Effect of Pipe Materials and Interspecific Interactions on Biofilm Formation and Chlorine Resistance: Turn Enemies into Friends
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Pipe Materials
2.2. Biofilm Formation and Chlorination Experiments
2.3. Biofilm Analysis Method
2.4. Analysis of Variance and Correlation
3. Results and Discussion
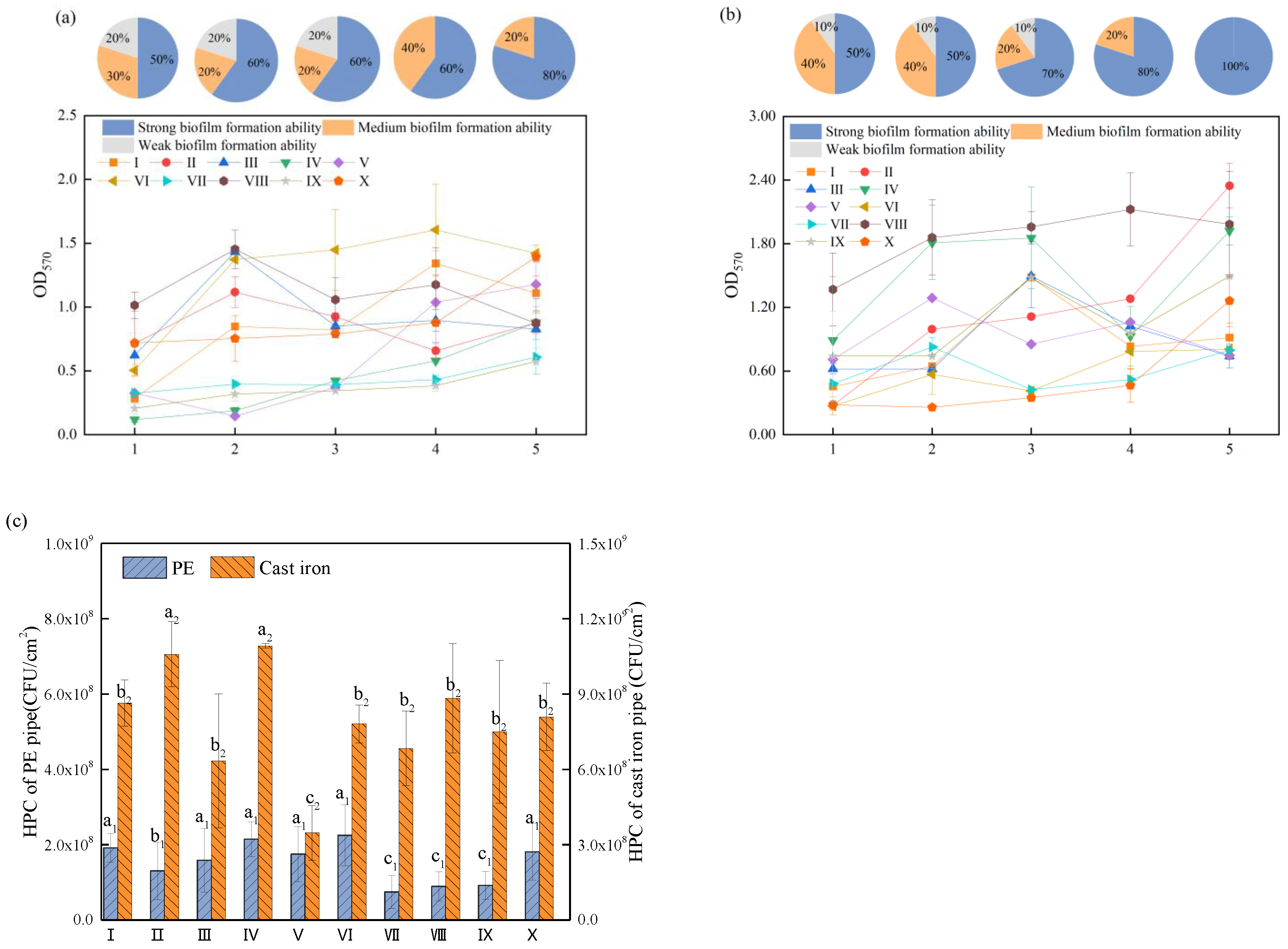
3.1. Biofilm Formation Ability
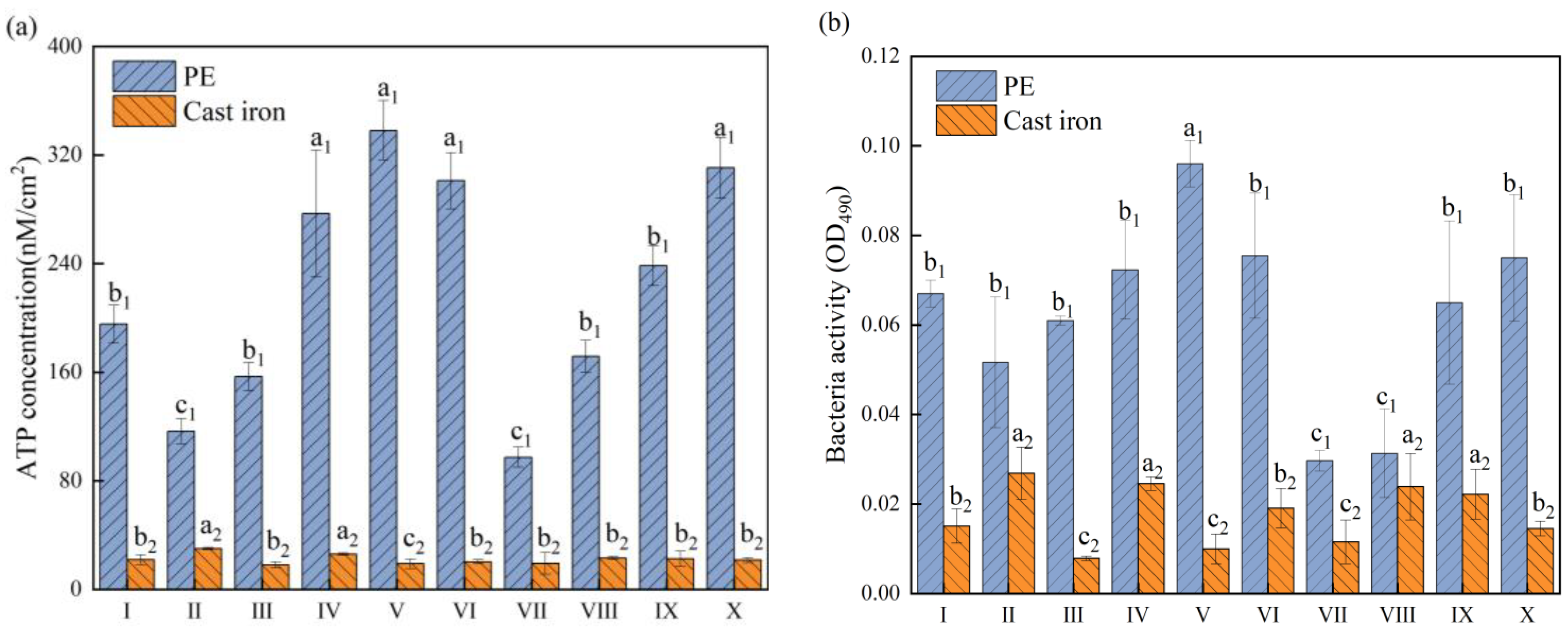
3.2. Dual-Species Biofilm Activity
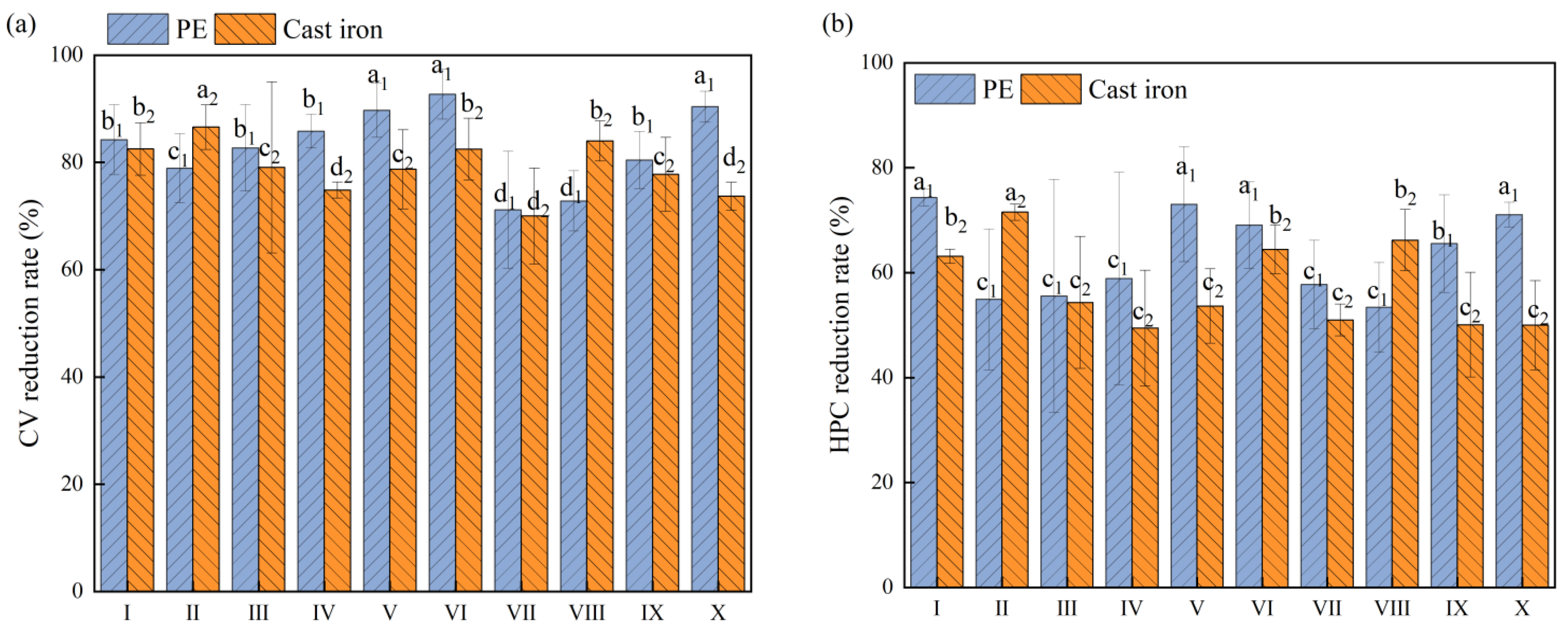
3.3. Dual-Species Biofilm Biomass Reduction after Chlorination
3.4. Dual-Species Biofilm Activity Reduction after Chlorination
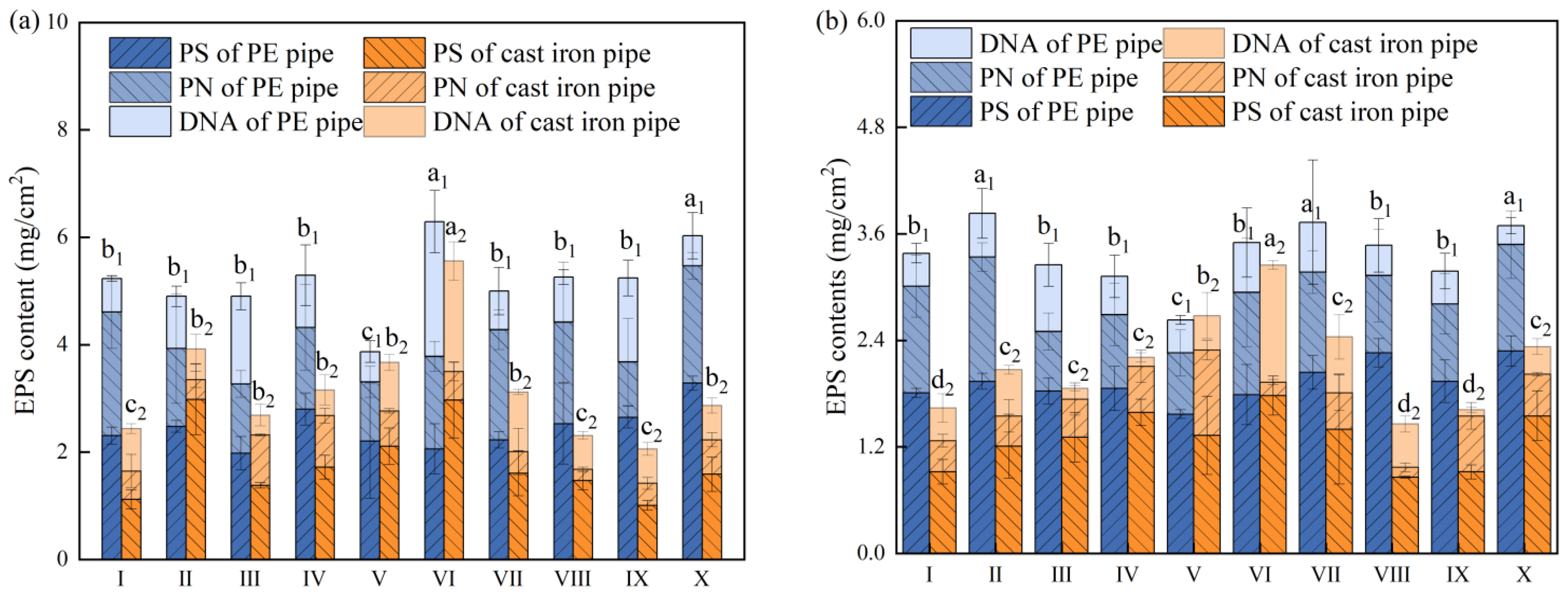
3.5. Dual-Species Biofilm EPS Content before and after Chlorination
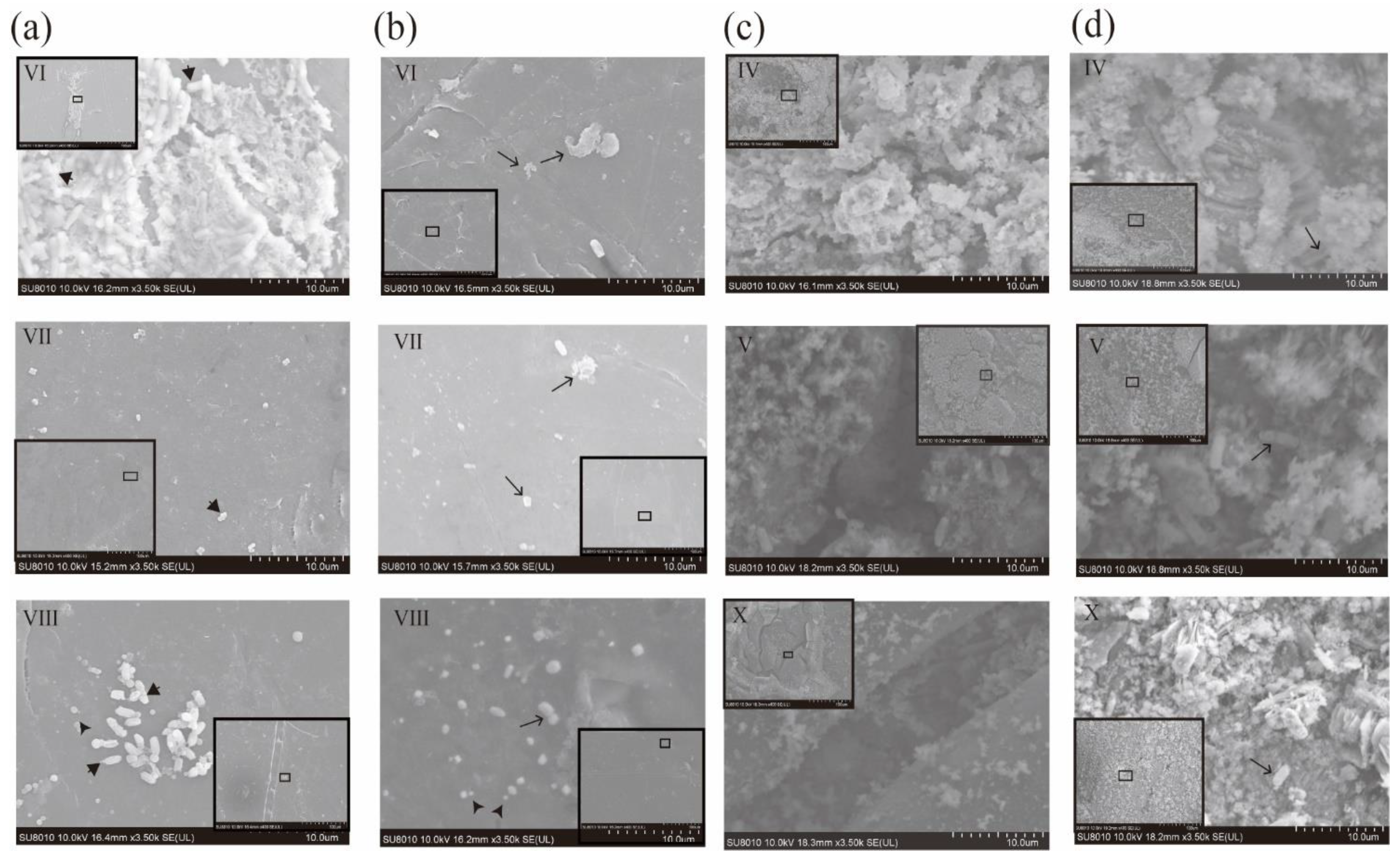
3.6. Biofilm Morphological Changes between Pre- and Post-Chlorinations
3.7. Mechanism of Dual-Species Biofilm Formation and Chlorine Resistance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, G.; Zhang, Y.; Van Der Mark, E.; Magic-Knezev, A.; Pinto, A.; Van Den Bogert, B.; Liu, W.; Van Der Meer, W.; Medema, G. Assessing the origin of bacteria in tap water and distribution system in an unchlorinated drinking water system by Source Tracker using microbial community fingerprints. Water Res. 2018, 138, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Bimakr, F.; Ginige, M.P.; Kaksonen, A.H.; Sutton, D.C.; Puzon, G.J.; Cheng, K.Y. Assessing graphite and stainless-steel for electrochemical sensing of biofilm growth in chlorinated drinking water systems. Sens. Actuators B-Chem. 2018, 277, 526–534. [Google Scholar] [CrossRef]
- Shan, L.; Bao, X.; Xu, S.; Zhu, Z.; Pei, Y.; Zheng, W.; Yuan, Y. Biofilm formation and chlorine resistance of microbial communities in household drinking water system: Preliminary idea of using bacteria to control bacteria. Process Biochem. 2024, 141, 179–189. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, W.; Nie, X.; Li, C.; Gu, J.; Zhang, C. Molecular Analysis of Bacterial Communities in Biofilms of a Drinking Water Clearwell. Microbes Environ. 2012, 27, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Simoes, L.C.; Simoes, M. Biofilms in drinking water: Problems and solutions. RSC Adv. 2013, 3, 2520–2533. [Google Scholar] [CrossRef]
- Liu, R.; Yu, Z.; Guo, H.; Liu, M.; Zhang, H.; Yang, M. Pyrosequencing analysis of eukaryotic and bacterial communities in faucet biofilms. Sci. Total Environ. 2012, 435, 124–131. [Google Scholar] [CrossRef]
- Szabo, J.G.; Meiners, G.; Heckman, L.; Rice, E.W.; Hall, J. Decontamination of Bacillus spores adhered to iron and cement-mortar drinking water infrastructure in a model system using disinfectants. J. Environ. Manag. 2017, 187, 1–7. [Google Scholar] [CrossRef]
- Zhu, Z.; Xu, S.; Bao, X.; Shan, L.; Pei, Y.; Zheng, W.; Yuan, Y. Effect of outdoor pipe materials and community-intrinsic properties on biofilm formation and chlorine resistance: Black sheep or team leader. J. Clean. Prod. 2023, 411, 137308. [Google Scholar] [CrossRef]
- Sadiq, F.A.; Burmølle, M.; Heyndrickx, M.; Flint, S.; Lu, W.; Chen, W.; Zhao, J.; Zhang, H. Community-wide changes reflecting bacterial interspecific interactions in multispecies biofilms. Crit. Rev. Microbiol. 2021, 47, 338–358. [Google Scholar] [CrossRef]
- Xu, X.; Liu, S.; Smith, K.; Cui, Y.; Wang, Z. An overview on corrosion of iron and steel components in reclaimed water supply systems and the mechanisms involved. J. Clean. Prod. 2020, 276, 124079. [Google Scholar] [CrossRef]
- Shan, L.; Zheng, W.; Xu, S.; Zhu, Z.; Pei, Y.; Bao, X.; Yuan, Y. Effect of household pipe materials on formation and chlorine resistance of the early-stage biofilm: Various interspecific interactions exhibited by the same microbial biofilm in different pipe materials. Arch. Microbiol. 2024, 206, 295. [Google Scholar] [CrossRef] [PubMed]
- Price, A.; Macey, M.C.; Miot, J.; Olsson-Francis, K. Draft Genome Sequences of the Nitrate-Dependent Iron-Oxidizing Proteobacteria Acidovorax sp. Strain BoFeN1 and Paracoccus pantotrophus Strain KS1. Microbiol. Resour. Announc. 2018, 7, e01050-18. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Luo, W.; Qin, Y.; Li, Z. Effects of the addition of nitrogen and phosphorus on anaerobic ammonium oxidation coupled with iron reduction (Feammox) in the farmland soils. Sci. Total Environ. 2020, 737, 139849. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, D.; Huang, X.; Qin, W. Acinetobacter harbinensis sp nov., isolated from river water. Int. J. Syst. Evol. Microbiol. 2014, 64, 1507–1513. [Google Scholar] [CrossRef] [PubMed]
- Rangjaroen, C.; Rerkasem, B.; Teaumroong, N.; Noisangiam, R.; Lumyong, S. Promoting plant growth in a commercial rice cultivar by endophytic diazotrophic bacteria isolated from rice landraces. Ann. Microbiol. 2015, 65, 253–266. [Google Scholar] [CrossRef]
- Tan, C.H.; Lee KW, K.; Burmølle, M.; Kjelleberg, S.; Rice, S.A. All together now: Experimental multispecies biofilm model systems. Environ. Microbiol. 2017, 19, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Shan, L.; Li, X.; Hu, F.; Yuan, Y.; Zhong, D.; Zhang, J. Effects of interspecific interactions on biofilm formation potential and chlorine resistance: Evaluation of dual-species biofilm observed in drinking water distribution systems. J. Water Process Eng. 2020, 38, 101564. [Google Scholar] [CrossRef]
- Wei, G.; Li, M.; Li, F.; Li, H.; Gao, Z. Distinct distribution patterns of prokaryotes between sediment and water in the Yellow River estuary. Appl. Microbiol. Biotechnol. 2016, 100, 9683–9697. [Google Scholar] [CrossRef]
- Wang, Y.; Lan, H.-Q.; Meng, T.; Chen, S.; Zuo, J.D.; Lin, N. A Lifetime Prediction Method of Pressured Gas Polyethylene Pipes by Thermal-Oxidative Aging Test and Tensile Test. J. Press. Vessel Technol. Trans. Asme 2018, 140, 011404. [Google Scholar] [CrossRef]
- Kilb, B.; Lange, B.; Schaule, G.; Flemming, H.C.; Wingender, J. Contamination of drinking water by coliforms from biofilms grown on rubber-coated valves. Int. J. Hyg. Environ. Health 2003, 206, 563–573. [Google Scholar] [CrossRef]
- Liu, R.; Zhu, J.; Yu, Z.; Joshi, D.; Zhang, H.; Lin, W.; Yang, M. Molecular analysis of long-term biofilm formation on PVC and cast iron surfaces in drinking water distribution system. J. Environ. Sci. 2014, 26, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Simoes, M.; Simoes, L.C.; Vieira, M.J. Species association increases biofilm resistance to chemical and mechanical treatments. Water Res. 2009, 43, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, D.; Brozel, V.S.; Mostert, J.F.; Von Holy, A. Differential efficacy of a chlorine dioxide-containing sanitizer against single species and binary biofilms of a dairy-associated Bacillus cereus and a Pseudomonas fluorescens isolate. J. Appl. Microbiol. 2002, 92, 352–361. [Google Scholar] [CrossRef]
- Hemdan, A.B.; Azab El-Liethy, M.; El-Taweel, G.E. The destruction of Escherichia coli adhered to pipe surfaces in a model drinking water distribution system via various antibiofilm agents. Water Environ. Res. 2020, 92, 2155–2167. [Google Scholar] [CrossRef] [PubMed]
- Leslie, E.; Hinds, J.; Hai, F.I. Causes, Factors, and Control Measures of Opportunistic Premise Plumbing Pathogens—A Critical Review. Appl. Sci. 2021, 11, 4474. [Google Scholar] [CrossRef]
- Douterelo, I.; Husband, S.; Boxall, J.B. The bacteriological composition of biomass recovered by flushing an operational drinking water distribution system. Water Res. 2014, 54, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Shi, B.; Bai, Y.; Wang, D. Bacterial community of biofilms developed under different water supply conditions in a distribution system. Sci. Total Environ. 2014, 472, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Simoes, L.C.; Simoes, M.; Oliveira, R.; Vieira, M.J. Potential of the adhesion of bacteria isolated from drinking water to materials. J. Basic Microbiol. 2007, 47, 174–183. [Google Scholar] [CrossRef]
- Zhu, Z.; Shan, L.; Hu, F.; Li, Z.; Zhong, D.; Yuan, Y.; Zhang, J. Biofilm formation potential and chlorine resistance of typical bacteria isolated from drinking water distribution systems. RSC Adv. 2020, 10, 31295–31304. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Shan, L.; Zhang, X.; Hu, F.; Zhong, D.; Yuan, Y.; Zhang, J. Effects of bacterial community composition and structure in drinking water distribution systems on biofilm formation and chlorine resistance. Chemosphere 2021, 264, 128410. [Google Scholar] [CrossRef]
- Stepanović, S.; Vuković, D.; Dakić, I.; Savić, B.; Švabić-Vlahović, M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Simoes, L.C.; Simoes, M.; Vieira, M.J. Biofilm interactions between distinct bacterial genera isolated from drinking water. Appl. Environ. Microbiol. 2007, 73, 6192–6200. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, M.; Yao, X.; Fei, Y.; Lin, Z.; Li, Z.; Cai, K.; Zhao, Y.; Luo, Z. HCAR1/MCT1 Regulates Tumor Ferroptosis through the Lactate-Mediated AMPK-SCD1 Activity and Its Therapeutic Implications. Cell Rep. 2020, 33, 108487. [Google Scholar] [CrossRef]
- Siddam, A.D.; Zaslow, S.J.; Wang, Y.; Phillips, K.S.; Silverman, M.D.; Regan, P.M.; Amarasinghe, J.J. Characterization of Biofilm Formation by Mycobacterium chimaera on Medical Device Materials. Front. Microbiol. 2021, 11, 586657. [Google Scholar] [CrossRef]
- Ivone, V.M.; Conceição, E.; Olga, C.N.; Célia, M.M. Bacterial diversity from the source to the tap: A comparative study based on 16S rRNA gene-DGGE and culture-dependent methods. FEMS Microbiol. Ecol. 2013, 83, 361–374. [Google Scholar]
- Jin, J.; Guan, Y. The mutual co-regulation of extracellular polymeric substances and iron ions in biocorrosion of cast iron pipes. Bioresour. Technol. 2014, 169, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, M.O.; Horvath, T.L. The role of mitochondrial uncoupling proteins in lifespan. Pflug. Arch. Eur. J. Physiol. 2010, 459, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Chen, Q.; Zhang, X.; Peng, J.; Zhang, M.; Zhong, Q. The elimination effects of lavender essential oil on Listeria monocytogenes biofilms developed at different temperatures and the induction of VBNC state. Lett. Appl. Microbiol. 2022, 74, 1016–1026. [Google Scholar] [CrossRef]
- Grass, G.; Rensing, C.; Solioz, M. Metallic Copper as an Antimicrobial Surface. Appl. Environ. Microbiol. 2011, 77, 1541–1547. [Google Scholar] [CrossRef]
- Huang, Z.; Feng, S.; Tong, Y.; Yang, H. Enhanced “contact mechanism” for interaction of extracellular polymeric substances with low-grade copper-bearing sulfide ore in bioleaching by moderately thermophilic Acidithiobacillus caldus. J. Environ. Manag. 2019, 242, 11–21. [Google Scholar] [CrossRef]
- Simoes, L.C.; Simoes, M.; Vieira, M.J. The effects of metabolite molecules produced by drinking water-isolated bacteria on their single and multispecies biofilms. Biofouling 2011, 27, 685–699. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.; Lee, C.-H.; Lee, S.; Bae, H. Intermittent chlorination shifts the marine biofilm population on reverse osmosis membranes. Membr. Water Treat. 2019, 10, 395–404. [Google Scholar]
- Uhl, W.; Schaule, G. Establishment of HPC(R2A) for regrowth control in non-chlorinated distribution systems. Int. J. Food Microbiol. 2004, 92, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Peng, H.; Liu, J.; Nguyen, T.H.; Hashmi, M.Z.; Shen, C. Occurrence and quantification of culturable and viable but non-culturable (VBNC) pathogens in biofilm on different pipes from a metropolitan drinking water distribution system. Sci. Total Environ. 2021, 764, 142851. [Google Scholar] [CrossRef]
- Wang, J.; Li, G.; Yin, H.; An, T. Bacterial response mechanism during biofilm growth on different metal material substrates: EPS characteristics, oxidative stress and molecular regulatory network analysis. Environ. Res. 2020, 185, 109451. [Google Scholar] [CrossRef]
- Xue, Z.; Sendamangalam, V.R.; Gruden, C.L.; Seo, Y. Multiple Roles of Extracellular Polymeric Substances on Resistance of Biofilm and Detached Clusters. Environ. Sci. Technol. 2012, 46, 13212–13219. [Google Scholar] [CrossRef]
- Maddela, N.R.; Meng, F. Discrepant roles of a quorum quenching bacterium (Rhodococcus sp. BH4) in growing dual-species biofilms. Sci. Total Environ. 2020, 713, 136402. [Google Scholar] [CrossRef]
- Bridier, A.; Briandet, R.; Thomas, V.; Dubois-Brissonnet, F. Resistance of bacterial biofilms to disinfectants: A review. Biofouling 2011, 27, 1017–1032. [Google Scholar] [CrossRef]
- Pan, R.; Zhang, K.; Cen, C.; Zhou, X.; Xu, J.; Wu, J.; Wu, X. Characteristics of biostability of drinking water in aged pipes after water source switching: ATP evaluation, biofilms niches and microbial community transition. Environ. Pollut. 2021, 271, 116293. [Google Scholar] [CrossRef]
- Wang, H.; Hu, C.; Shen, Y.; Shi, B.; Zhao, D.; Xing, X. Response of microorganisms in biofilm to sulfadiazine and ciprofloxacin in drinking water distribution systems. Chemosphere 2019, 218, 197–204. [Google Scholar] [CrossRef]
- Cloete, T.E.J.I.B. Biodegradation. Resist. Mech. Bact. Antimicrob. Compd. 2003, 51, 277–282. [Google Scholar]
- Alcántara, J.; Chico, B.; Simancas, J.; Díaz, I.; De la Fuente, D.; Morcillo, M. An attempt to classify the morphologies presented by different rust phases formed during the exposure of carbon steel to marine atmospheres. Mater. Charact. 2016, 118, 65–78. [Google Scholar] [CrossRef]
- Atram, R.R.; Bhuse, D.V.; Bhuse, V.M.; Atram, R.G.; Kondawar, S.B. Evolution of waste iron rust into alpha-Fe2O3/CNF and alpha-Fe2O3/PANI composites as an efficient positive electrode for sustainable hybrid supercapacitor. J. Mater. Sci. Mater. Electron. 2021, 32, 13787–13802. [Google Scholar] [CrossRef]
- Zhu, Z.; Xu, S.; Pei, Y.; Shan, L.; Zheng, W.; Bao, X.; Yuan, Y. Assessment of the microbiological safety of drinking water in outdoor pipe materials: Biofilm formation and chlorine resistance of typical bacteria. Environ. Sci. Water Res. Technol. 2023, 9, 1738. [Google Scholar] [CrossRef]
- Riyadh, A.; Peleato, N.M. Natural Organic Matter Character in Drinking Water Distribution Systems: A Review of Impacts on Water Quality and Characterization Techniques. Water 2024, 16, 446. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shan, L.; Pei, Y.; Xu, S.; Cui, Y.; Liu, Z.; Zhu, Z.; Yuan, Y. Effect of Pipe Materials and Interspecific Interactions on Biofilm Formation and Chlorine Resistance: Turn Enemies into Friends. Water 2024, 16, 2930. https://doi.org/10.3390/w16202930
Shan L, Pei Y, Xu S, Cui Y, Liu Z, Zhu Z, Yuan Y. Effect of Pipe Materials and Interspecific Interactions on Biofilm Formation and Chlorine Resistance: Turn Enemies into Friends. Water. 2024; 16(20):2930. https://doi.org/10.3390/w16202930
Chicago/Turabian StyleShan, Lili, Yunyan Pei, Siyang Xu, Yuhong Cui, Zhengqian Liu, Zebing Zhu, and Yixing Yuan. 2024. "Effect of Pipe Materials and Interspecific Interactions on Biofilm Formation and Chlorine Resistance: Turn Enemies into Friends" Water 16, no. 20: 2930. https://doi.org/10.3390/w16202930
APA StyleShan, L., Pei, Y., Xu, S., Cui, Y., Liu, Z., Zhu, Z., & Yuan, Y. (2024). Effect of Pipe Materials and Interspecific Interactions on Biofilm Formation and Chlorine Resistance: Turn Enemies into Friends. Water, 16(20), 2930. https://doi.org/10.3390/w16202930






