An Evaluation of Metal Binding Constants to Cell Surface Receptors in Freshwater Organisms, and Their Application in Biotic Ligand Models to Predict Metal Toxicity
Abstract
:1. Introduction
- (i)
- The metal’s binding affinity to ligands at cell surface receptors: a higher affinity means toxicity will occur at a lower metal concentration;
- (ii)
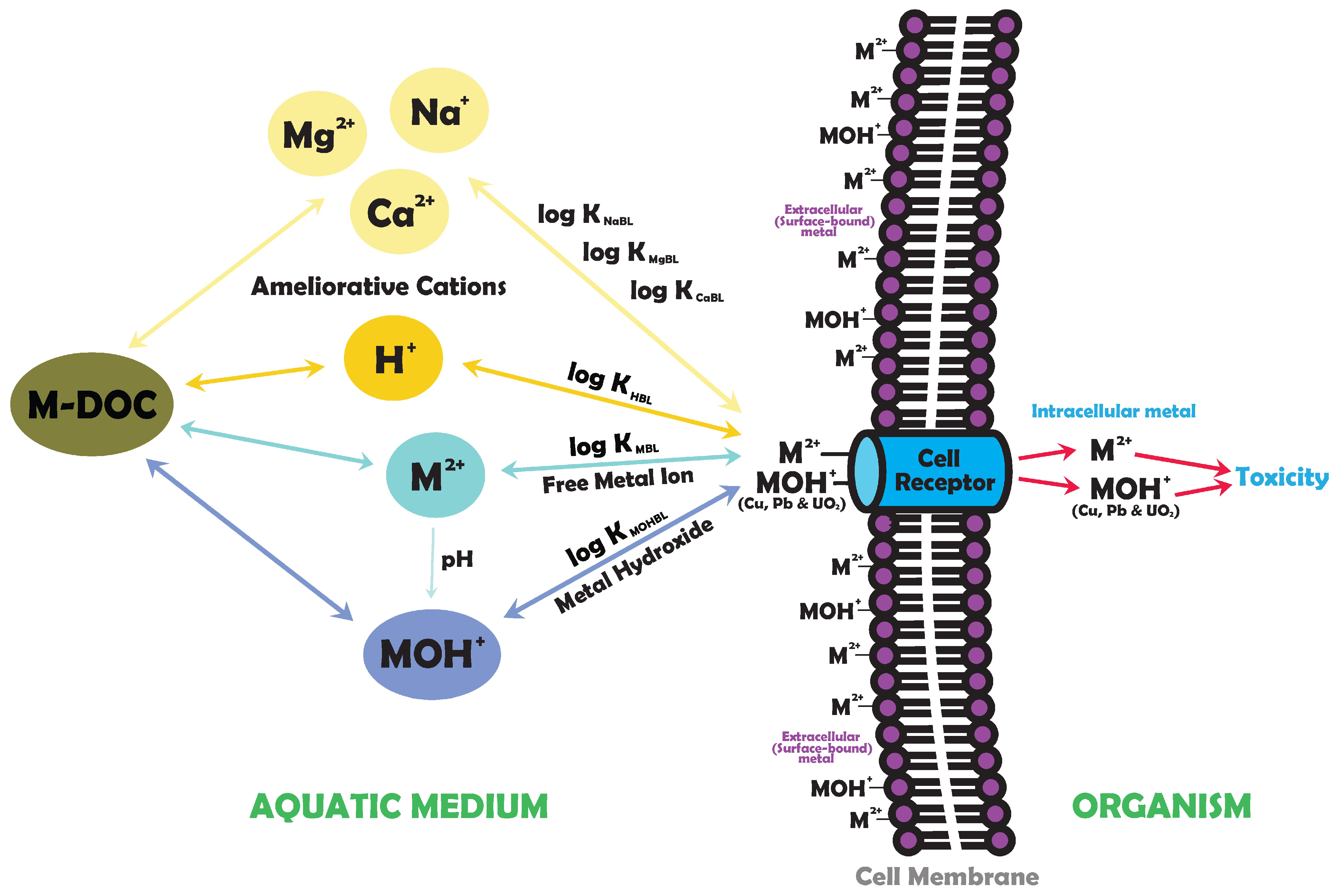
- Metal speciation in water: Free metal ions (Mz+) are typically toxic, but other positively charged species (MOH(z−1)+, MF(z−1)+) can also cause toxicity (Figure 1), especially for metals that hydrolyse within the pH range of natural freshwaters (pH 5.5–8.5), such as uranium (as uranyl, UO22+), copper (Cu2+), and lead (Pb2+). Predicting toxicity is challenging since total or dissolved metal concentrations are not reliable indicators of metal bioavailability [9]. Binding with anions, like bicarbonate or dissolved organic matter, reduces the concentration of free metal ions and reduces toxicity;
- (iii)
- Metals disrupt essential element uptake at cell surface receptors: however, the presence of essential elements and similar cations, such as Ca2+, magnesium (Mg2+), sodium (Na+), and protons (H+), in freshwater can ameliorate metal toxicity (Figure 1);
- (iv)
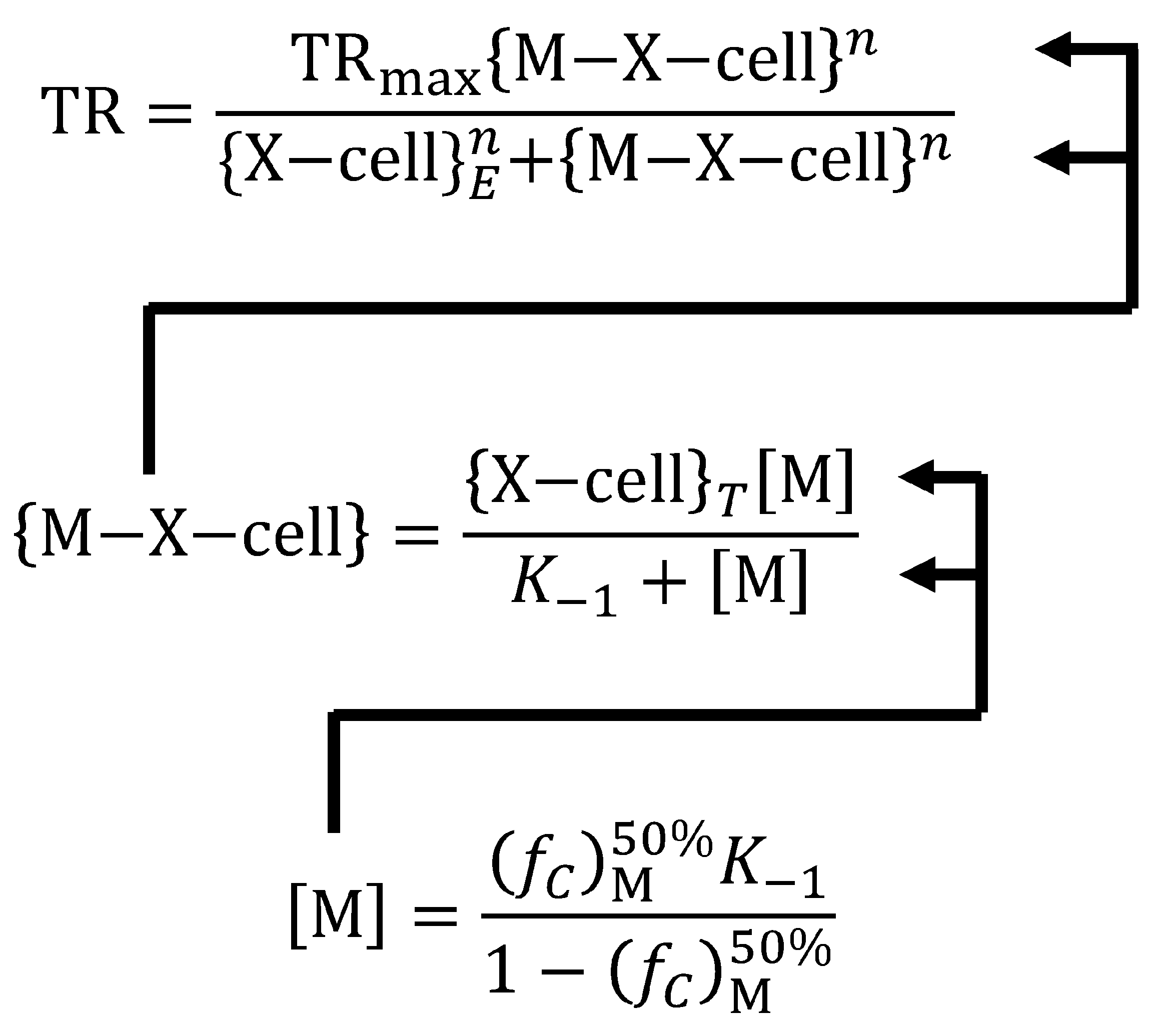
- Toxicity depends on the proportion of cell surface receptor sites bound by metals: If a toxic response occurs when 50% of sites are occupied, the TR50 can be calculated from the inverse of the metal concentration causing the effect. If less than 50% of sites are occupied at the TR50, then the metal’s binding affinity depends on both the metal concentration and the fraction of occupied sites.
2. Conceptual Models of Metal Binding to Biotic Ligands
3. Metal Binding Constants
3.1. Methodology
3.2. Zinc
3.3. Nickel
3.4. Cadmium
3.5. Cobalt
3.6. Copper
3.7. Uranium
3.8. Lead
3.9. Calcium
3.10. Magnesium
3.11. Sodium
3.12. Protons (H+)
3.13. Overview
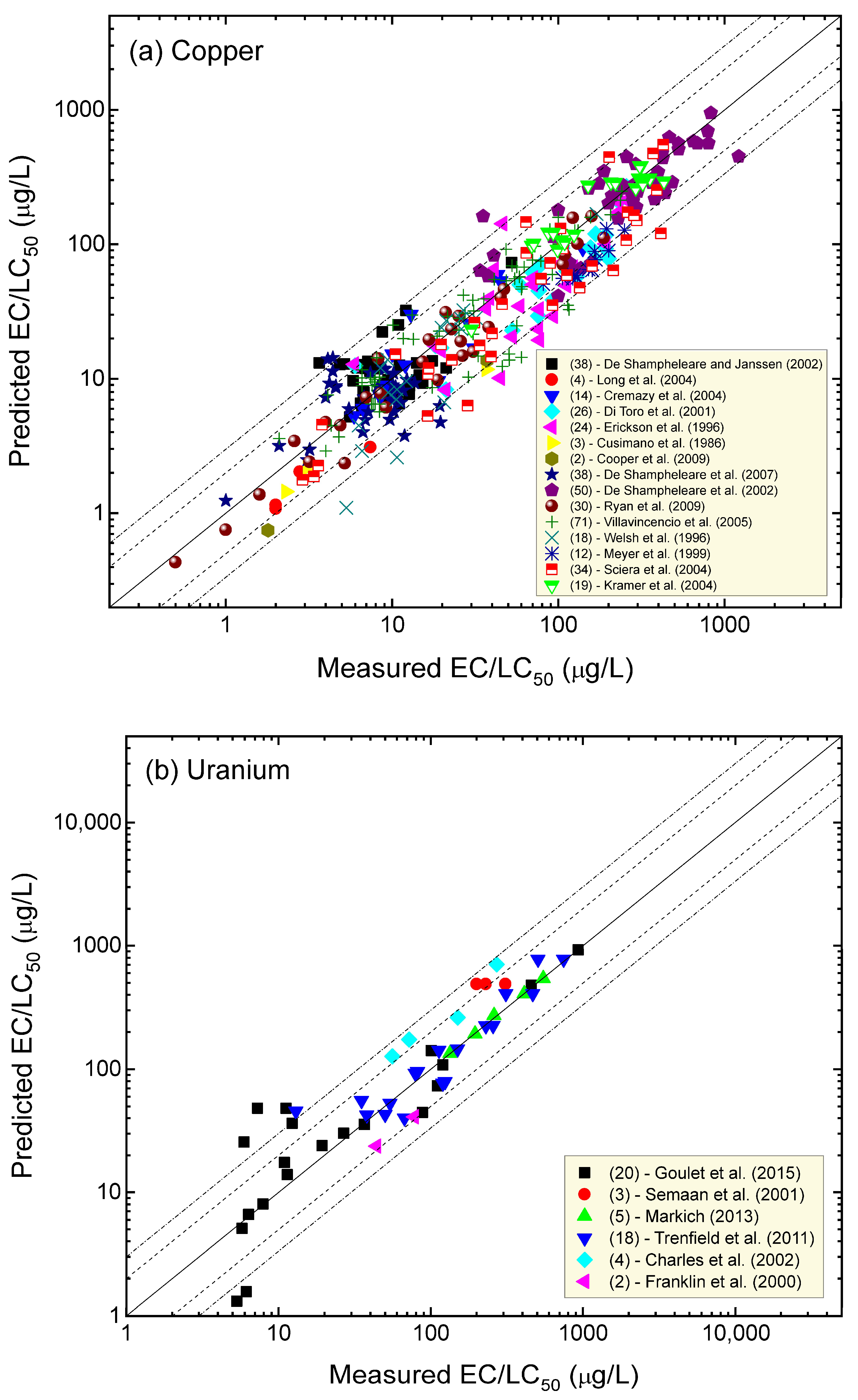
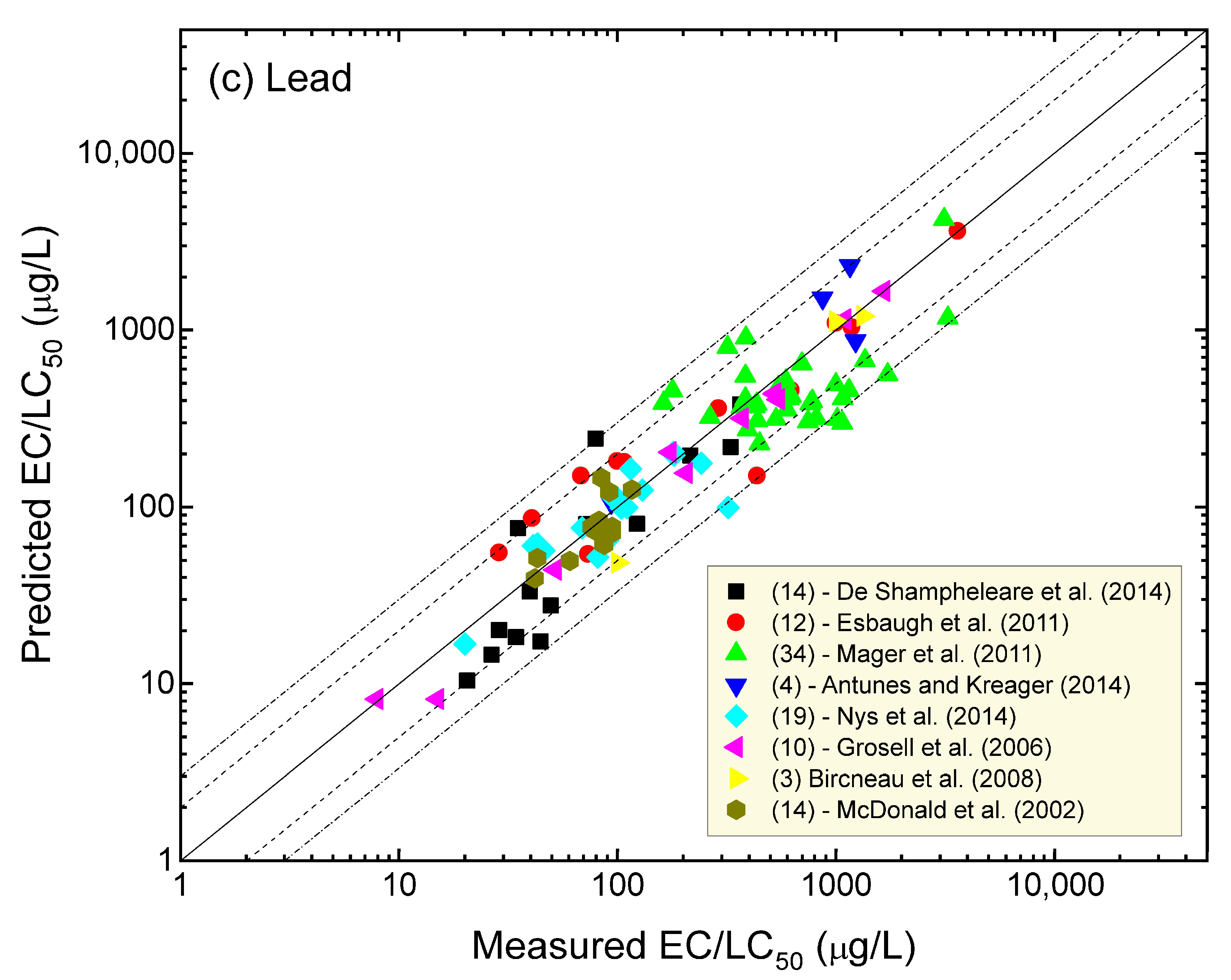
4. Comparison of Measured and Predicted Metal Toxicity
4.1. Zinc
4.2. Nickel
4.3. Cadmium
4.4. Cobalt
4.5. Copper
4.6. Uranium
4.7. Lead
5. Relationships between Metal Binding Constants and Toxicity
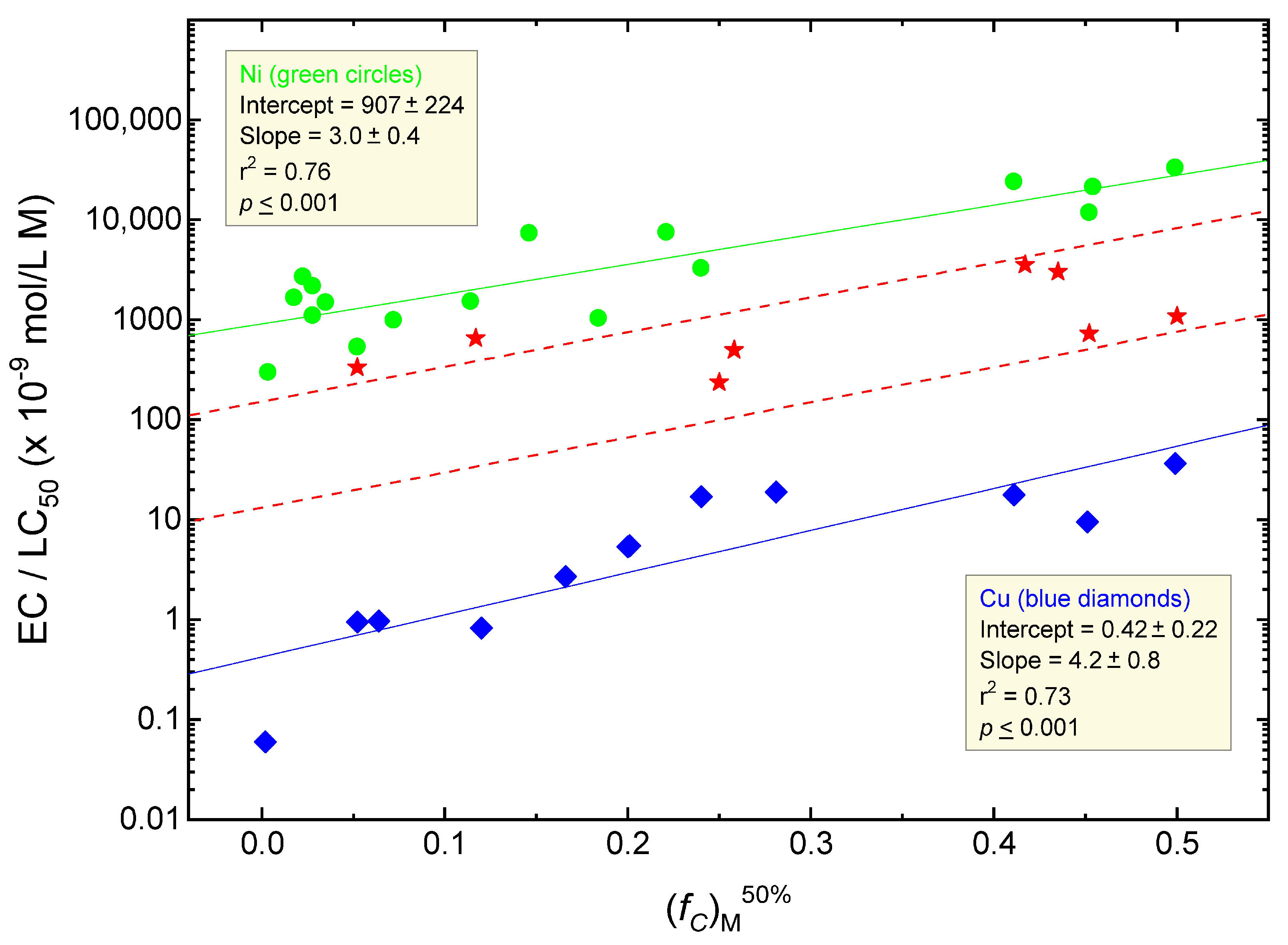
6. Organism Sensitivity and Its Relationship to the Fraction of Cells Bound at the TR50 ((fC)M50%)
7. The Binding Mechanism of MOH+
8. A Second Cell Surface Binding Site at a Moderately Alkaline pH?
9. Conclusions and Recommendations
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martinez-Finley, E.J.; Chakraborty, S.; Fretham, S.J.B.; Aschner, M. Cellular transport and homeostasis of essential and nonessential metals. Metallomics 2012, 4, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Weishaupt, A.K.; Lamann, K.; Tallarek, E.; Pezacki, A.T.; Matier, C.D.; Schwerdtle, T.; Aschner, M.; Chang, C.J.; Stürzenbaum, S.R.; Bornhorst, J. Dysfunction in atox-1 and ceruloplasmin alters labile Cu levels and consequently Cu homeostasis in C. elegans. Front. Mol. Biosci. 2024, 11, 1354627. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.G. Interactions between trace metals and aquatic organisms: A critique of the free-ion activity model in metal speciation and bioavailability. In Aquatic Systems; Tessier, A., Turner, D.R., Eds.; John Wiley and Sons: New York, NY, USA, 1995; pp. 45–102. [Google Scholar]
- Brown, P.L.; Markich, S.J. Evaluation of the free ion activity model of metal-organism interaction: Extension of the conceptual model. Aquat. Toxicol. 2000, 51, 177–194. [Google Scholar] [CrossRef] [PubMed]
- Di Toro, D.M.; Allen, H.E.; Bergman, H.L.; Meyer, J.S.; Paquin, P.R.; Santore, R.C. Biotic ligand model of the acute toxicity of metals. I. Technical basis. Environ. Toxicol. Chem. 2001, 20, 2383–2396. [Google Scholar] [CrossRef]
- Campbell, P.G.; Fortin, C. Biotic ligand model. In Encyclopedia of Aquatic Ecotoxicology; Férard, J.F., Blaise, C., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 237–245. [Google Scholar]
- Wang, P.; Kinraide, T.B.; Zhou, D.; Kopittke, P.M.; Peijnenburg, W.J.G.M. Plasma membrane surface potential: Dual effects upon ion uptake and toxicity. Plant Physiol. 2011, 155, 808–820. [Google Scholar] [CrossRef]
- Zhou, D.M.; Wang, P. A novel approach for predicting the uptake and toxicity of metallic and metalloid ions. Plant Signal. Behav. 2011, 6, 461–465. [Google Scholar] [CrossRef]
- De Schamphelaere, K.A.C.; Janssen, C.R. A biotic ligand model predicting acute copper toxicity for Daphnia magna: The effects of calcium, magnesium, sodium, potassium, and pH. Environ. Sci. Technol. 2002, 36, 48–54. [Google Scholar] [CrossRef]
- Ardestani, M.M.; van Straalen, N.M.; van Gestel, C.A.M. The relationship between metal toxicity and biotic ligand binding affinities in aquatic and soil organisms: A review. Environ. Pollut. 2014, 195, 133–147. [Google Scholar] [CrossRef]
- Slaveykova, V.; Wilkinson, K.J. Predicting the bioavailability of metals and metal complexes: Critical review of the biotic ligand model. Environ. Chem. 2005, 2, 9–24. [Google Scholar] [CrossRef]
- Campbell, P.G.C.; Errécalde, O.; Fortin, C.; Hiriart-Baer, V.P.; Vigneault, B. Metal bioavailability to phytoplankton—Applicability of the biotic ligand model. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2002, 133, 189–206. [Google Scholar] [CrossRef]
- Rand, G.M.; Wells, P.G.; McCarty, L.S. Introduction to aquatic toxicology. In Fundamentals of Aquatic Toxicology, 2nd ed.; Rand, G.M., Ed.; Taylor and Francis: Washington, DC, USA, 1995; pp. 3–66. [Google Scholar]
- Markich, S.J.; Brown, P.L.; Jeffree, R.A.; Lim, R.P. Valve movement responses of Velesunio angasi (Bivalvia: Hyriidae) to manganese and uranium: An exception to the free ion activity model. Aquat. Toxicol. 2000, 51, 155–175. [Google Scholar] [CrossRef] [PubMed]
- Markich, S.J.; Brown, P.L.; Jeffree, R.A.; Lim, R.P. The effects of pH and dissolved organic carbon on the toxicity of cadmium and copper to a freshwater bivalve: Further support for the extended free ion activity model. Arch. Environ. Contam. Toxicol. 2003, 45, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Crémazy, A.; Campbell, P.G.; Fortin, C. The biotic ligand model can successfully predict the uptake of a trivalent ion by a unicellular alga below pH 6.5 but not above: Possible role of hydroxo-species. Environ. Sci. Technol. 2013, 47, 2408–2415. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, K.J.; Campbell, P.G.C.; Couture, P. Effect of fluoride complexation on aluminum toxicity towards juvenile Atlantic salmon (Salmo salar). Can. J. Fish. Aquat. Sci. 1990, 47, 1446–1452. [Google Scholar] [CrossRef]
- Crémazy, A.; Campbell, P.G.; Fortin, C. In the presence of fluoride, free Sc3+ is not a good predictor of Sc bioaccumulation by two unicellular algae: Possible role of fluoro-complexes. Environ. Sci. Technol. 2014, 48, 9754–9761. [Google Scholar] [CrossRef]
- Koopal, L.K.; van Riemsdijk, W.H.; Kinniburgh, D.G. Humic matter and contaminants. General aspects of modeling metal ion binding. Pure Appl. Chem. 2001, 72, 2005–2016. [Google Scholar] [CrossRef]
- Wang, P.; Zhou, D.-M.; Li, L.-Z.; Luo, X.-S. Evaluating the biotic ligand model for toxicity and the alleviation of toxicity in terms of cell membrane surface potential. Environ. Toxicol. Chem. 2010, 29, 1503–1511. [Google Scholar] [CrossRef]
- Bánki, O.; Roskov, Y.; Döring, M.; Ower, G.; Hernández Robles, D.R.; Plata Corredor, C.A.; Stjernegaard Jeppesen, T.; Örn, A.; Vandepitte, L.; Pape, T.; et al. Catalogue of Life (Version 2024-08-29); Catalogue of Life: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Deleebeeck, N.M.; Muyssen, B.T.; De Laender, F.; Janssen, C.R.; De Schamphelaere, K.A. Comparison of nickel toxicity to cladocerans in soft versus hard surface waters. Aquat. Toxicol. 2007, 84, 223–235. [Google Scholar] [CrossRef]
- Warne, M.; Batley, G.E.; van Dam, R.A.; Chapman, J.C.; Fox, D.R.; Hickey, C.W.; Stauber, J.L. Revised Method for Deriving Australian and New Zealand Water Quality Guideline Values for Toxicants; Australian Government Department of Agriculture and Water Resources: Canberra, Australia, 2018. [Google Scholar]
- Tipping, E.; Lofts, S.; Sonke, J.E. Humic ion-binding model VII: A revised parameterisation of cation-binding by humic substances. Environ. Chem. 2011, 8, 225–235. [Google Scholar] [CrossRef]
- Markich, S.J.; Brown, P.L. Thermochemical Data (log K) for Metals and Metalloids, ASI C4/24; Aquatic Solutions International: Sydney, Australia, 2024. [Google Scholar]
- Bryan, S.E.; Tipping, E.; Hamilton-Taylor, J. Comparison and measured and modelled copper binding by natural organic matter in freshwaters. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2002, 133, 37–49. [Google Scholar] [CrossRef]
- Unsworth, E.R.; Wanken, K.W.; Zhang, H.; Davison, W.; Black, F.; Buffle, J.; Cao, J.; Cleven, R.; Galceran, J.; Gunkel, P.; et al. Model predictions of metal speciation in freshwaters compared to measurements made by in situ techniques. Environ. Sci. Technol. 2006, 40, 1942–1949. [Google Scholar] [CrossRef] [PubMed]
- Van Laer, L.; Smolders, E.; Degryse, F.; Janssen, C.; De Schamphelaere, K.A. Speciation of nickel in surface waters measured with the Donnan membrane technique. Anal. Chim. Acta 2006, 578, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Mueller, K.K.; Lofts, S.; Fortin, C.; Campbell, P.G. Trace metal speciation predictions in natural aquatic systems: Incorporation of dissolved organic matter (DOM) spectroscopic quality. Environ. Chem. 2012, 9, 356–368. [Google Scholar] [CrossRef]
- Mueller, K.K.; Couture, R.M.; Fortin, C.; Campbell, P.G. Nickel and copper complexation by natural dissolved organic matter—Titration of two contrasting lake waters and comparison of measured and modelled free metal ion concentrations. Environ. Chem. 2024, 21, EN23021. [Google Scholar] [CrossRef]
- Chen, W.; Guéguen, C.; Smith, D.S.; Galceran, J.; Puy, J.; Companys, E. Metal (Pb, Cd and Zn) binding to diverse organic matter samples and implications for speciation modeling. Environ. Sci. Technol. 2018, 52, 4163–4172. [Google Scholar] [CrossRef]
- Wang, P.; Ding, Y.; Liang, Y.; Liu, M.; Lin, X.; Ye, Q.; Shi, Z. Linking molecular composition to proton and copper binding ability of fulvic acid: A theoretical modeling approach based on FT-ICR-MS analysis. Geochim. Cosmochim. Acta 2021, 312, 279–298. [Google Scholar] [CrossRef]
- Mebane, C.A. Bioavailability and toxicity models of copper to freshwater life: The state of regulatory science. Environ. Toxicol. Chem. 2023, 42, 2529–2563. [Google Scholar] [CrossRef]
- Town, R.M.; van Leeuwen, H.P. Chemodynamic features of nickel(II) and its complexes: Implications for bioavailability in freshwaters. Ecotoxicol. Environ. Saf. 2022, 241, 113840. [Google Scholar] [CrossRef]
- Hassler, C.S.; Slaveykova, V.I.; Wilkinson, K.J. Discriminating between intra- and extracellular metals using chemical extractions. Limnol. Oceanogr. Methods 2004, 2, 237–247. [Google Scholar] [CrossRef]
- Warner, R.M. Applied Statistics I: Basic Bivariate Techniques, 3rd ed.; Sage Publications: Los Angeles, CA, USA, 2020. [Google Scholar]
- Peters, A.; Merrington, G.; Schlekat, C.; De Schampelaere, K.; Stauber, J.; Batley, G.; Harford, A.; van Dam, R.; Pease, C.; Mooney, T.; et al. Validation of the nickel biotic ligand model for locally relevant species in Australian freshwaters. Environ. Toxicol. Chem. 2018, 37, 2566–2574. [Google Scholar] [CrossRef]
- Price, G.A.; Stauber, J.L.; Stone, S.; Koppel, D.J.; Holland, A.; Jolley, D. Does toxicity test variability support bioavailability model predictions being within a factor of 2? Environ. Chem. 2022, 19, 177–182. [Google Scholar] [CrossRef]
- Meyer, J.S.; Traudt, E.M.; Ranville, J.F. Is the factor-of-2-rule broadly applicable for evaluating the prediction accuracy of metal-toxicity models? Bull. Environ. Contam. Toxicol. 2018, 100, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Nys, C.; Janssen, C.R.; Blust, R.; Smolders, E.; De Schamphalaere, K.A. Reproductive toxicity of binary and ternary mixture combinations of nickel, zinc, and lead to Ceriodaphnia dubia is best predicted with the independent action model. Environ. Toxicol. Chem. 2016, 35, 1796–1805. [Google Scholar] [CrossRef]
- Schubauer-Berigan, M.K.; Dierkes, J.R.; Monson, P.D.; Ankley, G.T. pH-dependent toxicity of Cd, Cu, Ni, Pb and Zn to Ceriodaphnia dubia, Pimephales promelas, Hyalella azteca and Lumbriculus variegates. Environ. Toxicol. Chem. 1993, 12, 1261–1266. [Google Scholar]
- Keithly, J.; Brooker, J.A.; DeForest, D.K.; Wu, B.K.; Brix, K.V. Acute and chronic toxicity of nickel to a cladoceran (Ceriodaphnia dubia) and an amphipod (Hyalella azteca). Environ. Toxicol. Chem. 2004, 23, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Santore, R.C.; Croteau, K.; Ryan, A.C.; Schlekat, C.; Middleton, E.; Garman, E.; Hoang, T. A review of water quality factors that affect nickel bioavailability to aquatic organisms: Refinement of the biotic ligand model for nickel in acute and chronic exposures. Environ. Toxicol. Chem. 2021, 40, 2121–2134. [Google Scholar] [CrossRef]
- Kozlova, T.; Wood, C.M.; McGeer, J.C. The effect of water chemistry on the acute toxicity of nickel to the cladoceran Daphnia pulex and the development of a biotic ligand model. Aquat. Toxicol. 2009, 91, 221–228. [Google Scholar] [CrossRef]
- Clifford, M.; McGeer, J.C. Development of a biotic ligand model to predict the acute toxicity of cadmium to Daphnia pulex. Aquat. Toxicol. 2010, 98, 1–7. [Google Scholar] [CrossRef]
- Markich, S.J. Sensitivity of the glochidia (larvae) of freshwater mussels (Bivalvia: Unionida: Hyriidae) to cadmium, cobalt, copper, lead, nickel and zinc: Differences between metals, species and exposure time. Sci. Total Environ. 2017, 601–602, 1427–1436. [Google Scholar] [CrossRef]
- Stubblefield, W.A.; Van Genderen, E.; Cardwell, A.S.; Heijerick, D.G.; Janssen, C.R.; De Schamphelaere, K.A.C. Acute and chronic toxicity of cobalt to freshwater organisms: Using a species sensitivity distribution approach to establish international water quality standards. Environ. Toxicol. Chem. 2020, 39, 799–811. [Google Scholar] [CrossRef]
- DeForest, D.K.; Santore, R.C.; Ryan, A.C.; Church, B.; Chowdhury, M.J.; Brix, K.V. Development of biotic ligand model–based freshwater aquatic life criteria for lead following US environmental protection agency guidelines. Environ. Toxicol. Chem. 2017, 36, 2695–2973. [Google Scholar] [CrossRef] [PubMed]
- Nys, C.; Van Sprang, P.; Lofts, S.; Baken, S.; Delbeke, K.; De Schamphelaere, K. Updated chronic copper biovailability models for invertebrates and algae. Environ. Toxicol. Chem. 2024, 43, 450–467. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, A.; Yamamuro, M.; Tatarazako, N. Acute toxicity of 50 metals to Daphnia magna. J. Appl. Toxicol. 2015, 35, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Kleinhenz, L.S.; Nugegoda, D.; Trenfield, M.A.; van Dam, R.A.; Humphrey, C.L.; Mooney, T.J.; Harford, A.J. Acute and chronic toxicity of magnesium to the early life stages of two tropical freshwater mussel species. Ecotoxicol. Environ. Saf. 2019, 184, 109638. [Google Scholar] [CrossRef]
- Lee, C.E.; Charmantier, G.; Lorin-Nebel, C. Mechanisms of Na+ uptake from freshwater habitats in animals. Front. Physiol. 2022, 13, 1006113. [Google Scholar] [CrossRef]
- Carbonaro, R.F.; Di Toro, D.M. Linear free energy relationships for metal-ligand complexation: Monodentate binding to negatively-charged oxygen donor atoms. Geochim. Cosmochim. Acta 2007, 71, 3958–3968. [Google Scholar] [CrossRef]
- Bringolf, R.B.; Morris, B.A.; Boese, C.J.; Santore, R.C.; Allen, H.E.; Meyer, J.S. Influence of dissolved organic matter on acute toxicity of zinc to larval fathead minnows (Pimephales promelas). Arch. Environ. Contam. Toxicol. 2006, 51, 438–444. [Google Scholar] [CrossRef]
- De Schamphelaere, K.A.; Janssen, C.R. Bioavailability and chronic toxicity of zinc to juvenile rainbow trout (Oncorhynchus mykiss): Comparison with other fish species and development of a biotic ligand model. Environ. Sci. Technol. 2004, 38, 6201–6209. [Google Scholar] [CrossRef]
- De Schamphelaere, K.A.; Janssen, C.R. Development and Validation of Biotic Ligand Models for Predicting Chronic Zinc Toxicity to Fish, Daphnids and Algae; Report ZEB-WA-01; Ghent University: Ghent, Belgium, 2004. [Google Scholar]
- Heijerick, D.G.; De Schamphelaere, K.A.C.; Van Sprang, P.A.; Janssen, C.R. Development of a chronic zinc biotic ligand model for Daphnia magna. Ecotoxicol. Environ. Saf. 2005, 62, 1–10. [Google Scholar] [CrossRef]
- Cooper, N.L.; Bidwell, J.R.; Kumar, A. Toxicity of copper, lead, and zinc mixtures to Ceriodaphnia dubia and Daphnia carinata. Ecotoxicol. Environ. Saf. 2009, 72, 1523–1528. [Google Scholar] [CrossRef]
- Van Regenmortel, T.; Berteloot, O.; Janssen, C.R.; De Schamphelaere, K.A.C. Analysing the capacity of the Daphnia magna and Pseudokirchneriella subcapitata bioavailability models to predict chronic zinc toxicity at high pH and low calcium concentrations and formation of a generalised bioavailability model for D. magna. Environ. Toxicol. Chem. 2017, 36, 2781–2798. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.; McGeer, J.C. Development of a biotic ligand model for the acute toxicity of zinc to Daphnia pulex in soft waters. Aquat. Toxicol. 2009, 91, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Heijerick, D.G.; De Schamphelaere, K.A.C.; Janssen, C.R. Biotic ligand model development predicting Zn toxicity to the alga Pseudokirchneriella subcapitata: Possibilities and limitations. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2002, 133, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Hyne, R.V.; Pablo, F.; Julli, M.; Markich, S.J. Influence of water chemistry on the acute toxicity of copper and zinc to the cladoceran Ceriodaphnia dubia. Environ. Toxicol. Chem. 2005, 24, 1667–1675. [Google Scholar] [CrossRef] [PubMed]
- Paulauskis, J.D.; Winner, R.W. Effects of water hardness and humic acid on zinc toxicity to Daphnia magna Straus. Aquat. Toxicol. 1988, 12, 273–290. [Google Scholar] [CrossRef]
- Cusimano, R.F.; Brakke, D.F.; Chapman, G.A. Effects of pH on the toxicities of cadmium, copper and zinc to steelhead trout (Salmo gairdneri). Can. J. Fish. Aquat. Sci. 1986, 43, 1497–1503. [Google Scholar] [CrossRef]
- Stauber, J.L.; Gadd, J.; Price, G.A.V.; Evans, A.; Holland, A.; Albert, A.; Batley, G.E.; Binet, M.T.; Golding, L.A.; Hickey, C.; et al. Applicability of chronic multiple linear regression models for predicting zinc toxicity in Australian and New Zealand freshwaters. Environ. Toxicol. Chem. 2023, 42, 2614–2629. [Google Scholar] [CrossRef]
- Deleebeeck, N.M.E.; De Schamphelaere, K.A.C.; Heijerick, D.G.; Bossuyt, B.T.A.; Janssen, C.R. The acute toxicity of nickel to Daphnia magna: Predictive capability of bioavailability models in artificial and natural waters. Ecotoxicol. Environ. Saf. 2008, 70, 67–78. [Google Scholar] [CrossRef]
- Schlekat, C.E.; Van Genderen, E.; De Schamphelaere, K.A.C.; Artunes, P.M.C.; Rogevich, E.C.; Stubblefield, W.A. Cross-species extrapolation of chronic nickel biotic ligand models. Sci. Total Environ. 2010, 408, 6148–6157. [Google Scholar] [CrossRef]
- Deleebeeck, N.M.E.; De Schamphelaere, K.A.C.; Janssen, C.R. Effects of Mg2+ and H+ on the toxicity of Ni2+ to the unicellular green alga Pseudokirchneriella subcapitata: Model development and validation with surface waters. Sci. Total Environ. 2009, 407, 1901–1914. [Google Scholar] [CrossRef]
- Deleebeeck, N.M.E.; De Schamphelaere, K.A.C.; Janssen, C.R. A bioavailability model predicting the toxicity of nickel to rainbow trout (Oncorhynchus mykiss) and fathead minnow (Pimephales promelas) in synthetic and natural waters. Ecotoxicol. Environ. Saf. 2007, 67, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mano, H.; Shinohara, N. Acute toxicity of nickel to Daphnia magna: Validation of bioavailability models in Japanese rivers. Water Air Soil Pollut. 2020, 231, 459. [Google Scholar] [CrossRef]
- Meyer, J.S.; Santore, R.C.; Bobbitt, J.P.; Debrey, L.D.; Boese, C.J.; Paquin, P.R.; Allen, H.E.; Bergman, H.L.; DiToro, D.M. Binding of nickel and copper to fish gills predicts toxicity when water hardness varies, but free-ion activity does not. Environ. Sci. Technol. 1999, 33, 913–916. [Google Scholar] [CrossRef]
- Schroeder, J.E. Development of Models for the Prediction of Short-term and Long-term Toxicity to Hyalella azteca from Separate Exposures to Nickel and Cadmium. Ph.D. Thesis, University of Waterloo, Waterloo, ON, Canada, 2008. [Google Scholar]
- Nys, C.; Janssen, C.R.; Van Sprang, P.; De Schamphalaere, K.A. The effect of pH on chronic aquatic nickel toxicity is dependent on the pH itself: Extending the chronic nickel bioavailability models. Environ. Toxicol. Chem. 2016, 35, 1097–1106. [Google Scholar] [CrossRef]
- Hoang, T.C.; Tomasso, J.R.; Klaine, S.J. Influence of water quality and age on nickel toxicity to fathead minnows (Pimephales promelas). Environ. Toxicol. Chem. 2004, 23, 86–92. [Google Scholar] [CrossRef]
- He, J.; Wang, C.; Schlekat, C.E.; Wu, F.; Middleton, E.; Garman, E.; Peters, A. Validation of nickel bioavailability models for algae, invertebrates, and fish in Chinese surface waters. Environ. Toxicol. Chem. 2023, 42, 1257–1265. [Google Scholar] [CrossRef]
- Chan, K. The Influence of Calcium and Dissolved Organic Matter on the Acute and Chronic Toxicity of Nickel to Hyalella azteca. Master’s Thesis, Wilfrid Laurier University, Waterloo, ON, Canada, 2013. [Google Scholar]
- Niyogi, S.; Kent, R.; Wood, C.M. Effects of water chemistry variables on gill binding and acute toxicity of cadmium in rainbow trout (Oncorhynchus mykiss): A biotic ligand model (BLM) approach. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2008, 148, 305–314. [Google Scholar] [CrossRef]
- Tan, Q.-G.; Wang, W.-X. Acute toxicity of cadmium in Daphnia magna under different calcium and pH conditions: Importance of influx rate. Environ. Sci. Technol. 2011, 45, 1970–1976. [Google Scholar] [CrossRef]
- Källqvist, T. Effect of water hardness on the toxicity of cadmium to the green alga Pseudokirchneriella subcapitata in an artificial growth medium and nutrient-spiked natural lake waters. J. Toxicol. Environ. Health 2009, 72, 277–283. [Google Scholar] [CrossRef]
- Clifford, M.S. A Study of the Waterborne and Dietary Toxicity of Cadmium to Hydra attenuata and Daphnia pulex in Soft Waters and the Development of Biotic Ligand Models to Predict such Toxicity. Ph.D. Thesis, Wilfrid Laurier University, Waterloo, ON, Canada, 2009. [Google Scholar]
- Jackson, B.P.; Lasier, P.J.; Miller, W.P.; Winger, P.W. Effects of calcium, magnesium and sodium on alleviating cadmium toxicity to Hyalella azteca. Bull. Environ. Contam. Toxicol. 2000, 64, 279–286. [Google Scholar] [CrossRef]
- Marr, J.C.A.; Hansen, J.A.; Meyer, J.S.; Cacela, D.; Podrabsky, T.; Lipton, J.; Bergman, H.L. Toxicity of cobalt and copper to rainbow trout: Application of a mechanistic model for predicting survival. Aquat. Toxicol. 1998, 43, 225–238. [Google Scholar] [CrossRef]
- dos Reis, L.L.; de Abreu, C.B.; Gebara, R.C.; Rocha, G.S.; Longo, E.; da Silva Mansano, A.; da Graça Gama Melão, M. Isolated and combined effects of cobalt and nickel on the microalga Raphidocelis subcapitata. Ecotoxicology 2024, 33, 104–118. [Google Scholar] [CrossRef] [PubMed]
- Alsop, D.H.; Wood, C.M. Kinetic analysis of zinc accumulation in the gills of juvenile rainbow trout: Effects of zinc acclimation and implications for biotic ligand modelling. Environ. Toxicol. Chem. 2000, 19, 1911–1918. [Google Scholar] [CrossRef]
- Long, K.E.; Van Genderen, E.J.; Klaine, S.J. The effects of low hardness and pH on copper toxicity to Daphnia magna. Environ. Toxicol. Chem. 2004, 23, 72–75. [Google Scholar] [CrossRef]
- Crémazy, A.; Wood, C.M.; Ng, T.Y.-T.; Smith, D.S.; Chowdhury, M.J. Experimentally derived acute and chronic copper biotic ligand models for rainbow trout. Aquat. Toxicol. 2017, 192, 224–240. [Google Scholar] [CrossRef]
- Erickson, R.J.; Benoit, D.A.; Mattson, V.R.; Nelson, H.P.; Leonard, E.N. The effects of water chemistry on the toxicity of copper to fathead minnows. Environ. Toxicol. Chem. 1996, 15, 181–193. [Google Scholar] [CrossRef]
- De Schamphelaere, K.A.C.; Bossuyt, B.T.A.; Janssen, C.R. Variability of the protective effect of sodium on the acute toxicity of copper to freshwater cladocerans. Environ. Toxicol. Chem. 2007, 26, 535–542. [Google Scholar] [CrossRef]
- De Schamphelaere, K.A.C.; Heijerick, D.G.; Janssen, C.R. Refinement and field validation of a biotic ligand model predicting acute copper toxicity to Daphnia magna. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2002, 133, 243–258. [Google Scholar] [CrossRef]
- Ryan, A.C.; Tomasso, J.R.; Klaine, S.J. Influence of pH, hardness, dissolved organic carbon concentration, and dissolved organic matter source on the acute toxicity of copper to Daphnia magna in soft waters: Implications for the biotic ligand model. Environ. Toxicol. Chem. 2009, 28, 1663–1670. [Google Scholar] [CrossRef]
- Villavicencio, G.; Urrestarazu, P.; Carvajal, C.; De Schamphelaere, K.A.C.; Janssen, C.R.; Torres, J.C.; Rodriguez, P.H. Biotic ligand model prediction of copper toxicity to Daphnids in a range of natural waters in Chile. Environ. Toxicol. Chem. 2005, 24, 1287–1299. [Google Scholar] [CrossRef]
- Welsh, P.G.; Parrott, J.L.; Dixon, D.G.; Hodson, P.V.; Spry, D.J.; Mierle, G. Estimating acute copper toxicity to larval fathead minnow (Pimephales promelas) in soft water from measurements of dissolved organic carbon, calcium and pH. Can. J. Fish. Aquat. Sci. 1996, 53, 1263–1271. [Google Scholar] [CrossRef]
- Sciera, K.L.; Isely, J.J.; Tomasso, J.R.; Klaine, S.J. Influence of multiple water-quality characteristics on copper toxicity to fathead minnows (Pimephales promelas). Environ. Toxicol. Chem. 2004, 23, 2900–2905. [Google Scholar] [CrossRef] [PubMed]
- Kramer, K.J.M.; Jak, R.G.; van Hattum, B.; Hooftman, R.N.; Zwolsman, J.J.G. Copper toxicity in relation to surface water-dissolved organic matter: Biological effects to Daphnia magna. Environ. Toxicol. Chem. 2004, 23, 2971–2980. [Google Scholar] [CrossRef] [PubMed]
- Goulet, R.R.; Thompson, P.A.; Serben, K.C.; Eickhoff, C.V. Impact of environmentally based chemical hardness on uranium speciation and toxicity in six aquatic species. Environ. Toxicol. Chem. 2015, 34, 562–574. [Google Scholar] [CrossRef]
- Semaan, M.; Holdway, D.A.; van Dam, R.A. Comparative sensitivity of three populations of the cladoceran Moinodaphnia macleayi to acute and chronic uranium exposure. Environ. Toxicol. 2001, 16, 365–376. [Google Scholar] [CrossRef]
- Markich, S.J. Water hardness reduces the accumulation and toxicity of uranium in a freshwater macrophyte (Ceratophyllum demersum). Sci. Total Environ. 2013, 443, 582–589. [Google Scholar] [CrossRef]
- Trenfield, M.A.; Ng, J.C.; Noller, B.N.; Markich, S.J.; van Dam, R.A. Dissolved organic carbon reduces uranium bioavailability and toxicity. 2. Uranium(VI) speciation and toxicity to three tropical freshwater organisms. Environ. Sci. Technol. 2011, 45, 3082–3089. [Google Scholar] [CrossRef]
- Charles, A.L.; Markich, S.J.; Stauber, J.L.; De Filippis, L.F. The effect of water hardness on the toxicity of uranium to a tropical freshwater alga (Chlorella sp.). Aquat. Toxicol. 2002, 60, 61–73. [Google Scholar] [CrossRef]
- Franklin, N.M.; Stauber, J.L.; Markich, S.J.; Lim, R.P. pH-dependent toxicity of copper and uranium to a tropical freshwater alga (Chlorella sp.). Aquat. Toxicol. 2000, 48, 275–289. [Google Scholar] [CrossRef]
- De Schamphelaere, K.A.; Nys, C.; Janssen, C.R. Toxicity of lead (Pb) to freshwater green algae: Development and validation of a bioavailability model and inter-species sensitivity comparison. Aquat. Toxicol. 2014, 155, 348–359. [Google Scholar] [CrossRef]
- Esbaugh, A.J.; Brix, K.V.; Mager, E.M.; Grosell, M. Multi-linear regression models predict the effects of water chemistry on acute lead toxicity to Ceriodaphnia dubia and Pimephales promelas. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2011, 154, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Mager, E.M.; Esbaugh, A.J.; Brix, K.V.; Ryan, A.C.; Grosell, M. Influences of water chemistry on the acute toxicity of lead to Pimephales promelas and Ceriodaphnia dubia. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2011, 153, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Antunes, P.M.C.; Kreager, N.J. Lead toxicity to Lemna minor predicted using a metal speciation chemistry approach. Environ. Toxicol. Chem. 2014, 33, 2225–2233. [Google Scholar] [CrossRef] [PubMed]
- Nys, C.; Janssen, C.R.; Mager, E.M.; Esbaugh, A.J.; Brix, K.V.; Grosell, M.; Stubblefield, W.A.; Holtze, K.; De Schamphelaere, K.A.C. Development and validation of a biotic ligand model for predicting chronic toxicity of lead to Ceriodaphnia dubia. Environ. Toxicol. Chem. 2014, 33, 394–403. [Google Scholar] [CrossRef]
- Grosell, M.; Gerdes, R.; Brix, K.V. Influence of Ca, humic acid and pH on lead accumulation and toxicity in the fathead minnow during prolonged water-borne lead exposure. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2006, 143, 473–483. [Google Scholar] [CrossRef]
- Bircneau, O.; Chowdhury, M.J.; Gillis, P.L.; McGeer, J.C.; Wood, C.M.; Wilkie, M.P. Modes of metal toxicity and impaired branchial ionregulation in rainbow trout exposed to mixtures of Pb and Cd in soft water. Aquat. Toxicol. 2008, 89, 222–231. [Google Scholar] [CrossRef]
- Macdonald, A.; Silk, L.; Schwartz, M.; Playle, R.C. A lead–gill binding model to predict acute lead toxicity to rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2002, 133, 227–242. [Google Scholar] [CrossRef]
- Liao, C.-M.; Ju, Y.-R.; Chen, W.Y. Subcelllular partitioning links BLM-based toxicokinetics for assessing cadmium toxicity to rainbow trout. Environ. Toxicol. 2010, 26, 600–609. [Google Scholar] [CrossRef]
- Altszyler, E.; Ventura, A.C.; Colman-Lerner, A.; Chernomoretz, A. Ultrasensitivity in signaling cascades revisited: Linking local and global ultrasensitivity estimations. PLoS ONE 2017, 12, e0180083. [Google Scholar]
- Niyogi, S.; Couture, P.; Pyle, G.; McDonald, D.G.; Wood, C.M. Acute cadmium biotic ligand model characteristics of laboratory-reared and wild yellow perch (Perca flavescens) relative to rainbow trout (Oncorhynchus mykiss). Can. J. Fish. Aquat. Sci. 2004, 61, 942–953. [Google Scholar] [CrossRef]
- Taylor, L.N.; Wood, C.M.; McDonald, D.G. An evaluation of sodium loss and gill metal binding properties to explain species differences in copper tolerance. Environ. Toxicol. Chem. 2003, 22, 2159–2166. [Google Scholar] [CrossRef] [PubMed]
- Leonard, E.M.; Marentette, J.R.; Balshine, S.; Wood, C.M. Critical body residues, Michaelis-Menten analysis of bioaccumulation, lethality and behaviour as endpoints of waterborne Ni toxicity in two teleosts. Ecotoxicology 2014, 23, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.L.; Sylva, R.N. Unified Theory of Metal-Ion Complex Formation Constants; Australian Atomic Energy Commission: Sydney, Australia, 1987. [Google Scholar]
- Wilde, K.L.; Stauber, J.L.; Markich, S.J.; Franklin, N.M.; Brown, P.L. The effect of pH on the uptake and toxicity of copper and zinc in a tropical freshwater alga (Chlorella sp.). Arch. Environ. Contam. Toxicol. 2006, 51, 174–185. [Google Scholar] [CrossRef] [PubMed]





| Taxa | Organism a | log KMBL | ||||||
|---|---|---|---|---|---|---|---|---|
| Zn2+ | Ni2+ | Cd2+ | Co2+ | Cu2+ | UO22+ | Pb2+ | ||
| Fish c | Acipenser transmontanus | 6.1 | 7.3 | 8.2 | 7.3 | |||
| Cottus bairdii | 6.1 (3) b | 7.2 | 8.1 | |||||
| Ctenopharyngodon idella | 5.7 | |||||||
| Danio rerio | 7.7 | |||||||
| Mogurnda mogurnda | 6.1 | 7.3 | 7.4 | |||||
| Neogobius melanostomus | 4.7 | |||||||
| Oncorhynchus clarkii | 5.8 | 7.8 | ||||||
| Oncorhynchus kisutch | 7.8 | |||||||
| Oncorhynchus mykiss | 5.9 (13) | 4.7 (3) | 7.5 (13) | 5.3 (3) | 7.9 (12) | 7.3 | 7.1 (3) | |
| Oncorhynchus nerka | 7.7 | |||||||
| Oncorhynchus tshawytscha | 6.1 | 7.6 | 7.7 (2) | |||||
| Oreochromis mossambicus | 7.0 | |||||||
| Perca flavescens | 7.2 | |||||||
| Pimephales promelas | 5.7 (3) | 4.7 (3) | 7.1 (3) | 5.7 | 7.6 (11) | 7.4 | 6.8 (5) | |
| Prosopium williamsoni | 5.5 | |||||||
| Salmo trutta | 7.6 | |||||||
| Salvelinus confluentus | 5.7 | 7.2 | ||||||
| Average (fish) | 5.9 | 4.7 | 7.3 | 5.5 | 7.8 | 7.4 | 7.1 | |
| Crustaceans (cladocerans) | Acantholeberis curvirostris | 7.8 | ||||||
| Acroperus elongatus | 8.2 | |||||||
| Acroperus harpae | 8.3 | |||||||
| Alona sp. | 8.1 | |||||||
| Alona affinis | 4.7 | |||||||
| Alona quadrangularis | 8.0 | |||||||
| Bosmina coregoni | 5.0 | |||||||
| Bosmina longirostris | 7.9 (2) | |||||||
| Camptocercus lilljeborgi | 4.9 | |||||||
| Ceriodaphnia cornuta | 7.9 | |||||||
| Ceriodaphnia dubia | 5.9 (8) | 5.2 (4) | 7.0 (3) | 5.5 | 8.0 (15) | 7.4 | 7.0 (7) | |
| Ceriodaphnia pulchella | 4.8 | 7.7 (2) | ||||||
| Ceriodaphnia quadrangula | 4.8 | |||||||
| Crustaceans (cladocerans) | Ceriodaphnia reticulata | 6.2 | 6.5 | 8.1 (3) | ||||
| Ceriodaphnia rigaudi | 7.0 | |||||||
| Ceriodaphnia silvestri | 6.9 | |||||||
| Chydorus ovalis | 4.7 | 7.9 | ||||||
| Chydorus sphearicus | 8.1 (2) | |||||||
| Daphnia ambigua | 7.9 | |||||||
| Daphnia carinata | 5.7 | 7.4 | 6.9 (2) | |||||
| Daphnia exilis | 7.8 | |||||||
| Daphnia galeata | 7.6 (3) | |||||||
| Daphnia longispina | 4.9 | 7.8 (2) | ||||||
| Daphnia magna | 5.8 (9) | 4.9 (6) | 6.9 (7) | 5.4 (2) | 7.8 (13) | 8.0 | 6.7 (4) | |
| Daphnia obtusa | 7.8 | |||||||
| Daphnia pulex | 5.9 (2) | 4.7 (2) | 6.6 (2) | 7.9 (4) | ||||
| Daphnia similis | 6.8 | |||||||
| Daphnia thomsoni | 6.3 | |||||||
| Disparalona rostrata | 7.7 | |||||||
| Eurycercus lamellatus | 8.1 | |||||||
| Moina affinis | 6.2 | 7.0 | 8.0 | |||||
| Moinodaphnia macleayi | 6.3 | 7.7 | 8.2 | |||||
| Peracantha truncata | 4.6 | |||||||
| Pleuroxus truncatus | 7.7 | |||||||
| Pseudosida ramosa | 7.2 | |||||||
| Pseudosida variabilis | 8.3 | |||||||
| Scapholeberis microcephala | 8.2 | |||||||
| Scapholeberis mucronata | 8.1 | |||||||
| Simocephalus expinosus | 7.8 (2) | |||||||
| Simocephalus serrulatus | 5.0 | 6.9 | ||||||
| Simocephalus vetulus | 4.8 | 6.9 | 7.9 (3) | |||||
| Average (cladocerans) | 6.0 | 4.8 | 6.9 | 5.5 | 7.9 | 7.9 | 6.8 | |
| Crustaceans (amphipods) | Gammarus sp. | 7.5 | ||||||
| Gammarus pseudolimnaeus | 6.5 | |||||||
| Gammarus pulex | 4.7 | |||||||
| Hyalella azteca | 5.9 (2) | 5.1 (4) | 7.3 (2) | 5.8 | 7.8 | 7.4 | 6.9 | |
| Crustaceans (copepod) | Notodiaptomus iheringi | 7.4 | 7.5 | |||||
| Crustaceans (ostracod) | Cypridopsis vidua | 7.8 | ||||||
| Crustaceans (decapod) | Macrobrachium rosenbergi | 5.6 | ||||||
| Average (crustaceans) | 6.0 | 4.9 | 6.9 | 5.6 | 7.9 | 7.7 | 6.9 | |
| Molluscs d (bivalves) | Alathyria profuga | 5.9 | 4.8 | 6.6 | 5.9 | 7.4 | 6.4 | |
| Cambarunio iris | 8.1 (3) | |||||||
| Corbicula fluminea | 7.7 | 7.6 | ||||||
| Cucumerunio novaehollandiae | 6.0 | 5.0 | 6.8 | 6.1 | 7.7 | 6.6 | ||
| Echyridella menziesii | 5.7 | 8.1 | ||||||
| Epioblasma rangiana | 7.9 | |||||||
| Epioblasma triquetra | 8.2 | |||||||
| Hyridella australis | 6.0 | 5.0 | 6.8 | 6.1 | 7.6 | 6.6 | ||
| Hyridella depressa | 6.0 | 4.9 | 6.7 | 6.0 | 7.5 | 6.5 | ||
| Molluscs (bivalves) | Hyridella drapeta | 5.9 | 4.9 | 6.6 | 6.0 | 7.5 | 6.5 | |
| Lampsilis abrupta | 7.9 | |||||||
| Lampsilis fasciola | 8.0 (2) | |||||||
| Lampsilis rafinesqueana | 6.3 | 7.4 | 7.8 | 6.8 | ||||
| Lampsilis siliquoidea | 6.3 | 7.5 | 7.8 (3) | 6.7 | ||||
| Obovaria subrotunda | 7.8 | |||||||
| Ortmanniana ligamentina | 7.6 (2) | |||||||
| Paetulunio fabalis | 8.2 | |||||||
| Potamilus ohiensis | 7.9 | |||||||
| Velesunio sp. | 8.2 | |||||||
| Velesunio anbiguus | 5.8 | 4.8 | 6.6 | 5.9 | 7.4 | 6.4 | ||
| Velesunio angasi | 6.3 | |||||||
| Venustaconcha ellipsiformis | 8.1 | |||||||
| Average (bivalves) | 6.0 | 4.9 | 6.9 | 6.0 | 7.8 | 7.6 | 6.6 | |
| Molluscs (gastropods) | Amerianna cumingi | 6.2 | 7.9 | 8.1 | ||||
| Filopaludina bengalensis | 7.9 | |||||||
| Fluminicola sp. | 8.2 | |||||||
| Fontigens aldrichi | 7.9 | |||||||
| Lymnaea stagnalis | 4.9 (3) | 6.6 (2) | 5.9 | 8.0 (4) | ||||
| Physella acuta | 8.3 | |||||||
| Physella gyrina | 7.7 | |||||||
| Planorbella pilsbryi | 8.2 | |||||||
| Pomacea paludosa | 5.8 | |||||||
| Potamopyrgus antipodarum | 7.3 | |||||||
| Pyrgulopsis robusta | 8.0 | |||||||
| Racesina luteola | 6.5 | 8.2 | ||||||
| Taylorconcha serpenticola | 8.1 | |||||||
| Average (gastropods) | 6.0 | 4.9 | 6.8 | 5.9 | 8.0 | 8.1 | — | |
| Average (molluscs) | 6.0 | 4.9 | 6.8 | 6.0 | 7.9 | 7.9 | 6.6 | |
| Rotifers | Anuraeopsis fissa | 5.8 | ||||||
| Brachionus calicyflorus | 4.6 | 7.4 | ||||||
| Brachionus rubens | 5.5 | |||||||
| Euchlanis dilatata | 6.1 | 7.9 | ||||||
| Lecane inermis | 6.3 | 8.0 | ||||||
| Lecane quadridentata | 6.2 | 7.5 | ||||||
| Average (rotifers) | 6.0 | 4.6 | — | — | 8.0 | — | 7.4 | |
| Microalgae (Chlorophyceae) | Ankistrodesmus arcuatus | 4.6 | 8.0 | |||||
| Chlamydomonas reinhardtii | 7.7 (2) | |||||||
| Chlorella sp. | 5.8 (4) | 5.0 (2) | 7.8 (11) | 7.8 (4) | ||||
| Raphidocelis subcapitata | 6.1 (9) | 4.7 (6) | 7.0 (7) | 5.7 (2) | 7.8 (8) | 8.1 | 7.0 (2) | |
| Average (microalgae) | 6.0 | 4.8 | 7.0 | 5.7 | 7.9 | 7.9 | 7.0 | |
| Macrophytes | Ceratophyllum demersum | 7.2 | 7.7 | |||||
| Lemna aequinoctialis | 7.7 | 7.4 (3) | ||||||
| Lemna minor | 6.2 | 4.7 (3) | 7.1 | 6.1 (2) | 7.4 | 7.8 (2) | 7.2 | |
| Hydra | Hydra circumcincta | 7.1 | ||||||
| Hydra viridissima | 6.1 | 5.2 | 7.7 (2) | 7.3 (3) | ||||
| Insects | Chironomus tentans | 7.6 | ||||||
| Tanytarsus dissimilis | 6.1 | 7.3 | 7.5 | 6.6 | ||||
| Euglenid | Euglena gracilis | 7.7 | ||||||
| Annelid | Aeolosoma sp. | 5.4 | ||||||
| Bacteria | Erwinia sp. | 7.7 | ||||||
| Average (all) | 6.0 | 4.8 | 7.0 | 5.8 | 7.9 | 7.7 | 6.8 | |
| 95% confidence limit | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 | |
| Number of organisms (n) | 42 | 31 | 37 | 15 | 84 | 20 | 21 | |
| Taxa | Organism a | log KMBL | |||
|---|---|---|---|---|---|
| Ca2+ | Mg2+ | Na+ | H+ | ||
| Fish | Ctenopharyngodon idella | 2.8 | |||
| Mogurnda mogurnda | 5.8 | ||||
| Oncorhynchus mykiss | 3.5 (7) b | 3.0 | 2.4 | 5.8 (5) | |
| Oreochromis mossambicus | 3.5 | ||||
| Perca flavescens | 3.7 | ||||
| Pimephales promelas | 3.2 (4) | 5.6 (2) | |||
| Average (fish) | 3.5 | 2.9 | 2.4 | 5.7 | |
| Crustaceans (cladocerans) | Ceriodaphnia dubia | 3.2 (2) | 2.6 | 2.4 (2) | 5.6 |
| Ceriodaphnia pulchella | 2.4 | ||||
| Ceriodaphnia reticulata | 2.1 | ||||
| Daphnia galeata | 2.2 | ||||
| Daphnia longispina | 2.1 | ||||
| Daphnia magna | 3.2 (5) | 2.8 (6) | 2.2 (5) | 5.8 (3) | |
| Daphnia pulex | 3.5 (2) | 3.0 (2) | 2.1 | 5.7 | |
| Simocephalus expinosus | 2.0 | ||||
| Simocephalus vetulus | 2.4 | ||||
| Ten species of cladocerans c | 3.2 | 2.6 | |||
| Average (cladocerans) | 3.3 | 2.7 | 2.2 | 5.7 | |
| Crustaceans (amphipod) | Hyalella azteca | 3.4 (3) | 2.8 (2) | 5.8 | |
| Average (crustaceans) | 3.3 | 2.8 | 2.2 | 5.7 | |
| Molluscs (bivalves) | Corbicula fluminea | 3.5 | |||
| Velesunio angasi | 2.7 | ||||
| Average (molluscs) | 3.5 | 2.7 | — | — | |
| Micoalgae | Chlamydomonas reinhardtii | 3.7 | 2.8 | 6.2 | |
| Chlorella sp. | 5.8 (2) | ||||
| Raphidocelis subcapitata | 3.1 | 2.9 (2) | 2.2 | 5.7 (3) | |
| Average (microalgae) | 3.4 | 2.8 | 2.2 | 5.9 | |
| Macrophyte | Ceratophyllum demersum | 3.2 | 2.7 | ||
| Hydra | Hydra circumcincta | 3.7 | 2.8 | ||
| Average | 3.4 | 2.8 | 2.2 | 5.8 | |
| 95% confidence limit | 0.1 | 0.1 | 0.1 | 0.1 | |
| Number of organisms (n) | 14 | 12 | 11 | 10 | |
| Metal | % of Data within a Factor of Two of the 1:1 Agreement Line | % of Data within a Factor of Three of the 1:1 Agreement Line | Number of Water Quality Scenarios | Number of Organisms (Species) | Number of Studies |
|---|---|---|---|---|---|
| Zinc | 81 | 94 | 142 | 7 | 13 |
| Nickel | 83 | 97 | 256 | 10 | 14 |
| Cadmium | 88 | 98 | 83 | 6 | 7 |
| Cobalt | 75 | 92 | 12 | 9 | 4 |
| Copper | 79 | 91 | 383 | 12 | 15 |
| Uranium | 75 | 88 | 52 | 10 | 6 |
| Lead | 80 | 95 | 110 | 5 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown, P.L.; Markich, S.J. An Evaluation of Metal Binding Constants to Cell Surface Receptors in Freshwater Organisms, and Their Application in Biotic Ligand Models to Predict Metal Toxicity. Water 2024, 16, 2999. https://doi.org/10.3390/w16202999
Brown PL, Markich SJ. An Evaluation of Metal Binding Constants to Cell Surface Receptors in Freshwater Organisms, and Their Application in Biotic Ligand Models to Predict Metal Toxicity. Water. 2024; 16(20):2999. https://doi.org/10.3390/w16202999
Chicago/Turabian StyleBrown, Paul L., and Scott J. Markich. 2024. "An Evaluation of Metal Binding Constants to Cell Surface Receptors in Freshwater Organisms, and Their Application in Biotic Ligand Models to Predict Metal Toxicity" Water 16, no. 20: 2999. https://doi.org/10.3390/w16202999
APA StyleBrown, P. L., & Markich, S. J. (2024). An Evaluation of Metal Binding Constants to Cell Surface Receptors in Freshwater Organisms, and Their Application in Biotic Ligand Models to Predict Metal Toxicity. Water, 16(20), 2999. https://doi.org/10.3390/w16202999





