1. Introduction
Rainfall is a significant cause of watershed runoff and soil erosion that takes away nutrient-rich topsoil, leading to soil infertility and surface water contamination. Surface runoff and water percolation from annual precipitation are important events in agriculture and water management. Soil erosion, nutrient runoff, loss of soil organic matter, and the impairment of environmental quality from sedimentation and pollution of natural water resources by agrochemicals, heavy metals, and other environmental contaminants (ammonia, nitrate, and phosphate, etc.) have stimulated interest in the appropriate management of agricultural production systems. Increased exposure to ammonia (NH
4+) released from the application of animal manures used as organic fertilizers in agriculture alters the metabolic rank in marine vertebrates, impairs muscle tightening, and competes with potassium ions (K+) in the muscle sheath of aquatic organisms [
1]. NH
4+ also affects neurotoxicity by depolarizing the nerve cells and reducing ATP, which can cause cell fatality [
2]. In addition, nitrate (NO
3−) accumulation in water systems from agricultural runoff and discharge from animal manure farms cause growth retardation, death of living species, and problems in the food chain. According to Singh et al. [
3], the maximum concentration of NO
3-N in the United States municipal water intake measures approximately 10 mg L
-1, which is near the World Health Organization’s recommended threshold of 11.3 mg L
−1.NO
3-N. In addition, several investigators [
4] have reported that PO
4−3 ions transported by runoff water or leached to groundwater were the smallest (3 and 2%, respectively). However, this minimum amount of PO
4−3 varied from 0.1 to 0.2 mg L
−1 in the water mass, and these amounts surpassed the suggested values of 0.01 mg L
−1 for the protection of aquatic life. Accordingly, contamination of natural water resources and the percolation of water from agricultural production systems is currently an important issue.
The lack of field dissipation data on agricultural chemicals and the increasing concerns about the presence of hazardous chemicals and their impacts on soil quality, aquatic animals, and surface runoff water quality following the use of animal manure-amended soil and/or inorganic fertilizers require innovative solutions. Ammonia (NH
4+) is transferred to nitrites (NO
2-N) and nitrates (NO
3-N) [
5] by the naturally occurring bacteria
Nitosomonas and
Nitobacter as follows:
Generally, it is important to minimize and slow down the surface runoff-water volume and to support the percolation of water into soil columns to increase the retention capability of water towards the plant root zone (a desirable attribute of water conservation).
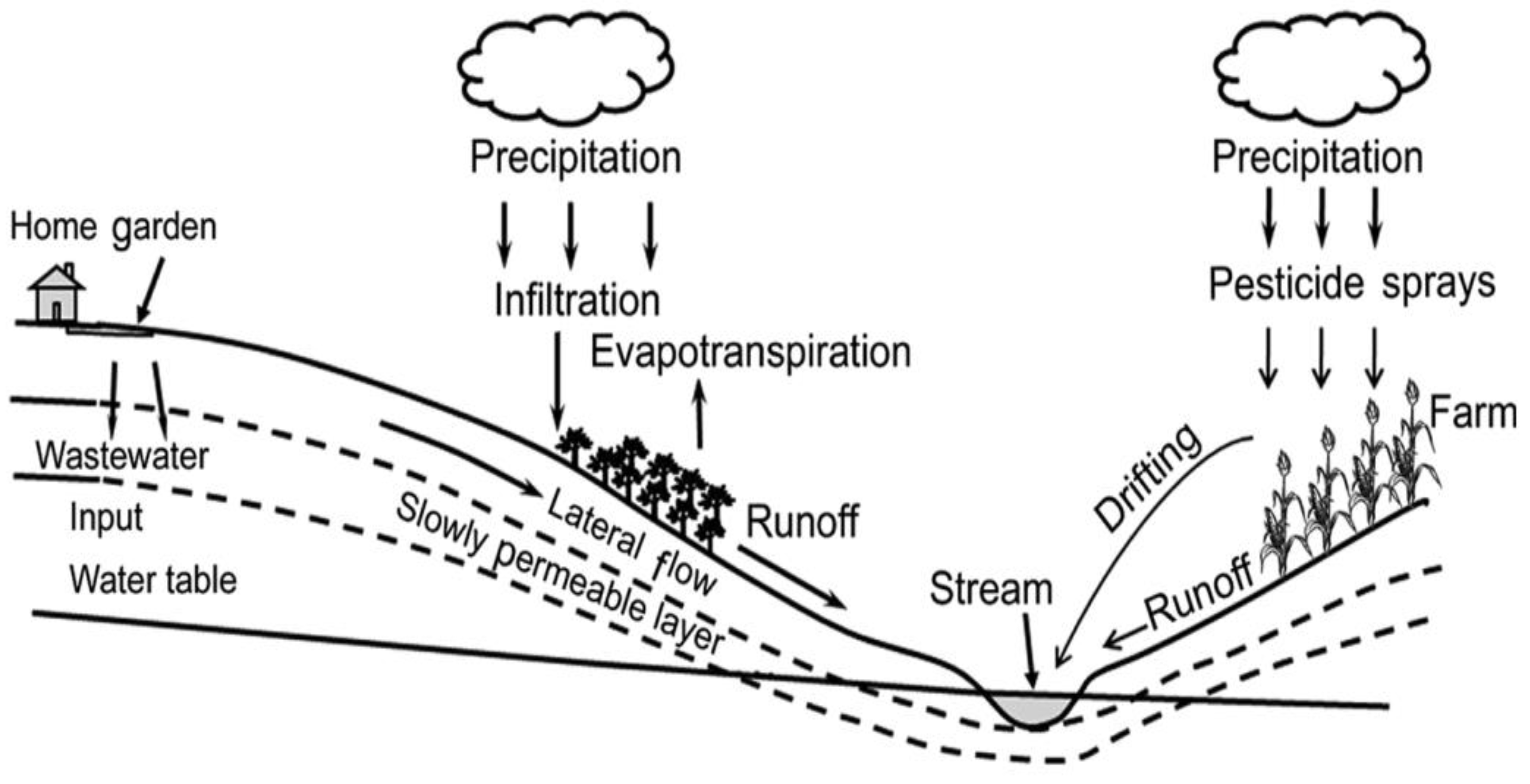
Phosphorus (P) is an essential nutrient needed for the growth of plants and animals. However, it is a common nonpoint source pollutant, contributing to the eutrophication of natural water resources. The mobility of P and N from anthropogenic activities and non-point and point sources can result in increased eutrophication and potentially the expansion of poisonous algal blooms, which can significantly influence natural water quality. Accordingly, quantitative and qualitative measures of runoff water volume and quality are of great importance. Fertilizers in runoff cause water pollution in agricultural fields and domestic gardens by running off into nearby natural water sources (
Figure 1). Therefore, technological and infrastructural solutions are required to reduce runoff water contaminant releases. Agriculture in Kentucky is a major industry, which means the use of organic and inorganic fertilizers is a common practice. The Kentucky River (Coordinates: 38°41′0″ N 85°11′18″ W, Wikipedia Encyclopedia) is a 260 mile (418 km)-long tributary of the Ohio River, located in the Commonwealth of Kentucky [
6]. The river and its tributaries drain much of the central region of the state, with its headwaters passing through the coal-mining regions of the Cumberland Mountains and its lower course passing through the Bluegrass Region in the central part of the state. The Kentucky River’s watershed encompasses approximately 7000 square miles (18,000 km
2), and it supplies drinking water to approximately one-sixth of the population of the Commonwealth of Kentucky. In highly erodible arable lands, such as most of the arable lands in Kentucky, soil erosion reduces productivity by decreasing soil depth, removing plant nutrients, and changing soil physical properties, resulting in less water infiltration, poorer crop establishment and root penetration, and potential contamination of surface water.
Accordingly, farming needs research efforts to develop new mitigation techniques and reduce the impact of contaminated water on surface and groundwater quality. Surface and drinking water NO
3− concentrations have increased in many parts of the world, mainly owing to the use of animal dung and elemental mineral fertilizers in agricultural production systems [
7]. High concentrations of NH
4+, NO
3−, and PO
4−3 in surface water increase algal blooms, and oxygen depletion, deteriorate aquatic organisms’ life cycles, and cause environmental stress and biodiversity loss. According to Antonious et al. [
7], recycling animal manure for use as a low-cost organic fertilizer can promote the restoration of ecologic and economic functions of soil, but it can also increase exposure to NH
4+, NO
3−, and PO
4−3 in surface and subsurface water and accumulation in edible plants.
Investigators have reported that biochar prepared via thermochemical conversion of wood, manure, and plant leaves has a hygroscopic and porous structure that is effective at retaining water and water-soluble nutrients, making it a good shelter and habitat for beneficial soil microorganisms [
8,
9]. These biochar characteristics improve nutrient availability to growing plants [
10] due to the high surface area, high surface functional groups, and cation exchange capacity of biochar. The biomass of organic wastes can be thermally transferred to produce biochar that has physicochemical properties that are suitable for use as soil amendments for agricultural use, promoting soil fertility and crop yield. Biochar has gained significant interest in recent years as a means of lessening the drought and salinity stresses of plants. Biochar immobilizes heavy metals in soils and reduces their uptake by plants [
11]. In addition, the porous structures of biochar can enhance the aeration of the compost pile and provide shelter for microorganisms [
7,
12]. In addition, animal manures (sewage sludge, SS; chicken manure, CM; etc.) represent a valuable source of N available at a low cost as an alternative to the high cost of inorganic fertilizers used to promote soil fertility and crop yield [
13,
14]. A chemical analysis of soil amended with CM and SS revealed a significant increase in organic matter, including N, P, and K content; the primary nutrients required to achieve target crop yields [
15]. CM generation [
16] and application in agriculture production systems have been significantly growing in recent years due to the rapid growth in the poultry industry. Poultry litter contains essential plant nutrients (N, P, K, S, Ca, Mg, B, Cu, Fe, Mn, Mo, and Zn) required for an excellent fertilizer [
17]. Phosphorus (P) fertilizers promote the growth of roots, flowers, seeds, and fruits [
18]. Chitin-containing waste materials from the seafood industry are also proposed to improve crop yield [
19].
Sweet potato flourishes during summer’s warm weather, which is required for optimal growth and root development. Profitable sweet potato production practices include using good seed stock or purchasing certified slips, well-drained, light, sandy loam or silt loam, soil fertility, irrigation, pest management, and handling of sweet potato roots [
20]. In the U.S., the commercially popular variety is the orange-fleshed sweet potato called “yam”, which contains significant β-carotene and sugar contents [
21].
The current investigation hypothesizes that the use of soil amendments, such as animal manures, chitin, and biochar, that contain high levels of organic matter and nutrients is an inexpensive way to improve crop yield and runoff water quality. In addition, animal manure is expected to reduce the need for synthetic inorganic fertilizers and offer amendments that are useful for improving soil structure and crop yield, providing significant savings to small farmers. The overall goal of the best management practices is to develop sustainable agricultural techniques that strike an acceptable balance between crop production benefits and ecological conservation by reducing the impact of excess nutrients on environmental quality and protecting watersheds from the mobility of water contaminants. Accordingly, the objectives of this investigation were: (1) to assess the impact of soil mixed with animal manure (chicken manure, CM or sewage sludge, SS) and organic products (chitin CH and biochar Bio) on runoff and seepage water volume following natural rainfall events, and (2) to monitor the impact of ammonia (NH4+), nitrate (NO3), and phosphate (PO4−3) ions released from animal manure and organic amendments used in agricultural production systems on runoff and the infiltration water quality standards.
3. Results and Discussion
The results revealed that the runoff water volume released from the CM-amended soil (172.6 L plot
−1) was significantly greater compared to runoff water released from biochar, chitin, and SS (77.86, 68.15, and 62.89 L plot
−1, respectively), including the control treatment (98.6 L plot
−1) (
Figure 4A). This volume from the CM-amended soil represents a 75% increase in runoff water compared to the runoff from the control plots.
Figure 4A also indicates that the biochar, chitin, and SS treatments significantly (
p ≤ 0.05) reduced runoff water volume compared to the control. Water infiltration into the vadose zone also varied among the soil treatments. Generally, a substantial volume of seepage water percolated through the soil column collected from CM- and SS-amended soil compared to the control, biochar, and chitin treatments (
Figure 4B). This might be due to reduced soil bulk density and increased soil interspaces (soil pores) after the addition of CM or SS amendments to the native soil. The soil amendments increased water infiltration into the soil column toward the vadose zone (the unsaturated water layer below the plant root), reduced the surface water runoff, and directed percolated water molecules toward the plant roots, significantly promoting crop yield. One should also consider that the significant precipitation during the July and September events (
Figure 5) resulted in an increased impact on the overall runoff water volume. There is an important advantage of using soil amendments such as CM and SS that are available at a low cost for limited-resource farmers, as they minimize runoff water volume and maximize percolated water.
Regardless of the soil treatment, the overall runoff water volume collected on 19 July 2021, was 183.83 L, and that on 1 September 2021, reached 202.82 L. These values were not significantly different (
p ≥ 0.05) from each other, whereas the volume of percolated water was significantly greater on 12 August, 1 September, 27 September, and 27 October compared to 19 July 2021 (0.50 L). On the contrary, the overall rainfall events were low on 12 August, 27 September, and 27 October 2021, but the percolated water volume was significantly greater compared to the percolated water collected on 19 July (
Figure 5). This variability in the water volume percolated through the soil column could be due to the duration of the rainfall, which was not measured in this study. Precipitation is not an adjacent process, and even on days with extreme precipitation, there are some hours without rainfall. Conversely, rainfall may also occur for short durations that are not enough to cause runoff; these events are most frequent in summer.
The addition of SS to native soil increased the concentration of NH
4+, NO
3−, and PO
4−3 compared to their concentrations in the native soil (the control treatment) (
Figure 6). When these nutrients are tightly bound to soil particles, they do not pose a threat to surface water. The application of organic amendments to soil, such as SS, is a current environmental and agricultural practice used to maintain and/or increase soil organic matter and nutrient contents, thus reclaiming degraded soils and supplying plant nutrients. This practice affects the C content of soil and the chemical nature and function of native soil humic acids that carry a negative pH-dependent charge, derived mainly from the range of dissociated carboxyl and phenol groups. This consequently substantially affects the fate of trace metal cations and inorganic contaminants, such as NO
3−, indicating that organic matter has an important effect on the bioavailability and persistence of soil nutrients.
The animal production area significantly alters the biogeochemical cycles of N, P, and C. Zhou et al. [
27] reported that approximately 70% of the NO
3-N in the rhizosphere of growing maize, wheat, and vegetable plants is scattered in soil levels greater than 1 m from the root area due to the application of manure N in amounts exceeding crop needs. As a result, the NO
3-N reaching the soil column area below the root region could not be exploited by the growing crops due to leaching through the soil column to lower soil levels through strong precipitation or irrigation incidents. NO
3− contamination of ground and surface water bodies is associated with increasing global fertilizer nitrogen (N) applications to growing plants [
28]. At levels exceeding the permissible limits of 10 mg L
−1, nitrate-N makes the groundwater unhealthy for drinking purposes. Surface water is adversely affected by elevated levels of NO
3-N originating from agricultural fertilizer, which is soluble and easily transported. This leads to eutrophication, as well as widespread hypoxia and anoxia, resulting in biodiversity loss and detrimental algal blooms that pose a threat to fisheries. Typically, approximately 10–30% of applied fertilizer N is lost due to water movement in modern agricultural systems [
29]. The destiny of nitrogen (N) in the sub-surface environment is determined by various factors, including land use practices, the management of N fertilizer, precipitation, irrigation techniques, soil N dynamics, and soil composition. In addition, composted animal manures stimulate the activity of soil microorganisms and increase the availability of NPK nutrients, which can significantly contribute to the growth of crops due to the increased microbial ability to decompose complex forms of organic matter into simple nutrients for plant uptake.
Regardless of the runoff dates, the overall concentration of NH
4+ in the runoff water collected from the chitin treatment (3.67 µg mL
−1) was significantly greater (
p ≤ 0.05) than in any of the other soil treatments (
Figure 7A). The NH
4+ in the runoff water volume collected from the control treatment (0.18 µg mL
−1), where no soil amendment was added, was significantly lower than other treatments. (
Figure 6). The results in
Figure 7A also indicate that the concentrations of NO
3− and phosphate PO
4−3 (1.8 and 1.021 µg mL
−1, respectively) were significantly lower in the runoff water collected from the CM-amended soil compared to the chitin treatment. In addition, the SS-amended soil treatment was effective in reducing the concentrations of NO
3 in the runoff water from 1.0 µg mL
−1 in the control treatment to 0.42 µg mL
−1 in the SS treatments, representing a 58% NO
3− reduction. This might be due to the binding of these nutrients to the SS and CM organic matter. On the contrary, the biochar was not effective in reducing the concentration of NH
4+ and NO
3− ions in the runoff water compared to the control.
Figure 7B reveals that the overall water percolated from the CM-amended soil into the vadose zone contained the greatest concentrations of NH
4+ and NO
3− (0.19 and 20.39 µg mL
−1, respectively) compared to the control treatment (0.074 and 9.52 µg mL
−1, respectively). The concentration of PO
4−3 ions in the percolated water from the biochar treatment was significantly greater compared to the other treatments, including the control treatment, indicating that the PO
4 −3 ions did not bind to the biochar particles.
When calculating the number of tipping buckets tips and the total volume of the runoff, the results revealed that the number of tips obtained from the CM-amended soil (57.5 tips) was significantly greater (
p ≤ 0.05) compared to the Bio-, CH-, and SS-amended soils (
Table 2).
Figure 8 shows the total concentrations of N as nitrate (NO
3-N), ammonia (NH
4+ N), and phosphate (PO
4−3) in the runoff water collected during the growing season from five rainfall events under the five soil management practices. The total runoff volume was obtained by multiplying the number of runoff water tips (calculated by the digit mechanical resettable counter) of each treatment by the volume of water in each tip. These values varied among the treatments and were all much higher than the limit of 10 mg L
−1 nitrates allowed in drinking water. Animal manure used as organic fertilizers contains significant amounts of nutrients released in runoff following rainfall events. However, it is emphasized that the concentrations of NO
3-N, NH
4-N, and PO
4−3 detected in the runoff down the land slope were contained within the runoff and not the receiving water. Significant portions of the runoff water volume and nutrient concentrations in runoff will be intercepted, with the remaining water often diluted by cleaner waters upon reaching streams, ponds, and lakes down the land slope.
On the contrary, it is noteworthy to mention that freshwater fish absorb nitrate ions (NO
3−) through their gills, which can result in a significant accumulation. Seawater fish, on the other hand, acquire NO
3− through both their gills and intestines [
30]. The intake of NO
3− from polluted drinking water can impact the functioning of the thyroid gland, leading to changes in thyroid hormone levels. Ward et al. [
31] discovered an elevated risk of thyroid cancer among individuals who were exposed to high concentrations of NO
3− (>5 mg L
−1 NO
3-N) through the public water supply over an extended period. Additionally, an increased risk of type 1 diabetes mellitus has been observed in individuals exposed to high NO
3− concentrations (>40–80 mg L
−1) [
32]. Kincheloe et al. [
33] reported a significant increase in the mortality rate of Chinook salmon eggs exposed to 20 mg L
−1 of NO
3−, while rainbow trout exhibited increased mortality at an exposure of 10 mg L
−1. Yildiz et al. [
34] also noted that exposure to NO
3− in the amount of 0.50 to 1.38 mg L
−1 caused an elevation in methemoglobin levels in Nile tilapia,
Oreochromis niloticus. Moreover, Davidson et al. [
35] observed reduced growth and survival rates in juvenile rainbow trout,
Oncorhynchus mykiss, when exposed to high concentrations of NO
3− (80–100 mg L
−1). Luo et al. [
36] observed decreased growth rates, developmental retardation, and increased mortality rates in rare minnow
Gobiocypris rarus when exposed to an elevated amount of NO
3−.
4. Conclusions
The application of organic and inorganic N fertilizers in agricultural production systems in the U.S. needs to be regulated, as N application rates are mainly related to a grower’s expertise in crop management practices. Excess amounts of N fertilizers used in agricultural production systems harm natural water resources. Improved N management strategies by corresponding the amount of N fertilizer needed for optimum plant yield while minimizing the NO3− concentration in runoff remains a major challenge. Oxidation of NH4+ to NO3− in water systems from agricultural runoff and discharge from animal manure farms cause growth retardation and death of aquatic living species. Extensive studies on N mobility in soil, runoff, and percolated water under field conditions are needed to enhance the adsorption of NO3 on soil particles and reduce nonpoint NO3 leakage and mobility to surface water for the safety of human and aquatic species.
The SS-amended soil was effective in reducing the concentrations of NO3− in the runoff water from 1.0 µg mL−1 in the control treatment to 0.42 µg mL-1, representing a 58% NO3− reduction. On the contrary, biochar was not effective in reducing the concentrations of NO3-N and NH4-N in the runoff water compared to the control. The concentration of PO4−3 ions in the percolated water from the biochar treatment was significantly greater compared to the other treatments, including the control treatment, indicating there was no impact of biochar in binding the PO4−3 ions.
Optimizing the application of N fertilizer rates stands out as the most impactful approach to managing fertilizers. It effectively regulates the release of nitrate-N from the soil–plant system. By adjusting the timing of N fertilizer application to avoid rainfall events and aligning it with the crop’s N uptake pattern, we can substantially decrease the leaching of nitrate-N beyond the crop’s root zone and reduce its mobility to surface water resources and the eutrophication process. The nonpoint of NO3− from agricultural fields to natural water resources is not caused by a single factor. It is due to a combination of several factors involving soil tillage, soil drainage, crop type, soil texture, soil organic matter composition, soil hydrology, temperature, rainfall schemes, and most importantly, N application rate. Monitoring weather precipitation patterns and improving animal manure and N application rates are recommended. Future objectives will include the chemical analysis of each animal manure used in this investigation, focusing on heavy metal contents and their impact on soil enzyme activity and crop yield.