Abstract
Heavy metal pollution is a critical issue affecting the safety of drinking water sources. However, the impact of human activities on heavy metal risk levels in water-carrying lakes remains unclear. This study aims to explore the risk mechanisms of heavy metals in Luoma Lake, an important water-carrying lake for the South-to-North Water Diversion Project. We explored the spatial and temporal differences in the distribution of heavy metals in Lake Luoma using methods such as the heavy metal pollution index (HPI) and assessed the risk variations using a health assessment model. The results indicated that heavy metal concentrations in water-carrying lakes generally decreased during the dry season, with Mn and Zn levels decreasing by 89.3% and 56.2%, respectively. The comprehensive score of HPI decreased by 13.16% following the retreating polder compared to the control area (Non-retreating polder area). Furthermore, the HPI at the drinking water intake was lower, which is closely associated with the elevated dissolved oxygen (DO) and oxidation–reduction potential (ORP) resulting from water diversion. The annual average health risk across the entire lake was not significant, with higher levels observed in the control area. The annual non-carcinogenic risk levels of Mn, Ni, Cu, Zn, and Pb range from 10−13 to 10−9, which are considered negligible risk levels. Notably, the carcinogenic risk posed by arsenic (As) through the drinking pathway reached 10−5 a−1, exceeding the maximum levels recommended by certain organizations. These findings provide a critical foundation for managing heavy metals in water-carrying drinking water sources.
1. Introduction
Drinking water sources in rivers and lakes are essential for human survival and development, with their water quality being directly linked to human health [1,2,3]. However, rapid urbanization and industrialization in recent years have led to the influx of numerous pollutants into natural water bodies via surface runoff and groundwater recharge, posing serious threats to the safety of these water sources. These pollutants comprise conventional contaminants like nitrogen and phosphorus, as well as toxic and hazardous substances such as heavy metals, antibiotics, and other emerging contaminants [4,5,6]. Among them, heavy metals, compared to other pollutants, have typical characteristics such as a wide range of sources, persistence, high bioaccumulation, and significant toxicity. These properties prevent their degradation during the material cycle, making them likely to cause long-lasting effects on river and lake ecosystems [7,8]. Moreover, intensive aquaculture practices in these lakes have aggravated the problem by introducing additional heavy metals, primarily through fish feed, which can bioaccumulate within the aquatic food web. This underscores the need to understand the interactions between aquaculture activities and heavy metal pollution in such ecosystems.
Numerous studies have shown that the assessment of heavy metal health risks in rivers and lakes has become an essential part of water environment management [9,10,11,12]. As research on heavy metal pollution in rivers and lakes continues to deepen, the previous methods of characterizing and evaluating heavy metal pollution are gradually becoming insufficient to meet the needs of ecological environmental protection. Consequently, biotoxicology and human health assessments are increasingly being introduced as additional methods for evaluating the impact of heavy metal pollution in these water bodies [13,14,15]. Currently, the common practice for health risk assessment is to determine the health risk levels of heavy metals in the water bodies of the study area based on models and standards proposed by the U.S. Environmental Protection Agency (EPA) [16,17,18,19]. Heavy metals not only directly impact the distribution of invertebrates such as mayflies, stoneflies, and wiggler mosquitoes but also inhibit the growth, metabolism, and reproduction of vertebrates like freshwater tilapia, posing a threat to all aspects of freshwater ecosystems [20,21,22,23]. Beyond the toxicological effects of direct contact, the indirect harm is evident as some affected organisms at the base of the food chain are preyed upon, thereby introducing heavy metals into the biological network. The complex trophic relationships within these ecosystems exacerbate the extent and spread of heavy metal contamination [24,25]. It is worth noting that to quantify the harm caused by pollutants in water sources to exposed populations, experts and scholars worldwide have conducted extensive research on the health risks associated with water environments [26,27,28]. Dong et al. (2020) assessed the human health risks of seven targeted heavy metals in the main drinking water sources of Dalian, China, and found that Hg poses potential carcinogenic and non-carcinogenic risks to nearby populations [29]. Heavy metal carcinogens such as cadmium and lead have been detected as carcinogenic in the water of six major rivers and their associated drinking water sources in Korea, with lead levels exceeding the EPA’s acceptable threshold by more than 10 times [30]. Yinghuan Qin and Yuqiang Tao (2022) reviewed the distribution of heavy metals in Chinese lakes and found that 36.4% of the lakes had excessive levels of Cd, Hg, and Ni. Meanwhile, ecotoxicological studies on Eisenia fetida have revealed high bioaccumulation factors for Cd, Cu, and Zn, with significant bioaccumulation effects observed [20]. The study by Sivakumar Rajeshkumar et al. (2018) [31] demonstrated that the elevated concentrations of heavy metals in the water and sediments of Taihu Lake during winter and summer resulted in significantly higher accumulation of heavy metals in the tissues of fish and oysters during these seasons. Therefore, heavy metals in the aquatic environments of lakes and reservoirs can significantly impact plants and fish through bioaccumulation in the food chain, leading to the stunted growth or even death of aquatic organisms and amplifying risks to human health via the food chain [32,33]. While small amounts of heavy metal intake can be beneficial to human health, exceeding the threshold can seriously compromise the safety of water sources [34].
Currently, numerous studies have focused on drinking water sources in closed lakes, exploring the potential threat of heavy metals from various perspectives [29,35,36]. However, due to the slow flow rates and limited exchange capacity of closed lakes, the spatial distribution and transport dynamics of heavy metals and other pollutants in these environments are often less complex, leading to a more predictable and uniform dispersion pattern [37]. With the implementation of China’s South-to-North Water Diversion Project, many lakes along the route have transitioned from relatively closed lakes to water-carrying lakes. This transformation has strengthened the hydraulic connections between the lakes and increased the frequency of water exchange, resulting in comprehensive changes in the environmental behavior, storage characteristics, and transport patterns of heavy metals and other pollutants [38,39]. Meanwhile, to restore the storage function of water-carrying lakes, large-scale retreating polder projects have been implemented. The reshaping of topography and the expansion of water surface area have further increased the uncertainty in the distribution of heavy metals [40]. However, there is limited research on the health risk assessment of heavy metals in water-carrying lakes, particularly in the context of water exchange and seasonal changes. Additionally, studies investigating the impact of retreating polder projects on the spatial distribution and dynamic behavior of heavy metals in water-carrying lakes remain scarce. These gaps provide a basis for further exploration. Therefore, the objectives of this study are (1) to investigate the spatial and temporal characteristics of heavy metal pollution in water-carrying lakes under the influence of water diversion and transfer; (2) to examine the impact of retreating polders on the heavy metal content in the water body; and (3) to assess the risks posed by heavy metals in water-carrying lakes to human health, providing a theoretical basis for ensuring the safety of drinking water in these lakes under the influence of retreating polders.
2. Materials and Methods
2.1. Research Area
Luoma Lake is a typical water-carrying lake located in Jiangsu Province, spanning the cities of Xuzhou and Suqian (34°00′–34°11′ N, 118°04′–118°18′ E). The lake covers a total area of approximately 260 square kilometers, with a volume of about 920 million cubic meters and an average depth of 4.4 m, classifying it as a shallow lake (Figure 1). Compared to deep lakes, shallow lakes are more susceptible to external factors such as rainfall and runoff, which can influence the distribution of nutrients and pollutants, such as heavy metals. Additionally, seasonal temperature changes can rapidly affect the entire water body. In summer, shallow lakes may warm up more quickly, promoting the growth of algae and other plankton; in winter, the cooling effects are more evenly distributed across the entire water body. Luoma Lake is the seventh-largest freshwater lake in China and a crucial node in the eastern route of the South-to-North Water Diversion Project. Situated in the lower reaches of the Yishusi Basin, it receives inflows from the upper Nansi Lake, the mainstream of the Yi River, and the Pizhou–Cangshan area, covering approximately 58,000 square kilometers. As a vital flood control reservoir in the lower Yishusi Basin, Luoma Lake regulates water flow into and out of the lake, ensuring the smooth operation of the South-to-North Water Diversion Project. Over seven years, Luoma Lake has transferred a total of 4.61 billion cubic meters of water, serving as a primary input channel and storage reservoir for the project. In addition to its role in the water diversion project, Luoma Lake is an essential water source for the surrounding urban areas, including the Huang-Huai-Hai Plain and the Shandong Peninsula. Historical land reclamation activities have significantly reduced the lake’s surface area, leading to severe ecological pollution, especially heavy metal contamination. To ensure drinking water safety and restore the lake’s storage and regulatory functions, the retreating polder initiative involved removing embankments and aquaculture enclosures across 49.5 square kilometers. This effort cleared approximately 25.33 square kilometers of nets and cages, excavated around 35.33 square kilometers of dikes, and restored about 30 square kilometers of open water surface, thereby reducing nitrogen and phosphorus pollution and enhancing the lake’s water mobility and self-purification capacity.
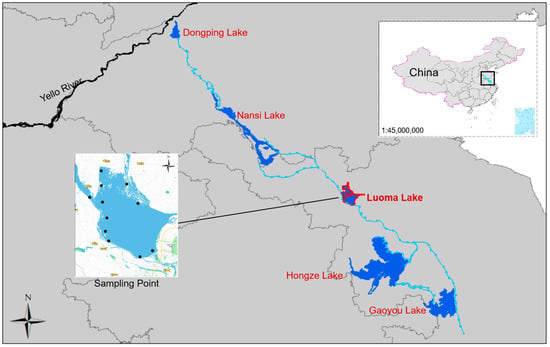
Figure 1.
Research area of Luoma Lake and sampling sites.
2.2. Sampling and Analysis
To systematically assess the impact of land reclamation on heavy metal pollution in the water body, 11 sampling points were established across various functional areas and geographical locations, including retreating polder areas, control areas, water source areas, and lake inlets and outlets (Figure 1). These sampling points were carefully chosen to represent key functional zones of Luoma Lake, such as the northern shore (retreating polder area), the southwestern shore (control area), and critical water inlets and outlets (as detailed in Table S1). The number of samples was determined based on the diversity of functional areas and geographical locations to ensure representativeness. Although no statistical method was used to calculate the sample size, the selection of sampling points was guided by the study’s objectives and the specific functions of the lake’s different regions. A total of 44 samples were collected, with grab sampling (instantaneous sampling) being employed at each point during two separate periods (July and December 2023), where two 1-L surface water samples were taken from each point—one for water quality analysis and the other for heavy metal concentration measurement. The samples were immediately stored in a refrigerated box at 4 °C to prevent any biological or chemical reactions that could alter the sample composition, ensuring the integrity of the samples during transport. To ensure representativeness and data accuracy, polyethylene bottles pre-cleaned and rinsed three times with sample water were used. For the determination of heavy metal concentrations, 10% nitric acid (HNO3) was added to the water samples on-site to lower the pH below 2. This acidification prevents the precipitation of metals and inhibits microbial activity, which could otherwise alter the concentrations of heavy metals during transport and storage. Laboratory analyses included water quality indicators and heavy metal concentrations. Water quality indicators were measured using national standard methods, including ammonia nitrogen (NH3-N), chlorophyll a (Chl-a), nitrate nitrogen (NO3-N), permanganate index (CODmn), total phosphorus (TP), turbidity (NTU), total nitrogen (TN), and total suspended solids (TSS) [41]. Field measurements included dissolved oxygen (DO), pH, electrical conductivity (EC), and oxidation–reduction potential (ORP), using portable instruments to ensure accuracy and timeliness. Heavy metal concentrations, including manganese (Mn), copper (Cu), lead (Pb), arsenic (As), nickel (Ni), and zinc (Zn), were analyzed using inductively coupled plasma mass spectrometry (ICP-MS, HJ 700-2014).
2.3. Evaluation Methods
2.3.1. Single Factor Pollution Index
The Single Factor Pollution Index (SFPI) is used to determine the degree of individual pollutants in water relative to their respective standard limits [42]. This index compares the actual concentration of a specific pollutant in a water sample with the corresponding environmental quality standard to determine the pollutant’s impact on the environment. The formula of SFPI is as follows:
where Pi is the SFPI of the i-th heavy metal element, Ci is the measured concentration of the i-th heavy metal element in the water sample, and Si is the environmental quality standard limit for the i-th pollutant based on the standards for drinking water sources of China (Table S2). When Pi ≤ 1, the concentration of the heavy metal is within the standard. When Pi > 1, the concentration exceeds the standard limit, indicating pollution risk and potential environmental impact, with higher values indicating more severe pollution. By calculating the Pi values of different heavy metal elements at various sampling points, the pollution status of these heavy metals in Luoma Lake can be intuitively assessed.
2.3.2. Heavy Metal Pollution Index
The Heavy Metal Pollution Index (HPI) is used to comprehensively evaluate the pollution status of multiple heavy metals in water bodies [43]. This method calculates a weighted concentration composite index of multiple heavy metal pollutants to measure the overall pollution level of the water body [44]. HPI is a comprehensive evaluation method that reflects the overall water quality status. The formula of HPI is as follows:
where Wi is the weight of the i-th parameter, Qi is the sub-index of the i-th parameter, and n is the number of parameters used.
The HPI was evaluated on the following basis: low pollution (HPI < 19), moderate pollution (19 ≤ HPI ≤ 38), and high pollution (HPI > 38).
2.3.3. Human Health Risks Assessment
According to the evaluation methods and health assessment models published by the EPA of the US, heavy metals in water bodies primarily expose humans through drinking water and skin contact [18,19]. Human health risks are assessed for both adults and children and are categorized into chemical carcinogenic risks and non-carcinogenic risks [1].
The formula of Di is as follows:
where Di is the average daily exposure dose per unit body weight of metal element w through drinking water, mg·(kg·d)−1; ρw is the average mass concentration of metal element w, mg·L−1; V is the daily water intake of humans, with an average of 2.2 L·d−1 for adults and 1 L·d−1 for children; tw is the exposure duration of metal element w, 70 years for carcinogenic elements and 35 years for non-carcinogenic elements; γ is the exposure frequency of metal element w, 365 d·a−1; m is the average body weight, 60 kg for adults and 25 kg for children; ta is the average exposure time, 25,550 days for carcinogenic metals and 12,775 days for non-carcinogenic metals.
The formula of the chemical carcinogenic health risk is as follows:
When is greater than 0.01, it is calculated as high-dose exposure:
The formula of the chemical non-carcinogenic health risk is as follows:
where and are the chemical carcinogenic and non-carcinogenic health risks of metal elements through drinking water exposure, a−1; fi is the carcinogenic slope factor of chemical carcinogenic metal element w through drinking water exposure, (kg·d)·mg−1; T is the human lifespan, calculated as an average of 78.18 years according to the Suqian Statistical Yearbook; is the daily intake reference dose of chemical non-carcinogenic metal element w through drinking water exposure, mg (kg·d)−1.
The formula of the average daily exposure dose per unit body weight through skin absorption is as follows:
where Dd is the average daily exposure dose per unit body weight of metal element w through skin absorption, mg·(kg·d)−1; S is the contact area of water with the skin, 18,000 cm2 for adults and 8000 cm2 for children; C is the permeation constant of heavy metal elements in water on the skin, cm·h−1; te is the exposure time, 0.6333 h·d−1 for adults and 0.4167 h·d−1 for children.
The formula of the chemical carcinogenic health risk of metal elements through skin absorption exposure is:
When is greater than 0.01, it is calculated as high-dose exposure:
The formula for the chemical non-carcinogenic health risk of metal elements through skin absorption exposure is:
where and are the chemical carcinogenic and non-carcinogenic health risks of metal elements through skin absorption exposure, a−1; fd is the carcinogenic slope factor of chemical carcinogenic metal element w through skin absorption exposure, (kg·d)·mg−1; is the daily intake reference dose of chemical non-carcinogenic metal element w through skin absorption exposure, mg (kg·d)−1. All reference values for heavy metal are listed in Table S3.
3. Results and Discussion
3.1. Temporal and Spatial Distribution of Heavy Metals
Table 1 presents the concentrations of heavy metals in various regions of Luoma Lake. Compared to the wet season (July), heavy metal concentrations in the water body significantly decreased during the dry season (December), with Mn and Zn showing reductions of 89.3% and 56.2%, respectively. The highest concentration of Mn (142.72 μg/L) was observed in the control area in July, while the lowest concentration (1.10 μg/L) occurred in the water source area in December, indicating that Mn levels are subject to significant seasonal regulation. The control area exhibited greater seasonal variability in heavy metal concentrations, particularly for Zn, which increased significantly during the dry season. In the control area, Zn (53.24 μg/L), Ni (1.60 μg/L), Cu (2.94 μg/L), and As (5.06 μg/L) reached their highest concentrations, which is likely attributable to human activities such as feed input. This increase is likely attributable to human activities such as feed input. Allah et al. (2024) confirmed that fish feed contains substantial amounts of heavy metals, including Pb, Cd, Cr, and Cu [45]. These heavy metals not only exacerbate concentration differences during feed application but also accumulate in the kidneys of fish, being excreted into the water through urination, defecation, and eventually released during decomposition after death [46,47]. In contrast, the lowest concentrations of Ni, Cu, Zn, As, and Pb were observed in the inlet/outlet areas, likely due to more frequent water exchange in these regions. This frequent water flow reduces the accumulation of heavy metals in the water body and facilitates their diffusion and migration, thus lowering local concentrations. The retreating polder area showed less influence from seasonal water conditions, indicating that the removal of aquaculture enclosures greatly benefits the control of heavy metal pollution. Notably, the concentrations of Zn and As in the water source area were relatively high and exhibited significant fluctuations, requiring special attention and further management.

Table 1.
Statistics of heavy metal concentrations (μg/L) in water bodies of the retreating polder area and various regions in Luoma Lake.
GIS technology is widely applied in environmental science, particularly in water quality monitoring. It allows for the visualization of discrete sampling data to illustrate spatial water quality distribution [48]. In this study, GIS combined with ordinary kriging interpolation was used to map the concentration distribution of Mn, Ni, Cu, Zn, As, and Pb in Luoma Lake during the rainy season (July) and dry season (December) (Figure 2). The figure indicates that heavy metal concentrations were higher in the northern and eastern regions during the wet season, but these levels decreased in the dry season. This pattern is likely related to surface runoff and precipitation. During the wet season, increased rainfall and surface runoff transport pollutants into the lake, whereas during the dry season, the reduced input of pollutants allows the lake’s self-purification capacity to become more evident. This also suggests that the restoration of the lake’s natural state has had a progressively more significant impact over time [49].
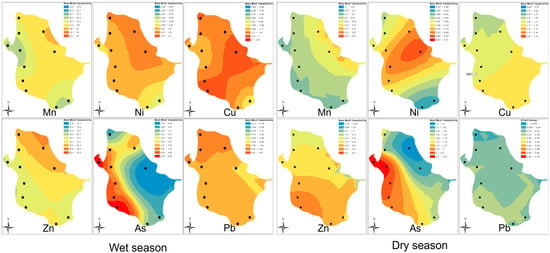
Figure 2.
Spatial distribution of heavy metal concentrations in Luoma Lake during the wet and dry seasons.
On the northern shore (retreating polder area), concentrations of all heavy metals decreased, particularly Ni, Zn, and Pb, shifting from higher levels during the wet season to lower levels in the dry season. This indicates that the retreating polder area has been effective in managing these heavy metal pollutants. Additionally, As and Cu concentrations were consistently higher on the western shore (control area) under both hydrological conditions, suggesting that fish farming activities may contribute to more severe As and Cu pollution [50]. Meanwhile, As concentrations were lower on the eastern shore, possibly due to easier outflow through the Yi River outlet. Higher concentrations of Cu and Ni near the lake’s inlets and outlets might indicate that these heavy metals are more likely to enter the lake through the Yi River and remain due to sedimentation and bioaccumulation processes [20].
In summary, the concentrations of heavy metals in Luoma Lake are influenced by both seasonal variations and the impact of the retreating polder project. During the wet season (July), increased rainfall and surface runoff bring a large amount of pollutants into the lake, causing a rise in heavy metal concentrations. In contrast, during the dry season (December), the reduction in pollutant input allows the lake’s self-purification processes to significantly reduce concentrations of Mn and Zn. In the control area, human activities, such as feed input, lead to peak concentrations of Zn, Ni, Cu, and As during the dry season, causing more variability. Meanwhile, the inlet/outlet areas show relatively low concentrations due to frequent water exchanges, which prevent heavy metal accumulation. In the retreating polder area, seasonal fluctuations are minimal, reflecting effective control of heavy metal pollution and improvements in water quality following the removal of aquaculture enclosures.
3.2. Heavy Metal Contamination Levels of Water-Carrying Lakes
Table 2 presents the Single Factor Pollution Index (SFPI) and Heavy Metal Pollution Index (HPI) for various regions under two hydrological conditions in Luoma Lake. The results indicate that all Pi values are below 1, signifying that the concentrations of individual heavy metals in Luoma Lake are within the acceptable limits set by China’s drinking water quality standards and do not reach the threshold for severe pollution. Notably, the Pi values for Mn were generally higher during the wet season (July), with a peak value of 0.858, suggesting that the wet season facilitates the mobilization of Mn into the water body, potentially leading to related environmental issues. During the wet season, the composite HPI was highest in the retreating polder area and control area, measuring 25.47 and 29.33, respectively, indicating a moderate level of pollution. The other regions exhibited low pollution levels. In contrast to the wet season, the HPI during the dry season (December) generally decreased, with a significant reduction observed in the retreating polder area, where the HPI dropped from the second-highest level in the wet season to the lowest in the dry season, decreasing from 25.47 to 7.15. This suggests that the management measures implemented in the retreating polder area effectively reduced heavy metal pollution during the dry season [51]. In contrast, the control area displayed distinct trends. Despite significant fluctuations in Mn and Zn concentrations between the wet and dry seasons, the composite HPI remained consistently high across both periods. This persistence might be attributed to aquaculture activities within the control area, where the application of feed could exacerbate heavy metal accumulation. The heavy metal pollution indices in the water source and lake inlet/outlet regions remained low throughout both seasons, indicating relatively stable pollution levels. However, Zn and As concentrations exhibited significant variability due to the influence of water diversion. Overall, the heavy metal pollution in Luoma Lake exhibits marked temporal and spatial variations, particularly driven by water diversion and polder retreat, which further exacerbates the distribution and concentration of heavy metals [35].

Table 2.
Single factor pollution index and heavy metal pollution index for different regions in Luoma Lake.
3.3. Impact of Human Activities on Heavy Metal
The study of correlations between heavy metals and water quality indicators during the wet and dry seasons in Luoma Lake (Figure 3) revealed significant temporal and spatial variations. In the wet season, Mn, Ni, Cu, Zn, and Pb exhibited strong correlations with most water quality indicators, particularly with NTU, TSS, and CODmn. These heavy metals were negatively correlated with DO and pH, with the correlation being stronger for DO. This indicates that heavy metal pollution during the wet season is likely closely linked to water turbidity and suspended solids content, and the decrease in dissolved oxygen is also a contributing factor to the increase in heavy metal concentrations [37]. In the dry season, Mn displayed significant negative correlations with DO, ORP, and NTU, suggesting that the dissolved oxygen levels and redox state of the water significantly influence the behavior and distribution of heavy metals. With reduced water mobility in the dry season, heavy metals are more prone to settling and accumulation, particularly under poor redox conditions [52]. Zn had significant positive correlations with TN and NTU, indicating that Zn pollution is closely associated with nutrient content and water turbidity. As exhibited a significant positive correlation with ORP, suggesting that the form and concentration of As in the water may be influenced by the redox environment.
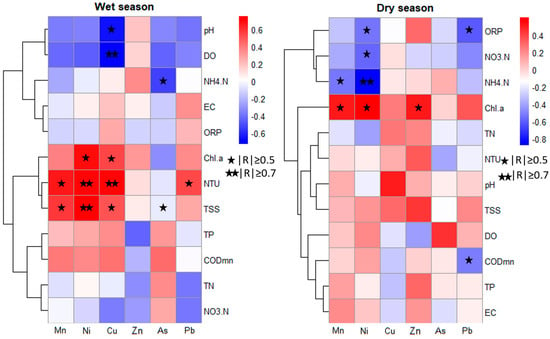
Figure 3.
Correlation analysis between heavy metals and environmental factors in Luoma Lake.
Luoma Lake, serving as a crucial regulating reservoir for the eastern route of the South-to-North Water Diversion Project, receives a substantial inflow from the upstream Yishusi River Basin. Water diversion activities significantly influence its hydrodynamics and water quality [33]. Figure 4 illustrates the daily water level and storage capacity of Luoma Lake in 2023. The water level of Luoma Lake exhibits marked monthly fluctuations, peaking in January (up to 23.19 m) and reaching its lowest in April (a minimum of 21.87 m), with water levels during the dry season (December) being lower compared to the wet season (July). Water diversion alters the lake’s residence time, water mobility, hydrological processes, temperature, dissolved oxygen, and nutrient distribution [40]. In our previous research, Luoma Lake’s water quality demonstrated distinct seasonal dynamics, with poorer water quality and a higher risk of eutrophication during the wet season (summer and autumn) and relatively better water quality during the dry season (winter and spring). The water quality in the southern part of the lake is significantly better than in the northern part, with pollution loads from the Yi River and the Grand Canal increasing with inflow volume, further driving spatial variations in Luoma Lake’s water quality. The water diversion process may introduce exogenous heavy metal pollution, altering the input-output balance of heavy metals in the lake. When the diverted water quality is good, it can reduce heavy metal concentrations in certain areas through dilution effects [35]. Changes in water flow speed and direction can lead to variations in the distribution and migration of heavy metals within the lake, potentially increasing concentrations in areas around the water inlets and outlets. Additionally, changes in the mixing degree of the water body can affect the vertical and horizontal distribution of heavy metals, potentially releasing them from sediments and increasing their concentration in the water.
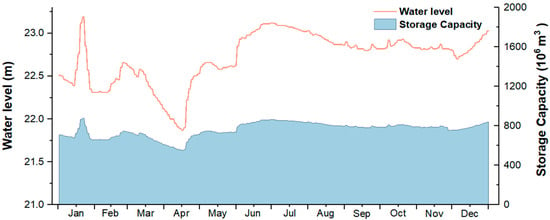
Figure 4.
Daily water level and storage capacity of Luoma Lake in 2023.
Luoma Lake’s heavy metal pollution originates from various sources; our previous research indicates that direct discharges from northern inflowing rivers and aquaculture-related agricultural activities are the primary pathways for heavy metals to enter the lake. Therefore, the retreating polder measures implemented in the northern lake areas play a critical role in directly mitigating heavy metal pollution. Meanwhile, the retreating polder strategy restored the lake and its wetlands, improving water environmental factors and indirectly affecting the concentration and distribution of heavy metals in the water. Jiayi et al. (2020) predicted, using a two-dimensional unsteady water environment mathematical model, that after implementing the retreating polder strategy, the annual average concentrations of COD, TN, and TP in the lake area would decrease by 6.8%, 5.2%, and 10%, respectively [53]. This improvement in water quality substantially reduces organic pollutants and nutrients in the water, thereby enhancing overall water quality. Juhua Luo et al. (2020) [53] found that the expansion of fish farming areas was positively correlated with NH3-N, TN, chl-a, TP, and COD and negatively correlated with SDD and DO. The retreating polder strategy can reverse these trends by increasing dissolved oxygen content and water transparency, thus enhancing the water body’s self-purification capacity. The retreating polder strategy significantly affects the concentration and distribution of heavy metals in the water body by improving water environment factors. The increase in dissolved oxygen alters the redox environment of the water body, changing the oxidation states of certain heavy metals (e.g., iron, manganese), leading to their precipitation and reduced dissolved concentrations in the water. Increased transparency indicates a reduction in suspended particles, which are typically carriers of heavy metals; thus, reducing suspended particles can decrease the mobility and bioavailability of heavy metals [52]. Furthermore, enhanced water mobility and improved water quality help stabilize sediments, reducing the resuspension of heavy metals and consequently lowering their concentration and toxicity. The reduction in TN and TP decreases the excessive accumulation of nutrients, which inhibits algal blooms and biomass growth, indirectly reducing the bioaccumulation and trophic transfer of heavy metals [54]. Through these mechanisms, the retreating polder strategy contributes to improving the ecological condition of the water body and mitigating the risk of heavy metal pollution [39]. This strategy also significantly enhances the hydrodynamic conditions of the lake, facilitating smoother water flow. These changes affect the distribution of heavy metals within the water body. Specifically, areas with higher flow velocities tend to promote the diffusion and migration of heavy metals, reducing their accumulation in both water and sediments thereby lowering their concentrations in local areas [55]. Additionally, the shortened water exchange cycle enhances the lake’s self-purification capacity, accelerating the natural dilution and removal processes of heavy metals, further improving water quality. However, stronger hydrodynamic conditions may also have some negative impacts. In high-flow areas, the biomass and diversity of aquatic plants are typically lower, as these plants struggle to establish stable growth in such environments. Despite this, the overall improvement in environmental capacity due to the retreating polder strategy positively impacts the diversity of aquatic plants. Aquatic plants play a crucial role in improving water quality by adsorbing and accumulating heavy metals through their roots, stems, and leaves. Studies have shown that aquatic plants have a high adsorption capacity for heavy metals such as Pb, Cd, Cu, and Zn [32]. These plants significantly reduce the concentrations of heavy metals in the water through bioaccumulation and biosorption processes, contributing to the maintenance and restoration of the lake ecosystem’s health and stability.
3.4. Health Risk of Heavy Metals in Water-Carrying Lakes
Figure 5 presents the annual average health risks of Mn, Ni, Cu, Zn, As, and Pb for adults and children at each sampling point. The health risks associated with heavy metals exhibit significant temporal and spatial variations. During the wet season, the non-carcinogenic health risks of heavy metals at all sampling points generally fall within the order of 10−10, which is higher than the dry season’s order of 10−11. For carcinogenic risks, the levels are similar under both hydrological conditions, around 10−6, but slightly higher during the wet season, consistent with the findings of Awaz et al. (2022) [56]. Notably, the health risk from Pb at sampling point R1 during the wet season is relatively high, reaching 2.17 × 10−9 for adults and 2.37 × 10−9 for children. Additionally, the non-carcinogenic health risks from Mn at points R1, C1, and I1 during the wet season are elevated, with these points concentrated on the northern shore of Luoma Lake. Excessive intake of Mn could lead to neurological disorders, liver damage, and cardiovascular diseases, highlighting the need to address the Mn health risk in this area [57,58]. There is considerable fluctuation in carcinogenic health risks among sampling points during the wet season, with the control area exhibiting significantly higher risks than the retreating polder area [59]. During the dry season, the health risks of Pb at C1 and W1 are relatively high, as well as Zn at C1. Although overall risk levels during the dry season are lower, specific heavy metal risks at certain points still warrant close attention.
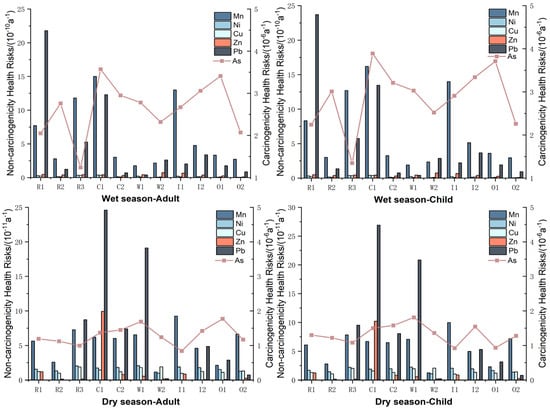
Figure 5.
Annual health risks for humans in Luoma Lake.
Table 3 presents the annual average health risks for adults and children caused by heavy metals (Mn, Ni, Cu, Zn, As, and Pb) in Luoma Lake through drinking water and dermal pathways. The annual average health risks for these metals via the drinking pathway are higher than those via dermal absorption, indicating that drinking water is the primary route of exposure. For the drinking pathway, the annual average health risks posed by these metals are lower for adults than for children. In contrast, for the dermal pathway, the annual average health risks are higher for adults than for children, highlighting age-related differences in the health risks associated with metal exposure.

Table 3.
Per capita annual health risks caused by metals through the drinking and dermal pathways, respectively.
The annual average health risks for the carcinogenic metal element As generally range from 10−8 to 10−5, with the highest level observed during the wet season for children via the drinking pathway (3.15 × 10−5 a−1). Table S4 lists the maximum acceptable risk levels and negligible risk levels recommended by various global institutions. It is evident that the carcinogenic health risks from As through the drinking pathway have already exceeded the maximum acceptable risk levels recommended by some institutions, emphasizing the need for special attention to the cancer risk posed by As, particularly since Luoma Lake is a crucial water source for the surrounding area [28]. The carcinogenic health risks from the dermal pathway are generally in the order of 10−8, remaining within the maximum acceptable risk levels. The annual average health risks for non-carcinogenic metal elements Mn, Ni, Cu, Zn, and Pb for both adults and children generally range from 10−13 to 10−9, considered negligible risk levels by the Netherlands Ministry of Housing, Spatial Planning and the Environment and the Royal Society (UK), posing no significant harm to the exposed population in the basin [58]. As remains the primary contributor to the annual average health risks for the population. Various methods have been developed to control arsenic levels in water bodies, with ion exchange [60], modified coagulation [61,62], adsorption-colloid-flotation [63], and photocatalysis [64] being widely studied. In Yangzonghai Lake, China, a direct atomization method involving ferric chloride (FeCl3) was used to reduce arsenic levels, achieving a removal efficiency of up to 82% [65], providing an example of large-scale arsenic mitigation efforts that can serve as a model for policy-based control of arsenic in lakes. Additionally, while the current health risks posed by other heavy metals such as Mn, Ni, Cu, and Zn are relatively low, they could become significant threats in the near future if not properly controlled. Although the annual average non-carcinogenic risks for these metals are considered negligible, the fluctuations in their concentrations at certain sampling points, particularly in areas affected by anthropogenic activities and dynamic hydrological conditions, warrant close attention. Over time, the accumulation of these heavy metals could exacerbate their spread and bioaccumulation in the ecosystem. Therefore, it is recommended that proactive management strategies be implemented, including regular monitoring, early warning systems, and targeted control measures for specific pollution sources, to prevent these metals from posing greater risks to the lake’s ecosystem and the surrounding population in the future.
4. Conclusions
This study provides valuable insights into the temporal and spatial distribution, contamination levels, and health risks associated with heavy metals in water-carrying lakes. The findings emphasize the influence of seasonal variations, human activities, and hydrodynamic conditions on heavy metal pollution. Key conclusions are summarized as follows:
- (a)
- Temporal and Spatial Distribution of Heavy Metals
This study found that the heavy metal content in water-carrying lakes generally decreased during the dry season compared to the wet season, with Mn and Zn showing reductions of 89.3% and 56.2%, respectively. Additionally, the control area showed the greatest seasonal variability in heavy metal concentrations. The retreating polder area showed minimal seasonal influence, suggesting that the removal of aquaculture enclosures has helped control heavy metal pollution;
- (b)
- Heavy metal contamination levels of water-carrying lakes
The highest integrated heavy metal pollution index (HPI) in the retreating polder and control areas during the wet season was 25.47 and 29.33, respectively, indicating medium pollution levels, while other areas exhibited low pollution levels. This suggests that human activities, such as polder construction, significantly increase heavy metal pollution in drinking water sources. Compared to the control area, the retreating polder area not only exhibited lower heavy metal concentrations and variability but also showed a substantial reduction in HPI;
- (c)
- Impact of Human Activities on Heavy Metal
The water transfer project greatly enhanced the hydrodynamic conditions and water exchange in the western part of the lake, significantly reducing heavy metal content, particularly at water intakes and the inlets and outlets of the water source. However, significant positive correlations were found between heavy metals (Mn, Ni, Cu, Zn, and Pb) and turbidity and total suspended solids, indicating that increased water transfer during the season could elevate heavy metal content and risk in areas with poor hydrodynamic conditions;
- (d)
- Health Risk of Heavy Metals in water-carrying lakes
The non-carcinogenic health risk for the entire lake during the wet season was on the order of 10−10, which is one order of magnitude higher than during the dry season. The carcinogenic health risk in the control area was significantly higher than in the retreating polder area. Except for As, the average annual health risks posed by heavy metals in the entire lake were not significant for adults and children. However, the average annual health risk from As exceeded the maximum acceptable risk recommended by some organizations, particularly the carcinogenic risk via the drinking pathway during the wet season, making it a key factor in drinking water safety. Notably, the mean annual health risks of all metal elements via the drinking water route were higher than those via the dermal pathway. Adults had lower mean annual health risks than children via the drinking water route but higher risks than children via the dermal pathway, suggesting variability in health risks across different age groups. Future studies should focus on key risk factors such as arsenic (As), particularly its environmental behavior and the factors influencing its distribution under varying hydrodynamic conditions, which are crucial for ensuring drinking water safety.
This study has several limitations. Firstly, it does not consider the distribution of heavy metals at varying water depths, which could significantly affect their migration and deposition patterns. Secondly, the analysis is based on short-term data, limiting the ability to observe long-term trends in heavy metal contamination. Lastly, the insufficient exploration of key risk factors such as arsenic (As), particularly its environmental behavior and the factors influencing its distribution under varying hydrodynamic conditions. These limitations highlight areas for further investigation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w16182699/s1, Table S1: Sampling Point Information of Luoma Lake; Table S2: Evaluation Standards for Heavy Metal Concentrations in Water; Table S3: Values of parameters related to the health risk assessment [66]; Table S4: Recommended maximum acceptable risk levels and negligible risk levels by various global institutions.
Author Contributions
J.W.; writing—original draft preparation, X.Z.; writing—original draft preparation and investigation, Y.D.; formal analysis, M.X.; resources, D.W.; conceptualization, Y.H.; resources, W.L.; methodology, Y.S.; software, F.S.; conceptualization and supervision, L.Y.; validation, Y.Z.; resources, Q.Z.; conceptualization, supervision, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was jointly supported by the National Key Research and Development Program of China (2023YFC3208804), the National Natural Science Foundation of China (52121006, 52309096, and U2040209), the China Postdoctoral Science Foundation (2023TQ0163 and 2024M751470), the Jiangsu Water Conservancy Science and Technology Project (2021005), the Central Public-interest Scientific Institution Basal Research Fund of China (Y923007), and the Water Resources Young Top Talent Grant Program (2022026).
Data Availability Statement
The datasets used or analysed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
Author Li Yao and Yiming Zhen were employed by the company China Water Resources Pearl River Planning Surveying & Designing Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Wang, J.; Yu, Y.; Jiang, J.; Li, B.; Xie, W.; Li, G.; Song, H.; Zhai, W.; Li, Y. Study on the Distribution Characteristics and Risk Assessment of Antibiotics and Resistance Genes in Water Sources of Wuhan. Toxics 2024, 12, 507. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.; Singh, A.K. Risk Assessment, Statistical Source Identification and Seasonal Fluctuation of Dissolved Metals in the Subarnarekha River, India. J. Hazard. Mater. 2014, 265, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Wang, X.; Li, J.; Gao, X.; Zhang, J.; Liu, Z. Ecological and Health Risk Assessments and Water Quality Criteria of Heavy Metals in the Haihe River. Environ. Pollut. 2021, 290, 117971. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, Q.; Ren, B.; Luo, J.; Yuan, J.; Ding, X.; Bian, H.; Yao, X. Trends and Health Risks of Dissolved Heavy Metal Pollution in Global River and Lake Water from 1970 to 2017. In Reviews of Environmental Contamination and Toxicology Volume 251; de Voogt, P., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–24. ISBN 978-3-030-27149-7. [Google Scholar]
- Lu, H.; Yu, S. Spatio-Temporal Variational Characteristics Analysis of Heavy Metals Pollution in Water of the Typical Northern Rivers, China. J. Hydrol. 2018, 559, 787–793. [Google Scholar] [CrossRef]
- Barakat, M.A. New Trends in Removing Heavy Metals from Industrial Wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Varol, M. Assessment of Heavy Metal Contamination in Sediments of the Tigris River (Turkey) Using Pollution Indices and Multivariate Statistical Techniques. J. Hazard. Mater. 2011, 195, 355–364. [Google Scholar] [CrossRef]
- Azeez, N.A.; Dash, S.S.; Gummadi, S.N.; Deepa, V.S. Nano-Remediation of Toxic Heavy Metal Contamination: Hexavalent Chromium [Cr(VI)]. Chemosphere 2021, 266, 129204. [Google Scholar] [CrossRef]
- Fang, X.; Peng, B.; Wang, X.; Song, Z.; Zhou, D.; Wang, Q.; Qin, Z.; Tan, C. Distribution, Contamination and Source Identification of Heavy Metals in Bed Sediments from the Lower Reaches of the Xiangjiang River in Hunan Province, China. Sci. Total Environ. 2019, 689, 557–570. [Google Scholar] [CrossRef]
- Liu, B.; Xu, M.; Wang, J.; Wang, Z.; Zhao, L. Ecological Risk Assessment and Heavy Metal Contamination in the Surface Sediments of Haizhou Bay, China. Mar. Pollut. Bull. 2021, 163, 111954. [Google Scholar] [CrossRef]
- Kalani, N.; Riazi, B.; Karbassi, A.; Moattar, F. Measurement and Ecological Risk Assessment of Heavy Metals Accumulated in Sediment and Water Collected from Gomishan International Wetland, Iran. Water Sci. Technol. 2021, 84, 1498–1508. [Google Scholar] [CrossRef]
- El Azhari, A.; Rhoujjati, A.; El Hachimi, M.L.; Ambrosi, J. Pollution and Ecological Risk Assessment of Heavy Metals in the Soil-Plant System and the Sediment-Water Column around a Former Pb/Zn-Mining Area in NE Morocco. Ecotoxicol. Environ. Saf. 2017, 144, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Al-Hashim, M.H.; El-Sorogy, A.S.; Al Qaisi, S.; Alharbi, T. Contamination and Ecological Risk of Heavy Metals in Al-Uqair Coastal Sediments, Saudi Arabia. Mar. Pollut. Bull. 2021, 171, 112748. [Google Scholar] [CrossRef] [PubMed]
- Zhaoyong, Z.; Abuduwaili, J.; Fengqing, J. Heavy Metal Contamination, Sources, and Pollution Assessment of Surface Water in the Tianshan Mountains of China. Environ. Monit. Assess. 2015, 187, 33. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.; Wu, Q.; Liu, P.; Hu, W.; Huang, B.; Shi, B.; Zhou, Y.; Kwon, B.-O.; Choi, K.; Ryu, J.; et al. Ecological Risk Assessment of Heavy Metals in Sediments and Water from the Coastal Areas of the Bohai Sea and the Yellow Sea. Environ. Int. 2020, 136, 105512. [Google Scholar] [CrossRef] [PubMed]
- Saha, N.; Rahman, M.S.; Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Industrial Metal Pollution in Water and Probabilistic Assessment of Human Health Risk. J. Environ. Manag. 2017, 185, 70–78. [Google Scholar] [CrossRef]
- Jiang, Y.; Chao, S.; Liu, J.; Yang, Y.; Chen, Y.; Zhang, A.; Cao, H. Source Apportionment and Health Risk Assessment of Heavy Metals in Soil for a Township in Jiangsu Province, China. Chemosphere 2017, 168, 1658–1668. [Google Scholar] [CrossRef]
- Yu, H.; Lin, M.; Peng, W.; He, C. Seasonal Changes of Heavy Metals and Health Risk Assessment Based on Monte Carlo Simulation in Alternate Water Sources of the Xinbian River in Suzhou City, Huaibei Plain, China. Ecotoxicol Env. Saf 2022, 236, 113445. [Google Scholar] [CrossRef]
- Andersen, M.E.; Meek, M.E.; Boorman, G.A.; Brusick, D.J.; Cohen, S.M.; Dragan, Y.P.; Frederick, C.B.; Goodman, J.I.; Hard, G.C.; O’Flaherty, E.J.; et al. Lessons Learned in Applying the U.S. EPA Proposed Cancer Guidelines to Specific Compounds. Toxicol. Sci. 2000, 53, 159–172. [Google Scholar] [CrossRef]
- Coelho, C.; Foret, C.; Bazin, C.; Leduc, L.; Hammada, M.; Inácio, M.; Bedell, J.P. Bioavailability and Bioaccumulation of Heavy Metals of Several Soils and Sediments (from Industrialized Urban Areas) for Eisenia Fetida. Sci. Total Environ. 2018, 635, 1317–1330. [Google Scholar] [CrossRef]
- Miranda, L.S.; Wijesiri, B.; Ayoko, G.A.; Egodawatta, P.; Goonetilleke, A. Water-Sediment Interactions and Mobility of Heavy Metals in Aquatic Environments. Water Res. 2021, 202, 117386. [Google Scholar] [CrossRef]
- Shang, G.; Wang, X.; Zhu, L.; Liu, S.; Li, H.; Wang, Z.; Wang, B.; Zhang, Z. Heavy Metal Pollution in Xinfengjiang River Sediment and the Response of Fish Species Abundance to Heavy Metal Concentrations. Int. J. Environ. Res. Public Health 2022, 19, 11087. [Google Scholar] [CrossRef] [PubMed]
- Harguinteguy, C.A.; Cirelli, A.F.; Pignata, M.L. Heavy Metal Accumulation in Leaves of Aquatic Plant Stuckenia Filiformis and Its Relationship with Sediment and Water in the Suquía River (Argentina). Microchem. J. 2014, 114, 111–118. [Google Scholar] [CrossRef]
- Rizk, R.; Juzsakova, T.; Ben Ali, M.; Rawash, M.A.; Domokos, E.; Hedfi, A.; Almalki, M.; Boufahja, F.; Shafik, H.M.; Rédey, Á. Comprehensive Environmental Assessment of Heavy Metal Contamination of Surface Water, Sediments and Nile Tilapia in Lake Nasser, Egypt. J. King Saud Univ. Sci. 2022, 34, 101748. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, N.; Liu, D.; Zhang, Y. Assessment and Source Analysis of Heavy Metal Contamination in Water and Surface Sediment in Dongping Lake, China. Chemosphere 2022, 307, 136016. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Mazumder, M.A.J.; Al-Attas, O.; Husain, T. Heavy Metals in Drinking Water: Occurrences, Implications, and Future Needs in Developing Countries. Sci. Total Environ. 2016, 569–570, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Cai, N.; Li, L.; Zhu, H.; Chen, L.; Li, S.; Meng, F.; Zhang, X. Multiple Evaluations, Risk Assessment, and Source Identification of Heavy Metals in Surface Water and Sediment of the Golmud River, Northeastern Qinghai-Tibet Plateau, China. Front. Environ. Sci. 2023, 10. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, C.; Zhao, X.; Dong, J.; Zheng, B. Risk Assessment of Heavy Metals in the Surface Sediment at the Drinking Water Source of the Xiangjiang River in South China. Environ. Sci. Eur. 2020, 32, 23. [Google Scholar] [CrossRef]
- Dong, W.; Zhang, Y.; Quan, X. Health Risk Assessment of Heavy Metals and Pesticides: A Case Study in the Main Drinking Water Source in Dalian, China. Chemosphere 2020, 242, 125113. [Google Scholar] [CrossRef]
- Yong, C.; Dongchun, S.; Seongeun, P.; Yeongwook, L.; Yoonho, C.; Seongjoon, C.; Jeeyeon, Y.; Mansik, H.; Yeosin, P.; Hyun, L. Risk Assessment and Management of Drinking Water Pollutants in Korea. Water Sci. Technol. 1997, 36, 309–323. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Liu, Y.; Zhang, X.; Ravikumar, B.; Bai, G.; Li, X. Studies on seasonal pollution of heavy metals in water, sediment, fish and oyster from the Meiliang Bay of Taihu Lake in China. Chemosphere 2018, 191, 626–638. [Google Scholar]
- Kong, W.; Xu, Q.; Lyu, H.; Kong, J.; Wang, X.; Shen, B.; Bi, Y. Sediment and Residual Feed from Aquaculture Water Bodies Threaten Aquatic Environmental Ecosystem: Interactions among Algae, Heavy Metals, and Nutrients. J. Environ. Manage 2023, 326, 116735. [Google Scholar] [CrossRef] [PubMed]
- Castro, L.N.; Rendina, A.E.; Orgeira, M.J. Assessment of Toxic Metal Contamination Using a Regional Lithogenic Geochemical Background, Pampean Area River Basin, Argentina. Sci. Total Environ. 2018, 627, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Zhou, F.; Zhang, X.; Qin, J.; Li, H. Distribution of Heavy Metals in Soils and Vegetables and Health Risk Assessment in the Vicinity of Three Contaminated Sites in Guangdong Province, China. Hum. Ecol. Risk Assess. Int. J. 2018, 24, 1901–1915. [Google Scholar] [CrossRef]
- Nyantakyi, A.J.; Akoto, O.; Fei-Baffoe, B. Seasonal Variations in Heavy Metals in Water and Sediment Samples from River Tano in the Bono, Bono East, and Ahafo Regions, Ghana. Environ. Monit. Assess. 2019, 191, 570. [Google Scholar] [CrossRef]
- Duodu, G.O.; Goonetilleke, A.; Ayoko, G.A. Comparison of Pollution Indices for the Assessment of Heavy Metal in Brisbane River Sediment. Environ. Pollut. 2016, 219, 1077–1091. [Google Scholar] [CrossRef]
- Popa, C.L.; Dontu, S.I.; Levei, E.A.; Ioja, C.I.; Popa, A.-M.; Miclean, M.; Hoaghia, M.-A.; Cadar, O.; Carstea, E.M. Spatial Variation of Organochlorine Pesticides and Dissolved Organic Matter in Urban Closed Lakes. J. Environ. Sci. Health Part B 2020, 55, 329–341. [Google Scholar] [CrossRef]
- Kong, M.; Zhu, Y.; Han, T.; Zhang, S.; Li, J.; Xu, X.; Chao, J.; Zhang, Y.; Gao, Y. Interactions of Heavy Metal Elements across Sediment-Water Interface in Lake Jiaogang. Environ. Pollut. 2021, 286, 117578. [Google Scholar] [CrossRef]
- Bi, B.; Liu, X.; Guo, X.; Lu, S. Occurrence and Risk Assessment of Heavy Metals in Water, Sediment, and Fish from Dongting Lake, China. Environ. Sci. Pollut. Res. 2018, 25, 34076–34090. [Google Scholar] [CrossRef]
- Wang, J.; Gao, M.; Guo, H.; Chen, E. Spatiotemporal Distribution and Historical Evolution of Polders in the Dongting Lake Area, China. J. Geogr. Sci. 2016, 26, 1561–1578. [Google Scholar] [CrossRef]
- Rice, E.W.; Bridgewater, L. American Public Health Association Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012; Volume 10. [Google Scholar]
- Reta, G.; Dong, X.; Li, Z.; Bo, H.; Yu, D.; Wan, H.; Su, B. Application of Single Factor and Multi-Factor Pollution Indices Assessment for Human-Impacted River Basins: Water Quality Classification and Pollution Indicators. Nat. Environ. Pollut. Technol. 2019, 18, 1063–1072. [Google Scholar]
- Sajil Kumar, P.J.; Davis Delson, P.; Thomas Babu, P. Appraisal of Heavy Metals in Groundwater in Chennai City Using a HPI Model. Bull. Environ. Contam. Toxicol. 2012, 89, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Vasistha, P.; Ganguly, R. Assessment of spatio-temporal variations in lake water body using indexing method. Environ. Sci. Pollut. Res. 2020, 27, 41856–41875. [Google Scholar] [CrossRef] [PubMed]
- Rajar, A.B.; Malik, Z.; Ujan, J.A.; Rind, K.H.; Ullah, R.; Naz, S.; Ullah, M.; Zahid, M.; Khan, K.; Khayyam, K.; et al. Implications of Heavy Metal Accumulation in Fish Feed, Water, Sediment, and Different Fish Species in a Polyculture System. Biol. Trace Elem. Res. 2024. [Google Scholar] [CrossRef] [PubMed]
- Habib, S.S.; Batool, A.I.; Rehman, M.F.U.; Naz, S. Evaluation and Association of Heavy Metals in Commonly Used Fish Feed with Metals Concentration in Some Tissues of O. Niloticus Cultured in Biofloc Technology and Earthen Pond System. Biol. Trace Elem. Res. 2023, 201, 3006–3016. [Google Scholar] [CrossRef]
- Habib, S.S.; Batool, A.I.; Rehman, M.F.U.; Naz, S. Assessment and Bioaccumulation of Heavy Metals in Fish Feeds, Water, and Some Tissues of Cyprinus Carpio Cultured in Different Environments (Biofloc Technology and Earthen Pond System). Biol. Trace Elem. Res. 2023, 201, 3474–3486. [Google Scholar] [CrossRef] [PubMed]
- Vasistha, P.; Ganguly, R. Water quality assessment in two lakes of Panchkula, Haryana, using GIS: Case study on seasonal and depth wise variations. Environ. Sci. Pollut. Res. 2022, 29, 43212–43236. [Google Scholar]
- Pratiwi, D.; Sumiarsa, D.; Oktavia, D.; Sunardi, S. Water Quality Influences Self-Purification in the Cihawuk and Majalaya Segments Upstream of the Citarum River, West Java, Indonesia. Water 2023, 15, 2998. [Google Scholar] [CrossRef]
- Ehiemere, V.C.; Ihedioha, J.N.; Ekere, N.R.; Ibeto, C.N.; Abugu, H.O. Pollution and Risk Assessment of Heavy Metals in Water, Sediment and Fish (Clarias Gariepinus) in a Fish Farm Cluster in Niger Delta Region, Nigeria. J. Water Health 2022, 20, 927–945. [Google Scholar] [CrossRef]
- Haidery, A.; Umar, R.; Khan, I. Seasonal Variation and Spatial Distribution of Heavy Metal (Loid)s Concentration in Groundwater and Surface Water from Hard-Rock Terrain, Ranchi, India. Environ. Dev. Sustain. 2024. [Google Scholar] [CrossRef]
- Wang, K.; Aji, D.; Li, P.; Hu, C. Characterization of Heavy Metal Contamination in Wetland Sediments of Bosten Lake and Evaluation of Potential Ecological Risk, China. Front. Environ. Sci. 2024, 12. [Google Scholar] [CrossRef]
- Luo, J.; Pu, R.; Ma, R.; Wang, X.; Lai, X.; Mao, Z.; Zhang, L.; Peng, Z.; Sun, Z. Mapping Long-Term Spatiotemporal Dynamics of Pen Aquaculture in a Shallow Lake: Less Aquaculture Coming along Better Water Quality. Remote Sens. 2020, 12, 1866. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E. Trophic Transfer, Bioaccumulation, and Biomagnification of Non-Essential Hazardous Heavy Metals and Metalloids in Food Chains/Webs—Concepts and Implications for Wildlife and Human Health. Hum. Ecol. Risk Assess. Int. J. 2019, 25, 1353–1376. [Google Scholar] [CrossRef]
- Mok, W.J.; Ghaffar, M.A.; Noor, M.I.; Lananan, F.; Azra, M.N. Understanding Climate Change and Heavy Metals in Coastal Areas: A Macroanalysis Assessment. Water 2023, 15, 891. [Google Scholar] [CrossRef]
- Mohammed, A.B.; Goran, S.M.A.; Tarafdar, A. Profiling of Seasonal Variation in and Cancer Risk Assessment of Benzo(a)Pyrene and Heavy Metals in Drinking Water from Kirkuk City, Iraq. Environ. Sci. Pollut. Res. 2022, 29, 22203–22222. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Agrawal, M.; Sagar, R. Assessment of Potential Health Risks Due to Heavy Metals through Vegetable Consumption in a Tropical Area Irrigated by Treated Wastewater. Environ. Syst. Decis. 2015, 35, 375–388. [Google Scholar] [CrossRef]
- Wang, X.; Han, X.; Guo, S.; Ma, Y.; Zhang, Y. Associations between Patterns of Blood Heavy Metal Exposure and Health Outcomes: Insights from NHANES 2011–2016. BMC Public Health 2024, 24, 558. [Google Scholar] [CrossRef]
- Miletić, A.; Lučić, M.; Onjia, A. Exposure Factors in Health Risk Assessment of Heavy Metal(Loid)s in Soil and Sediment. Metals 2023, 13, 1266. [Google Scholar] [CrossRef]
- Vagliasindi, F.G.A.; Benjami, M.M. Arsenic removal in fresh and nom-pre-loaded ion exchange packed bed adsorption reactors. Water Sci. Technol. 1998, 38, 337–343. [Google Scholar] [CrossRef]
- Chwirka, J.D.; Colvin, C.; Gomez, J.D.; Mueller, P.A. Arsenic removal from drinking water using the coagulation/microfiltration process. J. Am. Water Works Assoc. 2004, 96, 106–114. [Google Scholar] [CrossRef]
- Andrianisa, H.A.; Ito, A.; Sasaki, A.; Aizawa, J.; Umitaet, T. Biotransformation of arsenic species by activated sludge and removal of bio-oxidised arsenate from wastewater by coagulation with ferric chloride. Water Res. 2008, 42, 4809–4817. [Google Scholar] [CrossRef]
- Peng, F.F.; Di, P.K. Removal of arsenic from aqueous solution by adsorbing colloid. Ind. Eng. Chem. Res. 1994, 33, 922–928. [Google Scholar]
- Bissen, M.; Vieillard-Baron, M.M.; Schindelin, A.J.; Frimmel, F.H. TiO2-cata-lyzed photooxidation of arsenite to arsenate in aqueous samples. Chemosphere 2001, 44, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, S.; Zhang, S.; Yang, X.; Huang, Z.; Wang, C.; Wei, Q.; Zhang, G.; Xiao, J.; Jiang, F.; et al. Arsenic pollution and its treatment in Yangzonghai lake in China: In situ remediation. Ecotoxicol. Environ. Saf. 2015, 122, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Zhang, Y.; Zhang, X.; Shan, Z.; Zhang, C. Characteristics of heavy metal pollution and health risk assessment of surface water in the Guanzhong section of the Weihe River Basin. J. Ecol. Environ. 2022, 31, 131–141. (In Chinese) [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).