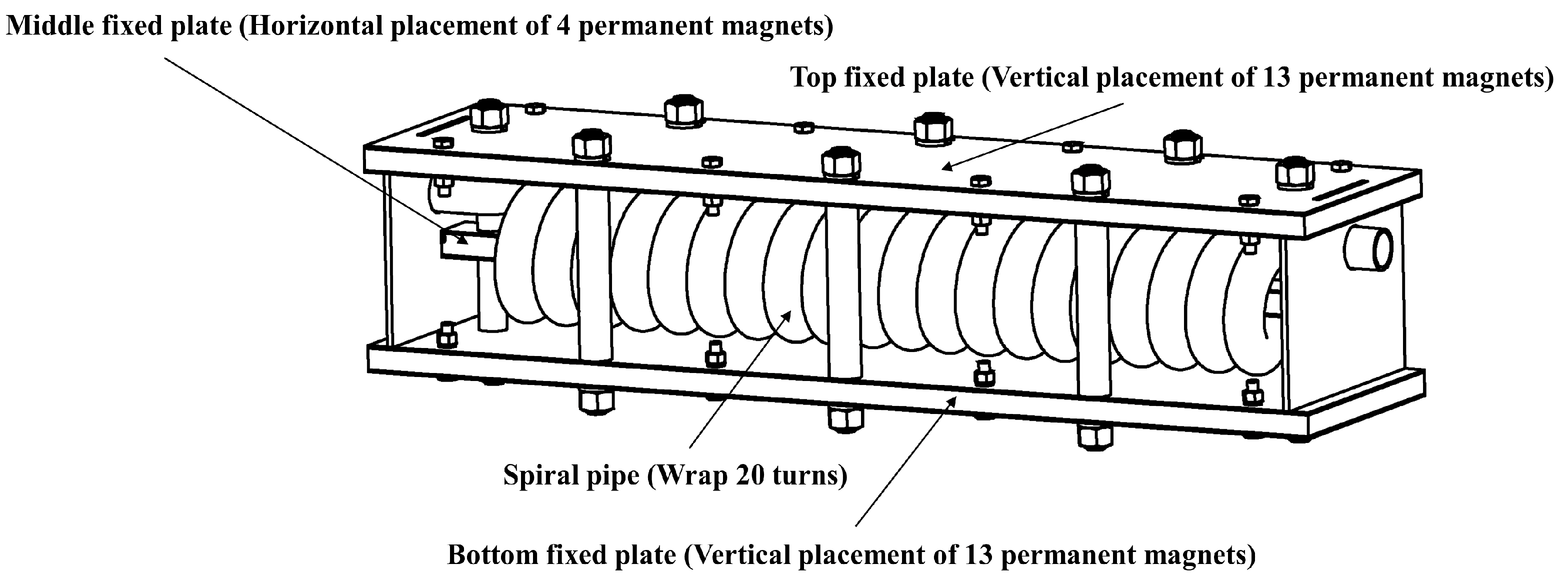
2.2.2. Magnetizing Device
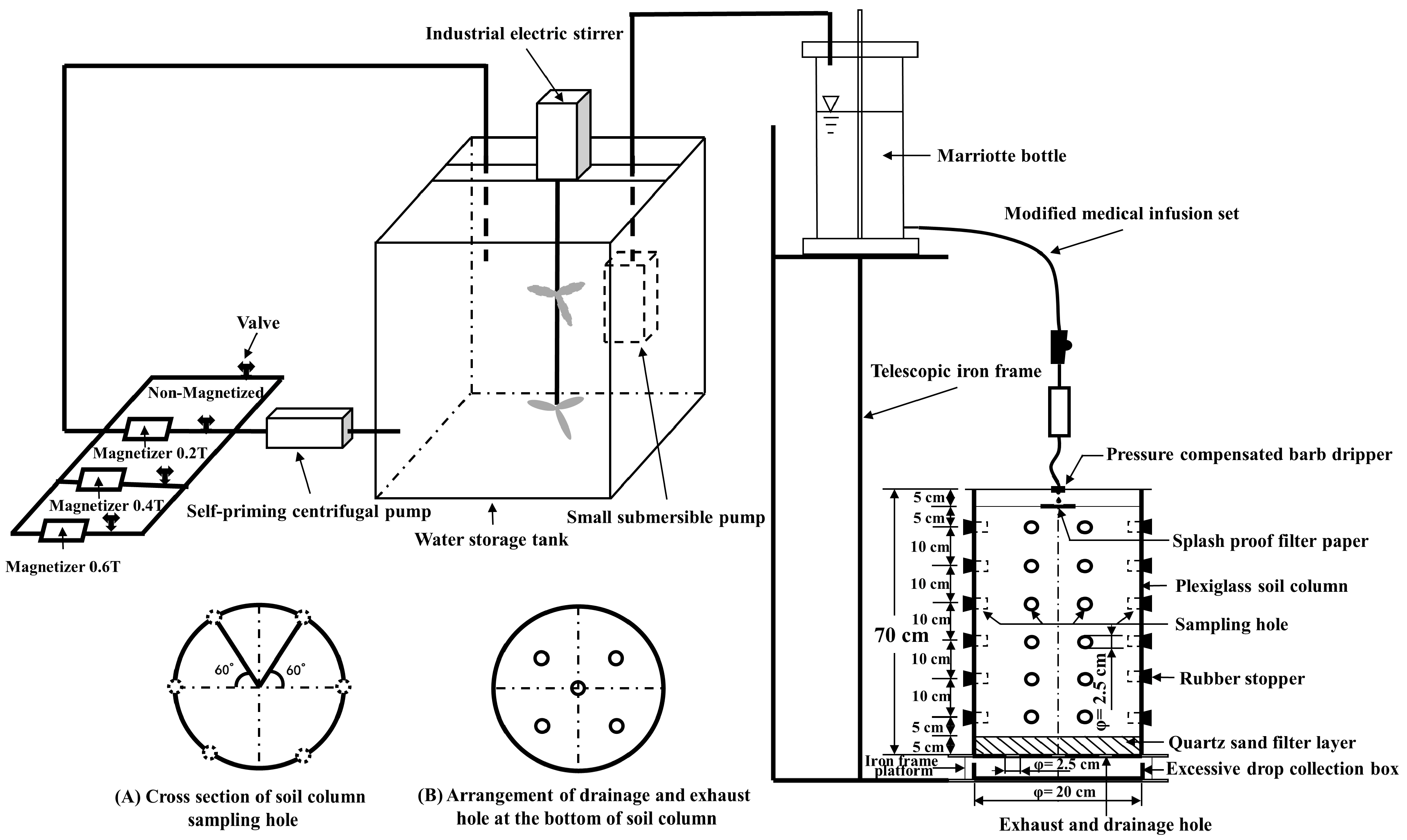
2.2.3. Soil Water-Salt Transport Experimental System
- (1)
- Saline water circulation magnetization treatment device: The artificially prepared saline water was passed through the magnetizer multiple times to achieve complete circulation of magnetization. The device consisted of a water storage tank, an electric stirrer, a self-priming pump, magnetizers, a pipeline, and pipe fittings. The water storage tank utilized a 150 L polyethylene plastic container, with an industrial electric stirrer installed at the water inlet. The magnetizer’s spiral pipe diameter, as well as the PVC pipe and all connecting fittings, were of the branch pipe size (32 mm) commonly used in drip irrigation systems for agricultural production.
- (2)
- Soil columns: These were constructed using a 70 cm plexiglass pipe with an inner diameter of 20 cm and a wall thickness of 5 mm. The bottom was sealed with a 10 mm thick acrylic plate, and five holes were added to ensure proper exhaust and drainage. Additionally, six sampling holes were arranged in a honeycomb pattern at 10 cm intervals from the bottom up, each with an inner diameter of 2.5 cm. The sampling holes were evenly spaced on the same horizontal plane to facilitate soil sample collection. Rubber plugs were used to block the holes during the experiment, as described by Zhao et al. [26]. The soil columns were placed on an iron frame with a hollow upper end and a certain height. An excessive droplet collection box was arranged in the middle of the iron frame.
- (3)
- Simulated self-pressure drip irrigation device: The water supply equipment used a cylindrical Marriotte bottle with an inner diameter of 15 cm and a height of 70 cm. The bypass scale pipe allowed for easy reading of the water level, and the water supply head of the telescopic frame was adjusted to be controlled at 93 cm. The Marriotte bottle was connected to a modified medical infusion set to drip MSW into the soil column. The infusion set’s drip pot was inserted into a pressure-compensated barb dripper (designed to have a flow rate of 40 L h−1, NETAFIM Agricultural Science and Technology Co., Ltd. Guangzhou, China). The dripper’s flow rate was controlled by the flow regulator to be (2.36 ± 0.08) L h−1.
2.2.4. Soil Column Filling and Infiltration-Redistribution Test
2.3. Calculation and Statistical Analysis
2.3.1. Calculation of Wetting Front Migration, Water-Salt and Ion Storage
2.3.2. Statistical Analysis
3. Results
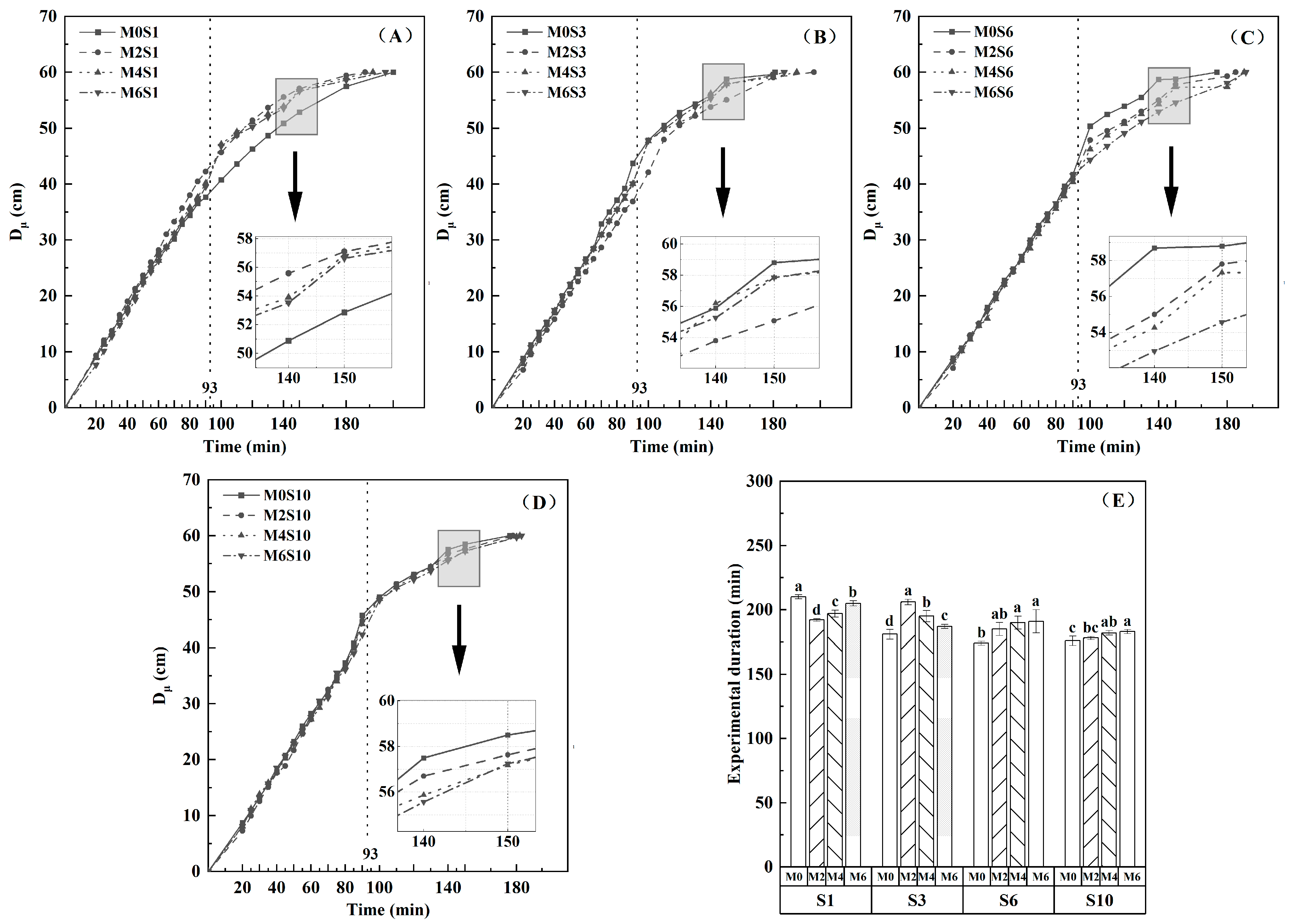
3.1. Effects of Magnetized Saline Water Drip Irrigation on Wetting Front Migration
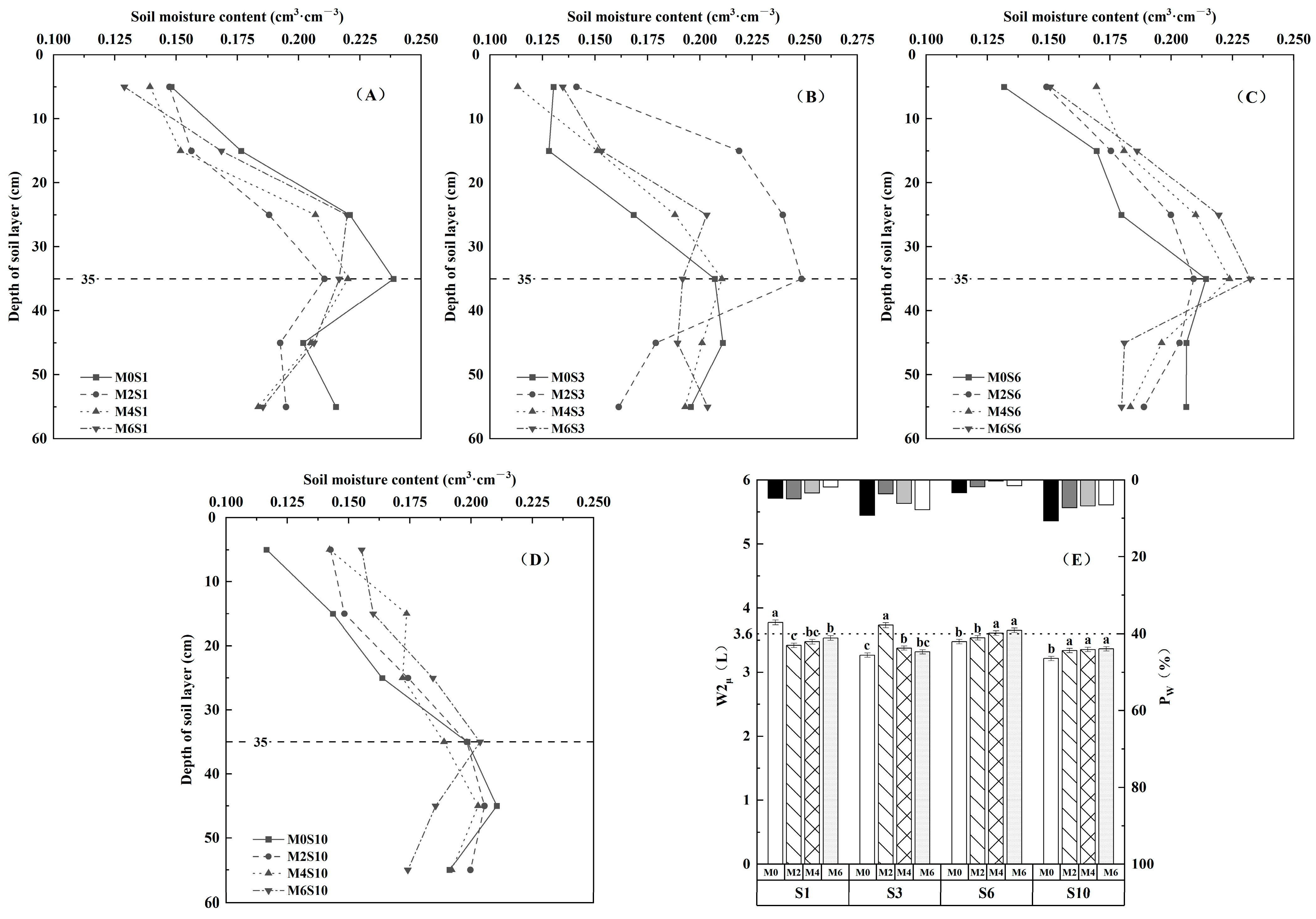
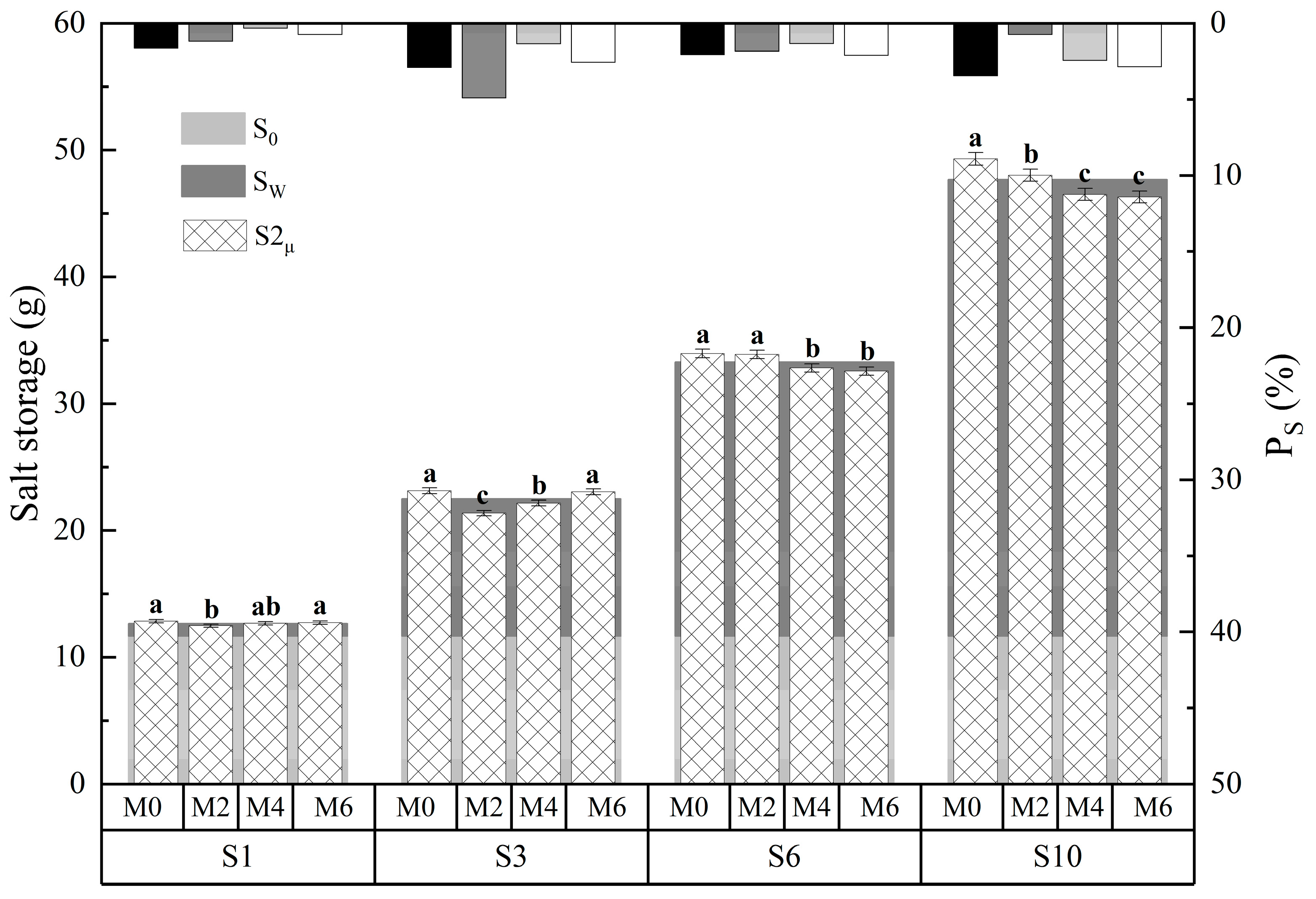
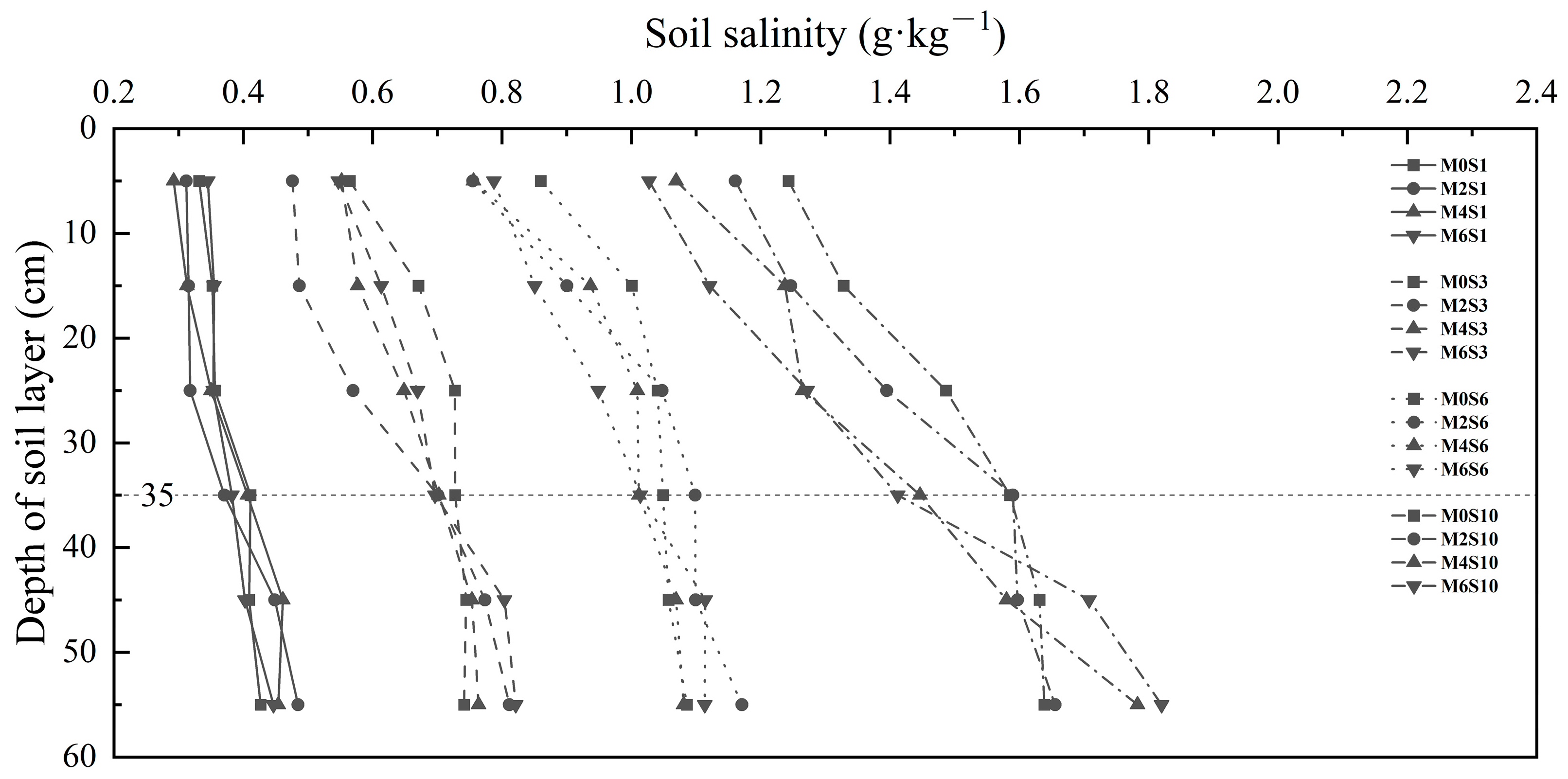
3.2. Effects of Magnetized Saline Water Drip Irrigation on Water-Salt Content in Soil
3.3. Effects of Magnetized Saline Water Drip Irrigation on Soil Salt Ion Content
4. Discussion
4.1. Relationship between Magnetization Mechanism and Wetting Front Migration
4.2. Relationship between the Magnetization Mechanism and Water-Salt-Ion Content in Soil
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jangir, R.P.; Yadav, B.S. Management of saline irrigation water for enhancing crop productivity. J. Sci. Ind. Res. 2011, 70, 622–627. [Google Scholar] [CrossRef]
- Oster, J.D. Irrigation with poor quality water. Agric. Water Manag. 1994, 25, 271–297. [Google Scholar] [CrossRef]
- Sakadevan, K.; Nguyen, M.L. Extent, impact, and response to soil and water salinity in arid and semiarid regions. Adv. Agron. 2010, 109, 55–74. [Google Scholar] [CrossRef]
- Hussain, M.I.; Farooq, M.; Muscolo, A.; Rehman, A. Crop diversification and saline water irrigation as potential strategies to save freshwater resources and reclamation of marginal soils-a review. Environ. Sci. Pollut. Res. 2020, 27, 28695–28729. [Google Scholar] [CrossRef]
- Zhao, G.Q.; Zhou, B.B.; Mu, Y.; Wang, Y.H.; Liu, Y.Q.; Wang, L. Irrigation with activated water promotes root growth and improves water use of winter wheat. Agronomy 2021, 11, 2459. [Google Scholar] [CrossRef]
- Dobránszki, J. From mystery to reality: Magnetized water to tackle the challenges of climate change and for cleaner agricultural production. J. Clean Prod. 2023, 425, 139077. [Google Scholar] [CrossRef]
- Amiri, M.C.; Dadkhah, A.A. On reduction in the surface tension of water due to magnetic treatment. Colloids Surf. A Physicochem. Eng. Asp. 2006, 278, 252–255. [Google Scholar] [CrossRef]
- Otsuka, I.; Ozeki, S. Does magnetic treatment of water change its properties? J. Phys. Chem. B 2006, 110, 1509–1512. [Google Scholar] [CrossRef]
- Wang, Y.K.; Wei, H.N.; Li, Z.W. Effect of magnetic field on the physical properties of water. Results Phys. 2018, 8, 262–267. [Google Scholar] [CrossRef]
- Al-Shrouf, A.M. The effect of magnetic treatment of irrigation water on cucumber production and water productivity. Acta. Hortic. 2013, 1054, 111–117. [Google Scholar] [CrossRef]
- Khoshravesh-Miangoleh, M.; Kiani, A.R. Effect of magnetized water on infiltration capacity of different soil textures. Soil Use Manag. 2015, 30, 588–594. [Google Scholar] [CrossRef]
- Higashitani, K.; Kage, A.; Katamura, S.; Imai, K.; Hatade, S. Effects of a magnetic-field on the formation of CaCO3 particles. J. Colloid Interface Sci. 1993, 156, 90–95. [Google Scholar] [CrossRef]
- Wang, C.F.; Lu, S.Q.; Li, M.J.; Zhang, Y.L.; Sa, Z.Y.; Liu, J.; Wang, H.; Wang, S.C. Study on the dust removal and temperature reduction coupling performances of magnetized water spray. Environ. Sci. Pollut. Res. 2021, 29, 6151–6165. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Yang, H.W.; He, J.S.; Zhu, W.P. The effects of magnetic fields on water molecular hydrogen bonds. J. Mol. Struct. 2009, 938, 15–19. [Google Scholar] [CrossRef]
- Mascolo, M.C. Effect of magnetic field on calcium carbonate precipitated in natural waters with prevalent temporary hardness. J. Water Process Eng. 2021, 41, 102087. [Google Scholar] [CrossRef]
- Sronsri, C.; Sittipol, W.; U-yen, K. Quantity and quality of lettuce (Lactuca sativa L.) grown by a circulating hydroponic method with a Halbach array magnetizer. J. Food Compos. Anal. 2022, 108, 104460. [Google Scholar] [CrossRef]
- Alattar, E.; Elwasife, K.; Radwan, E. Growth characteristics of chilli pepper (Capsicum annuum) under the effect of magnetizing water with neodymium magnets (NdFeB). AGRIVITA J. Agricu. Sci. 2021, 43, 398–408. [Google Scholar] [CrossRef]
- Pang, X.F.; Deng, B. Investigation of changes in properties of water under the action of a magnetic field. Sci. China Ser. G Phys. Mech. Astro. 2008, 51, 1621–1632. [Google Scholar] [CrossRef]
- Absalan, Y.; Gholizadeh, M.; Choi, H.J. Magnetized solvents: Characteristics and various applications. J. Mol. Liq. 2021, 335, 116167. [Google Scholar] [CrossRef]
- Wang, Q.J.; Xu, Z.Y.; Shang, Y.Y.; Zhang, J.H. Effect of salinity of magnetized brackish water on salt and water movement. Trans. Chin. Soc. Agric. Mach. 2017, 48, 198–206. Available online: https://link.cnki.net/urlid/11.1964.s.20170531.1612.008 (accessed on 21 July 2024).
- Mohamed, A.I.; Ebead, B.M. Effect of magnetic treated irrigation water on salt removal from a sandy soil and on the availability of certain nutrients. Int. J. Eng. Appl. Sci. 2013, 2, 36–44. Available online: https://www.magtech.com.pk/pdf/agriculture-7.pdf (accessed on 25 July 2024).
- Al-Ogaidi, A.A.M.; Wayayok, A.; Rowshon, M.K.; Abdullah, A.F. The influence of magnetized water on soil water dynamics under drip irrigation systems. Agric. Water Manag. 2017, 180, 70–77. [Google Scholar] [CrossRef]
- Hanks, R.J.; Klute, A.; Bresler, D.F. A numeric method for estimating infiltration, redistribution, drainage and evaporation of water from soil. Water Resour. Res. 1969, 5, 1064–1069. [Google Scholar] [CrossRef]
- Zlotopolski, V. The Impact of magnetic water treatment on salt distribution in a large unsaturated soil column. Int. Soil Water Conserv. Res. 2017, 5, 253–257. [Google Scholar] [CrossRef]
- Xin, M.L.; Zhao, Q.; Ma, Y.J.; Qiao, Y. A type of fluid magnetizer: China Pate-nt. 2022, CN217868241U. Available online: https://pss-system.cponline.cnipa.gov.cn/documents/detail?prevPageTit=changgui (accessed on 26 July 2024).
- Zhao, Y.G.; Li, Y.; Wang, S.J.; Wang, J.; Xu, L.Z. Combined application of a straw layer and flue gas desulphurization gypsum to reduce soil salinity and alkalinity. Pedosphere 2020, 30, 226–235. [Google Scholar] [CrossRef]
- Tang, Q.Y.; Zhang, C.X. Data Processing System (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Sci. 2013, 20, 254–260. [Google Scholar] [CrossRef]
- Chibowski, E.; Szczes, A. Magnetic water treatment–A review of the latest approaches. Chemosphere 2018, 203, 54–67. [Google Scholar] [CrossRef]
- Luzar, A.; Chandler, D. Hydrogen-bond kinetics in liquid water. Nature 1996, 379, 55–57. [Google Scholar] [CrossRef]
- Henchman, R.H. Water’s dual nature and its continuously changing hydrogen bonds. J. Phys. Condens. Matter. 2016, 28, 384001. [Google Scholar] [CrossRef]
- Toledo, E.J.L.; Ramalho, T.C.; Magriotis, Z.M. Influence of magnetic field on physical–chemical properties of the liquid water: Insights from experimental and theoretical models. J. Mol. Struct. 2008, 888, 409–415. [Google Scholar] [CrossRef]
- Sidorenko, G.; Brilly, M.; Laptev, B.; Gorlenko, N.; Antoshkin, L.; Vidmar, A.; Kryzanowski, A. The role of modification of the structure of water and water-containing systems in changing their biological, therapeutic, and other properties overview. Water 2021, 13, 2441. [Google Scholar] [CrossRef]
- Wang, Y.H.; Yang, X.G. Magnetized water treatment technology and its application in agriculture. Agric. Eng. 2014, 4, 74–77. Available online: https://d.wanfangdata.com.cn/periodical/ChlQZXJpb2RpY2FsQ0hJTmV3UzIwMjQwNzA0Eg5ueWdjaDIwMTQwNTAyMxoIeG5uaG1yNWw%3D (accessed on 5 August 2024).
- Holysz, L.; Szczes, A.; Chibowski, E. Effects of a static magnetic field on water and electrolyte solutions. J. Colloid Interface Sci. 2007, 316, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Sarimov, R.M.; Simakin, A.V.; Matveeva, T.A.; Gudkov, S.V.; Lyakhov, G.A.; Pustovoy, V.I.; Troitskii, A.V.; Shcherbakov, I.A. Influence of Magnetic Fields with Induction of 7 T on Physical and Chemical Properties of Aqueous NaCl Solutions. Appl. Sci. 2021, 11, 11466. [Google Scholar] [CrossRef]
- Wang, Q.J.; Zhang, J.H.; Men, Q.; Tan, S.; Zhou, L.W.; Liu, X.Y. Experiment on physical and chemical characteristics of activated brackish water by magnetization or ionization. Trans. Chin. Soc. Agric. Eng. 2016, 32, 60–66. Available online: http://www.tcsae.org/cn/article/doi/10.11975/j.issn.1002-6819.2016.10.009 (accessed on 6 August 2024).
- Pang, X.F.; Deng, B.; Tang, B. Influences of magnetic field on macroscopic properties of water. Mod. Phys. Lett. B 2012, 26, 1250069. [Google Scholar] [CrossRef]
- Basset, C.; Abou Najm, M.; Ghezzehei, T.; Hao, X.X.; Daccache, A. How does soil structure affect water infiltration? A meta-data systematic review. Soil Tillage Res. 2023, 226, 105577. [Google Scholar] [CrossRef]
- Niaz, N.; Tang, C.; Zhang, R.X.; Chu, G.X. Application of magnetic treated water irrigation increased soil salt leachate by altering water property. Eurasian Soil Sci. 2021, 54, S26–S32. [Google Scholar] [CrossRef]
- Ishii, K.; Yamamoto, S.; Yamamoto, M.; Nakayama, H. Relative change of viscosity of water under a transverse magnetic field of 10 T is smaller than 10−4. Chem. Lett. 2005, 34, 874–875. [Google Scholar] [CrossRef]
- Yi, G.; Wang, Q.J.; Kang, W.; Zhang, J.H.; Kai, W.; Yang, L. Spring irrigation with magnetized water affects soil water-salt distribution, emergence, growth, and photosynthetic characteristics of cotton seedlings in Southern Xinjiang, China. BMC Plant Biol. 2023, 23, 174. [Google Scholar] [CrossRef]
- Abdelghany, A.E.; Abdo, A.I.; Alashram, M.G.; Eltohamy, K.M.; Li, J.B.; Xiang, Y.Z.; Zhang, F.C. Magnetized saline water irrigation enhances soil chemical and physical properties. Water 2022, 14, 4048. [Google Scholar] [CrossRef]
- Khoshravesh, M.; Mostafazadeh-Fard, B.; Mousavi, S.F.; Kiani, A.R. Effects of magnetized water on the distribution pattern of soil water with respect to time in trickle irrigation. Soil Use Manag. 2011, 27, 515–522. [Google Scholar] [CrossRef]
- Cai, R.; Yang, H.W.; He, J.S.; Zhu, W.P. Effects of magnetic fields on the water molecular structure in calcium chloride solutions. J. Tsinghua Univ. (Sci. Tech.) 2010, 50, 1404–1407. Available online: https://link.cnki.net/doi/10.16511/j.cnki.qhdxxb.2010.09.006 (accessed on 7 August 2024).
- Hamza, J.N.; Al-Sulttani, A.O. Impact of using magnetic water on the micro structure of leached saline-sodic soil. Environ. Monit. Assess. 2022, 194, 608. [Google Scholar] [CrossRef] [PubMed]
- Bu, D.S.; Feng, W.G.; Cai, L.H.; Zhou, L. Effects of magnetization water on desalinization in cotton farmland of under-film dripping irrigation in XinJiang Province. Trans. Chin. Soc. Agric. Eng. 2010, 26, 163–166. Available online: http://www.tcsae.org/cn/article/id/20101431 (accessed on 10 August 2024).
- Guo, B.; Han, H.B.; Chai, F. Influence of magnetic field on microstructural and dynamic properties of sodium, magnesium and calcium ions. Trans. Nonferrous Met. Soc. China 2011, 21, S494–S498. [Google Scholar] [CrossRef]
- Mostafazadeh-Fard, B.; Khoshravesh, M.; Mousavi, S.F.; Kiani, A.R. Effects of magnetized water on soil chemical components underneath trickle irrigation. J. Irrig. Drain. Eng. 2012, 138, 1075–1081. [Google Scholar] [CrossRef]
- Huang, C.Y.; Xu, J.M. Pedology, 3rd ed.; China Agric Press: Beijing, China, 2010. [Google Scholar]
- Liu, X.M.; Ma, F.Y.; Zhu, H.; Ma, X.S.; Guo, J.Y.; Wan, X.; Wang, L.; Wang, H.T.; Wang, Y.P. Effects of magnetized water treatment on growth characteristics and ion absorption, transportation and distribution in Populus × euramericana ‘Neva’ under NaCl stress. Can. J. For. Res. 2017, 47, 828–838. [Google Scholar] [CrossRef]
| Mineralization of Irrigation Water (g L−1) | Magnetization (T) | |||
|---|---|---|---|---|
| 0 (M0) | 0.2 (M2) | 0.4 (M4) | 0.6 (M6) | |
| 0.27 (S1) | M0S1 | M2S1 | M4S1 | M6S1 |
| 3.00 (S3) | M0S3 | M2S3 | M4S3 | M6S3 |
| 6.00 (S6) | M0S6 | M2S6 | M4S6 | M6S6 |
| 10.00 (S10) | M0S10 | M2S10 | M4S10 | M6S10 |
| Soluble Salt Ions | Content (mg kg−1) | ||||
|---|---|---|---|---|---|
| M0S1 | M2S1 | M0S3 | M2S3 | ||
| Cation | K+ | 18.80 ± 0.95 b | 21.42 ± 1.22 a | 22.70 ± 1.07 b | 26.24 ± 1.11 a |
| Na+ | 19.13 ± 2.63 a | 21.42 ± 1.29 a | 32.11 ± 1.52 b | 37.01 ± 1.80 a | |
| Ca2+ | 62.47 ± 3.15 b | 79.96 ± 3.49 a | 96.86 ± 4.57 b | 118.44 ± 3.04 a | |
| Mg2+ | 11.03 ± 1.52 b | 24.44 ± 3.36 a | 14.58 ± 0.69 b | 34.15 ± 4.69 a | |
| ∑ | 111.44 | 147.23 | 166.25 | 215.84 | |
| Anion | Cl− | 121.65 ± 7.88 a | 127.18 ± 6.48 a | 257.90 ± 12.18 a | 264.46 ± 12.29 a |
| HCO3− | 359.28 ± 9.92 b | 435.75 ± 19.14 a | 461.12 ± 11.89 b | 499.52 ± 16.44 a | |
| CO32− | 0.00 | 0.00 | 0.00 | 0.00 | |
| SO42− | 232.74 ± 5.53 a | 251.72 ± 8.12 a | 595.32 ± 28.11 b | 664.39 ± 31.74 a | |
| ∑ | 713.67 | 814.65 | 1314.34 | 1428.37 | |
| Soluble Salt Ions | Content (mg kg−1) | ||||
|---|---|---|---|---|---|
| M0S6 | M6S6 | M0S10 | M6S10 | ||
| Cation | K+ | 26.25 ± 1.27 a | 28.06 ± 3.10 a | 36.92 ± 1.60 b | 44.27 ± 2.22 a |
| Na+ | 92.18 ± 4.47 b | 110.61 ± 4.49 a | 184.85 ± 9.14 b | 214.64 ± 5.00 a | |
| Ca2+ | 184.83 ± 8.96 b | 237.00 ± 11.06 a | 294.95 ± 15.60 b | 358.70 ± 15.89 a | |
| Mg2+ | 50.12 ± 2.43 b | 77.69 ± 8.59 a | 53.93 ± 2.34 b | 64.77 ± 4.12 a | |
| ∑ | 353.39 | 453.36 | 570.64 | 682.39 | |
| Anion | Cl− | 510.31 ± 4.91 b | 554.38 ± 15.41 a | 734.67 ± 6.55 b | 772.21 ± 10.36 a |
| HCO3− | 620.62 ± 30.10 b | 680.91 ± 21.5 a | 840.77 ± 38.04 b | 963.73 ± 14.72 a | |
| CO32− | 0.00 | 0.00 | 0.00 | 0.00 | |
| SO42− | 538.91 ± 26.13 b | 654.59 ± 32.85 a | 1037.70 ± 29.07 b | 1124.55 ± 24.98 a | |
| ∑ | 1669.83 | 1889.87 | 2613.15 | 2860.48 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, M.; Zhao, Q.; Qiao, Y.; Ma, Y. Magnetized Saline Water Drip Irrigation Alters Soil Water-Salt Infiltration and Redistribution Characteristics. Water 2024, 16, 2693. https://doi.org/10.3390/w16182693
Xin M, Zhao Q, Qiao Y, Ma Y. Magnetized Saline Water Drip Irrigation Alters Soil Water-Salt Infiltration and Redistribution Characteristics. Water. 2024; 16(18):2693. https://doi.org/10.3390/w16182693
Chicago/Turabian StyleXin, Mingliang, Qiao Zhao, Ying Qiao, and Yingjie Ma. 2024. "Magnetized Saline Water Drip Irrigation Alters Soil Water-Salt Infiltration and Redistribution Characteristics" Water 16, no. 18: 2693. https://doi.org/10.3390/w16182693
APA StyleXin, M., Zhao, Q., Qiao, Y., & Ma, Y. (2024). Magnetized Saline Water Drip Irrigation Alters Soil Water-Salt Infiltration and Redistribution Characteristics. Water, 16(18), 2693. https://doi.org/10.3390/w16182693





