Integrated Constructed Wetland–Microbial Fuel Cell Systems Using Activated Carbon: Structure-Activity Relationship of Activated Carbon, Removal Performance of Organics and Nitrogen
Abstract
1. Introduction
2. Materials and Methods
2.1. Screening of Activated Carbon
2.1.1. Determination of the Physical and Chemical Properties of Activated Carbon
2.1.2. Experiment on the Nitrogen Adsorption of Activated Carbon
2.1.3. Characterization of the Electrochemical Properties of Activated Carbon
2.2. Construction of the Integrated CW–MFC Systems
2.3. Sampling and Analysis
3. Results and Discussion
3.1. Characterization of Activated Carbon
3.2. Nitrogen Adsorption Capacity of Activated Carbon
3.3. Electrochemical Properties of Activated Carbon
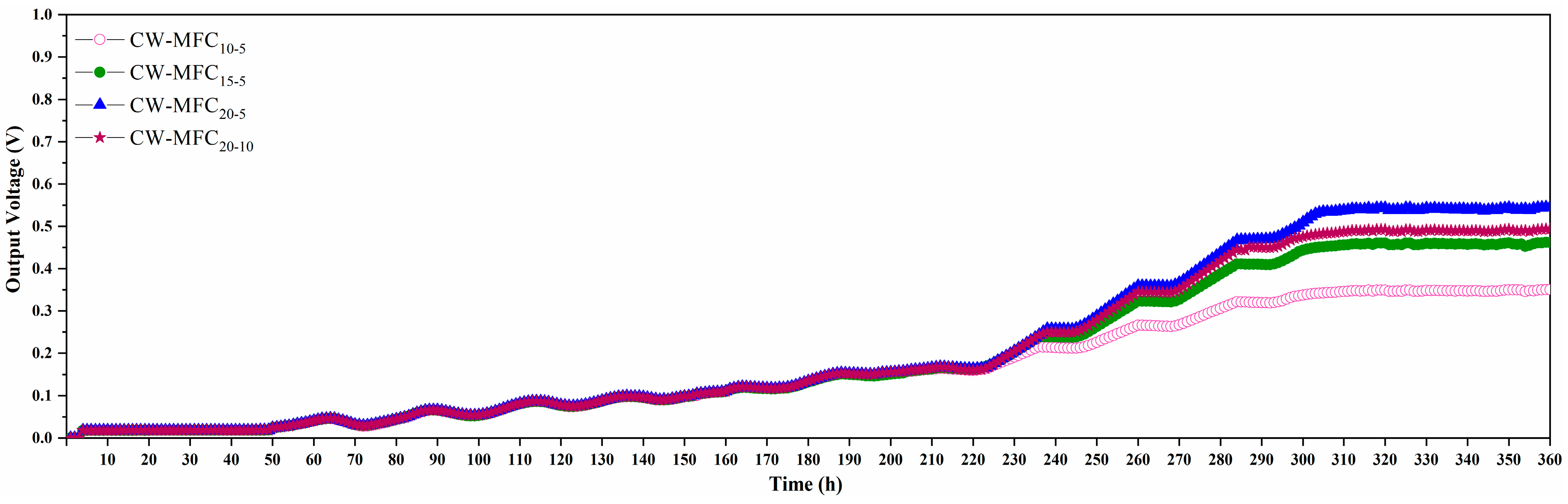
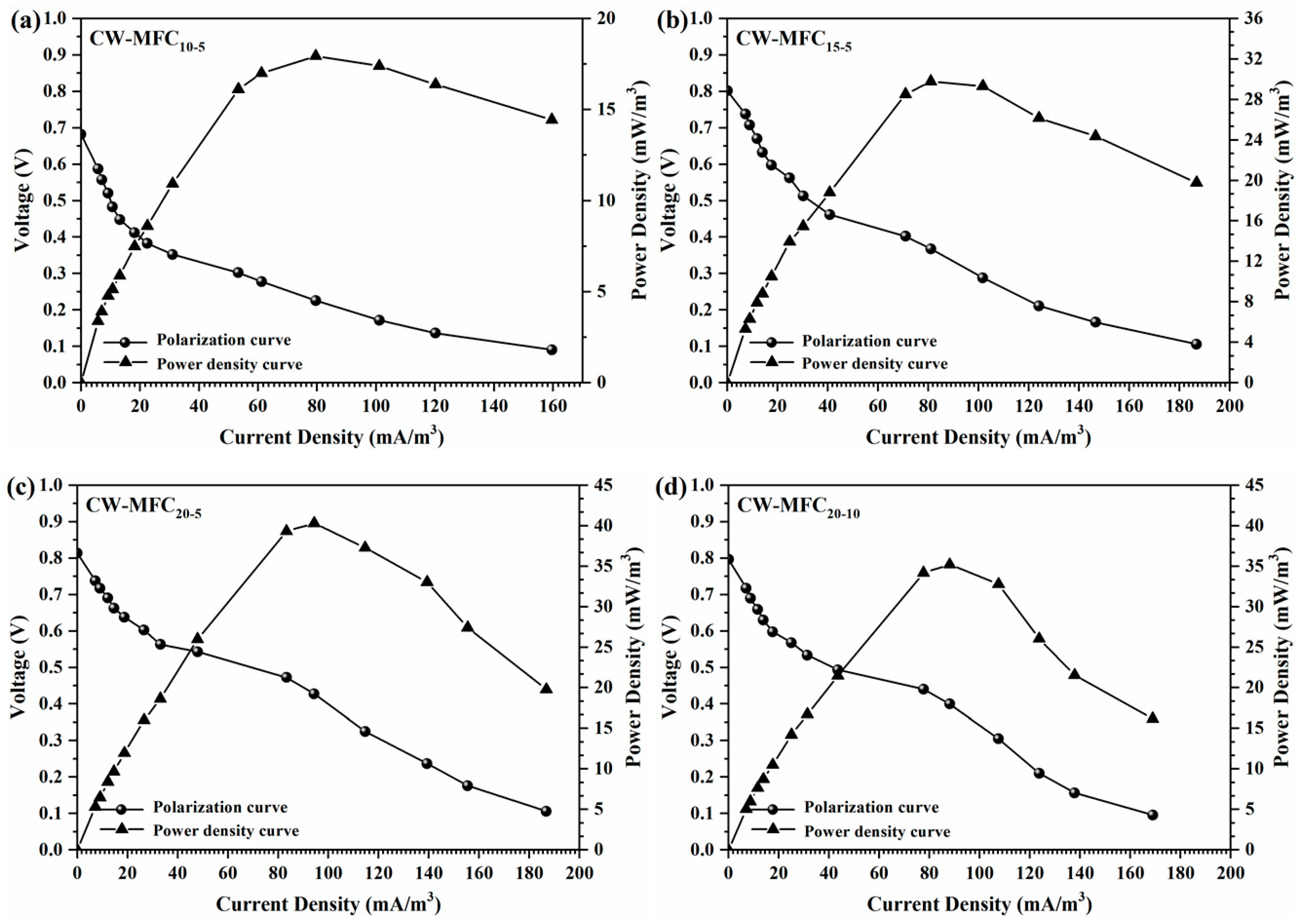
3.4. Electricity Generation and Coulombic Efficiency of CW–MFC Systems
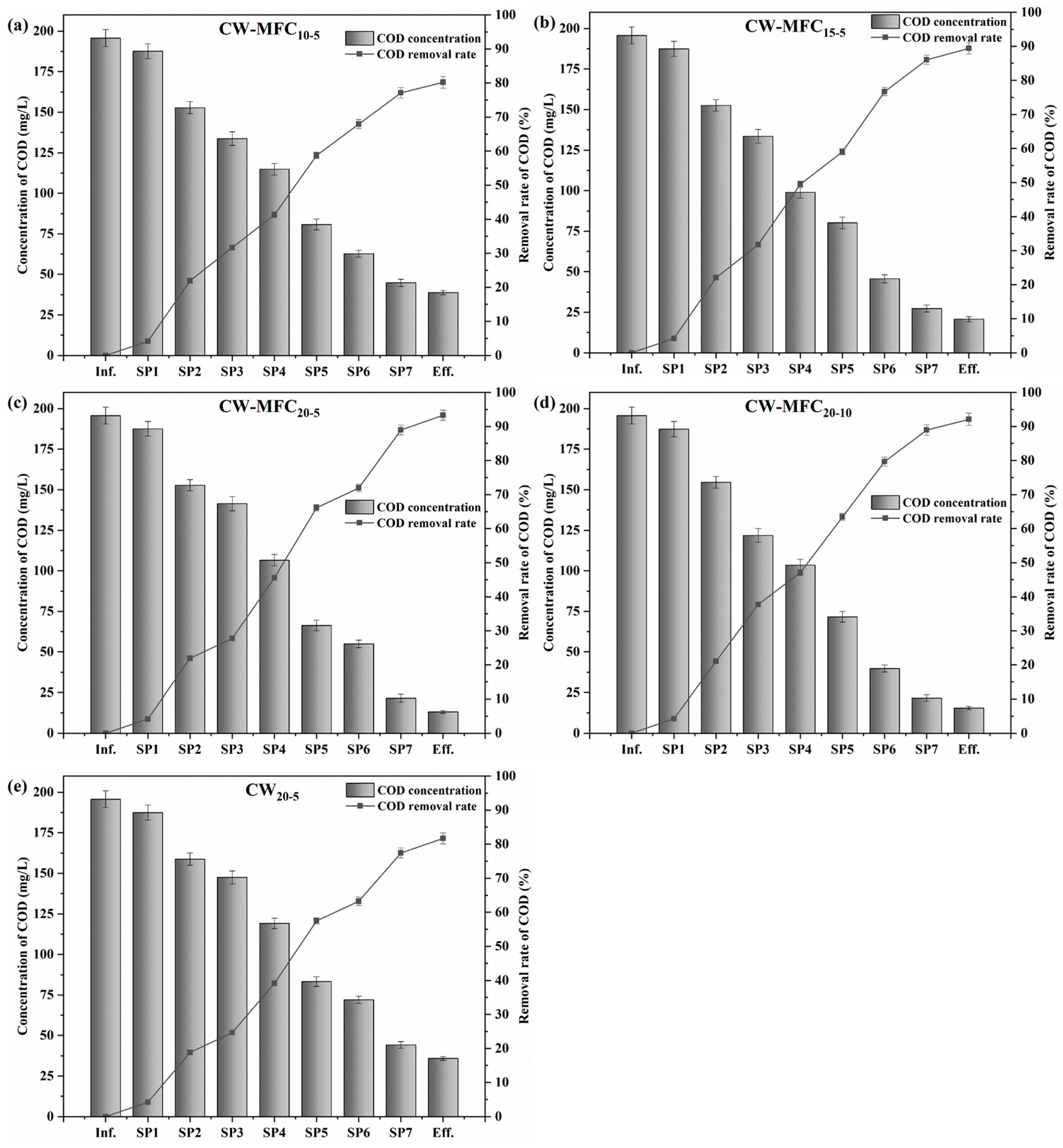
3.5. Organics Removal in CW–MFC Systems
3.6. Nitrogen Removal in CW–MFC Systems
3.7. Microbial Activity in CW–MFC Systems
3.7.1. Enzymatic Activity
3.7.2. Nitrification and Denitrification Potential
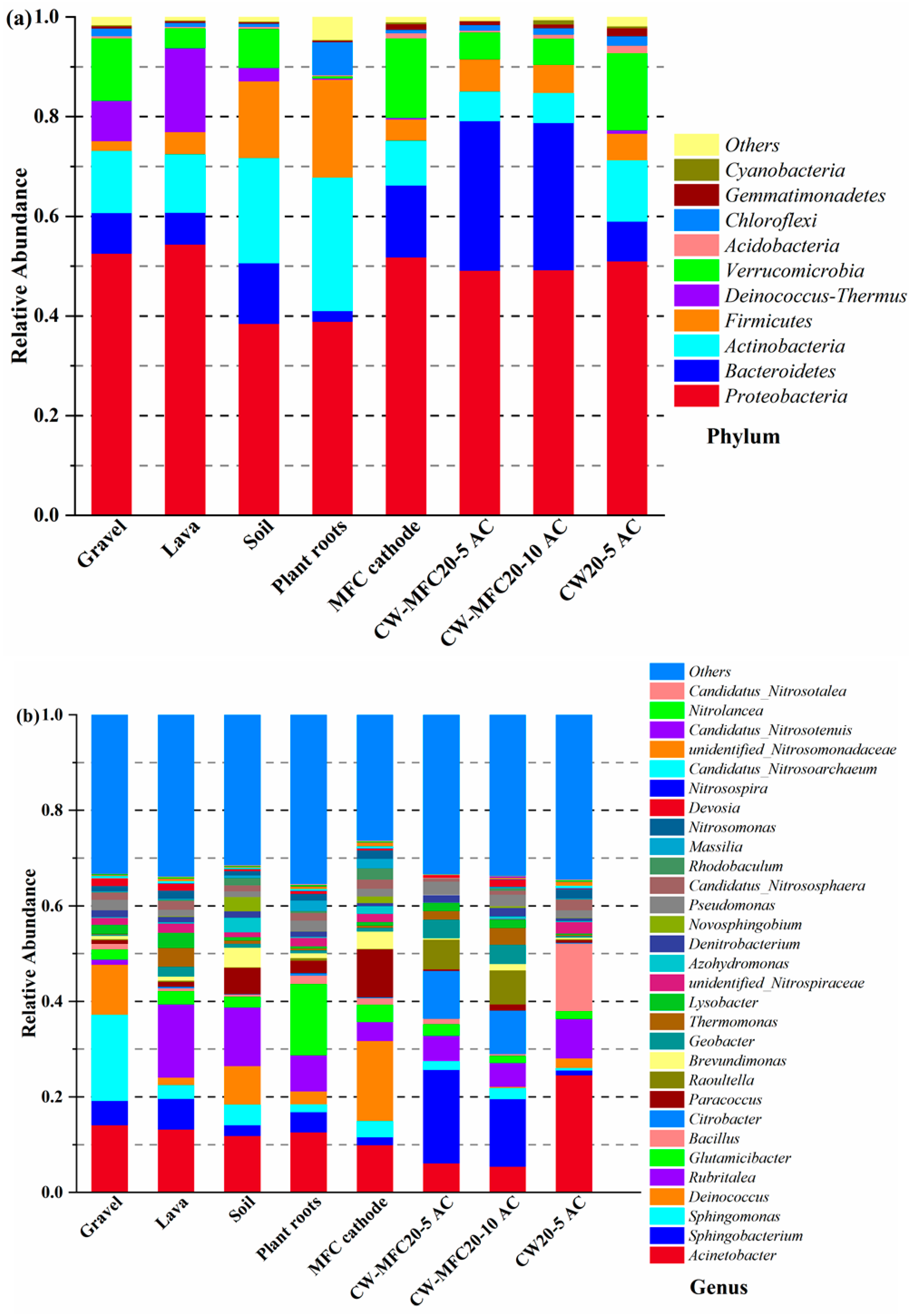
3.8. Microbial Diversity in CW–MFC Systems
4. Practical Applications and Future Research Prospects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Doherty, L.; Zhao, Y.; Zhao, X.; Hu, Y.; Hao, X.; Xu, L.; Liu, R. A Review of a recently emerged technology: Constructed wetland—Microbial fuel cells. Water Res. 2015, 85, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Guadarrama-Pérez, O.; Gutiérrez-Macías, T.; García-Sánchez, L.; Guadarrama-Pérez, V.H.; Estrada-Arriaga, E.B. Recent advances in constructed wetland-microbial fuel cells for simultaneous bioelectricity production and wastewater treatment: A review. Int. J. Energy Res. 2019, 43, 5106–5127. [Google Scholar] [CrossRef]
- Zhang, C.L.; Yu, Y.Y.; Fang, Z.; Naraginti, S.; Zhang, Y.; Yong, Y.-C. Recent advances in nitroaromatic pollutants bioreduction by electroactive bacteria. Process Biochem. 2018, 70, 129–135. [Google Scholar] [CrossRef]
- Sallam, E.R.; Khairy, H.M.; Elnouby, M.S.; Fetouh, H.A. Sustainable electricity production from seawater using Spirulina platensis microbial fuel cell catalyzed by silver nanoparticles-activated carbon composite prepared by a new modified photolysis method. Biomass Bioenergy 2021, 148, 106038. [Google Scholar] [CrossRef]
- Bruce, E. Logan. Microbial Fuel Cells. Wiley Online Library. 2008. Available online: https://aiche.onlinelibrary.wiley.com/doi/10.1002/aic.11634 (accessed on 7 November 2023).
- Ji, B.; Zhao, Y.; Vymazal, J.; Mander, Ü.; Lust, R.; Tang, C. Mapping the field of constructed wetland-microbial fuel cell: A review and bibliometric analysis. Chemosphere 2021, 262, 128366. [Google Scholar] [CrossRef] [PubMed]
- Naga Samrat, M.V.V.; Kesava Rao, K.; Ruggeri, B.; Tommasi, T. Denitrification of water in a microbial fuel cell (MFC) using seawater bacteria. J. Clean. Prod. 2018, 178, 449–456. [Google Scholar] [CrossRef]
- Wang, X.; Tian, Y.; Liu, H.; Zhao, X.; Wu, Q. Effects of influent COD/TN ratio on nitrogen removal in integrated constructed wetland–microbial fuel cell systems. Bioresour. Technol. 2019, 271, 492–495. [Google Scholar] [CrossRef]
- Corbella, C.; Puigagut, J.; Garfí, M. Life cycle assessment of constructed wetland systems for wastewater treatment coupled with microbial fuel cells. Sci. Total Environ. 2017, 584–585, 355–362. [Google Scholar] [CrossRef]
- Aelterman, P.; Verstraete, W. Bioanode performance in bioelectrochemical systems: Recent improvements and prospects. Trends Biotechnol. 2009, 27, 168–178. [Google Scholar]
- Janicek, A.; Fan, Y.; Liu, H. Design of microbial fuel cells for practical application: A review and analysis of scale-up studies. Biofuels 2014, 5, 79–92. [Google Scholar] [CrossRef]
- Sallam, E.R.; Fetouh, H.A. Comparative study for the production of sustainable electricity from marine sediment using recyclable low-cost solid wastes aluminum foil and graphite anodes. ChemistrySelect 2022, 7, e202103972. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, S.; Logan, B.E. Production of electricity from acetate or butyrate using a single-chamber microbial fuel cell. Environ. Sci. Technol. 2005, 39, 658–662. [Google Scholar] [CrossRef]
- Zhang, E.; Wang, F.; Yu, Q.; Scott, K.; Wang, X.; Diao, G. Durability and regeneration of activated carbon air-cathodes in long-term operated microbial fuel cells. J. Power Sources 2017, 360, 21–27. [Google Scholar] [CrossRef]
- Wang, J.L.; Guo, X. Adsorption isotherm models: Classification, physical meaning, application and solving method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.J.; Sajab, M.S.; Busu, W.N.W.; Misran, S.; Zakaria, S.; Chin, S.X.; Chia, C.H. Comparative adsorption mechanism of rice straw activated carbon activated with NaOH and KOH. Sains Malays. 2020, 49, 2721–2734. [Google Scholar] [CrossRef]
- Zhang, J.; Duan, Y.; Jiang, Z.; Chen, T.; Wang, K.; Wang, K.; Zhang, W.; Hu, J. Investigation of the supercapacitance of ruthenium-based/hemp stem activated carbon. J. Phys. Chem. Solids 2021, 153, 110019. [Google Scholar] [CrossRef]
- Sandoval, L.; Zamora-Castro, S.A.; Vidal-Alvarez, M.; Marín-Muñiz, J.L. Role of wetland plants and use of ornamental flowering plants in constructed wetlands for wastewater treatment: A review. Appl. Sci. 2019, 9, 685. [Google Scholar] [CrossRef]
- Yaglikci, S.; Gokce, Y.; Yagmur, E.; Aktas, Z. The performance of sulphur doped activated carbon supercapacitors prepared from waste tea. Environ. Technol. 2020, 41, 36–48. [Google Scholar] [CrossRef]
- Trazzi, P.A.; Leahy, J.J.; Hayes, M.H.B.; Kwapinski, W. Adsorption and desorption of phosphate on biochars. J. Environ. Chem. Eng. 2016, 4, 37–46. [Google Scholar] [CrossRef]
- Konneh, M.; Wandera, S.M.; Murunga, S.I.; Raude, J.M. Adsorption and desorption of nutrients from abattoir wastewater: Modelling and comparison of rice, coconut and coffee husk biochar. Heliyon 2021, 7, e08458. [Google Scholar] [CrossRef]
- Nguyen, B.T.; Dinh, G.D.; Dong, H.P.; Le, L.B. Sodium adsorption isotherm and characterization of biochars produced from various agricultural biomass wastes. J. Clean. Prod. 2022, 346, 131250. [Google Scholar] [CrossRef]
- Elanthamilan, E.; Jennifer, S.J.; Wang, S.-F.; Merlin, J.P. Effective conversion of cassia fistula dry fruits biomass into porous activated carbon for supercapacitors. Mater. Chem. Phys. 2022, 286, 126188. [Google Scholar] [CrossRef]
- Elaiyappillai, E.; Srinivasan, R.; Johnbosco, Y.; Devakumar, P.; Murugesan, K.; Kesavan, K.; Johnson, P.M. Low cost activated carbon derived from cucumis melo fruit peel for electrochemical supercapacitor application. Appl. Surf. Sci. 2019, 486, 527–538. [Google Scholar] [CrossRef]
- Hossain, M.N.; Chen, S.; Chen, A. Fabrication and electrochemical study of ruthenium-ruthenium oxide/activated carbon nanocomposites for enhanced energy storage. J. Alloys Compd. 2018, 751, 138–147. [Google Scholar] [CrossRef]
- Luo, X.Y.; Chen, Y.; Mo, Y. A review of charge storage in porous carbon-based supercapacitors. New Carbon Mater. 2021, 36, 49–68. [Google Scholar] [CrossRef]
- Endo, M.; Takeda, T.; Kim, Y.J.; Koshiba, K.; Ishii, K. High power electric double layer capacitor (EDLC’s); from operating principle to pore size control in advanced activated carbons. Carbon Lett. 2001, 1, 117–128. [Google Scholar]
- Gandla, D.; Wu, X.D.; Zhang, F.M.; Wu, C.R.; Tan, D.Q. High-performance and high-voltage supercapacitors based on N-doped mesoporous activated carbon derived from dragon fruit peels. ACS Omega 2021, 6, 7615–7625. [Google Scholar] [CrossRef]
- Min, B.; Kim, J.; Oh, S.; Regan, J.M.; Logan, B.E. Electricity generation from swine wastewater using microbial fuel cells. Water Res. 2005, 39, 4961–4968. [Google Scholar] [CrossRef]
- Yadav, A.K.; Dash, P.; Mohanty, A.; Abbassi, R.; Mishra, B.K. Performance assessment of innovative constructed wetland-microbial fuel cell for electricity production and dye removal. Ecol. Eng. 2012, 47, 126–131. [Google Scholar] [CrossRef]
- Zhao, Y.; Collum, S.; Phelan, M.; Goodbody, T.; Doherty, L.; Hu, Y. Preliminary investigation of constructed wetland incorporating microbial fuel cell: Batch and continuous flow trials. Chem. Eng. J. 2013, 229, 364–370. [Google Scholar] [CrossRef]
- Liu, S.; Song, H.; Li, X.; Yang, F. Power generation enhancement by utilizing plant photosynthate in microbial fuel cell coupled constructed wetland system. Int. J. Photoenergy 2013, 2013, 172010. [Google Scholar] [CrossRef]
- Fang, Z.; Song, H.-L.; Cang, N.; Li, X.-N. Performance of microbial fuel cell coupled constructed wetland system for decolorization of azo dye and bioelectricity generation. Bioresour. Technol. 2013, 144, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Villaseñor, J.; Capilla, P.; Rodrigo, M.A.; Cañizares, P.; Fernández, F.J. Operation of a Horizontal subsurface flow constructed wetland-microbial fuel cell treating wastewater under different organic loading rates. Water Res. 2013, 47, 6731–6738. [Google Scholar] [CrossRef] [PubMed]
- Doherty, L.; Zhao, Y.; Zhao, X.; Wang, W. Nutrient and organics removal from swine slurry with simultaneous electricity generation in an alum sludge-based constructed wetland incorporating microbial fuel cell technology. Chem. Eng. J. 2015, 266, 74–81. [Google Scholar] [CrossRef]
- Fang, Z.; Song, H.; Cang, N.; Li, X. Electricity production from azo dye wastewater using a microbial fuel cell coupled constructed wetland operating under different operating conditions. Biosens. Bioelectron. 2015, 68, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Oon, Y.-L.; Ong, S.-A.; Ho, L.-N.; Wong, Y.-S.; Dahalan, F.A.; Oon, Y.-S.; Lehl, H.K.; Thung, W.-E. Synergistic effect of up-flow constructed wetland and microbial fuel cell for simultaneous wastewater treatment and energy recovery. Bioresour. Technol. 2016, 203, 190–197. [Google Scholar] [CrossRef]
- Yakar, A.; Türe, C.; Türker, O.C.; Vymazal, J.; Saz, Ç. Impacts of various filtration media on wastewater treatment and bioelectric production in up-flow constructed wetland combined with microbial fuel cell (UCW-MFC). Ecol. Eng. 2018, 117, 120–132. [Google Scholar] [CrossRef]
- Vymazal, J. Removal of nutrients in various types of constructed wetlands. Sci. Total Environ. 2007, 380, 48–65. [Google Scholar] [CrossRef]
- Wang, J.; Song, X.; Wang, Y.; Abayneh, B.; Li, Y.; Yan, D.; Bai, J. Nitrate removal and bioenergy production in constructed wetland coupled with microbial fuel cell: Establishment of electrochemically active bacteria community on anode. Bioresour. Technol. 2016, 221, 358–365. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, Y.; Wang, X.; Yu, W. Applying multiple bio-cathodes in constructed wetland-microbial fuel cell for promoting energy production and bioelectrical derived nitrification-denitrification process. Chem. Eng. J. 2018, 344, 105–113. [Google Scholar] [CrossRef]
- Huang, J.; Li, R.; Ma, Y.X.; Cao, C.; Li, X.; Han, T.W.; Cao, M.F. Effects of macrophytes on micro–And nanoplastic retention and cycling in constructed wetlands. Environ. Pollut. 2023, 326, 121259. [Google Scholar] [CrossRef] [PubMed]
- Li, B.L.; Yan, W.K.; Wang, Y.; Wang, H.; Zhou, Z.; Li, Y.; Zhang, W.Q. Effects of key enzyme activities and microbial communities in a flocculent-granular hybrid complete autotrophic nitrogen removal over nitrite reactor under mainstream conditions. Bioresour. Technol. 2019, 280, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.L.; Yang, T.; Zhang, J.; Li, X.Z. The configuration, purification effect and mechanism of intensified constructed wetland for wastewater treatment from the aspect of nitrogen removal: A review. Bioresour. Technol. 2019, 293, 122086. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, S.; Yang, X.L.; Yang, Y.L.; Xu, H.; Li, X.N.; Song, H.L. Enhanced degradation of bisphenol A and ibuprofen by an up-flow microbial fuel cell-coupled constructed wetland and analysis of bacterial community structure. Chemosphere 2019, 217, 599–608. [Google Scholar] [CrossRef]
- Richter, H.; Nevin, K.P.; Jia, H.; Lowy, D.A.; Lovley, D.R.; Tender, L.M. Cyclic voltammetry of biofilms of wild type and mutant Geobacter Sulfurreducens on fuel cell anodes indicates possible roles of OmcB, OmcZ, type IV Pili, and protons in extracellular electron transfer. Energy Environ. Sci. 2009, 2, 506. [Google Scholar] [CrossRef]
- Jiang, M.; Zheng, X.; Chen, Y. Enhancement of denitrification performance with reduction of nitrite accumulation and N2O emission by Shewanella oneidensis MR-1 in microbial denitrifying process. Water Res. 2020, 169, 115242. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illuminaamplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Knight, R. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Robert, C.E.; Brian, J.H.; Jose, C.C. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar]
- Haas, B.J.; Gevers, D.; Earl, A.M.; Feldgarden, M.; Ward, D.V.; Giannoukos, G.; Ciulla, D.; Tabbaa, D.; Highlander, S.K.; Sodergren, E.; et al. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 2011, 21, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.; Tiedje, J. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]

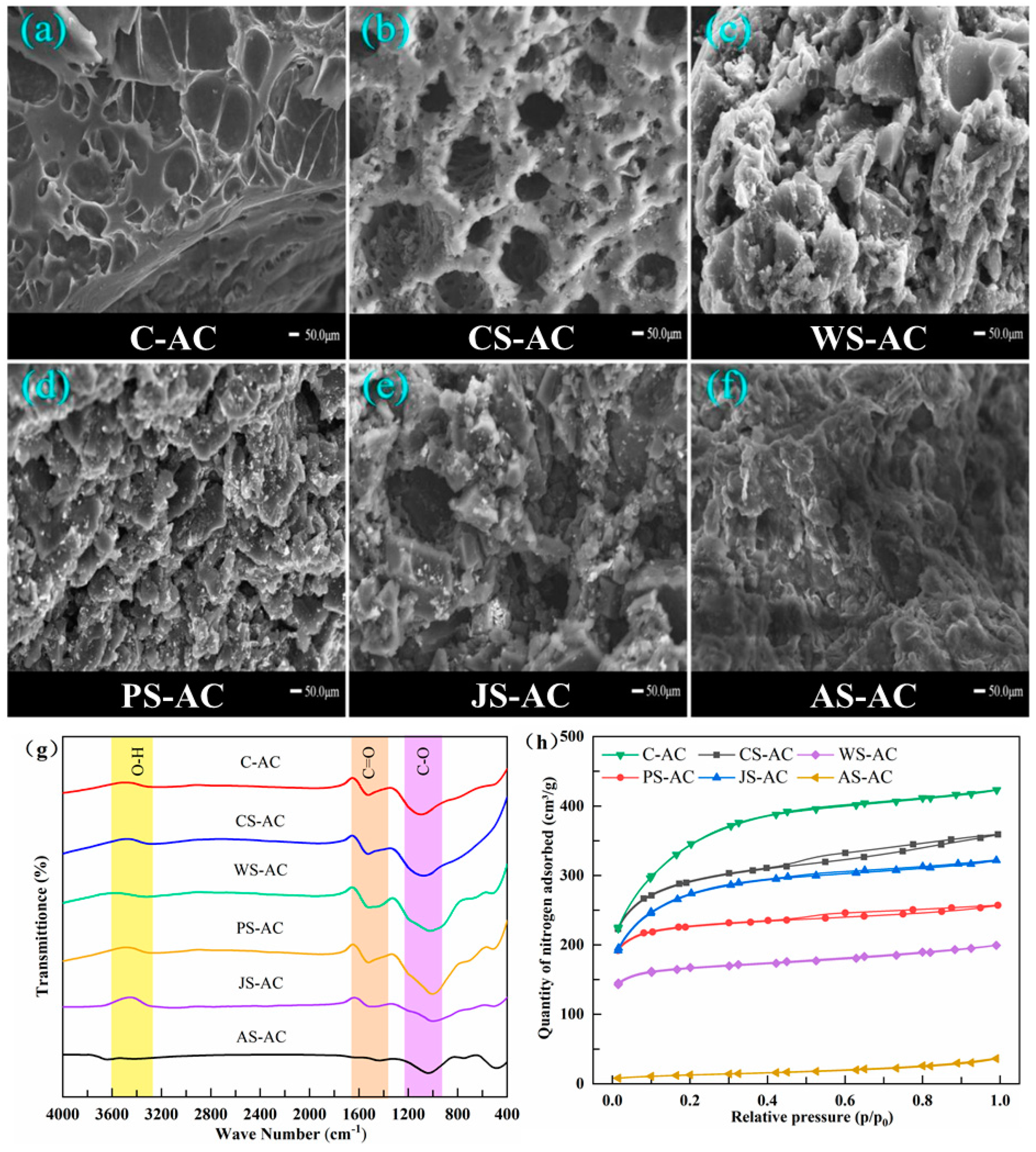





| AC | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Proportion of Pore Volume | Average Pore Size (nm) | Cost (US$/ton) | |||
|---|---|---|---|---|---|---|---|---|
| Vtotal | Vmicro | Vmeso | Vmicro/Vtotal | Vmeso/Vtotal | ||||
| C-AC | 852.3 | 0.651 | 0.095 | 0.556 | 0.146 | 0.854 | 2.50 | 530–850 |
| CS-AC | 833.1 | 0.551 | 0.265 | 0.286 | 0.481 | 0.519 | 2.51 | 1130–1510 |
| WS-AC | 632.1 | 0.394 | 0.275 | 0.119 | 0.698 | 0.302 | 2.49 | 810–1410 |
| PS-AC | 482.3 | 0.305 | 0.203 | 0.102 | 0.664 | 0.336 | 2.53 | 1050–1400 |
| JS-AC | 789.9 | 0.494 | 0.189 | 0.305 | 0.383 | 0.617 | 2.50 | 1020–1400 |
| AS-AC | 42.6 | 0.051 | 0.001 | 0.050 | 0.029 | 0.971 | 4.78 | 1500–2100 |
| AC | L/nH | Rs/mΩ | Rct/mΩ | C/F | Y0(W)/mMho×s^(1/2) | Y0(CPE)/mMho×s^N | N |
|---|---|---|---|---|---|---|---|
| C-AC | 891 | 565 | 312 | 1.1 × 105 | 816 | 4.68 | 0.742 |
| CS-AC | 746 | 495 | 306 | 7.9 | 487 | 3.94 | 0.804 |
| WS-AC | 943 | 473 | 295 | 1.16 | 790 | 4.18 | 0.778 |
| PS-AC | 275 | 415 | 449 | 1.16 | 695 | 4.76 | 0.78 |
| JS-AC | 0 | 376 | 489 | 0.731 | 2.06 | 14.6 | 0.627 |
| No. | CW–MFC (Volume) | Wastewater | Electrode | HRT (Day) | Pollutant Removal (%) | Maximum Power Density (mW/m3) | Coulombic Efficiency (%) | References |
|---|---|---|---|---|---|---|---|---|
| 1 | Up-flow subsurface (5 L) | Azo dye | Graphite plate | 4.0 | COD 75–76.5 Azo dye 81 TN 49.7 | 13.0 | 0.05–0.06 | [30] |
| 2 | Up-flow subsurface (3.7 L) | swine wastewater | Graphite plate | 0.9–1.25 | NH3-N 77.4 | 23.0 | 0.1–0. 6 | [31] |
| 3 | Up-flow subsurface (35.3 L) | synthetic wastewater (glucose) | Activated carbon stainless steel mesh | 2.0 | COD 94.8 TN 90.8 | 24.84 | 0.39–1.29 | [32] |
| 4 | Up-flow subsurface (35.3 L) | Azo dye | Activated carbon stainless steel mesh | 3.0 | Azo dye 91.2 COD 85.7 | 11 | 0.58–1.71 | [33] |
| 5 | Up-flow subsurface (270 L) | synthetic wastewater (glucose, acetate) | Graphite plate | 3.2 | COD 90–95 | 41.5 | 0.27–0.45 | [34] |
| 6 | Up-down flow subsurface (20.2 L) | swine wastewater | Graphite particles graphite rods | 1.0 | COD 64 NH3-N 75 | 15 | 0.25 | [35] |
| 7 | Up-flow subsurface (35.3 L) | Azo dye | Activated carbon stainless steel mesh | 3.0 | COD 85.7 Azo dye 92.8 | 3.7 | 0.037–1.89 | [36] |
| 8 | Up-flow subsurface (19 L) | synthetic wastewater (acetate) | Activated carbon | 1.0 | COD 99 NH3-N 96 | 12.5 | 0.06–1.42 | [37] |
| 9 | Up-flow subsurface (117 L) | synthetic wastewater (glucose) | Graphite plate | 4.0 | NH3-N 93.2 TP 96.7 | 0.7 | 0.88–1.64 | [38] |
| 10 | Up-flow subsurface (11.3 L) | synthetic wastewater (glucose) | Activated carbon stainless steel mesh | 2.0 | COD 80.2–93.3 NH3-N 79.1–97.9 TN 86.1–97.8 | 10.80–25.71 | 0.6708–0.8896 | The present study |
| CW–MFC20-5 | CW–MFC20-10 | CW20-5 | ||
|---|---|---|---|---|
| Dehydrogenase (IU/g) | gravel | 1.3247 ± 0.1875 | 1.3259 ± 0.1905 | 1.3268 ± 0.1822 |
| activated carbon (MFC anodes) | 1.7285 ± 0.2156 | 1.7053 ± 0.2062 | 1.5667 ± 0.2075 | |
| lava | 1.5389 ± 0.2189 | 1.5385 ± 0.1866 | 1.5394 ± 0.1959 | |
| soil | 1.5225 ± 0.1986 | 1.5230 ± 0.1867 | 1.5232 ± 0.1896 | |
| plant roots | 1.8586 ± 0.1859 | 1.8588 ± 0.1923 | 1.8592 ± 0.2054 | |
| MFC cathodes | 0.7992 ± 0.0859 | 0.8016 ± 0.0912 | ||
| Catalase (IU/g) | gravel | 0.8756 ± 0.0812 | 0.8761 ± 0.0843 | 0.8762 ± 0.0896 |
| activated carbon (MFC anodes) | 0.9514 ± 0.0926 | 0.9356 ± 0.0932 | 0.8751 ± 0.0921 | |
| lava | 0.8813 ± 0.0856 | 0.8824 ± 0.0861 | 0.8826 ± 0.0876 | |
| soil | 0.9125 ± 0.0921 | 0.9129 ± 0.0923 | 0.9138 ± 0.0954 | |
| plant roots | 1.1782 ± 0.1245 | 1.1779 ± 0.1242 | 1.1778 ± 0.1226 | |
| MFC cathodes | 1.1523 ± 0.1178 | 1.1547 ± 0.1189 | ||
| Ammonia monooxygenase (IU/g) | gravel | 2.5624 ± 0.2546 | 2.5620 ± 0.2512 | 2.5619 ± 0.2716 |
| activated carbon (MFC anodes) | 2.9468 ± 0.2713 | 2.9256 ± 0.2623 | 2.7451 ± 0.2643 | |
| lava | 2.6548 ± 0.2419 | 2.6564 ± 0.2537 | 2.6576 ± 0.2538 | |
| soil | 3.0759 ± 0.2785 | 3.0749 ± 0.2713 | 3.0751 ± 0.2841 | |
| plant roots | 3.7426 ± 0.2812 | 3.7435 ± 0.2746 | 3.7432 ± 0.2795 | |
| MFC cathodes | 3.5769 ± 0.2794 | 3.5772 ± 0.2723 | ||
| CW–MFC20-5 | CW–MFC20-10 | CW20-5 | ||
|---|---|---|---|---|
| Nitrification intensity/ 10−2 mg/(kg·h) | gravel | 5.41 ± 0.0796 | 5.43 ± 0.0758 | 5.40 ± 0.0726 |
| activated carbon (MFC anodes) | 6.55 ± 0.0811 | 6.38 ± 0.0805 | 5.98 ± 0.0795 | |
| lava | 5.33 ± 0.0678 | 5.36 ± 0.0698 | 5.35 ± 0.0702 | |
| soil | 6.14 ± 0.0724 | 6.12 ± 0.0785 | 6.15 ± 0.0746 | |
| plant roots | 7.81 ± 0.0923 | 7.84 ± 0.0921 | 7.82 ± 0.0915 | |
| MFC cathodes | 7.23 ± 0.0875 | 7.21 ± 0.0891 | ||
| Denitrification intensity/ mg/(kg·h) | gravel | 241.3 ± 2.54 | 240.5 ± 2.78 | 239.8 ± 2.62 |
| activated carbon (MFC anodes) | 287.4 ± 3.08 | 282.2 ± 2.91 | 269.4 ± 3.01 | |
| lava | 249.8 ± 2.71 | 248.6 ± 2.83 | 250.2 ± 2.85 | |
| soil | 241.7 ± 2.78 | 242.0 ± 2.52 | 242.3 ± 2.67 | |
| plant roots | 155.4 ± 2.23 | 154.8 ± 2.68 | 156.4 ± 2.05 | |
| MFC cathodes | 245.4 ± 2.35 | 245.9 ± 2.12 | ||
| Observed Species | Shannon | Simpson | Chaol | ACE | Good’s Coverage | PD_Whole Tree | |
|---|---|---|---|---|---|---|---|
| CW–MFC20-5 | |||||||
| gravel | 1525 | 7.065 | 0.962 | 1649.579 | 1651.378 | 0.993 | 139.759 |
| activated carbon (MFC anodes) | 1619 | 7.485 | 0.989 | 1742.421 | 1726.389 | 0.995 | 146.423 |
| lava | 1549 | 7.142 | 0.974 | 1682.385 | 1680.723 | 0.993 | 142.878 |
| soil | 1533 | 7.128 | 0.978 | 1701.169 | 1702.347 | 0.996 | 143.891 |
| plant roots | 1683 | 7.559 | 0.992 | 1785.693 | 1811.469 | 0.994 | 148.763 |
| MFC cathodes | 1316 | 6.671 | 0.953 | 1578.634 | 1587.634 | 0.993 | 134.569 |
| CW–MFC20-10 | |||||||
| gravel | 1529 | 7.072 | 0.961 | 1646.865 | 1652.246 | 0.994 | 139.462 |
| activated carbon (MFC anodes) | 1621 | 7.478 | 0.988 | 1742.247 | 1726.149 | 0.995 | 146.157 |
| lava | 1542 | 7.14 | 0.978 | 1680.897 | 1679.469 | 0.996 | 143.057 |
| soil | 1542 | 7.127 | 0.977 | 1700.579 | 1701.894 | 0.996 | 143.431 |
| plant roots | 1692 | 7.551 | 0.991 | 1784.896 | 1810.679 | 0.993 | 148.957 |
| MFC cathodes | 1321 | 6.667 | 0.951 | 1579.021 | 1585.897 | 0.995 | 134.754 |
| CW20-5 | |||||||
| gravel | 1523 | 7.07 | 0.958 | 1648.459 | 1651.894 | 0.992 | 138.963 |
| activated carbon (MFC anodes) | 1559 | 7.192 | 0.973 | 1685.589 | 1660.549 | 0.995 | 141.578 |
| lava | 1547 | 7.162 | 0.975 | 1681.769 | 1681.021 | 0.996 | 143.147 |
| soil | 1536 | 7.121 | 0.976 | 1701.548 | 1701.869 | 0.996 | 144.214 |
| plant roots | 1685 | 7.562 | 0.992 | 1783.897 | 1810.746 | 0.995 | 149.127 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Xue, M.; Wang, Z.; Xia, W.; Zhang, C. Integrated Constructed Wetland–Microbial Fuel Cell Systems Using Activated Carbon: Structure-Activity Relationship of Activated Carbon, Removal Performance of Organics and Nitrogen. Water 2024, 16, 278. https://doi.org/10.3390/w16020278
Wang X, Xue M, Wang Z, Xia W, Zhang C. Integrated Constructed Wetland–Microbial Fuel Cell Systems Using Activated Carbon: Structure-Activity Relationship of Activated Carbon, Removal Performance of Organics and Nitrogen. Water. 2024; 16(2):278. https://doi.org/10.3390/w16020278
Chicago/Turabian StyleWang, Xiaoou, Ming Xue, Zhaoyu Wang, Weiyi Xia, and Changping Zhang. 2024. "Integrated Constructed Wetland–Microbial Fuel Cell Systems Using Activated Carbon: Structure-Activity Relationship of Activated Carbon, Removal Performance of Organics and Nitrogen" Water 16, no. 2: 278. https://doi.org/10.3390/w16020278
APA StyleWang, X., Xue, M., Wang, Z., Xia, W., & Zhang, C. (2024). Integrated Constructed Wetland–Microbial Fuel Cell Systems Using Activated Carbon: Structure-Activity Relationship of Activated Carbon, Removal Performance of Organics and Nitrogen. Water, 16(2), 278. https://doi.org/10.3390/w16020278







