Occurrence, Bioaccumulation, and Potential Risks of Steroid Hormones in Freshwater Aquaculture Ponds in South China
Abstract
:1. Introduction
2. Material and Methods
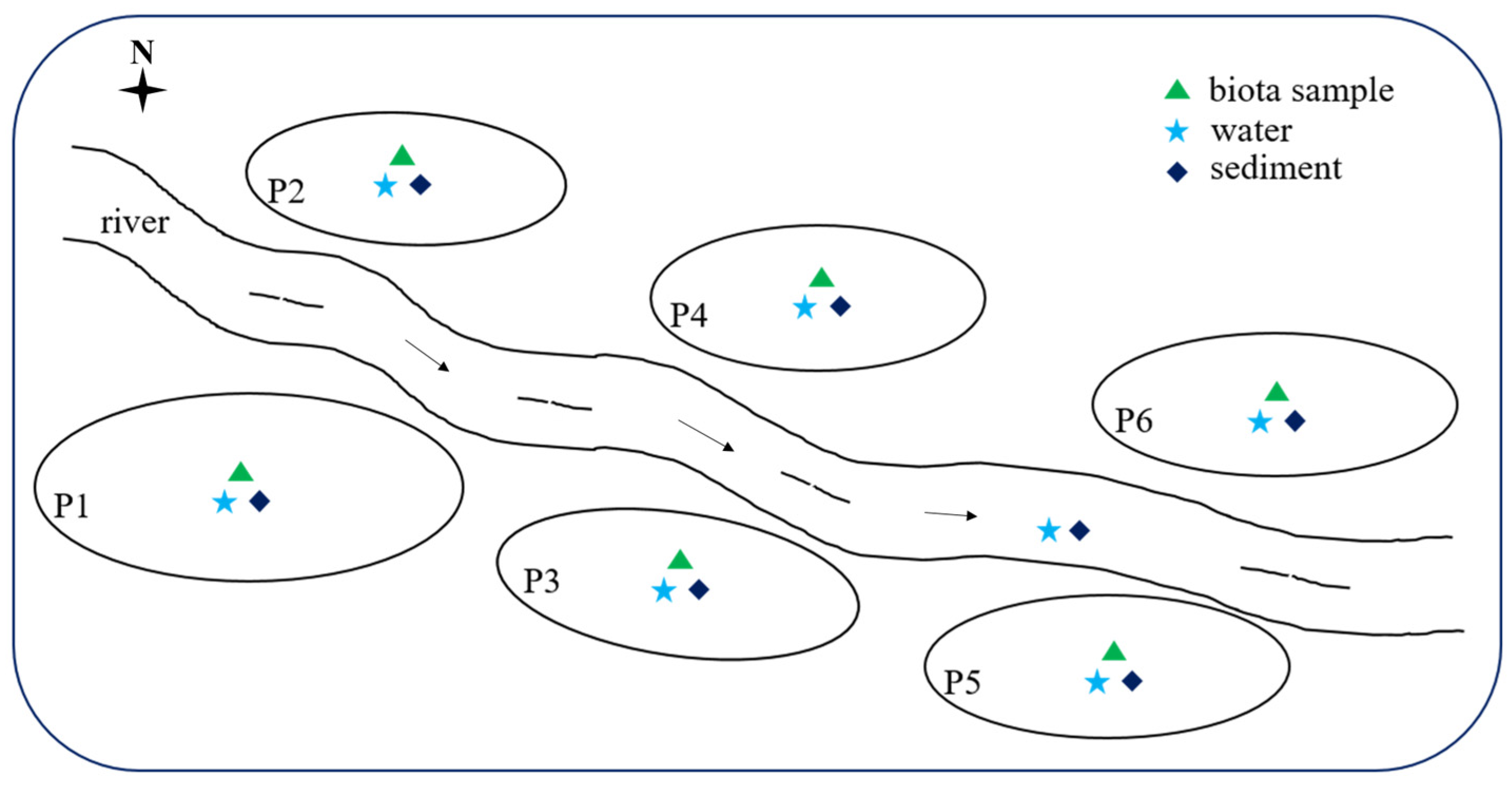
2.1. Chemicals and Sample Collection
2.2. Sample Extraction and Instrumental Analysis
2.3. Data Analysis
3. Results and Discussion
3.1. Occurrence of Steroid Hormones in Water and Sediment Samples
3.2. Occurrence of Steroid Hormones in Feed Samples
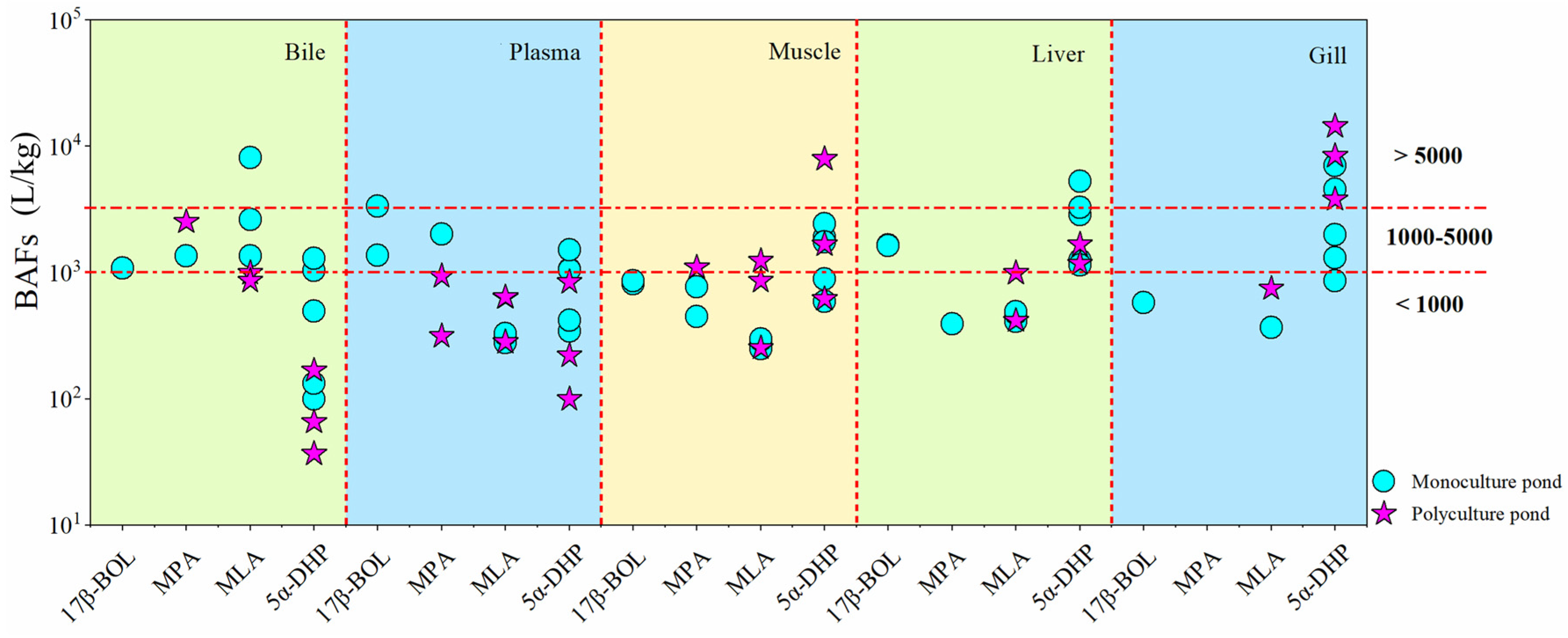
3.3. Occurrence of Steroid Hormones in Fish Samples
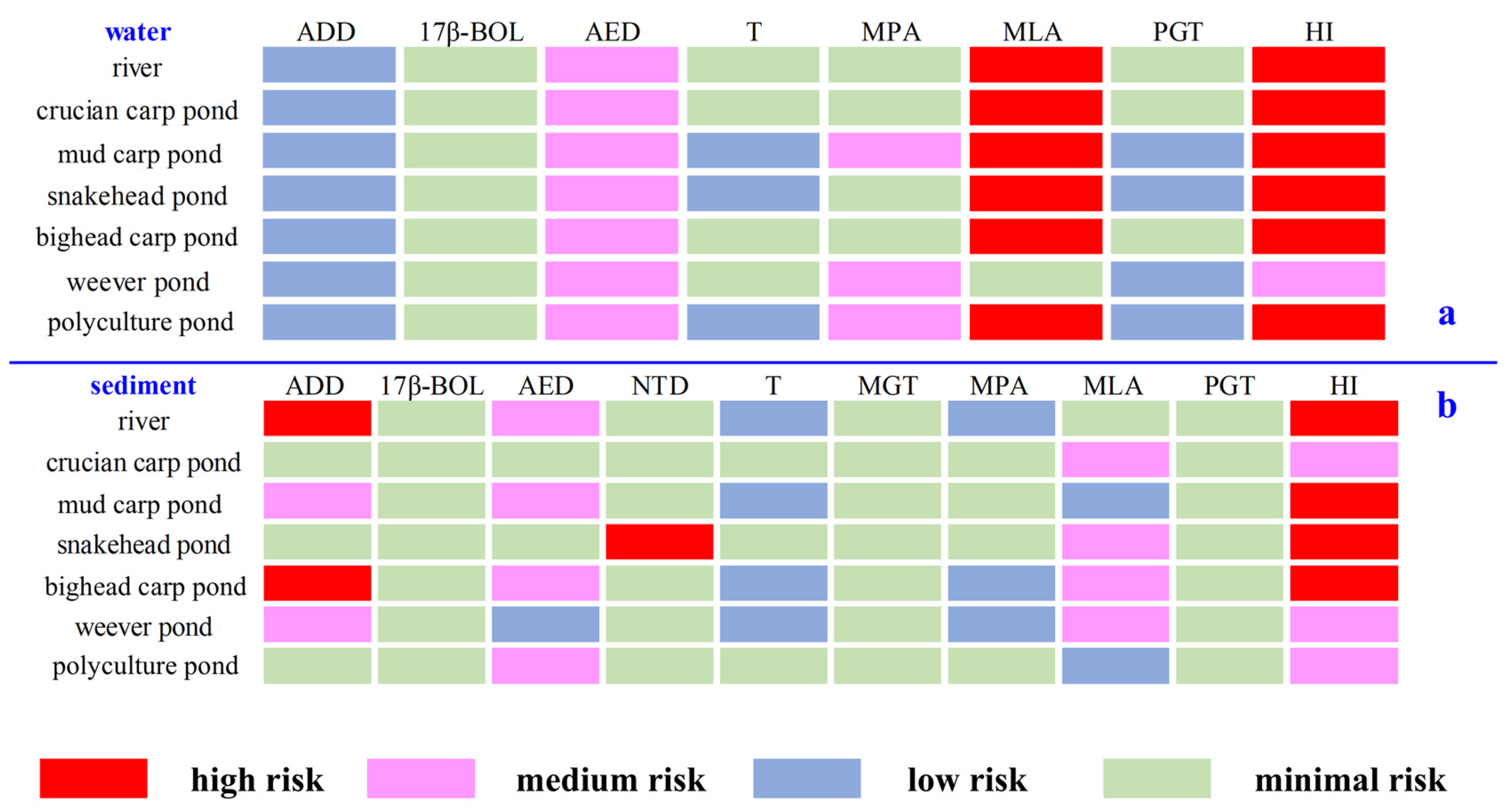
3.4. Ecological Risk Assessment
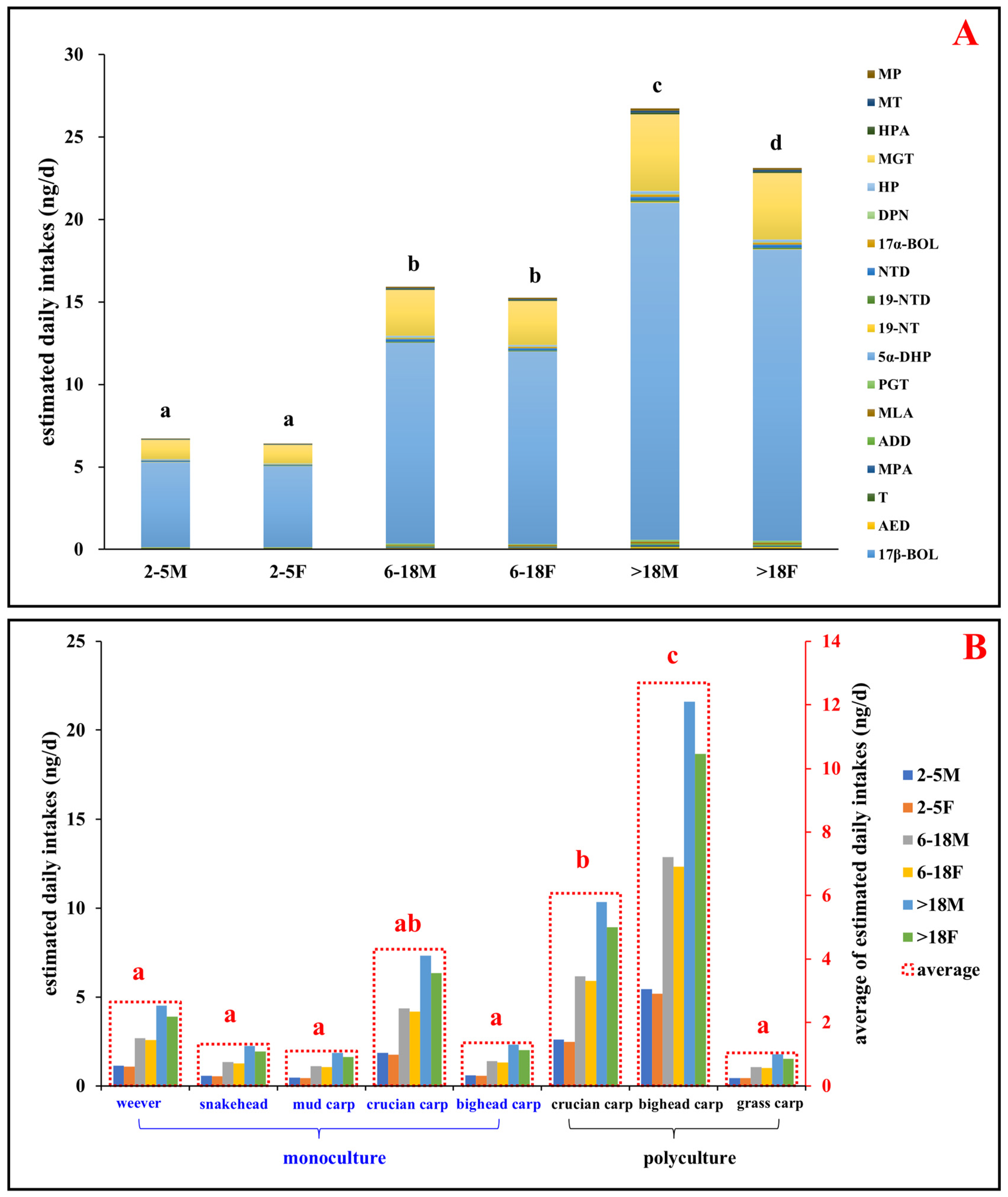
3.5. Human Health Risk Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cho, S.H.; Pyo, H.; Lee, J.J.; Zee, S.; Kim, E.; Park, J.W.; Park, C.B. Reproductive disorders linked to the interaction between sex steroid and thyroid hormonal activities, oxidative stress responses, and the rate of metabolism of tris (1,3-dichloro-2-propyl) phosphate (TDCPP) in zebrafish. Ecotoxicol. Environ. Saf. 2023, 265, 115535. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.Q.; Jing, Z.X.; Pan, C.G.; Lin, Z.; Zhen, Z.; Hou, L.P.; Dong, Z.D. The progestin norethindrone alters growth, reproductive histology and gene expression in zebrafish (Danio rerio). Chemosphere 2020, 242, 125285. [Google Scholar] [CrossRef] [PubMed]
- Grzegorzek, M.; Wartalska, K.; Kowalik, R. Occurrence and sources of hormones in water resources—Environmental and health impact. Environ. Sci. Pollut. Res. 2024, 31, 37907–37922. [Google Scholar] [CrossRef] [PubMed]
- Lai, D.Y.; Kacew, S.; Dekant, W. Tetrabromobisphenol A (TBBPA): Possible modes of action of toxicity and carcinogenicity in rodents. Food Chem. Toxicol. 2015, 80, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Kumar, V.; Vimal, S.; Umesh, M.; Sharma, P.; Thazeem, B.; Kaur, K.; Thomas, J.; Pasrija, R.; Utreja, D. Hazard identification of endocrine-disrupting carcinogens (EDCs) in relation to cancers in humans. Environ. Toxicol. Phar. 2024, 109, 104480. [Google Scholar] [CrossRef] [PubMed]
- Schmid, S.; Willi, R.A.; Salgueiro-González, N.; Fent, K. Effects of new generation progestins, including as mixtures and in combination with other classes of steroid hormones, on zebrafish early life stages. Sci. Total Environ. 2020, 709, 136262. [Google Scholar] [CrossRef]
- Almazrouei, B.; Islayem, D.; Alskafi, F.; Catacutan, M.K.; Amna, R.; Nasrat, S.; Sizirici, B.; Yildiz, I. Steroid hormones in wastewater: Sources, treatments, environmental risks, and regulations. Emerg. Contam. 2023, 9, 100210. [Google Scholar] [CrossRef]
- Unnikrishan, A.; Khalid, N.K.; Rayaroth, M.P.; Thomas, S.; Nazim, A.; Aravindakumar, C.T.; Aravind, U.K. Occurrence and distribution of steroid hormones (estrogen) and other contaminants of emerging concern in a south indian water body. Chemosphere 2024, 351, 141124. [Google Scholar] [CrossRef]
- Mohammadi, F.; Yavari, Z.; Nikoo, M.R.; Al-Nuaimi, A.; Karimi, H. Machine learning model optimization for removal of steroid hormones from wastewater. Chemosphere 2023, 343, 140209. [Google Scholar] [CrossRef]
- Liu, S.S.; Ying, G.G.; Liu, Y.S.; Yang, Y.Y.; He, L.Y.; Chen, J.; Liu, W.R.; Zhao, J.L. Occurrence and removal of progestagens in two representative swine farms: Effectiveness of lagoon and digester treatment. Water Res. 2015, 77, 146–154. [Google Scholar] [CrossRef]
- Liu, S.; Xu, X.R.; Qi, Z.H.; Chen, H.; Hao, Q.W.; Hu, Y.X.; Zhao, J.L.; Ying, G.G. Steroid bioaccumulation profiles in typical freshwater aquaculture environments of South China and their human health risks via fish consumption. Environ. Pollut. 2017, 228, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Toso, A.; Garoche, C.; Balaguer, P. Human and fish differences in steroid receptors activation: A review. Sci. Total Environ. 2024, 948, 174889. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Xie, H.W.; Junaid, M.; Xu, N.; Zhu, Y.C.; Tao, H.C.; Wong, M.H. Spatiotemporal distribution, source apportionment and risk assessment of typical hormones and phenolic endocrine disrupting chemicals in environmental and biological samples from the mariculture areas in the Pearl River Delta, China. Sci. Total Environ. 2022, 807, 150752. [Google Scholar] [CrossRef]
- Ojoghoro, J.O.; Scrimshaw, M.D.; Sumpter, J.P. Steroid hormones in the aquatic environment. Sci. Total Environ. 2021, 792, 148306. [Google Scholar] [CrossRef]
- Li, H.; Cui, Z.G.; Cui, H.W.; Bai, Y.; Yin, Z.D.; Qu, K.M. Hazardous substances and their removal in recirculating aquaculture systems: A review. Aquaculture 2023, 569, 739399. [Google Scholar] [CrossRef]
- Rodrigues, D.A.d.S.; Starling, M.C.V.; de Barros, A.L.C.; Santos, M.C.; da Silva, E.S.; Viana, G.C.C.; Ribeiro, L.F.d.S.; Simcik, M.F.; Amorim, C.C. Occurrence of antibiotics, hormones and PFAs in surface water from a Nile tilapia aquaculture facility in a Brazilian hydroelectric reservoir. Chemosphere 2024, 352, 141444. [Google Scholar] [CrossRef] [PubMed]
- Azizi-Lalabadi, M.; Pirsaheb, M. Investigation of steroid hormone residues in fish: A systematic review. Process Saf. Environ. 2021, 152, 14–24. [Google Scholar] [CrossRef]
- Xu, R.; Liu, S.; Pan, Y.F.; Wu, N.N.; Huang, Q.Y.; Li, H.X.; Lin, L.; Hou, R.; Xu, X.R.; Cheng, Y.Y. Steroid metabolites as overlooked emerging contaminants: Insights from multimedia partitioning and source–sink simulation in an estuarine environment. J. Hazard Mater. 2024, 461, 132673. [Google Scholar] [CrossRef]
- Runnalls, T.J.; Beresford, N.; Kugathas, S.; Margiotta-Casaluci, L.; Scholze, M.; Scott, A.P.; Sumpter, J.P. From single chemicals to mixtures—Reproductive effects of levonorgestrel and ethinylestradiol on the fathead minnow. Aquat. Toxicol. 2015, 169, 152–167. [Google Scholar] [CrossRef]
- Tran-Lam, T.-T.; Quan, T.C.; Bui, M.Q.; Dao, Y.H.; Le, G.T. Endocrine-disrupting chemicals in Vietnamese marine fish: Occurrence, distribution, and risk assessment. Sci. Total Environ. 2024, 908, 168305. [Google Scholar] [CrossRef]
- Rose, M.D.; Shearer, G.; Farrington, W.H. The effect of cooking on veterinary drug residues in food: 1. Clenbuterol. Food Addit. Contam. 1995, 12, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Pleadin, J.; Samardžija, M. Hormonally active substances in the food chain from farm animals to consumers. Vet. Stanica 2019, 50, 501–512. [Google Scholar]
- Zhou, X.Y.; Yang, Z.G.; Luo, Z.F.; Li, H.P.; Chen, G.Y. Endocrine disrupting chemicals in wild freshwater fishes: Species, tissues, sizes and human health risks. Environ. Pollut. 2019, 244, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Tan, X.; Huang, C.; Zhao, H.; Lan, W. Sources, pollution characteristics, and ecological risk assessment of steroids in Beihai bay, Guangxi. Water 2022, 14, 1399. [Google Scholar] [CrossRef]
- Liu, S.; Chen, H.; Xu, X.R.; Hao, Q.W.; Zhao, J.L.; Ying, G.G. Three classes of steroids in typical freshwater aquaculture farms: Comparison to marine aquaculture farms. Sci. Total Environ. 2017, 609, 942–950. [Google Scholar] [CrossRef] [PubMed]
- E.C. (European Commission) (Ed.) European Commission Technical Guidance Document in Support of Commission Directive 93/67/EEC on Risk Assessment for New Notified Substances and Commission Regulation No 1488/94 on Risk Assessment for Existing Substances, Part II; Office for Official Publications of the European Communities: Luxembourg, 2003. [Google Scholar]
- Guo, J.Y.; Wu, F.C.; Shen, R.L.; Zeng, E.Y. Dietary intake and potential health risk of DDTs and PBDEs via seafood consumption in South China. Ecotox. Environ. Safe 2010, 73, 1812–1819. [Google Scholar] [CrossRef]
- FAO. Food Supply-Livestock and Fish Primary Equivalent. 2011. Available online: http://www.fao.org/faostat/en/#data/CL/ (accessed on 4 July 2024).
- Yu, Q.M.; Yang, X.D.; Zhao, F.Z.; Hu, X.D.; Ren, H.Q.; Geng, J.J. Occurrence and removal of progestogens from wastewater treatment plants in China: Spatiotemporal variation and process comparison. Water Res. 2022, 211, 118038. [Google Scholar] [CrossRef]
- Liu, S.; Tian, F.; Pan, Y.F.; Li, H.X.; Lin, L.; Hou, R.; Zhang, L.B.; Zhang, Z.; Liu, S.S.; Xu, X.R. Contamination and ecological risks of steroid metabolites require more attention in the environment: Evidence from the fishing ports. Sci. Total Environ. 2022, 807, 150814. [Google Scholar] [CrossRef]
- Zhang, R.L.; Pei, J.Y.; Zhang, R.J.; Wang, S.P.; Zeng, W.B.; Huang, D.L.; Wang, Y.; Zhang, Y.Y.; Wang, Y.H.; Yu, K.F. Occurrence and distribution of antibiotics in mariculture farms, estuaries and the coast of the Beibu Gulf, China: Bioconcentration and diet safety of seafood. Ecotox. Environ. Safe 2018, 154, 27–35. [Google Scholar] [CrossRef]
- de Alda, M.J.L.; Gil, A.; Paz, E.; Barceló, D. Occurrence and analysis of estrogens and progestogens in river sediments by liquid chromatography-electrospray-mass spectrometry. Analyst 2002, 127, 1299–1304. [Google Scholar] [CrossRef]
- Omar, T.F.T.; Aris, A.Z.; Yusoff, F.M.; Mustafa, S. Occurrence, distribution, and sources of emerging organic contaminants in tropical coastal sediments of anthropogenically impacted Klang River estuary, Malaysia. Mar. Pollut. Bull. 2018, 131, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Mulabagal, V.; Wilson, C.; Hayworth, J.S. An ultrahigh-performance chromatography/tandem mass spectrometry quantitative method for trace analysis of potential endocrine disrupting steroid hormones in estuarine sediments. Rapid Commun. Mass Sp. 2017, 31, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Yarahmadi, H.; Duy, S.V.; Hachad, M.; Dorner, S.; Sauvé, S.; Prévost, M. Seasonal variations of steroid hormones released by wastewater treatment plants to river water and sediments: Distribution between particulate and dissolved phases. Sci. Total Environ. 2018, 635, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.S.; Ying, G.G.; Liu, S.; Lai, H.J.; Chen, Z.F.; Pan, C.G.; Zhao, J.L.; Chen, J. Analysis of 21 progestagens in various matrices by ultra-high-performance liquid chromatography tandem mass spectrometry (UHPLC-MS/MS) with diverse sample pretreatment. Anal. Bioanal. Chem. 2014, 406, 7299–7311. [Google Scholar] [CrossRef]
- Ministry of Agriculture and Rural Affairs of the People’s Republic of China, Aquaculture Medication Understanding Sheet No. 1 and 2. 2022. Available online: http://www.yyj.moa.gov.cn/gzdt/202211/t20221115_6415528.htm (accessed on 13 August 2024).
- Fent, K. Progestins as endocrine disrupters in aquatic ecosystems: Concentrations, effects and risk assessment. Environ. Int. 2015, 84, 115–130. [Google Scholar] [CrossRef]
- Bioaccumulation Criteria. 2012. Available online: http://www.pbtprofiler.net/criteria.asp/ (accessed on 2 March 2024).
- Chen, J.; Liu, Y.S.; Deng, W.J.; Ying, G.G. Removal of steroid hormones and biocides from rural wastewater by an integrated constructed wetland. Sci. Total Environ. 2019, 660, 358–365. [Google Scholar] [CrossRef]
- Hallgren, S.; Olsén, K.H. Effects on guppy brain aromatase activity following short-term steroid and 4-nonylphenol exposures. Environ. Toxicol. 2010, 25, 261–271. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Zhao, J.L.; Ying, G.G.; Liu, Y.S.; Pan, C.G. Emission estimation and multimedia fate modeling of seven steroids at the river basin scale in China. Environ. Sci. Technol. 2014, 48, 7982–7992. [Google Scholar] [CrossRef]


| Mode | Species | Gender | Testosterone | Progesterone |
|---|---|---|---|---|
| monoculture | weever | male | 4.19 × 107 | 4.51 × 108 |
| female | 3.62 × 107 | 3.90 × 108 | ||
| mud carp | male | 2.88 × 107 | 5.50 × 108 | |
| female | 2.49 × 107 | 4.75 × 108 | ||
| snakehead | male | 0 | 5.63 × 108 | |
| female | 0 | 4.87 × 108 | ||
| crucian carp | male | 2.86 × 107 | 5.52 × 108 | |
| female | 2.47 × 107 | 4.77 × 108 | ||
| bighead carp | male | 0 | 5.44 × 108 | |
| female | 0 | 4.71 × 108 | ||
| polyculture | crucian carp | male | 3.68 × 107 | 6.85 × 108 |
| female | 3.18 × 107 | 5.92 × 108 | ||
| bighead carp | male | 3.75 × 107 | 5.73 × 108 | |
| female | 3.24 × 107 | 4.96 × 108 | ||
| grass carp | male | 0 | 5.53 × 108 | |
| female | 0 | 4.78 × 108 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.-S.; Li, Y.-F.; Ning, J.-J.; Xu, L.; Wang, L.-G.; Huang, D.-L.; Wang, X.-H.; Tang, Q.-H.; Du, F.-Y. Occurrence, Bioaccumulation, and Potential Risks of Steroid Hormones in Freshwater Aquaculture Ponds in South China. Water 2024, 16, 2872. https://doi.org/10.3390/w16202872
Liu S-S, Li Y-F, Ning J-J, Xu L, Wang L-G, Huang D-L, Wang X-H, Tang Q-H, Du F-Y. Occurrence, Bioaccumulation, and Potential Risks of Steroid Hormones in Freshwater Aquaculture Ponds in South China. Water. 2024; 16(20):2872. https://doi.org/10.3390/w16202872
Chicago/Turabian StyleLiu, Shuang-Shuang, Ya-Fang Li, Jia-Jia Ning, Lei Xu, Liang-Gen Wang, De-Lian Huang, Xue-Hui Wang, Que-Hui Tang, and Fei-Yan Du. 2024. "Occurrence, Bioaccumulation, and Potential Risks of Steroid Hormones in Freshwater Aquaculture Ponds in South China" Water 16, no. 20: 2872. https://doi.org/10.3390/w16202872





