A Review on the Roles of Extracellular Polymeric Substances (EPSs) in Wastewater Treatment: Source, Mechanism Study, Bioproducts, Limitations, and Future Challenges
Abstract
:1. Introduction
2. EPS Properties and Production
2.1. Properties and Function of EPSs
2.2. Production of EPSs
2.3. Bioreactors for EPS Production
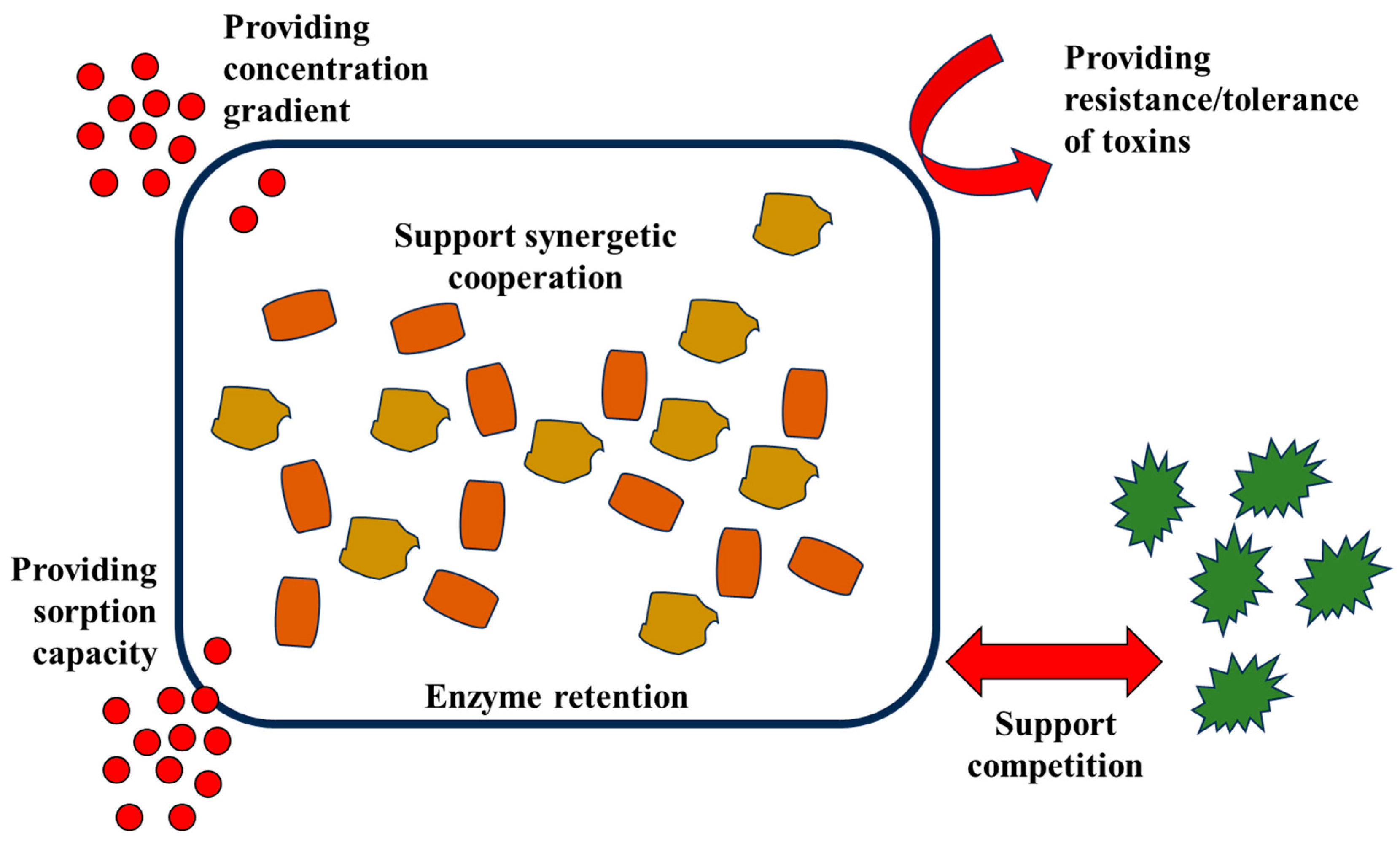
3. EPS Roles in Wastewater Treatment
4. Mechanisms Involving EPSs in Wastewater Treatment
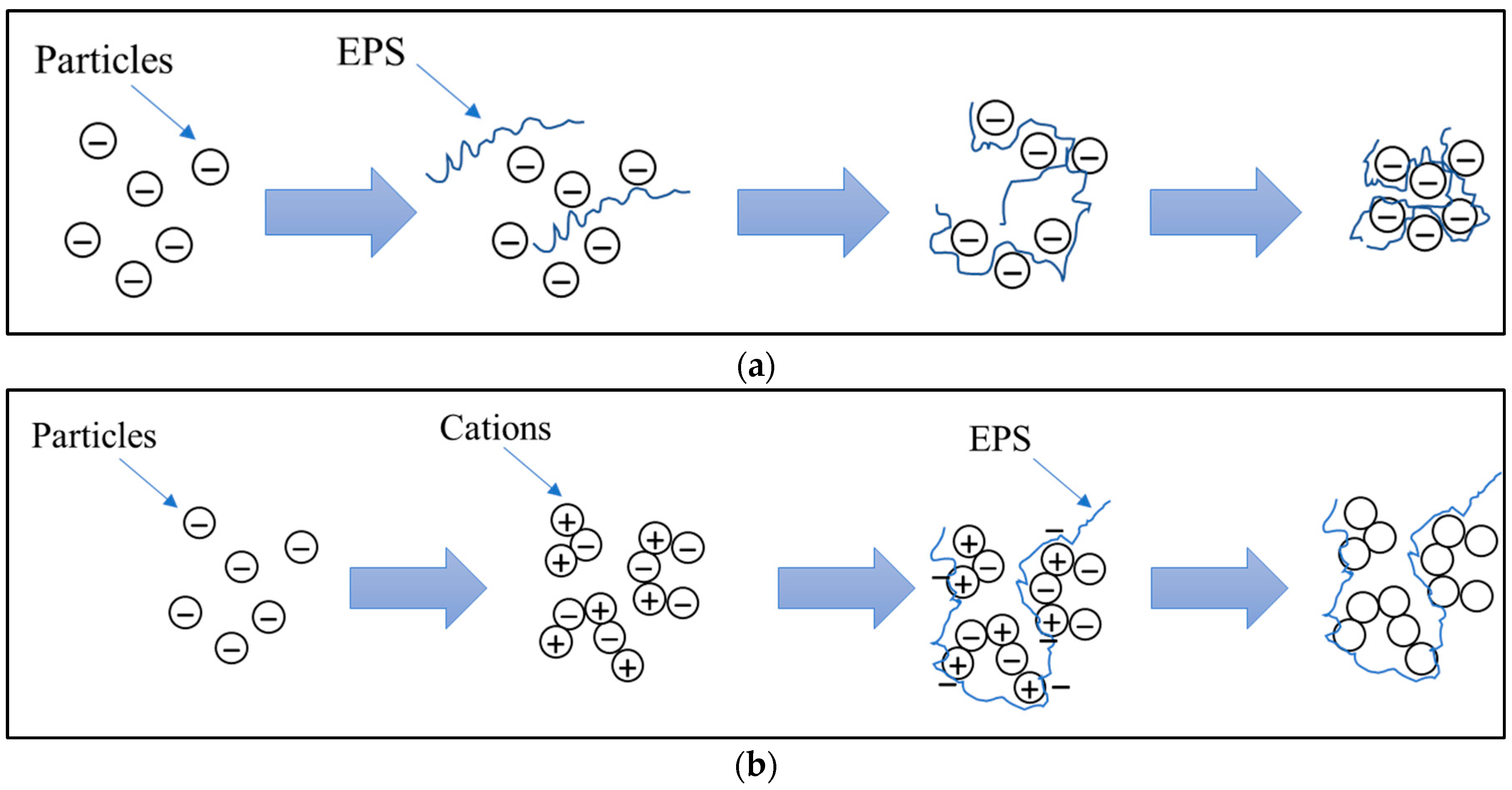
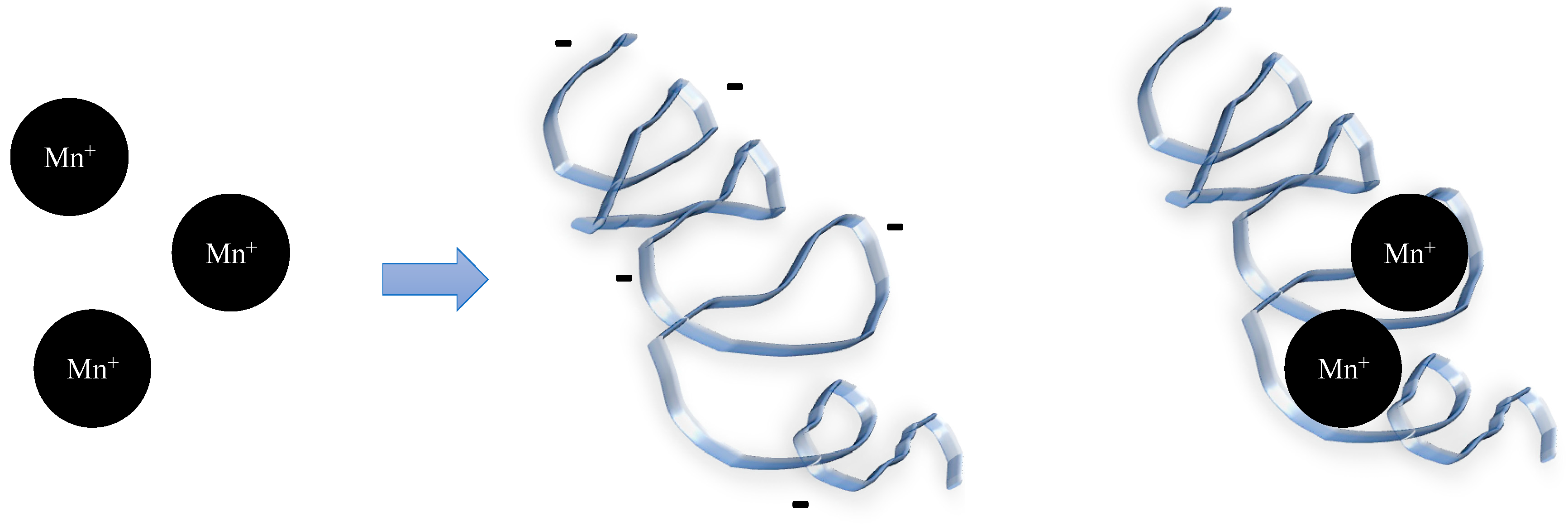
4.1. EPSs Promoting Aggregation and Flocculation
4.2. EPSs Promoting Adsorption
4.3. EPSs Promoting Decolorization
4.4. EPSs Promoting Degradation
5. Configuration of Bioreactors Used in Wastewater Treatment Involving EPSs
5.1. Membrane Bioreactors
5.2. Moving Bed Bioreactors
5.3. Fluidized Bed Bioreactors
5.4. Trickling Filter
6. Natural Bioproducts from EPSs for Wastewater Treatment
6.1. Bioflocculant
6.2. Coating Material
7. Limitations of the Current Study and Future Research Directions
7.1. Limitations of the Current Study
7.1.1. Limited Knowledge of Potential Species for EPS Production
7.1.2. EPS Extraction
7.1.3. Mass Production
7.2. Future Research Directions
7.2.1. Selection of New Potential EPS-Producing Species
7.2.2. Innovation in Simple EPS Extraction Method
7.2.3. Optimization and Innovation in Mass Production
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Z.; Smith, S.R. Enzyme Recovery from Biological Wastewater Treatment. Waste Biomass Valor. 2021, 12, 4185–4211. [Google Scholar] [CrossRef]
- Shi, Y.; Huang, J.; Zeng, G.; Gu, Y.; Chen, Y.; Hu, Y.; Tang, B.; Zhou, J.; Yang, Y.; Shi, L. Exploiting Extracellular Polymeric Substances (EPS) Controlling Strategies for Performance Enhancement of Biological Wastewater Treatments: An Overview. Chemosphere 2017, 180, 396–411. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Shen, Z.; Han, Z.; Zhou, Y. The Effect of Extracellular Polymeric Substances on Exogenous Highly Toxic Compounds in Biological Wastewater Treatment: An Overview. Bioresour. Technol. Rep. 2019, 5, 28–42. [Google Scholar] [CrossRef]
- Sheng, G.P.; Yu, H.Q.; Li, X.Y. Extracellular Polymeric Substances (EPS) of Microbial Aggregates in Biological Wastewater Treatment Systems: A Review. Biotechnol. Adv. 2010, 28, 882–894. [Google Scholar] [CrossRef]
- Salama, Y.; Chennaoui, M.; Sylla, A.; Mountadar, M.; Rihani, M.; Assobhei, O. Characterization, Structure, and Function of Extracellular Polymeric Substances (EPS) of Microbial Biofilm in Biological Wastewater Treatment Systems: A Review. Desalinat. Water Treat. 2016, 57, 16220–16237. [Google Scholar] [CrossRef]
- Elnahas, M.O.; Amin, M.A.; Hussein, M.M.D.; Shanbhag, V.C.; Ali, A.E.; Wall, J.D. Isolation, Characterization and Bioactivities of an Extracellular Polysaccharide Produced from Streptomyces Sp. MOE6. Molecules 2017, 22, 1396. [Google Scholar] [CrossRef]
- Vasilieva, Z.; Gaponenkov, I.; Vasekha, M.; Ivanova, T. Extraction of Extracellular Polymeric Substances of Activated Sludge and Their Application for Wastewater Treatment. IOP Conf. Ser. Earth Environ. Sci. 2019, 302, 012018. [Google Scholar] [CrossRef]
- Brunt, E.G.; Burgess, J.G. The Promise of Marine Molecules as Cosmetic Active Ingredients. Int. J. Cosmet. Sci. 2018, 40, 1–15. [Google Scholar] [CrossRef]
- Zannini, E.; Waters, D.M.; Coffey, A.; Arendt, E.K. Production, Properties, and Industrial Food Application of Lactic Acid Bacteria-Derived Exopolysaccharides. Appl. Microbiol. Biotechnol. 2016, 100, 1121–1135. [Google Scholar] [CrossRef]
- Awasthi, S. Application of EPS in Agriculture: An Important Natural Resource for Crop Improvement. Agric. Res. Technol. 2017, 8, 22–24. [Google Scholar] [CrossRef]
- Nouha, K.; Kumar, R.S.; Tyagi, R.D. Heavy Metals Removal from Wastewater Using Extracellular Polymeric Substances Produced by Cloacibacterium Normanense in Wastewater Sludge Supplemented with Crude Glycerol and Study of Extracellular Polymeric Substances Extraction by Different Methods. Bioresour. Technol. 2016, 212, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Dobrowolski, R.; Szcześ, A.; Czemierska, M.; Jarosz-Wikołazka, A. Studies of Cadmium(II), Lead(II), Nickel(II), Cobalt(II) and Chromium(VI) Sorption on Extracellular Polymeric Substances Produced by Rhodococcus Opacus and Rhodococcus Rhodochrous. Bioresour. Technol. 2017, 225, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Wang, Y.; Wang, X.; Li, M.; Han, F.; Ju, L.; Zhang, G.; Shi, L.; Li, K.; Wang, B.; et al. Toxicity Assessment of 4-Chlorophenol to Aerobic Granular Sludge and Its Interaction with Extracellular Polymeric Substances. J. Hazard. Mater. 2015, 289, 101–107. [Google Scholar] [CrossRef]
- Li, R.; Ning, X.a.; Sun, J.; Wang, Y.; Liang, J.; Lin, M.; Zhang, Y. Decolorization and Biodegradation of the Congo Red by Acinetobacter Baumannii YNWH 226 and Its Polymer Production’s Flocculation and Dewatering Potential. Bioresour. Technol. 2015, 194, 233–239. [Google Scholar] [CrossRef]
- Agunbiade, M.; Pohl, C.; Ashafa, O. Bioflocculant Production from Streptomyces Platensis and Its Potential for River and Waste Water Treatment. Braz. J. Microbiol. 2018, 49, 731–741. [Google Scholar] [CrossRef]
- Wang, L.F.; Qian, C.; Jiang, J.K.; Ye, X.D.; Yu, H.Q. Response of Extracellular Polymeric Substances to Thermal Treatment in Sludge Dewatering Process. Environ. Pollut. 2017, 231, 1388–1392. [Google Scholar] [CrossRef] [PubMed]
- Żur, J.; Wojcieszyńska, D.; Guzik, U. Metabolic Responses of Bacterial Cells to Immobilization. Molecules 2016, 21, 958. [Google Scholar] [CrossRef]
- Di Martino, P. Extracellular Polymeric Substances, a Key Element in Understanding Biofilm Phenotype. AIMS Microbiol. 2018, 4, 274–288. [Google Scholar] [CrossRef]
- Flemming, H.-C. EPS—Then and Now. Microorganisms 2016, 4, 41. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Ahmad, M.; Tufail, M.A.; Asghar, H.N.; Nazli, F.; Zahir, Z.A. Appraising the Potential of EPS-producing Rhizobacteria with ACC-deaminase Activity to Improve Growth and Physiology of Maize under Drought Stress. Physiol. Plant 2020, 172, 463–476. [Google Scholar] [CrossRef]
- Nishanth, S.; Bharti, A.; Gupta, H.; Gupta, K.; Gulia, U.; Prasanna, R. Cyanobacterial Extracellular Polymeric Substances (EPS): Biosynthesis and Their Potential Applications. In Microbial and Natural Macromolecules; Academic Press: Cambridge, MA, USA, 2021; pp. 349–369. [Google Scholar]
- Udaiyappan, A.F.M.; Hasan, H.A.; Takriff, M.S.; Abdullah, S.R.S.; Maeda, T.; Mustapha, N.A.; Mohd Yasin, N.H.; Nazashida Mohd Hakimi, N.I. Microalgae-Bacteria Interaction in Palm Oil Mill Effluent Treatment. J. Water Process Eng. 2020, 35, 101203. [Google Scholar] [CrossRef]
- Gientka, I.; Blazejak, S.; Stasiak-Rózanska, L.; Chlebowska-Smigiel, A. Exopolysaccharides from Yeast: Insight into Optimal Conditions for Biosynthesis, Chemical Composition and Functional Properties—Review. Acta Sci. Pol. Technol. Aliment. 2015, 14, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, S.; Banerjee, D. Fungal Exopolysaccharide: Production, Composition and Applications. Microbiol. Insights 2013, 6, MBI.S10957. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cui, X.; Wu, B.; Wang, Z.; Liu, Y.; Ren, T.; Xia, S.; Rittmann, B.E. Microalgal Extracellular Polymeric Substances (EPS) and Their Roles in Cultivation, Biomass Harvesting, and Bioproducts Extraction. Bioresour. Technol. 2024, 406, 131054. [Google Scholar] [CrossRef]
- Sun, Y.; Su, J.; Ali, A.; Zhang, S.; Zheng, Z.; Min, Y. Effect of Fungal Pellets on Denitrifying Bacteria at Low Carbon to Nitrogen Ratio: Nitrate Removal, Extracellular Polymeric Substances, and Potential Functions. Sci. Total Environ. 2022, 847, 157591. [Google Scholar] [CrossRef]
- Imron, M.F.; Kurniawan, S.B.; Ismail, N.I.; Abdullah, S.R.S. Future Challenges in Diesel Biodegradation by Bacteria Isolates: A Review. J. Clean. Prod. 2020, 251, 119716. [Google Scholar] [CrossRef]
- Chug, R.; Mathur, S.; Kothari, S.L.; Harish; Gour, V.S. Maximizing EPS Production from Pseudomonas Aeruginosa and Its Application in Cr and Ni Sequestration. Biochem. Biophys. Rep. 2021, 26, 100972. [Google Scholar] [CrossRef]
- Biswas, J.; Paul, A.K. Optimization of Factors Influencing Exopolysaccharide Production by Halomonas Xianhensis Sur308 under Batch Culture. AIMS Microbiol. 2017, 3, 564–579. [Google Scholar] [CrossRef]
- Prete, R.; Alam, M.K.; Perpetuini, G.; Perla, C.; Pittia, P.; Corsetti, A. Lactic Acid Bacteria Exopolysaccharides Producers: A Sustainable Tool for Functional Foods. Foods 2021, 10, 1653. [Google Scholar] [CrossRef]
- Park, Y.J.; Kim, Y.J.; Yu, H.H.; Lee, N.-K.; Paik, H.-D. Cell-Free Supernatants of Bacillus Subtilis and Bacillus Polyfermenticus Inhibit Listeria Monocytogenes Biofilm Formation. Food Control 2023, 144, 109387. [Google Scholar] [CrossRef]
- Chug, R.; Gour, V.S.; Mathur, S.; Kothari, S.L. Optimization of Extracellular Polymeric Substances Production Using Azotobacter Beijreinckii and Bacillus Subtilis and Its Application in Chromium (VI) Removal. Bioresour. Technol. 2016, 214, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Delbarre-Ladrat, C.; Sinquin, C.; Marchand, L.; Bonnetot, S.; Zykwinska, A.; Verrez-Bagnis, V.; Colliec-Jouault, S. Influence of the Carbon and Nitrogen Sources on Diabolican Production by the Marine Vibrio Diabolicus Strain CNCM I-1629. Polym. 2022, 14, 1994. [Google Scholar] [CrossRef] [PubMed]
- Costa, O.Y.A.; Raaijmakers, J.M.; Kuramae, E.E. Microbial Extracellular Polymeric Substances: Ecological Function and Impact on Soil Aggregation. Front. Microbiol. 2018, 9, 337094. [Google Scholar] [CrossRef]
- Nguyen, P.; Nguyen, T.; Bui, D.; Hong, P. Exopolysaccharide Production by Lactic Acid Bacteria: The Manipulation of Environmental Stresses for Industrial Applications. AIMS Microbiol. 2020, 6, 451–469. [Google Scholar] [CrossRef]
- Morcillo, R.; Manzanera, M. The Effects of Plant-Associated Bacterial Exopolysaccharides on Plant Abiotic Stress Tolerance. Metabolites 2021, 11, 337. [Google Scholar] [CrossRef]
- Yan, M.; Wang, B.; Xu, X.; der Meister, T.; Tabγač, H.; Hwang, F.; Liu, Z. Extrusion of Dissolved Oxygen by Exopolysaccharide from Leuconostoc Mesenteroides and Its Implications in Relief of the Oxygen Stress. Front. Microbiol. 2018, 9, 2467. [Google Scholar] [CrossRef]
- Ghosh, P.K.; Maiti, T.K. Structure of Extracellular Polysaccharides (EPS) Produced by Rhizobia and Their Functions in Legume–Bacteria Symbiosis—A Review. Achiev. Life Sci. 2016, 10, 136–143. [Google Scholar] [CrossRef]
- Mallikarjuna, N.; Yellamma, K. Genetic and Metabolic Engineering of Microorganisms for the Production of Various Food Products. In Recent Developments in Applied Microbiology and Biochemistry; Academic Press: Cambridge, MA, USA, 2019; pp. 167–182. [Google Scholar]
- Buhari, J.; Hasan, H.A.; Kurniawan, S.B.; Abdullah, S.R.S.; Othman, A.R. Future and Challenges of Co-Biofilm Treatment on Ammonia and Bisphenol A Removal from Wastewater. J. Water Process Eng. 2023, 54, 103969. [Google Scholar] [CrossRef]
- Pechaud, Y.; Peyre Lavigne, M.; Bessiere, Y.; Ochoa, J.C.; Queinnec, I.; Paul, E. Influence of Shear Stress, Organic Loading Rate and Hydraulic Retention Time on the Biofilm Structure and on the Competition between Different Biological Aggregate Morphotypes. J. Environ. Chem. Eng. 2022, 10, 107597. [Google Scholar] [CrossRef]
- Gangalla, R.; Sampath, G.; Beduru, S.; Sarika, K.; Kaveriyappan Govindarajan, R.; Ameen, F.; Alwakeel, S.; Thampu, R.K. Optimization and Characterization of Exopolysaccharide Produced by Bacillus Aerophilus Rk1 and Its in Vitro Antioxidant Activities. J. King Saud. Univ. Sci. 2021, 33, 101470. [Google Scholar] [CrossRef]
- Hasan, H.A.; Ezril Hafiz, R.; Muhamad, M.H.; Sheikh Abdullah, S.R.; Hasan, H.A.; Ezril Hafiz, R.; Muhamad, M.H.; Sheikh Abdullah, S.R.; Hassimi, A.H.; Ezril Hafiz, R.; et al. Bioflocculant Production Using Palm Oil Mill and Sago Mill Effluent as a Fermentation Feedstock: Characterization and Mechanism of Flocculation. J. Environ. Manag. 2020, 260, 110046. [Google Scholar] [CrossRef]
- Chen, S.; Zheng, Y.; Han, C.; Liu, H.; Chen, Y.; Zhou, J.; Su, S. Production of a Bioflocculant Using Old Polyester Fibre as a Fermentation Feedstock and Its Use in Treatment of Polyester Alkali-Peeling Wastewater. J. Environ. Chem. Eng. 2021, 9, 105455. [Google Scholar] [CrossRef]
- Sayyed, R.Z.; Jamadar, D.D.; Patel, P.R. Production of Exo-Polysaccharide by Rhizobium Sp. Indian. J. Microbiol. 2011, 51, 294–300. [Google Scholar] [CrossRef]
- Ghosh, P.K.; Ganguly, J.; Maji, P.; Maiti, T.K. Production and Composition of Extracellular Polysaccharide Synthesized by Rhizobium Undicola Isolated from Aquatic Legume, Neptunia Oleracea Lour. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2015, 85, 581–590. [Google Scholar] [CrossRef]
- Yan, Z.-R.; Meng, H.-S.; Yang, X.-Y.; Zhu, Y.-Y.; Li, X.-Y.; Xu, J.; Sheng, G.-P. Insights into the Interactions between Triclosan (TCS) and Extracellular Polymeric Substance (EPS) of Activated Sludge. J. Environ. Manag. 2019, 232, 219–225. [Google Scholar] [CrossRef]
- Kurniawan, S.B.S.B.S.B.; Imron, M.F.M.F.; Sługocki, Ł.; Nowakowski, K.; Ahmad, A.; Najiya, D.; Abdullah, S.R.S.S.R.S.; Othman, A.R.A.R.; Purwanti, I.F.I.F.; Hasan, H.A.H.A.; et al. Assessing the Effect of Multiple Variables on the Production of Bioflocculant by Serratia Marcescens: Flocculating Activity, Kinetics, Toxicity, and Flocculation Mechanism. Sci. Total Environ. 2022, 836, 155564. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Panesar, P.S.; Mehariya, S. Microbial Exopolysaccharides; CRC Press: Boca Raton, FL, USA, 2024; ISBN 9781003342687. [Google Scholar]
- Singh, N.K.; Pandey, S.; Singh, R.P.; Dahiya, S.; Gautam, S.; Kazmi, A.A. Effect of Intermittent Aeration Cycles on EPS Production and Sludge Characteristics in a Field Scale IFAS Reactor. J. Water Process Eng. 2018, 23, 230–238. [Google Scholar] [CrossRef]
- Miqueleto, A.P.; Dolosic, C.C.; Pozzi, E.; Foresti, E.; Zaiat, M. Influence of Carbon Sources and C/N Ratio on EPS Production in Anaerobic Sequencing Batch Biofilm Reactors for Wastewater Treatment. Bioresour. Technol. 2010, 101, 1324–1330. [Google Scholar] [CrossRef]
- Traina, F.; Capodici, M.; Torregrossa, M.; Viviani, G.; Corsino, S.F. PHA and EPS Production from Industrial Wastewater by Conventional Activated Sludge, Membrane Bioreactor and Aerobic Granular Sludge Technologies: A Comprehensive Comparison. Chemosphere 2024, 355, 141768. [Google Scholar] [CrossRef]
- Shao, Y.; Zhang, H.; Buchanan, I.; Mohammed, A.; Liu, Y. Comparison of Extracellular Polymeric Substance (EPS) in Nitrification and Nitritation Bioreactors. Int. Biodeterior. Biodegrad. 2019, 143, 104713. [Google Scholar] [CrossRef]
- More, T.T.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Biopolymers Production by Mixed Culture and Their Applications in Water and Wastewater Treatment. Water Environ. Res. 2015, 87, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Nouha, K. EPS Producing Microorganisms from Municipal Wastewater Activated Sludge. J. Pet. Environ. Biotechnol. 2015, 7, 1. [Google Scholar] [CrossRef]
- Ajao, V.; Bruning, H.; Rijnaarts, H.; Temmink, H. Natural Flocculants from Fresh and Saline Wastewater: Comparative Properties and Flocculation Performances. Chem. Eng. J. 2018, 349, 622–632. [Google Scholar] [CrossRef]
- Al-Dhabi, N.A.; Esmail, G.A.; Valan Arasu, M. Sustainable Conversion of Palm Juice Wastewater into Extracellular Polysaccharides for Absorption of Heavy Metals from Saudi Arabian Wastewater. J. Clean. Prod. 2020, 277, 124252. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, L.; Shang, H.; Li, Q.; Zhou, W. Treatment of Azo Dye Wastewater by the Self-Flocculating Marine Bacterium Aliiglaciecola Lipolytica. Environ. Technol. Innov. 2020, 19, 100810. [Google Scholar] [CrossRef]
- Zhang, B.; Ning, D.; Yang, Y.; Van Nostrand, J.D.; Zhou, J.; Wen, X. Biodegradability of Wastewater Determines Microbial Assembly Mechanisms in Full-Scale Wastewater Treatment Plants. Water Res. 2020, 169, 115276. [Google Scholar] [CrossRef]
- Bezawada, J.; Hoang, N.V.; More, T.T.; Yan, S.; Tyagi, N.; Tyagi, R.D.; Surampalli, R.Y. Production of Extracellular Polymeric Substances (EPS) by Serratia Sp.1 Using Wastewater Sludge as Raw Material and Flocculation Activity of the EPS Produced. J. Environ. Manag. 2013, 128, 83–91. [Google Scholar] [CrossRef]
- Ajao, V.; Fokkink, R.; Leermakers, F.; Bruning, H.; Rijnaarts, H.; Temmink, H. Bioflocculants from Wastewater: Insights into Adsorption Affinity, Flocculation Mechanisms and Mixed Particle Flocculation Based on Biopolymer Size-Fractionation. J. Colloid. Interface Sci. 2021, 581, 533–544. [Google Scholar] [CrossRef]
- Yellapu, S.K.; Klai, N.; Kaur, R.; Tyagi, R.D.; Surampalli, R.Y. Oleaginous Yeast Biomass Flocculation Using Bioflocculant Produced in Wastewater Sludge and Transesterification Using Petroleum Diesel as a Co-Solvent. Renew. Energy 2019, 131, 217–228. [Google Scholar] [CrossRef]
- Li, Z.; Lu, P.; Zhang, D.; Chen, G.; Zeng, S.; He, Q. Population Balance Modeling of Activated Sludge Flocculation: Investigating the Influence of Extracellular Polymeric Substances (EPS) Content and Zeta Potential on Flocculation Dynamics. Sep. Purif. Technol. 2016, 162, 91–100. [Google Scholar] [CrossRef]
- Kyung, O.; Hendren, Z.; Dong, G.; Dong, D.; Woo, J. In Fl Uence of Activated Sludge Derived-Extracellular Polymeric Substance (ASD-EPS) as Bio- Fl Occulation of Microalgae for Biofuel Recovery. Algal Res. 2020, 45, 101736. [Google Scholar] [CrossRef]
- Pu, L.; Zeng, Y.-J.; Xu, P.; Li, F.-Z.; Zong, M.-H.; Yang, J.-G.; Lou, W.-Y. Using a Novel Polysaccharide BM2 Produced by Bacillus Megaterium Strain PL8 as an Efficient Bioflocculant for Wastewater Treatment. Int. J. Biol. Macromol. 2020, 162, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Cunha, C.; Silva, L.; Paulo, J.; Faria, M.; Nogueira, N.; Cordeiro, N. Microalgal-Based Biopolymer for Nano- and Microplastic Removal: A Possible Biosolution for Wastewater Treatment. Environ. Pollut. 2020, 263, 114385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Xu, X.-Y.; Zhang, X.-L.; Zou, K.; Liu, B.-Z.; Qing, T.-P.; Feng, B. Nanoparticles-EPS Corona Increases the Accumulation of Heavy Metals and Biotoxicity of Nanoparticles. J. Hazard. Mater. 2020, 409, 124526. [Google Scholar] [CrossRef]
- Tyagi, B.; Gupta, B.; Thakur, I.S. Biosorption of Cr (VI) from Aqueous Solution by Extracellular Polymeric Substances (EPS) Produced by Parapedobacter sp. ISTM3 Strain Isolated from Mawsmai cave, Meghalaya, India. Environ. Res. 2020, 191, 110064. [Google Scholar] [CrossRef]
- Szewczuk-Karpisz, K. Flocculation efficiency of the Sinorhizobium meliloti 1021 exopolysaccharide relative to mineral oxide suspensions—A preliminary study for wastewater treatment. Sep. Purif. Technol. 2018, 201, 51–59. [Google Scholar] [CrossRef]
- Paulo, A.M.S.; Amorim, C.L.; Costa, J.; Mesquita, D.P.; Ferreira, E.C.; Castro, P.M.L. Science of the Total Environment Long-Term Stability of a Non-Adapted Aerobic Granular Sludge Process Treating Fi Sh Canning Wastewater Associated to EPS Producers in the Core Microbiome. Sci. Total Environ. 2020, 756, 144007. [Google Scholar] [CrossRef]
- Hu, Y.Q.; Wei, W.; Gao, M.; Zhou, Y.; Wang, G.X.; Zhang, Y. Effect of Pure Oxygen Aeration on Extracellular Polymeric Substances (EPS) of Activated Sludge Treating Saline Wastewater. Process Saf. Environ. Prot. 2019, 123, 344–350. [Google Scholar] [CrossRef]
- Govarthanan, M.; Jeon, C.; Jeon, Y.; Kwon, J.-H.; Bae, H.; Kim, W. Non-toxic nano approach for wastewater treatment using Chlorella vulgaris exopolysaccharides immobilized in iron-magnetic nanoparticles. Int. J. Biol. Macromol. 2020, 162, 1241–1249. [Google Scholar] [CrossRef]
- Cui, Y.-W.; Huang, J.-L.; Alam, F. Fast Granulation of Halophilic Activated Sludge Treating Low-Strength Organic Saline Wastewater via Addition of Divalent Cations. Chemosphere 2021, 264, 128396. [Google Scholar] [CrossRef]
- Alias, J.; Hasan, H.A.; Abdullah, S.R.S.; Othman, A.R. Properties of Bioflocculant-Producing Bacteria for High Flocculating Activity Efficiency. Environ. Technol. Innov. 2022, 27, 102529. [Google Scholar] [CrossRef]
- Almansoory, A.F.; Al-Baldawi, I.A.; Hazaimeh, M. Optimization of the EPS Production of a Bacterial Floc Consortium Using Different Parameters. Biocatal. Agric. Biotechnol. 2020, 23, 101466. [Google Scholar] [CrossRef]
- Lapointe, M.; Barbeau, B. Understanding the Roles and Characterizing the Intrinsic Properties of Synthetic vs. Natural Polymers to Improve Clarification through Interparticle Bridging: A Review. Sep. Purif. Technol. 2020, 231, 115893. [Google Scholar] [CrossRef]
- Choy, S.Y.; Prasad, K.M.N.; Wu, T.Y.; Raghunandan, M.E.; Phang, S.M.; Juan, J.C.; Ramanan, R.N. Starch-Based Flocculant Outperformed Aluminium Sulfate Hydrate and Polyaluminium Chloride through Effective Bridging for Harvesting Acicular Microalga Ankistrodesmus. Algal Res. 2018, 29, 343–353. [Google Scholar] [CrossRef]
- Alnawajha, M.M.; Kurniawan, S.B.; Abdullah, S.R.S.; Hasan, H.A.; Othman, A.R. Performance of Water-Extracted Leucaena Leucocephala Seeds as Coagulant and Alum in Treating Aquaculture Effluent: Effect of Dosage, Rapid Mixing Speed, and Settling Time. Int. J. Environ. Sci. Technol. 2023, 20, 9981–9994. [Google Scholar] [CrossRef]
- Alnawajha, M.M.; Kurniawan, S.B.; Imron, M.F.; Abdullah, S.R.S.; Hasan, H.A.; Othman, A.R. Plant-Based Coagulants/Flocculants: Characteristics, Mechanisms, and Possible Utilization in Treating Aquaculture Effluent and Benefiting from the Recovered Nutrients. Environ. Sci. Pollut. Res. 2022, 29, 58430–58453. [Google Scholar] [CrossRef]
- Ye, L.; Wu, J.; Huang, M.; Yan, J. The Role of Suspended Extracellular Polymeric Substance (EPS) on Equilibrium Flocculation of Clay Minerals in High Salinity Water. Water Res. 2023, 244, 120451. [Google Scholar] [CrossRef]
- Mohite, B.V.; Koli, S.H.; Narkhede, C.P.; Patil, S.N.; Patil, S.V. Prospective of Microbial Exopolysaccharide for Heavy Metal Exclusion. Appl. Biochem. Biotechnol. 2017, 183, 582–600. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, G.; Tan, Q.; Gao, M.; Chen, G.; Huang, X.; Xu, X.; Li, L.; Wang, J.; Zhang, Y.; et al. Polysaccharide-Based Biopolymer Hydrogels for Heavy Metal Detection and Adsorption. J. Adv. Res. 2023, 44, 53–70. [Google Scholar] [CrossRef]
- Fouda-Mbanga, B.G.; Prabakaran, E.; Pillay, K. Carbohydrate Biopolymers, Lignin Based Adsorbents for Removal of Heavy Metals (Cd2+, Pb2+, Zn2+) from Wastewater, Regeneration and Reuse for Spent Adsorbents Including Latent Fingerprint Detection: A Review. Biotechnol. Rep. 2021, 30, e00609. [Google Scholar] [CrossRef]
- Ramli, N.N.; Kurniawan, S.B.; Ighalo, J.O.; Mohd Said, N.S.; Marsidi, N.; Buhari, J.; Ramli Shah, R.A.; Zulkifli, M.; Alias, J.; Daud, N.M.; et al. A Review of the Treatment Technologies for Hexavalent Chromium Contaminated Water. BioMetals 2023, 36, 1189–1219. [Google Scholar] [CrossRef] [PubMed]
- Ramli, N.N.N.N.; Othman, A.R.A.R.; Kurniawan, S.B.S.B.S.B.; Abdullah, S.R.S.S.R.S.; Hasan, H.A.H.A. Metabolic Pathway of Cr(VI) Reduction by Bacteria: A Review. Microbiol. Res. 2023, 268, 127288. [Google Scholar] [CrossRef]
- Ren, L.; Hong, Z.; Qian, W.; Li, J.; Xu, R. Adsorption Mechanism of Extracellular Polymeric Substances from Two Bacteria on Ultisol and Alfisol. Environ. Pollut. 2018, 237, 39–49. [Google Scholar] [CrossRef]
- Khatri, A.; White, M. Sustainable Dyeing Technologies. In Sustainable Apparel; Woodhead Publishing: Sawston, UK, 2015; pp. 135–160. [Google Scholar]
- Brigé, A.; Motte, B.; Borloo, J.; Buysschaert, G.; Devreese, B.; Van Beeumen, J.J. Bacterial Decolorization of Textile Dyes Is an Extracellular Process Requiring a Multicomponent Electron Transfer Pathway. Microb. Biotechnol. 2008, 1, 40–52. [Google Scholar] [CrossRef]
- Li, Y.; Sun, L.-L.; Sun, Y.-Y.; Cha, Q.-Q.; Li, C.-Y.; Zhao, D.-L.; Song, X.-Y.; Wang, M.; McMinn, A.; Chen, X.-L.; et al. Extracellular Enzyme Activity and Its Implications for Organic Matter Cycling in Northern Chinese Marginal Seas. Front. Microbiol. 2019, 10, 2137. [Google Scholar] [CrossRef]
- Bozelli, J.C.; Epand, R.M. Determinants of Lipids Acyl Chain Specificity: A Tale of Two Enzymes. Biophys. Chem. 2020, 265, 106431. [Google Scholar] [CrossRef]
- Martínez, R.J.; Godínez, L.A.; Robles, I. Waste Resources Utilization for Biosorbent Preparation, Sorption Studies, and Electrocatalytic Applications. In Valorization of Wastes for Sustainable Development; Elsevier: Amsterdam, The Netherlands, 2023; pp. 395–418. [Google Scholar]
- Ikram, M.; Zahoor, M.; Naeem, M.; Islam, N.U.; Shah, A.B.; Shahzad, B. Bacterial Oxidoreductive Enzymes as Molecular Weapons for the Degradation and Metabolism of the Toxic Azo Dyes in Wastewater: A Review. Z. Phys. Chem. 2023, 237, 187–209. [Google Scholar] [CrossRef]
- Debnath, R.; Saha, T. An Insight into the Production Strategies and Applications of the Ligninolytic Enzyme Laccase from Bacteria and Fungi. Biocatal. Agric. Biotechnol. 2020, 26, 101645. [Google Scholar] [CrossRef]
- Chandanshive, V.V.; Rane, N.R.; Gholave, A.R.; Patil, S.M.; Jeon, B.H.; Govindwar, S.P. Efficient Decolorization and Detoxification of Textile Industry Effluent by Salvinia Molesta in Lagoon Treatment. Environ. Res. 2016, 150, 88–96. [Google Scholar] [CrossRef]
- Zhang, H.; Jia, Y.; Khanal, S.K.; Lu, H.; Fang, H.; Zhao, Q. Understanding the Role of Extracellular Polymeric Substances on Ciprofloxacin Adsorption in Aerobic Sludge, Anaerobic Sludge, and Sulfate-Reducing Bacteria Sludge Systems. Environ. Sci. Technol. 2018, 52, 6476–6486. [Google Scholar] [CrossRef]
- Wei, Z.; Niu, S.; Wei, Y.; Liu, Y.; Xu, Y.; Yang, Y.; Zhang, P.; Zhou, Q.; Wang, J.J. The Role of Extracellular Polymeric Substances (EPS) in Chemical-Degradation of Persistent Organic Pollutants in Soil: A Review. Sci. Total Environ. 2024, 912, 168877. [Google Scholar] [CrossRef] [PubMed]
- Lü, F.; Wang, J.; Shao, L.; He, P. Enzyme Disintegration with Spatial Resolution Reveals Different Distributions of Sludge Extracellular Polymer Substances. Biotechnol. Biofuels 2016, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-M.; Wu, H.-Z.; Wang, Y.-X.; Zhu, S.; Wei, C.-H. Enhancement of Phenol Biodegradation: Metabolic Division of Labor in Co-Culture of Stenotrophomonas Sp. N5 and Advenella Sp. B9. J. Hazard. Mater. 2020, 400, 123214. [Google Scholar] [CrossRef] [PubMed]
- Köhler, A.; Hellweg, S.; Escher, B.I.; Hungerbühler, K. Organic Pollutant Removal versus Toxicity Reduction in Industrial Wastewater Treatment: The Example of Wastewater from Fluorescent Whitening Agent Production. Environ. Sci. Technol. 2006, 40, 3395–3401. [Google Scholar] [CrossRef]
- De Sotto, R.; Bae, S. Nutrient Removal Performance and Microbiome of an Energy-Efficient Reciprocation MLE-MBR Operated under Hypoxic Conditions. Water Res. 2020, 182, 115991. [Google Scholar] [CrossRef]
- Lares, M.; Ncibi, M.C.; Sillanpää, M.; Sillanpää, M. Occurrence, Identification and Removal of Microplastic Particles and Fibers in Conventional Activated Sludge Process and Advanced MBR Technology. Water Res. 2018, 133, 236–246. [Google Scholar] [CrossRef]
- Gu, J.; Liu, H.; Wang, S.; Zhang, M.; Liu, Y. An Innovative Anaerobic MBR-Reverse Osmosis-Ion Exchange Process for Energy-Efficient Reclamation of Municipal Wastewater to NEWater-like Product Water. J. Clean. Prod. 2019, 230, 1287–1293. [Google Scholar] [CrossRef]
- Bin, Z.; Baosheng, S.; Min, J.; Taishi, G.; Zhenghong, G. Extraction and Analysis of Extracellular Polymeric Substances in Membrane Fouling in Submerged MBR. Desalination 2008, 227, 286–294. [Google Scholar] [CrossRef]
- Huang, H.; Fan, X.; Peng, P.; Peng, C.; Gao, Y.; Zhang, X.; Ren, H. Two Birds with One Stone: Simultaneous Improvement of Biofilm Formation and Nitrogen Transformation in MBBR Treating High Ammonia Nitrogen Wastewater via Exogenous N-Acyl Homoserine Lactones. Chem. Eng. J. 2020, 386, 124001. [Google Scholar] [CrossRef]
- Li, C.; Liang, J.; Lin, X.; Xu, H.; Tadda, M.A.; Lan, L.; Liu, D. Fast Start-up Strategies of MBBR for Mariculture Wastewater Treatment. J. Environ. Manag. 2019, 248, 109267. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Q.; Zhu, Y.; Zhao, T. Response of Wastewater Treatment Performance, Microbial Composition and Functional Genes to Different C/N Ratios and Carrier Types in MBBR Inoculated with Heterotrophic Nitrification-Aerobic Denitrification Bacteria. Bioresour. Technol. 2021, 336, 125339. [Google Scholar] [CrossRef] [PubMed]
- Shitu, A.; Zhu, S.; Qi, W.; Tadda, M.A.; Liu, D.; Ye, Z. Performance of Novel Sponge Biocarrier in MBBR Treating Recirculating Aquaculture Systems Wastewater: Microbial Community and Kinetic Study. J. Environ. Manag. 2020, 275, 111264. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Pakshirajan, K. Continuous Removal and Recovery of Metals from Wastewater Using Inverse Fluidized Bed Sulfidogenic Bioreactor. J. Clean. Prod. 2021, 284, 124769. [Google Scholar] [CrossRef]
- Kasonga, T.K.; Coetzee, M.A.A.; Kamika, I.; Momba, M.N.B. Data on the Degradation of Pharmaceuticals and Their Metabolites by a Fungal Consortium in a Non-Sterile Stirred Fluidized Bioreactor. Data Brief. 2020, 28, 105057. [Google Scholar] [CrossRef]
- Aboabboud, M.; Ibrahim, H.; Awad, A. Biological Ammonia Removal from Drinking Water in Fluidized Bed Reactors. WIT Trans. Ecol. Environ. 2008, 111, 453–463. [Google Scholar] [CrossRef]
- Gao, D.W.; Hu, Q.; Yao, C.; Ren, N.Q.; Wu, W.M. Integrated Anaerobic Fluidized-Bed Membrane Bioreactor for Domestic Wastewater Treatment. Chem. Eng. J. 2014, 240, 362–368. [Google Scholar] [CrossRef]
- Tekerlekopoulou, A.G.; Vayenas, D.V. Ammonia, Iron and Manganese Removal from Potable Water Using Trickling Filters. Desalination 2007, 210, 225–235. [Google Scholar] [CrossRef]
- Tang, W.; Li, X.; Liu, H.; Wu, S.; Zhou, Q.; Du, C.; Teng, Q.; Zhong, Y.; Yang, C. Sequential Vertical Flow Trickling Filter and Horizontal Flow Multi-Soil-Layering Reactor for Treatment of Decentralized Domestic Wastewater with Sodium Dodecyl Benzene Sulfonate. Bioresour. Technol. 2020, 300, 122634. [Google Scholar] [CrossRef]
- Giri, S.S.; Ryu, E.C.; Park, S.C. Characterization of the Antioxidant and Anti-Inflammatory Properties of a Polysaccharide-Based Bioflocculant from Bacillus Subtilis F9. Microb. Pathog. 2019, 136, 103642. [Google Scholar] [CrossRef]
- Giri, S.S.; Harshiny, M.; Sen, S.S.; Sukumaran, V.; Park, S.C. Production and Characterization of a Thermostable Bioflocculant from Bacillus Subtilis F9, Isolated from Wastewater Sludge. Ecotoxicol. Environ. Saf. 2015, 121, 45–50. [Google Scholar] [CrossRef]
- Cao, G.; Zhang, Y.; Chen, L.; Liu, J.; Mao, K.; Li, K.; Zhou, J. Production of a Bioflocculant from Methanol Wastewater and Its Application in Arsenite Removal. Chemosphere 2015, 141, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Chen, C. Removal of Arsenite by a Microbial Bioflocculant Produced from Swine Wastewater. Chemosphere 2017, 181, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Nancharaiah, Y.V.; Sarvajith, M. Aerobic Granular Sludge Process: A Fast Growing Biological Treatment for Sustainable Wastewater Treatment. Curr. Opin. Environ. Sci. Health 2019, 12, 57–65. [Google Scholar] [CrossRef]
- Seviour, T.; Yuan, Z.; van Loosdrecht, M.C.M.; Lin, Y. Aerobic Sludge Granulation: A Tale of Two Polysaccharides? Water Res. 2012, 46, 4803–4813. [Google Scholar] [CrossRef]
- Karakas, I.; Sam, S.B.; Cetin, E.; Dulekgurgen, E.; Yilmaz, G. Resource Recovery from an Aerobic Granular Sludge Process Treating Domestic Wastewater. J. Water Process Eng. 2020, 34, 101148. [Google Scholar] [CrossRef]
- Felz, S.; Kleikamp, H.; Zlopasa, J.; van Loosdrecht, M.C.M.; Lin, Y. Impact of Metal Ions on Structural EPS Hydrogels from Aerobic Granular Sludge. Biofilm 2020, 2, 100011. [Google Scholar] [CrossRef]
- Felz, S.; Vermeulen, P.; van Loosdrecht, M.C.M.; Lin, Y.M. Chemical Characterization Methods for the Analysis of Structural Extracellular Polymeric Substances (EPS). Water Res. 2019, 157, 201–208. [Google Scholar] [CrossRef]
- Felz, S.; Al-Zuhairy, S.; Aarstad, O.A.; van Loosdrecht, M.C.M.; Lin, Y.M. Extraction of Structural Extracellular Polymeric Substances from Aerobic Granular Sludge. J. Vis. Exp. 2016, 115, 54534. [Google Scholar] [CrossRef]
- Schambeck, C.M.; Magnus, B.S.; de Souza, L.C.R.; Leite, W.R.M.; Derlon, N.; Guimarães, L.B.; da Costa, R.H.R. Biopolymers Recovery: Dynamics and Characterization of Alginate-like Exopolymers in an Aerobic Granular Sludge System Treating Municipal Wastewater without Sludge Inoculum. J. Environ. Manag. 2020, 263, 110394. [Google Scholar] [CrossRef]
- Nancharaiah, Y.V.; Kiran Kumar Reddy, G. Aerobic Granular Sludge Technology: Mechanisms of Granulation and Biotechnological Applications. Bioresour. Technol. 2018, 247, 1128–1143. [Google Scholar] [CrossRef]
- Lin, Y.M.; Nierop, K.G.J.; Girbal-Neuhauser, E.; Adriaanse, M.; van Loosdrecht, M.C.M. Sustainable Polysaccharide-Based Biomaterial Recovered from Waste Aerobic Granular Sludge as a Surface Coating Material. Sustain. Mater. Technol. 2015, 4, 24–29. [Google Scholar] [CrossRef]
- Van Leeuwen, K.; de Vries, E.; Koop, S.; Roest, K. The Energy & Raw Materials Factory: Role and Potential Contribution to the Circular Economy of the Netherlands. Environ. Manag. 2018, 61, 786–795. [Google Scholar] [CrossRef]
- Shukla, A.; Mehta, K.; Parmar, J.; Pandya, J.; Saraf, M. Depicting the Exemplary Knowledge of Microbial Exopolysaccharides in a Nutshell. Eur. Polym. J. 2019, 119, 298–310. [Google Scholar] [CrossRef]
- Feng, C.; Lotti, T.; Canziani, R.; Lin, Y.; Tagliabue, C.; Malpei, F. Extracellular Biopolymers Recovered as Raw Biomaterials from Waste Granular Sludge and Potential Applications: A Critical Review. Sci. Total Environ. 2021, 753, 142051. [Google Scholar] [CrossRef]
- Kim, N.K.; Mao, N.; Lin, R.; Bhattacharyya, D.; van Loosdrecht, M.C.M.; Lin, Y. Flame Retardant Property of Flax Fabrics Coated by Extracellular Polymeric Substances Recovered from Both Activated Sludge and Aerobic Granular Sludge. Water Res. 2020, 170, 115344. [Google Scholar] [CrossRef]
- Gupta, A.; Thakur, I.S. Study of Optimization of Wastewater Contaminant Removal along with Extracellular Polymeric Substances (EPS) Production by a Thermotolerant Bacillus Sp. ISTVK1 Isolated from Heat Shocked Sewage Sludge. Bioresour. Technol. 2016, 213, 21–30. [Google Scholar] [CrossRef]
- Xia, L.; Tan, J.; Wu, P.; He, Q.; Song, S.; Li, Y. Biopolymers Extracted from Klebsiella Sp. and Bacillus Sp. in Wastewater Sludge as Superb Adsorbents for Aqueous Hg(II) Removal from Water. Chem. Phys. Lett. 2020, 754, 137689. [Google Scholar] [CrossRef]
- Qian, Y.; Zhang, K.; Jin, H.; Lei, L.; Zhang, H.; Gan, H. Removal of Acenaphthene from Wastewater by Pseudomonas Sp. in Anaerobic Conditions: The Effects of Extra and Intracellular Substances. Environ. Technol. 2020, 41, 1298–1306. [Google Scholar] [CrossRef]
- Vaningelgem, F.; Zamfir, M.; Mozzi, F.; Adriany, T.; Vancanneyt, M.; Swings, J.; De Vuyst, L. Biodiversity of Exopolysaccharides Produced by Streptococcus Thermophilus Strains Is Reflected in Their Production and Their Molecular and Functional Characteristics. Appl. Environ. Microbiol. 2004, 70, 900–912. [Google Scholar] [CrossRef]
- Daba, G.M.; Elnahas, M.O.; Elkhateeb, W.A. Contributions of Exopolysaccharides from Lactic Acid Bacteria as Biotechnological Tools in Food, Pharmaceutical, and Medical Applications. Int. J. Biol. Macromol. 2021, 173, 79–89. [Google Scholar] [CrossRef]
- Gupta, J.; Rathour, R.; Dupont, C.L.; Kaul, D.; Thakur, I.S. Genomic Insights into Waste Valorized Extracellular Polymeric Substances (EPS) Produced by Bacillus Sp. ISTL8. Environ. Res. 2021, 192, 110277. [Google Scholar] [CrossRef]
- Nagaraj, V.; Skillman, L.; Li, D.; Ho, G. Review—Bacteria and Their Extracellular Polymeric Substances Causing Biofouling on Seawater Reverse Osmosis Desalination Membranes. J. Environ. Manag. 2018, 223, 586–599. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Fang, H.H.P. Extraction of Extracellular Polymeric Substances (EPS) of Sludges. J. Biotechnol. 2002, 95, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, S.B.; Abdullah, S.R.S.; Othman, A.R.; Purwanti, I.F.; Imron, M.F.; Ismail, N.I.; Ahmad, A.; Hasan, H.A. Isolation and Characterisation of Bioflocculant-Producing Bacteria from Aquaculture Effluent and Its Performance in Treating High Turbid Water. J. Water Process Eng. 2021, 42, 102194. [Google Scholar] [CrossRef]
- Abu Bakar, S.N.H.; Abu Hasan, H.; Abdullah, S.R.S.; Kasan, N.A.; Muhamad, M.H.; Kurniawan, S.B. A Review of the Production Process of Bacteria-Based Polymeric Flocculants. J. Water Process Eng. 2021, 40, 101915. [Google Scholar] [CrossRef]
- Ahmad, A.; Kurniawan, S.B.; Abdullah, S.R.S.; Othman, A.R.; Hasan, H.A. Exploring the Extraction Methods for Plant-Based Coagulants and Their Future Approaches. Sci. Total Environ. 2022, 818, 151668. [Google Scholar] [CrossRef]
- Angelin, J.; Kavitha, M. Exopolysaccharides from Probiotic Bacteria and Their Health Potential. Int. J. Biol. Macromol. 2020, 162, 853–865. [Google Scholar] [CrossRef]
- Li, W.; Xia, X.; Tang, W.; Ji, J.; Rui, X.; Chen, X.; Jiang, M.; Zhou, J.; Zhang, Q.; Dong, M. Structural Characterization and Anticancer Activity of Cell-Bound Exopolysaccharide from Lactobacillus Helveticus MB2-1. J. Agric. Food Chem. 2015, 63, 3454–3463. [Google Scholar] [CrossRef]
- Xu, D.; Hu, Y.; Wu, F.; Jin, Y.; Xu, X.; Gänzle, M.G. Comparison of the Functionality of Exopolysaccharides Produced by Sourdough Lactic Acid Bacteria in Bread and Steamed Bread. J. Agric. Food Chem. 2020, 68, 8907–8914. [Google Scholar] [CrossRef]
- Ding, Z.; Bourven, I.; Guibaud, G.; van Hullebusch, E.D.; Panico, A.; Pirozzi, F.; Esposito, G. Role of Extracellular Polymeric Substances (EPS) Production in Bioaggregation: Application to Wastewater Treatment. Appl. Microbiol. Biotechnol. 2015, 99, 9883–9905. [Google Scholar] [CrossRef]
- Zeng, J.; Gao, J.-M.; Chen, Y.-P.; Yan, P.; Dong, Y.; Shen, Y.; Guo, J.-S.; Zeng, N.; Zhang, P. Composition and Aggregation of Extracellular Polymeric Substances (EPS) in Hyperhaline and Municipal Wastewater Treatment Plants. Sci. Rep. 2016, 6, 26721. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.S.; Amorim, C.L.; Ramos, M.A.; Mesquita, D.P.; Inocêncio, P.; Ferreira, E.C.; van Loosdrecht, M.; Castro, P.M.L. Variability in the Composition of Extracellular Polymeric Substances from a Full-Scale Aerobic Granular Sludge Reactor Treating Urban Wastewater. J. Environ. Chem. Eng. 2020, 8, 104156. [Google Scholar] [CrossRef]
- Samer, M. Biological and Chemical Wastewater Treatment Processes. Wastewater Treat. Eng. 2015, 247, 1128–1143. [Google Scholar] [CrossRef]
- Moghannem, S.A.M.; Farag, M.M.S.; Shehab, A.M.; Azab, M.S. Exopolysaccharide Production from Bacillus Velezensis KY471306 Using Statistical Experimental Design. Braz. J. Microbiol. 2018, 49, 452–462. [Google Scholar] [CrossRef]
- Hua, X.; Wu, Z.; Zhang, H.; Lu, D.; Wang, M.; Liu, Y.; Liu, Z. Degradation of Hexadecane by Enterobacter Cloacae Strain TU That Secretes an Exopolysaccharide as a Bioemulsifier. Chemosphere 2010, 80, 951–956. [Google Scholar] [CrossRef]
- Uthayasooriyan, M.; Pathmanathan, S.; Ravimannan, N.; Sathyaruban, S. Formulation of Alternative Culture Media for Bacterial and Fungal Growth. Pharm. Lett. 2016, 8, 431–436. [Google Scholar]
- Arulanantham, R.; Pathmanathan, S.; Ravimannan, N.; Niranjan, K. Alternative Culture Media for Bacterial Growth Using Different Formulation of Protein Sources. J. Nat. Prod. Plant Resour 2012, 2, 697–700. [Google Scholar]
- Lukwambe, B.; Yang, W.; Zheng, Y.; Nicholaus, R.; Zhu, J.; Zheng, Z. Bioturbation by the Razor Clam (Sinonovacula Constricta) on the Microbial Community and Enzymatic Activities in the Sediment of an Ecological Aquaculture Wastewater Treatment System. Sci. Total Environ. 2018, 643, 1098–1107. [Google Scholar] [CrossRef]



| Bacterial Species | Production Conditions | EPS Yield | References |
|---|---|---|---|
| Bacillus aerophilus rk1 | pH 7, 72 h incubation, 30 °C temperature | 3.73 g/L | [42] |
| Bacillus velezensis | Incubation 72 h, 150 rpm mixing, 30 °C temperature | 2 g/L | [43] |
| Diaphorobacter nitroreducens R9 | pH 9.5, 33.5 °C temperature | 4 g/L | [44] |
| Rhizobium sp. | Mannitol, sucrose, dextrose, fructose, maltose | 1.04–2.47 g/L | [45] |
| Rhizobium undicola strain N37 | Galactose/mannose = 94.17:5.83 | 0.515 g/L | [46] |
| Saccharomyces cerevisiae | Incubation 60 h | 0.97 g/L | [47] |
| Serratia marcescens | pH 7, 150 rpm mixing, 25–27 °C temperature, nutrient broth medium, 72 h incubation | 0.377 g/L | [48] |
| Streptomyces platensis | Initial inoculum 1%, pH 7, glucose, peptone | 0.461 g/L | [15] |
| Inoculum | Bioreactor | EPS Production | Reference |
|---|---|---|---|
| Consortium-activated sludge microorganism | Integrated fixed film-activated sludge | 83 mg/L | [50] |
| Consortium from scale up-flow anaerobic sludge blanket reactor | Anaerobic sequencing batch biofilm reactors | 23.6 mg/g C | [51] |
| Consortium-activated sludge microorganism | Activated sludge | 51% w/w | [52] |
| Consortium-activated sludge microorganism | Membrane bioreactor | 61% w/w | [52] |
| Consortium-activated sludge microorganism | Aerobic granular sludge | 50% w/w | [52] |
| Consortium-activated sludge microorganism | Nitrification condition-activated sludge | 396.9 mg/g MLVSS | [53] |
| Consortium-activated sludge microorganism | Nitritation condition-activated sludge | 82.5 mg/g MLVSS | [52] |
| Occurred Mechanism | Source of EPS | Type of Wastewater | Performance | References |
|---|---|---|---|---|
| Flocculation | Bacteria consortium (13 species) from secondary wastewater sludge | Kaolin suspension (5 g/L) + calcium ion (Ca+) |
| [54] |
| Flocculation | Bacteria consortium (13 species) from secondary wastewater sludge | River water, municipal wastewater, and brewery wastewater |
| [54] |
| Flocculation | Bacteria consortium (8 species) from wastewater sludge | Kaolin suspension (5 g/L) + calcium ion (Ca+) |
| [55] |
| Flocculation | Bacteria consortium from membrane bioreactor (MBR) sludges (after 50 days of operation) | Synthetic saline water: Kaolin suspension (5 g/L) + 30 g NaCl/L with addition Ca2+ 50 mg/L Synthetic freshwater: Kaolin suspension (5 g/L) with addition Ca2+ 100 mg/L |
| [57] |
| Flocculation | Serratia sp. 1 from wastewater sludge | Kaolin suspension (5 g/L) |
| [60] |
| Flocculation | Bacteria consortium from wastewater | Dual clay suspension (Kaolin and montmorillonite) |
| [61] |
| Flocculation | Cloacibacterium normanense from wastewater sludge | Oleaginous yeast fermented broth (20 g/L) |
| [62] |
| Flocculation | Bacteria consortium from activated sludge | Sludge suspension |
| [63] |
| Flocculation | Bacteria consortium from saline and freshwater MBR | Synthetic saline water: Kaolin suspension (5 g/L) + NaCl 30 g/L Synthetic freshwater: Kaolin suspension (5 g/L) with addition Ca2+ 100 mg/L |
| [55] |
| Flocculation | Bacteria consortium from activated sludge of municipal wastewater treatment plant | Freshwater algal culture (Chlorellavulgaris, Chlamydomonas asymmetrica, and Scenedesmus sp.) |
| [64] |
| Flocculation | Bacillus megaterium | Kaolin suspension (4 g/L) |
| [65] |
| Flocculation | Cyanothece sp. strain | Polystyrene nano- and microplastic stock |
| [66] |
| Adsorption | P. aeruginosa Al-Dhabi 144 from submerged palm juice wastewater fermentation | Date syrup industry wastewater |
| [57] |
| Adsorption | Purchased E. coli K12 | Cd2+, Cu2+, Ni2+, Pb2+ and Ag+ solution (1000 mg/L) |
| [67] |
| Adsorption | Parapedobacter sp. ISTM3 | Zn2+, Cu2+, Pb2+, Cr6+, Fe2+, and Cd2+ (20 mg/L) |
| [68] |
| Adsorption | Bacteria consortium from activated sludge of municipal wastewater treatment plant | Triclosan (TCS) solution |
| [47] |
| Adsorption | Sinorhizobium meliloti 1021 | Mineral oxide suspension (chromium (III) oxide, silica, zirconia) |
| [69] |
| Adsorption | Bacillus megaterium | Pb2+, Zn2+, Ni2+ solution |
| [65] |
| Decolorization | Bacillus megaterium | Congo red (CR) and methylene blue (MB) |
| [65] |
| Decolorization | Aliiglaciecola lipolytica | Congo red |
| [58] |
| Degradation: aerobic granular sludge process in sequencing batch reactor (AGS-SBR) | Rhodobacteraceae, Flavobacteriaceae, Sphingomonadaceae, Methylococcaceae, Comamonadaceae bacterial families | Fish canning industry effluent |
| [70] |
| Degradation: activated sludge process in sequencing batch reactor (SBR) | Bacteria consortium from activated sludge of municipal wastewater treatment plant | Synthetic wastewater |
| [71] |
| Ion exchange (using magnetic nanocomposite particle) | micro-algae Chlorella vulgaris | Wastewater samples from a wastewater treatment plant |
| [72] |
| Granulation of activated sludge | Flavobacteriaceae bacteria family | Saline wastewater |
| [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, H.A.; Rahim, N.F.M.; Alias, J.; Ahmad, J.; Said, N.S.M.; Ramli, N.N.; Buhari, J.; Abdullah, S.R.S.; Othman, A.R.; Jusoh, H.H.W.; et al. A Review on the Roles of Extracellular Polymeric Substances (EPSs) in Wastewater Treatment: Source, Mechanism Study, Bioproducts, Limitations, and Future Challenges. Water 2024, 16, 2812. https://doi.org/10.3390/w16192812
Hasan HA, Rahim NFM, Alias J, Ahmad J, Said NSM, Ramli NN, Buhari J, Abdullah SRS, Othman AR, Jusoh HHW, et al. A Review on the Roles of Extracellular Polymeric Substances (EPSs) in Wastewater Treatment: Source, Mechanism Study, Bioproducts, Limitations, and Future Challenges. Water. 2024; 16(19):2812. https://doi.org/10.3390/w16192812
Chicago/Turabian StyleHasan, Hassimi Abu, Nurul Farhana Mohd Rahim, Jahira Alias, Jamilah Ahmad, Nor Sakinah Mohd Said, Nur Nadhirah Ramli, Junaidah Buhari, Siti Rozaimah Sheikh Abdullah, Ahmad Razi Othman, Hajjar Hartini Wan Jusoh, and et al. 2024. "A Review on the Roles of Extracellular Polymeric Substances (EPSs) in Wastewater Treatment: Source, Mechanism Study, Bioproducts, Limitations, and Future Challenges" Water 16, no. 19: 2812. https://doi.org/10.3390/w16192812











