Heterogeneous Activation of Persulfate by Petal-Shaped Co3O4@BiOI to Degrade Bisphenol AF
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Co3O4@BiOI
2.3. Catalyst Characterization
3. Results and Discussion
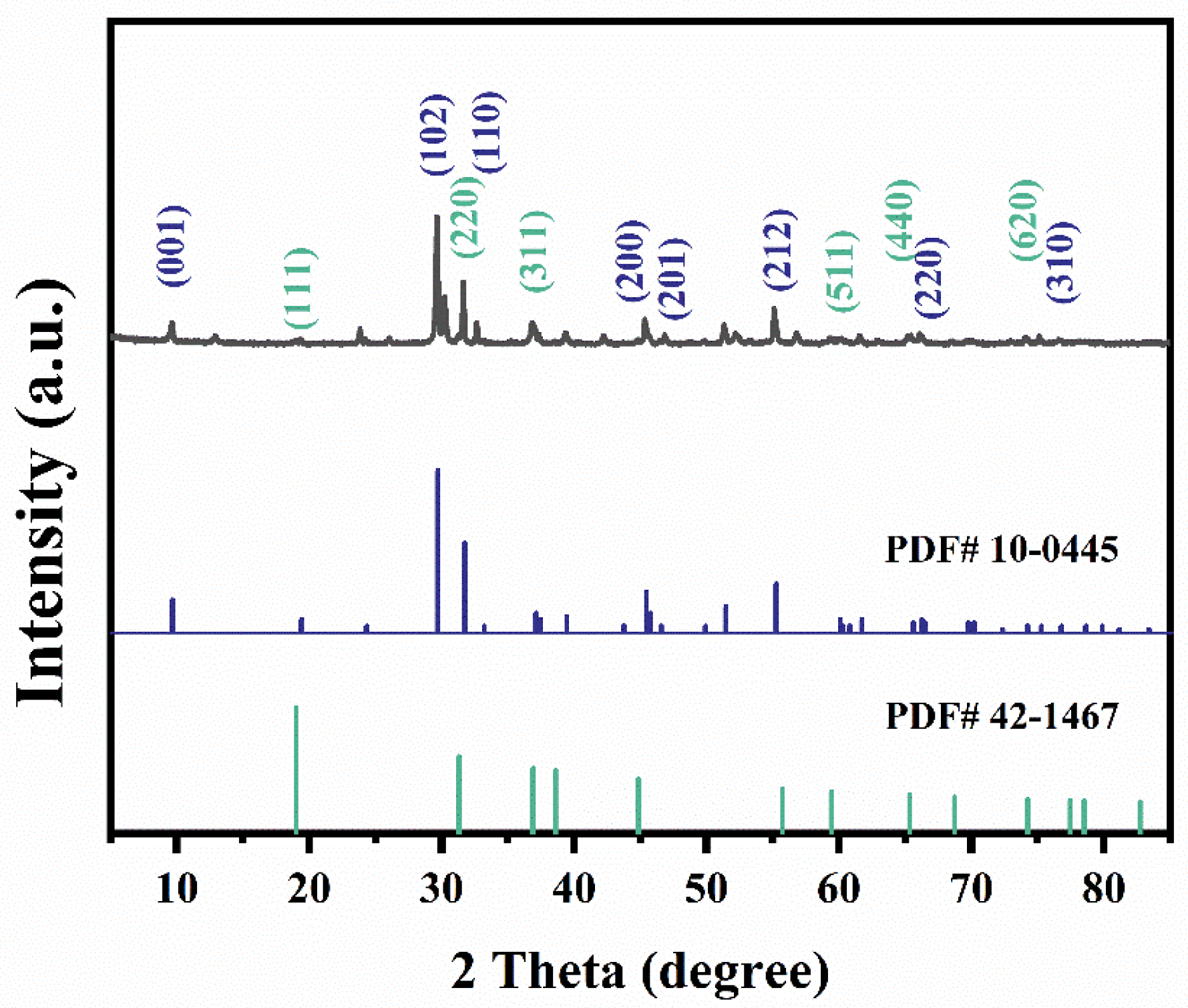
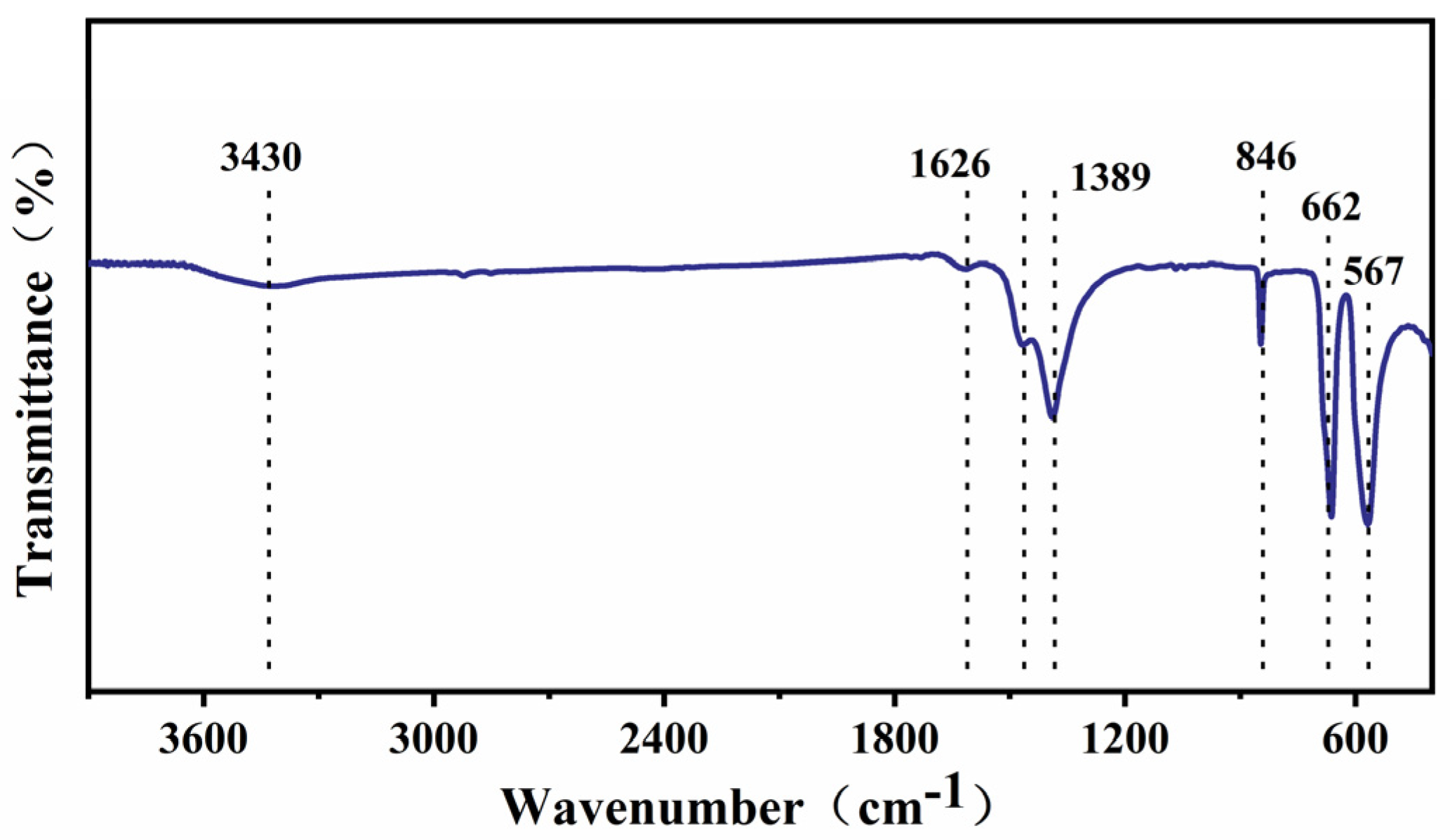
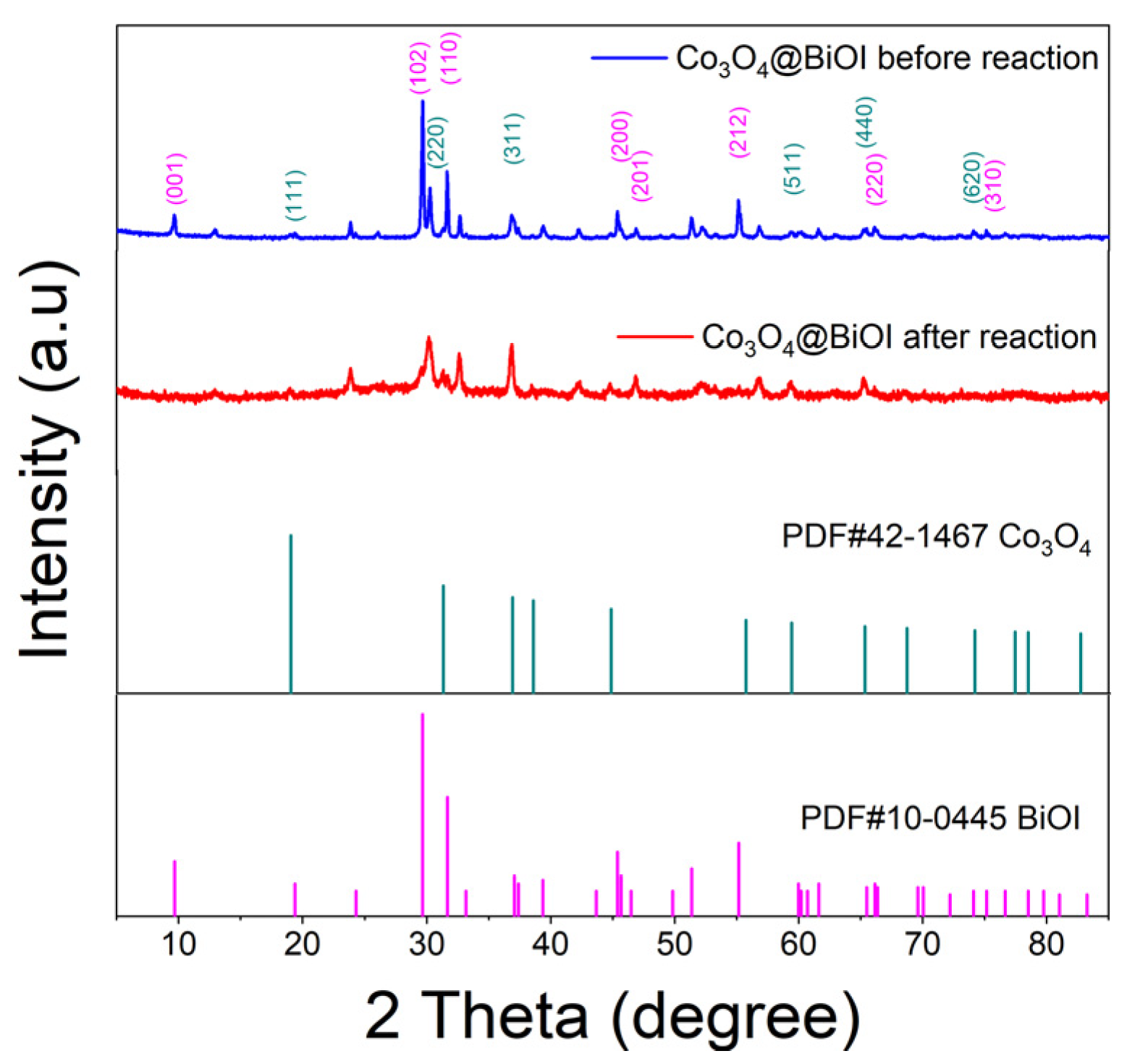
3.1. Characteristic of Co3O4@BiOI
3.2. Evaluation of Catalytic Efficiency
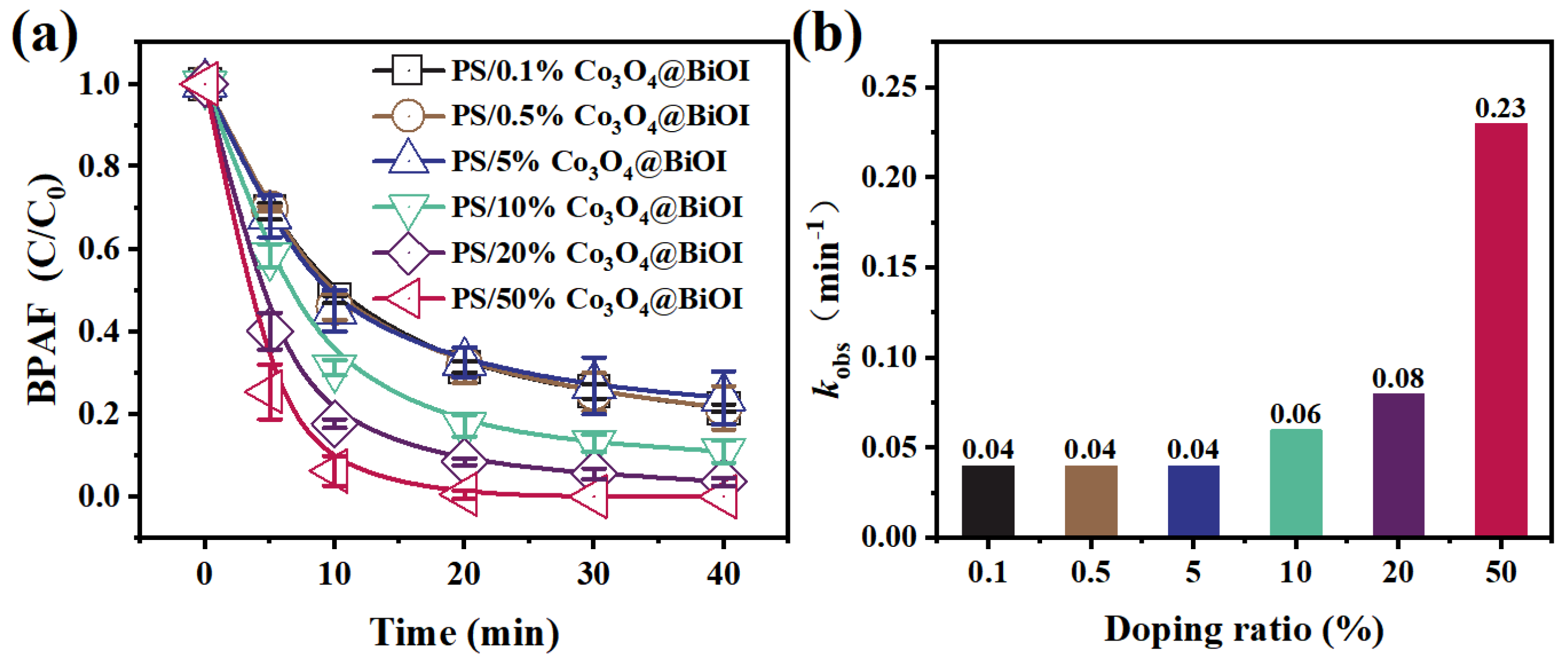
3.2.1. Effect of Doping
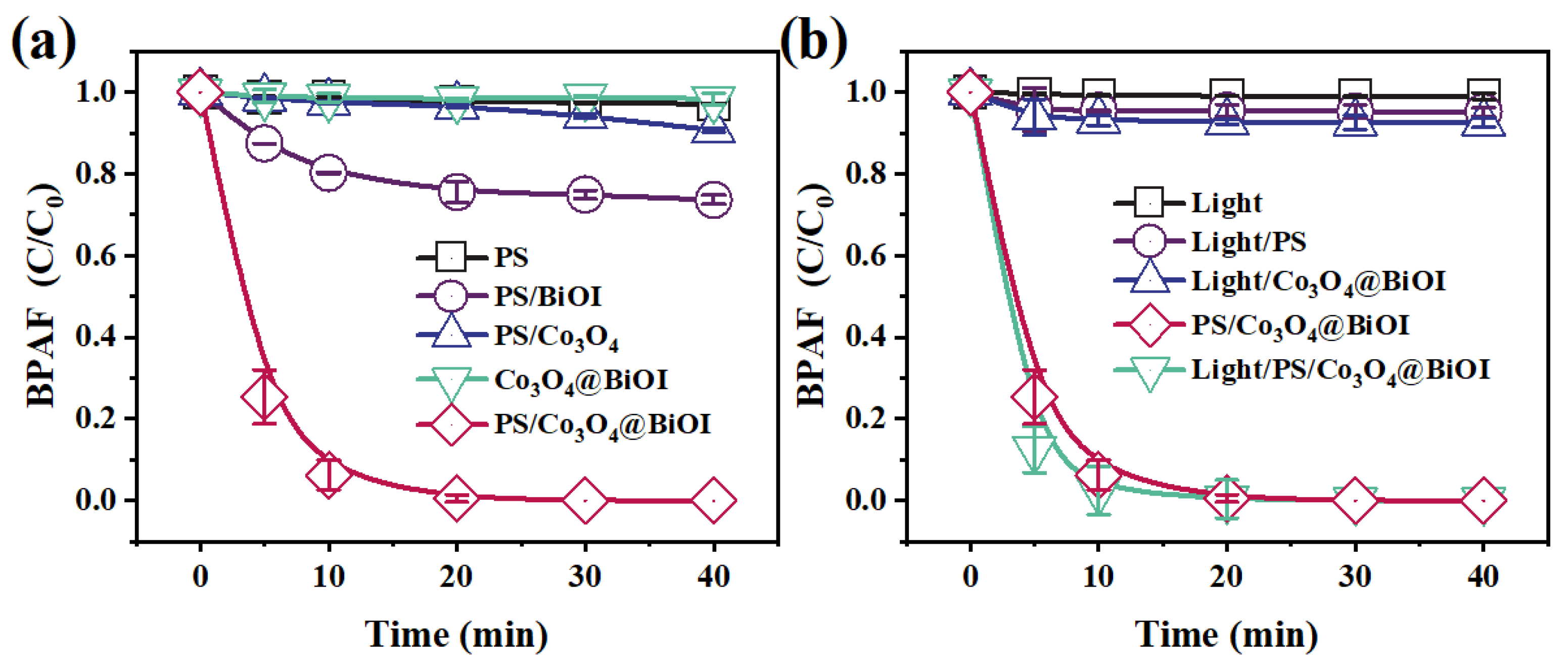
3.2.2. Comparison Experiment
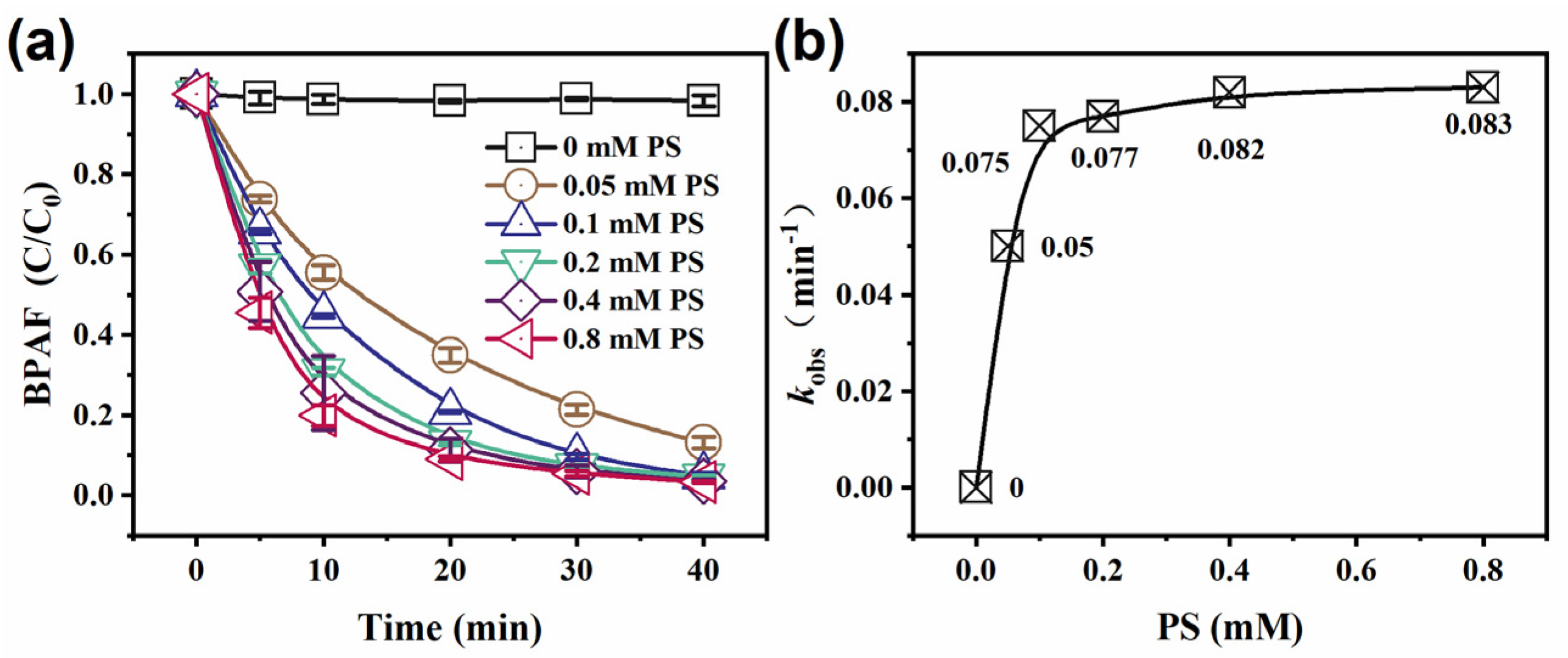
3.2.3. Effect of PS Concentration
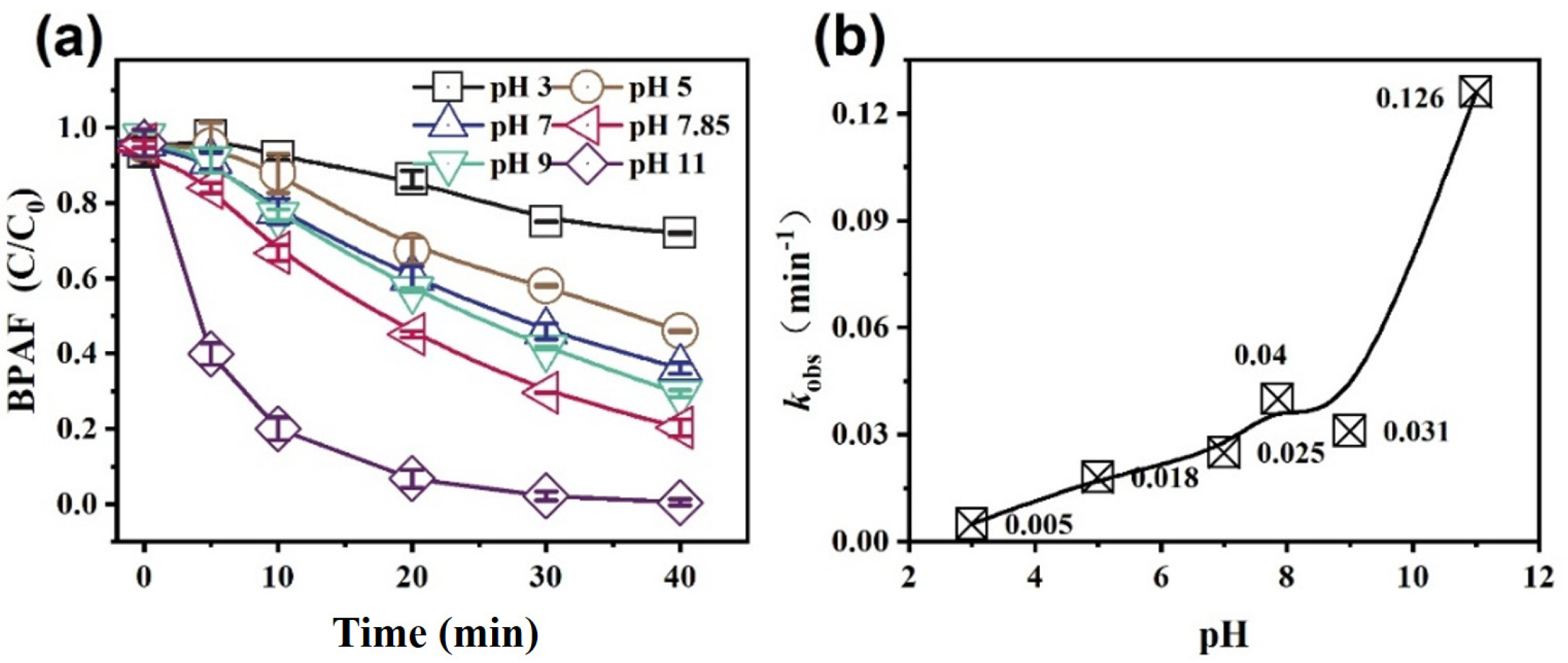
3.2.4. Effect of Initial pH
3.2.5. Effect of Inorganic Anions
3.3. Reusability of Co3O4@BiOI
3.4. PS Activation Mechanism of Co3O4@BiOI
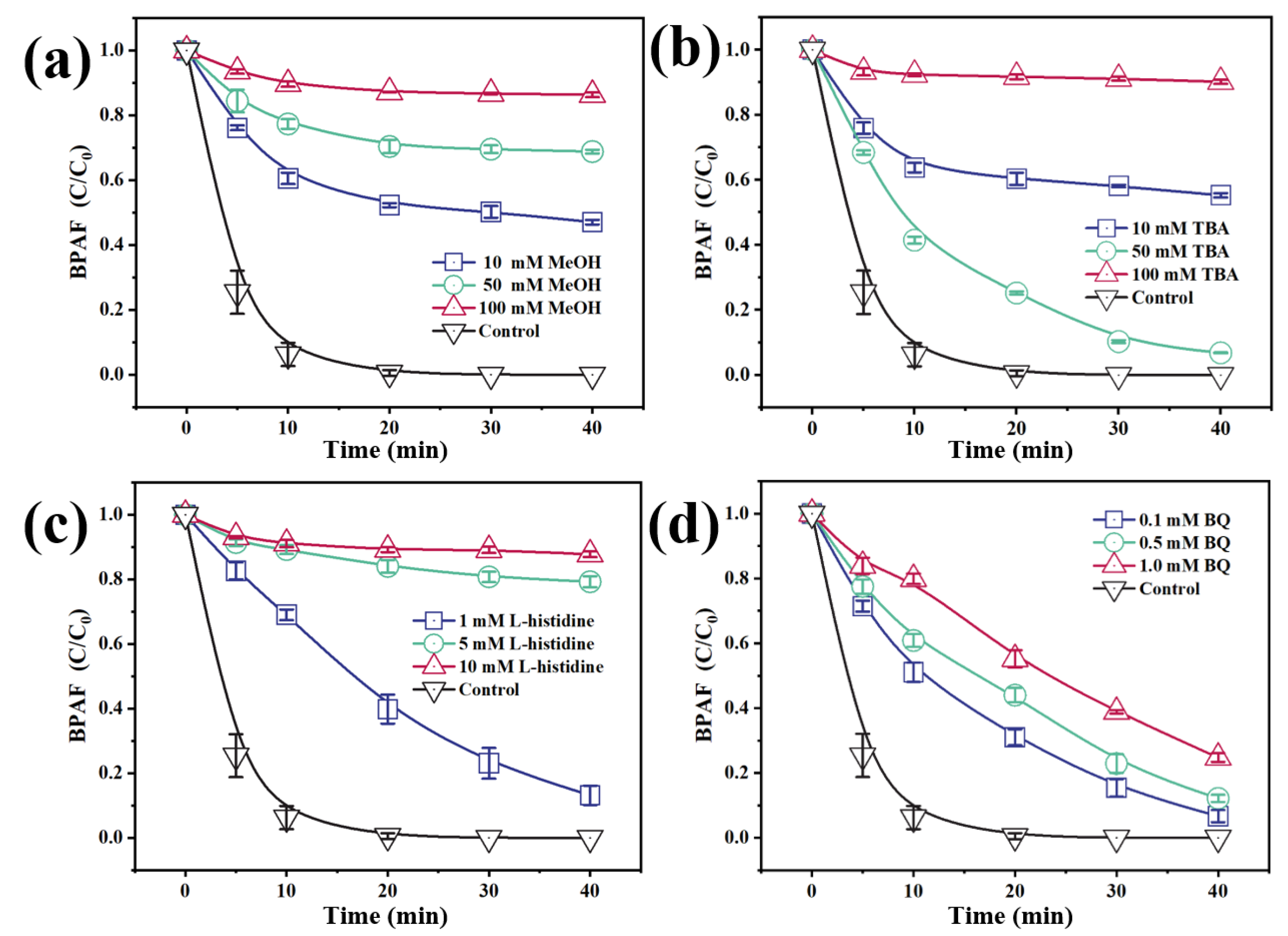
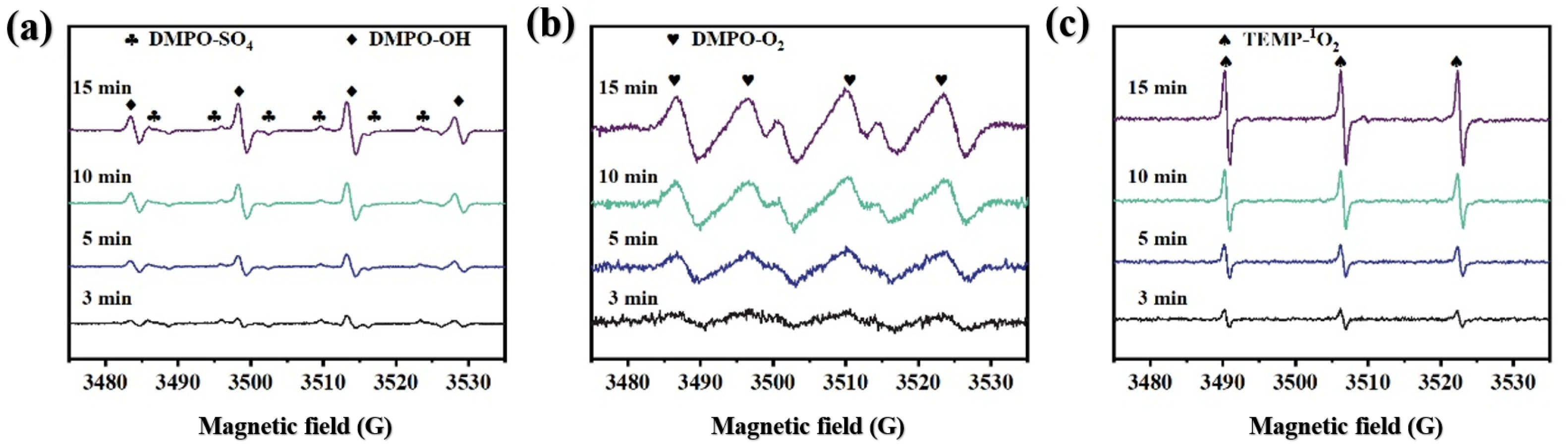
3.4.1. Identification of Active Species
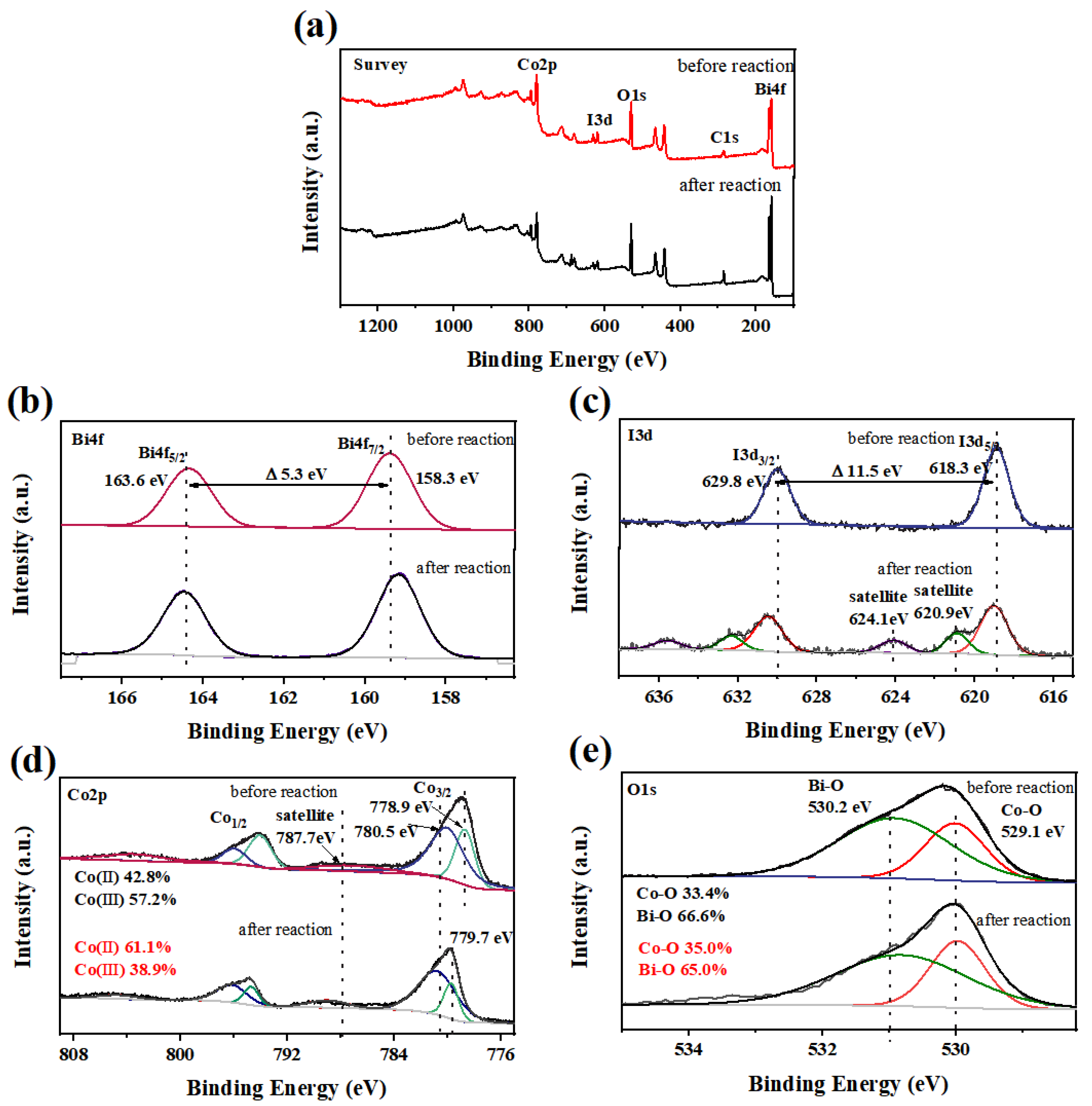
3.4.2. Catalysis Mechanism
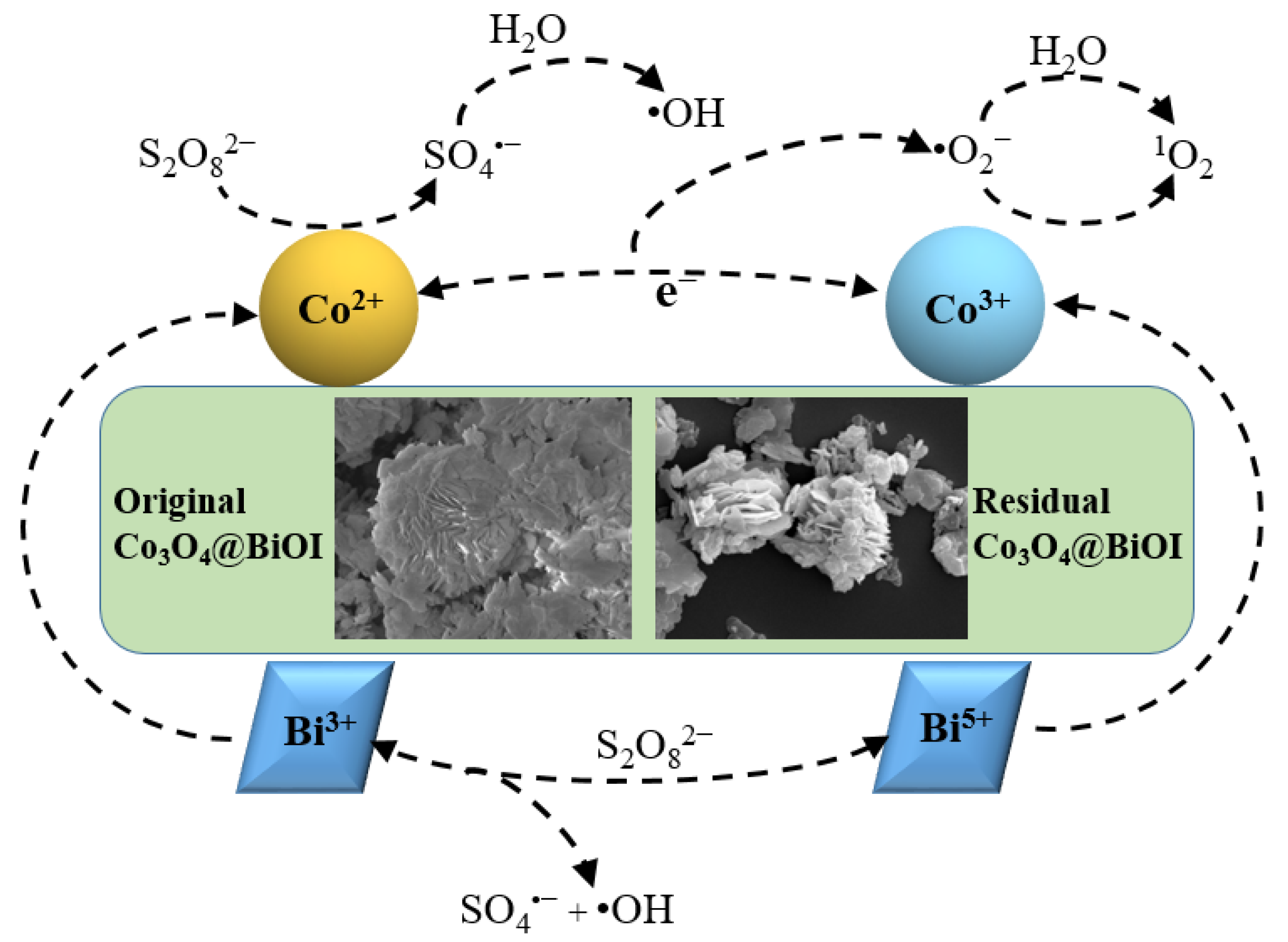
3.5. The Proposed Mechanism in Co3O4@BiOI
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Lin, Y.; Yen, C.; Miaw, C.; Chen, T.; Wu, M.; Hsieh, C. Identification, contribution, and estrogenic activity of potential EDCs in a river receiving concentrated livestock effluent in Southern Taiwan. Sci. Total Environ. 2018, 636, 464–476. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, I.; Fiory, F.; Perruolo, G.; Miele, C.; Beguinot, F.; Formisano, P.; Oriente, F. Potential Mechanisms of Bisphenol A (BPA) Contributing to Human Disease. Int. J. Mol. Sci. 2020, 21, 5761. [Google Scholar] [CrossRef] [PubMed]
- den Braver-Sewradj, S.P.; van Spronsen, R.; Hessel, E.V.S. Substitution of bisphenol A: A review of the carcinogenicity, reproductive toxicity, and endocrine disruption potential of alternative substances. Crit. Rev. Toxicol. 2020, 50, 128–147. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Ruan, T.; Wang, T.; Liu, R.; Jiang, G. Distribution and Preliminary Exposure Assessment of Bisphenol AF (BPAF) in Various Environmental Matrices around a Manufacturing Plant in China. Environ. Sci. Technol. 2012, 46, 13136–13143. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Huang, Y.; Li, X.; Lei, Y.; Teng, M.; Li, X.; Wang, C.; Li, Y. Developmental Effects and Estrogenicity of Bisphenol A Alternatives in a Zebrafish Embryo Model. Environ. Sci. Technol. 2018, 52, 3222–3231. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Lee, L.S. Partitioning Behavior of Bisphenol Alternatives BPS and BPAF Compared to BPA. Environ. Sci. Technol. 2017, 51, 3725–3732. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Wu, F. Photodegradation of bisphenol AF in montmorillonite dispersions: Kinetics and mechanism study. Appl. Clay Sci. 2010, 49, 182–186. [Google Scholar] [CrossRef]
- Yang, T.; Wang, L.; Liu, Y.; Huang, Z.; He, H.; Wang, X.; Jiang, J.; Gao, D.; Ma, J. Comparative study on ferrate oxidation of BPS and BPAF: Kinetics, reaction mechanism, and the improvement on their biodegradability. Water Res. 2019, 148, 115–125. [Google Scholar] [CrossRef]
- Wang, Q.; Zeng, H.; Liang, Y.; Cao, Y.; Xiao, Y.; Ma, J. Degradation of bisphenol AF in water by periodate activation with FeS (mackinawite) and the role of sulfur species in the generation of sulfate radicals. Chem. Eng. J. 2021, 407, 126738. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Cheng, X.; Wang, J.; Yang, B.; Guo, H. Amino-modified metal–organic frameworks as peroxymonosulfate catalyst for bisphenol AF decontamination: ROS generation, degradation pathways, and toxicity evaluation. Sep. Purif. Technol. 2022, 282, 119967. [Google Scholar] [CrossRef]
- Xu, X.; Liu, Q.; Bai, L.; Wang, L.; Wang, D.; Gao, S. Transformation of Bisphenol AF during Aqueous Chlorination: Kinetics, Mechanisms, and Influence of Ph. ACS EST Water 2021, 1, 449–458. [Google Scholar] [CrossRef]
- Khan, J.A.; He, X.; Shah, N.S.; Khan, H.M.; Hapeshi, E.; Fatta-Kassinos, D.; Dionysiou, D.D. Kinetic and mechanism investigation on the photochemical degradation of atrazine with activated H2O2, S2O82− and HSO5−. Chem. Eng. J. 2014, 252, 393–403. [Google Scholar] [CrossRef]
- Kilic, M.Y.; Abdelraheem, W.H.; He, X.; Kestioglu, K.; Dionysiou, D.D. Photochemical treatment of tyrosol, a model phenolic compound present in olive mill wastewater, by hydroxyl and sulfate radical-based advanced oxidation processes (AOPs). J. Hazard. Mater. 2019, 367, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Sajjadi, S.; Khataee, A.; Bagheri, N.; Kobya, M.; Şenocak, A.; Demirbas, E.; Karaoğlu, A.G. Degradation of diazinon pesticide using catalyzed persulfate with Fe3O4@MOF-2 nanocomposite under ultrasound irradiation. J. Ind. Eng. Chem. 2019, 77, 280–290. [Google Scholar] [CrossRef]
- Lei, Y.; Tian, Y.; Fang, C.; Zhan, W.; Duan, L.; Zhang, J.; Zuo, W.; Kong, X. Insights into the oxidation kinetics and mechanism of diesel hydrocarbons by ultrasound activated persulfate in a soil system. Chem. Eng. J. 2019, 378, 122253. [Google Scholar] [CrossRef]
- Bruton, T.A.; Sedlak, D.L. Treatment of perfluoroalkyl acids by heat-activated persulfate under conditions representative of in situ chemical oxidation. Chemosphere 2018, 206, 457–464. [Google Scholar] [CrossRef]
- Yang, L.; Xue, J.; He, L.; Wu, L.; Ma, Y.; Chen, H.; Li, H.; Peng, P.; Zhang, Z. Review on ultrasound assisted persulfate degradation of organic contaminants in wastewater: Influences, mechanisms and prospective. Chem. Eng. J. 2019, 378, 122146. [Google Scholar] [CrossRef]
- Guo, H.; Ke, T.; Gao, N.; Liu, Y.; Cheng, X. Enhanced degradation of aqueous norfloxacin and enrofloxacin by UV-activated persulfate: Kinetics, pathways and deactivation. Chem. Eng. J. 2017, 316, 471–480. [Google Scholar] [CrossRef]
- Huang, Y.; Sun, Y.; Xu, Z.; Luo, M.; Zhu, C.; Li, L. Removal of aqueous oxalic acid by heterogeneous catalytic ozonation with MnOx/sewage sludge-derived activated carbon as catalysts. Sci. Total Environ. 2017, 575, 50–57. [Google Scholar] [CrossRef]
- Qian, L.; Kopinke, F.; Scherzer, T.; Griebel, J.; Georgi, A. Enhanced degradation of perfluorooctanoic acid by heat-activated persulfate in the presence of zeolites. Chem. Eng. J. 2022, 429, 132500. [Google Scholar] [CrossRef]
- Li, Y.; Sun, J.; Sun, S.P. Mn2+-mediated homogeneous Fenton-like reaction of Fe(III)-NTA complex for efficient degradation of organic contaminants under neutral conditions. J. Hazard. Mater. 2016, 313, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Li, X.; Jiang, Z.; Li, C.; Zhao, J.; Wang, H.; Liao, Q. Structure-dependent catalysis of Co3O4 crystals in persulfate activation via nonradical pathway. Appl. Surf. Sci. 2020, 525, 146482. [Google Scholar] [CrossRef]
- Avetta, P.; Pensato, A.; Minella, M.; Malandrino, M.; Maurino, V.; Minero, C.; Hanna, K.; Vione, D. Activation of Persulfate by Irradiated Magnetite: Implications for the Degradation of Phenol under Heterogeneous Photo-Fenton-Like Conditions. Environ. Sci. Technol. 2015, 49, 1043–1050. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, M.; Zhu, L. Activation of persulfate by Co3O4 nanoparticles for orange G degradation. RSC Adv. 2016, 6, 758–768. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J. Bimetallic and nitrogen Co-doped biochar for peroxymonosulfate (PMS) activation to degrade emerging contaminants. Sep. Purif. Technol. 2023, 307, 122807. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, Y.; Zhou, Y.; Liu, X.; Wang, C.; Lan, Y.; Li, Y. Co3O4-MnO2 nanoparticles moored on biochar as a catalyst for activation of peroxymonosulfate to efficiently degrade sulfonamide antibiotics. Sep. Purif. Technol. 2022, 281, 119935. [Google Scholar] [CrossRef]
- Chen, X.; Li, F.; Zhang, M.; Liu, B.; Chen, H.; Wang, H. Highly dispersed and stabilized Co3O4/C anchored on porous biochar for bisphenol A degradation by sulfate radical advanced oxidation process. Sci. Total Environ. 2021, 777, 145794. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zheng, X.; Cheng, H.; Rao, M.; Chen, K.; Xia, J.; Lin, L.; Zhu, H. Mn-Fe3O4 nanoparticles anchored on the urushiol functionalized 3D-graphene for the electrochemical detection of 4-nitrophenol. J. Hazard. Mater. 2021, 409, 124926. [Google Scholar] [CrossRef]
- Li, X.; Niu, C.; Huang, D.; Wang, X.; Zhang, X.; Zeng, G.; Niu, Q. Preparation of magnetically separable Fe3O4/BiOI nanocomposites and its visible photocatalytic activity. Appl. Surf. Sci. 2013, 286, 40–46. [Google Scholar] [CrossRef]
- Bao, S.; Yan, N.; Shi, X.; Li, R.; Chen, Q. High and stable catalytic activity of porous Ag/Co3O4 nanocomposites derived from MOFs for CO oxidation. Appl. Catal. A Gen. 2014, 487, 189–194. [Google Scholar] [CrossRef]
- Qin, C.; Qi, Y.; Teng, X.; Ajarem, J.S.; Allam, A.A.; Qu, R. Degradation of Bisphonel AF (BPAF) by zero-valent iron activated persulfate: Kinetics, mechanisms, theoretical calculations, and effect of co-existing chloride. Chemosphere 2023, 316, 137774. [Google Scholar] [CrossRef]
- Chen, W.; Huang, J.; Yu, X.; Fu, X.; Zhu, Y.; Zhang, Y. The roles of graphene and sandwich structure in rGO/BiOI/rGO to enhance the photoelectrocatalytic activity. J. Solid State Chem. 2020, 289, 121480. [Google Scholar] [CrossRef]
- Xiong, J.; Zeng, H.; Xu, S.; Peng, J.; Liu, F.; Wang, L. Enhancing the intrinsic properties of flower-like BiOI by S-doping toward excellent photocatalytic performances. J. Mater. Sci. Technol. 2022, 118, 181–189. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, Y.; Guo, H.; Liu, Y. Heterogeneous activation of peroxymonosulfate for bisphenol AF degradation with BiOI0.5Cl0.5. RSC Adv. 2019, 9, 14060–14071. [Google Scholar] [CrossRef] [PubMed]
- Malefane, M.E.; Feleni, U.; Mafa, P.J.; Kuvarega, A.T. Fabrication of direct Z-scheme Co3O4/BiOI for ibuprofen and trimethoprim degradation under visible light irradiation. Appl. Surf. Sci. 2020, 514, 145940. [Google Scholar] [CrossRef]
- Song, Y.; Qi, J.; Tian, J.; Gao, S.; Cui, F. Construction of Ag/g-C3N4 photocatalysts with visible-light photocatalytic activity for sulfamethoxazole degradation. Chem. Eng. J. 2018, 341, 547–555. [Google Scholar] [CrossRef]
- Liang, C.; Wang, Z.; Mohanty, N. Influences of carbonate and chloride ions on persulfate oxidation of trichloroethylene at 20 °C. Sci. Total Environ. 2006, 370, 271–277. [Google Scholar] [CrossRef]
- Wu, C.; Linden, K.G. Phototransformation of selected organophosphorus pesticides: Roles of hydroxyl and carbonate radicals. Water Res. 2010, 44, 3585–3594. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Cao, Y.; Zeng, H.; Liang, Y.; Ma, J.; Lu, X. Ultrasound-enhanced zero-valent copper activation of persulfate for the degradation of bisphenol AF. Chem. Eng. J. 2019, 378, 122143. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Li, W.; Lan, Y.; Chen, C. Performance comparison and mechanism investigation of Co3O4-modified different crystallographic MnO2 (α, β, γ, and δ) as an activator of peroxymonosulfate (PMS) for sulfisoxazole degradation. Chem. Eng. J. 2022, 427, 130888. [Google Scholar] [CrossRef]
- Quang, N.D.; Majumder, S.; Van, P.C.; Jeong, J.; Kim, C.; Kim, D. Co3O4/reduced graphene oxide/BiVO4 nanorod as high performance photoanode for water oxidation. Electrochim. Acta 2020, 364, 137283. [Google Scholar] [CrossRef]
- Ren, Y.; Lin, L.; Ma, J.; Yang, J.; Feng, J.; Fan, Z. Sulfate radicals induced from peroxymonosulfate by magnetic ferrospinel MFe2O4 (M = Co, Cu, Mn, and Zn) as heterogeneous catalysts in the water. Appl. Catal. B Environ. 2015, 165, 572–578. [Google Scholar] [CrossRef]
- Yang, Q.; Choi, H.; Dionysiou, D.D. Nanocrystalline cobalt oxide immobilized on titanium dioxide nanoparticles for the heterogeneous activation of peroxymonosulfate. Appl. Catal. B Environ. 2007, 74, 170–178. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.; Han, E.; Zhao, L.; Lian, J. Reduced graphene oxide wrapped Fe3O4–Co3O4 yolk–shell nanostructures for advanced catalytic oxidation based on sulfate radicals. Appl. Surf. Sci. 2017, 396, 945–954. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D. Radical Generation by the Interaction of Transition Metals with Common Oxidants. Environ. Sci. Technol. 2004, 38, 3705–3712. [Google Scholar] [CrossRef]
- Wang, Y.; Indrawirawan, S.; Duan, X.; Sun, H.; Ang, H.M.; Tadé, M.O.; Wang, S. New insights into heterogeneous generation and evolution processes of sulfate radicals for phenol degradation over one-dimensional α-MnO2 nanostructures. Chem. Eng. J. 2015, 266, 12–20. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Rykov, A.I.; Han, H.; Jin, C.; Liu, X.; Wang, J. Excellent photo-Fenton catalysts of Fe–Co Prussian blue analogues and their reaction mechanism study. Appl. Catal. B Environ. 2015, 179, 196–205. [Google Scholar] [CrossRef]





| Product ID | M/Z | Formula | Structure |
|---|---|---|---|
| P1 | 336 | C15H10F6O2 | 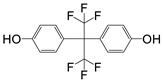 |
| P2 | 110 | C6H6O2 |  |
| P3 | 242 | C15H12F4O4 |  |
| P4 | 108 | C6H4O2 | 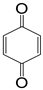 |
| P5 | 367 | C15H10F6O4 | 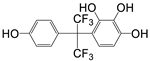 |
| P6 | 386 | C15H12F6O5 |  |
| P7 | 356 | C6H6O |  |
| P8 | 352 | C15H10F6O3 | 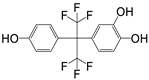 |
| P9 | 384 | C15H10F6O5 | 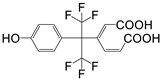 |
| P10 | 702 | C30H18F12O6 |  |
| P11 | 84 | C5H8O | 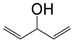 |
| P12 | 102 | C5H10O | 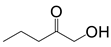 |
| P13 | 114 | C7H14O |  |
| P14 | 98 | C5H6O2 | 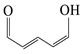 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Liu, C.; Lin, Z.; Chen, Q. Heterogeneous Activation of Persulfate by Petal-Shaped Co3O4@BiOI to Degrade Bisphenol AF. Water 2024, 16, 2887. https://doi.org/10.3390/w16202887
Zhang J, Liu C, Lin Z, Chen Q. Heterogeneous Activation of Persulfate by Petal-Shaped Co3O4@BiOI to Degrade Bisphenol AF. Water. 2024; 16(20):2887. https://doi.org/10.3390/w16202887
Chicago/Turabian StyleZhang, Jian, Changling Liu, Zheng Lin, and Qiang Chen. 2024. "Heterogeneous Activation of Persulfate by Petal-Shaped Co3O4@BiOI to Degrade Bisphenol AF" Water 16, no. 20: 2887. https://doi.org/10.3390/w16202887
APA StyleZhang, J., Liu, C., Lin, Z., & Chen, Q. (2024). Heterogeneous Activation of Persulfate by Petal-Shaped Co3O4@BiOI to Degrade Bisphenol AF. Water, 16(20), 2887. https://doi.org/10.3390/w16202887





