Adsorption Removal of Organophosphates from Water by Steel Slag: Modification, Performance, and Energy Site Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
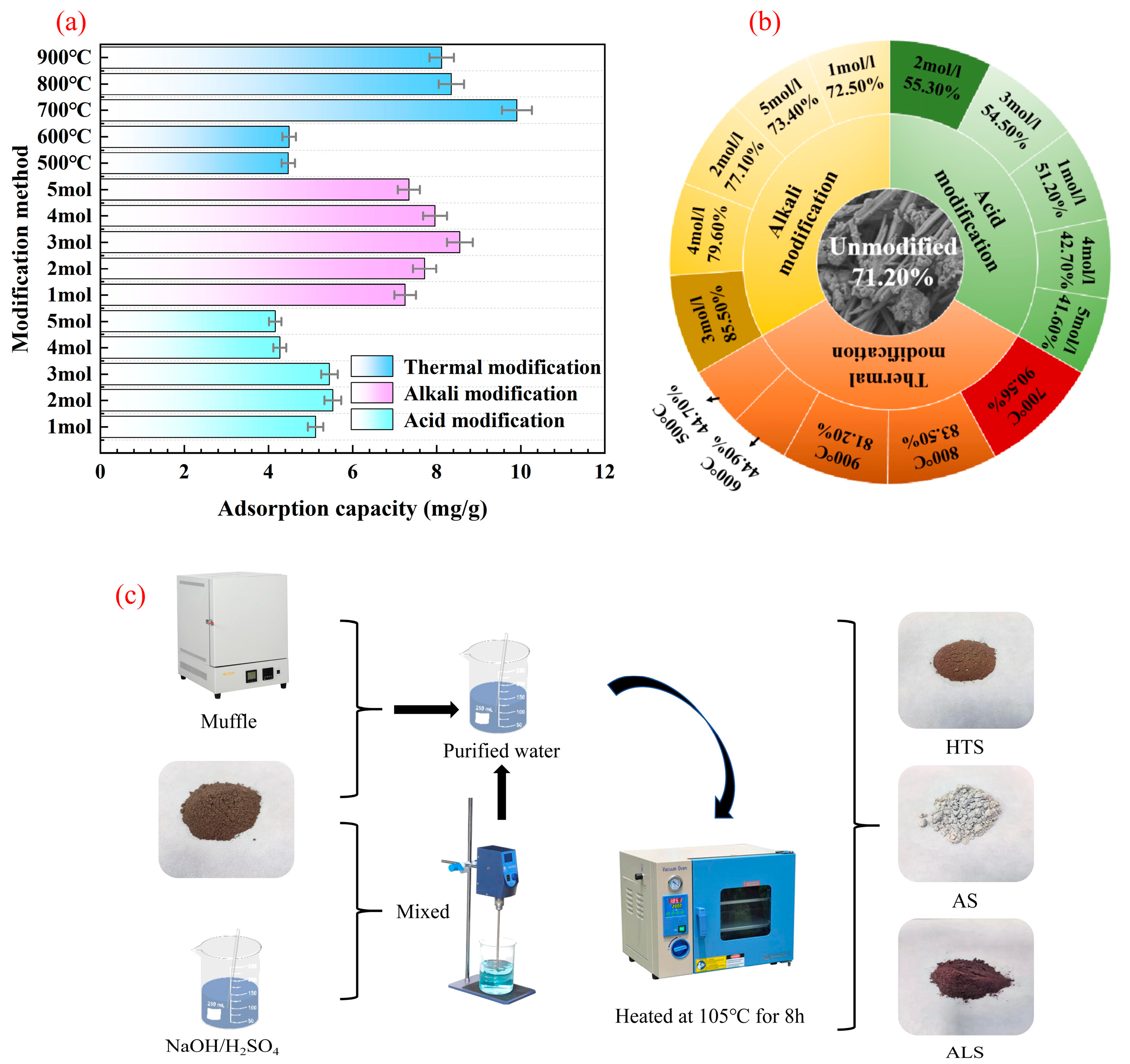
2.2. Modified Steel Slag Preparation
2.2.1. Acid-Modified Steel Slag
2.2.2. Alkali-Modified Steel Slag
2.2.3. Thermally Modified Steel Slag
2.3. Performance of Adsorbed HEDP
2.3.1. HEDP Adsorption Effect of Different Modified Steel Slags
2.3.2. Adsorption Kinetics Experiments
2.3.3. Adsorption Isotherms Experiments
2.3.4. Site Energy Distribution
2.3.5. Impact of pH
2.3.6. Regeneration Experiment
2.3.7. Analytical Methods
3. Results and Discussion
3.1. Selection of Modification Methods
3.2. SEM, BET, and XRD Analyses
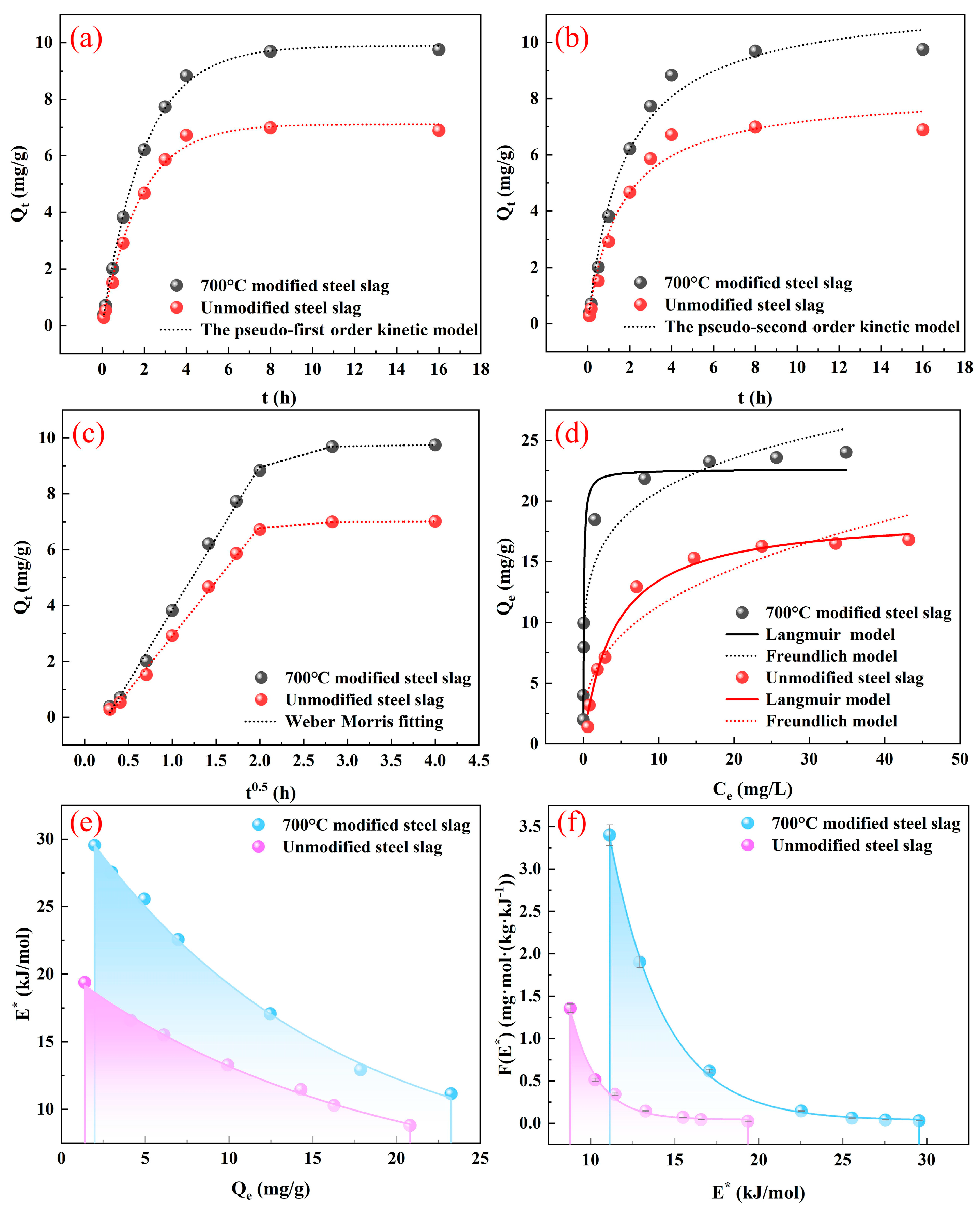
3.3. Adsorption Kinetics
3.4. Adsorption Isotherms
3.5. Adsorption Site Energy
3.6. Assessment of Practical Applications
3.6.1. Effect of pH
3.6.2. Effect of Co-Existing Ions
3.6.3. Adsorbent Regeneration
3.7. Adsorption Mechanism
- Hydrogen bonding interactions: the hydroxyl and phosphate groups in the HEDP molecule are able to form hydrogen bonds with the hydroxyl or hydrated metal ions on the surface of steel slag. Although these hydrogen bonds are not covalent and their strength is relatively low, they play a crucial role in the positioning and stabilization of the molecules in the early stage of adsorption, which helps to promote the initial attachment of molecules.
- Surface complexation: metal ions on the surface of steel slag, such as Fe and Ca, form stable complexes with the phosphate groups of HEDP through coordination. In this complexation process, the phosphate group acts as a ligand to provide lone-pair electrons to coordinate with the empty orbitals of the metal ions, thus building a strong chemical bond. This action significantly strengthens the adsorption strength, tunes the surface chemistry, and influences the subsequent adsorption behavior.
- Ligand exchange: during the adsorption process, HEDP molecules can effectively replace the original ligands on the steel slag surface, such as water molecules and carbonate ions. As the complexes formed by HEDP and metal ions are more stable, it can adjust the surface chemical environment and optimize the adsorption conditions, thus significantly enhancing the adsorption efficiency.
4. Conclusions
- Optimization of modification effect and conditions: High-temperature modification significantly enhanced the adsorption capacity of steel slag for HEDP, especially in the weak alkaline environment, and the removal efficiency of modified steel slag was up to more than 95%, which was significantly better than that of unmodified samples.
- Adsorption kinetic analysis: The adsorption kinetics followed a quasi-one-stage model, indicating that the adsorption rate was controlled by the surface-active sites, and the adsorption process was divided into two phases: fast adsorption and slow equilibrium.
- Adsorption mechanism and thermodynamics: The Langmuir model accurately described the adsorption behavior of modified steel slag on HEDP, and confirmed that the adsorption process was mainly completed by chemical precipitation and ligand exchange, which was a kind of chemical adsorption. The adsorption energy site analysis revealed the inhomogeneity of the energy distribution of the modified adsorption sites, which enhanced the affinity between the adsorbent and the adsorbate.
- Regeneration performance and cyclic stability: The regeneration experiments of the adsorbent showed that the modified steel slag maintained a high regeneration capacity despite the decrease in adsorption efficiency after several cycles, and its performance was significantly better than that of the unmodified sample, demonstrating good cyclic stability and regeneration potential.
- Microscopic characterization and elemental analysis: The SEM-EDS technique revealed significant changes in the surface morphology and elemental composition of the steel slag before and after adsorption, and the enrichment of phosphorus, carbon, oxygen, silicon, calcium, and other elements verified the validity of the adsorption process, and at the same time provided microscopic evidence of the adsorption mechanism.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rott, E.; Happel, O.; Armbruster, D.; Minke, R. Behavior of PBTC, HEDP, and Aminophosphonates in the Process of Wastewater Treatment. Water 2020, 12, 53. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, B.; Shan, C.; Yan, X.; Chen, H.; Pan, B. Occurrence and transformation of phosphonates in textile dyeing wastewater along full-scale combined treatment processes. Water Res. 2020, 184, 116173. [Google Scholar] [CrossRef] [PubMed]
- Rott, E.; Steinmetz, H.; Metzger, J.W. Organophosphonates: A review on environmental relevance, biodegradability and re-moval in wastewater treatment plants. Sci. Total Environ. 2018, 615, 1176–1191. [Google Scholar] [CrossRef]
- Lin, H.; Wang, Y.; Dong, Y. A review of methods, influencing factors and mechanisms for phosphorus recovery from sewage and sludge from municipal wastewater treatment plants. J. Environ. Chem. Eng. 2024, 12, 111657. [Google Scholar] [CrossRef]
- Melia, P.M.; Cundy, A.B.; Sohi, S.P.; Hooda, P.S.; Busquets, R. Trends in the recovery of phosphorus in bioavailable forms from wastewater. Chemosphere 2017, 186, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Hu, H.; Wang, C.; Shi, Q.; Zhang, Q.; Chen, M.; Wang, Q.; Zhang, T. New insight into the changes in met-al-phosphonate complexes from the addition of CaCO3 to enhance ferric flocculation for efficient phosphonate removal. Chemosphere 2023, 311, 137078. [Google Scholar] [CrossRef]
- Wu, B.; Wan, J.; Zhang, Y.; Pan, B.; Lo, I.M.C. Selective Phosphate Removal from Water and Wastewater using Sorption: Process Fundamentals and Removal Mechanisms. Environ. Sci. Technol. 2020, 54, 50–66. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, H.; Lu, J.; Li, Y.; Bao, M. Enhanced photocatalytic activity of glyphosate over a combination strategy of GQDs/TNAs heterojunction composites. J. Colloid Interface Sci. 2022, 607, 607–620. [Google Scholar] [CrossRef]
- Sahu, J.N.; Kapelyushin, Y.; Mishra, D.P.; Ghosh, P.; Sahoo, B.K.; Trofimov, E.; Meikap, B.C. Utilization of ferrous slags as coagulants, filters, adsorbents, neutralizers/stabilizers, catalysts, additives, and bed materials for water and wastewater treatment: A review. Chemosphere 2023, 325, 138201. [Google Scholar] [CrossRef]
- Claveau-Mallet, D.; Boutet, E.; Comeau, Y. Steel slag filter design criteria for phosphorus removal from wastewater in de-centralized applications. Water Res. 2018, 143, 28–37. [Google Scholar] [CrossRef]
- Cha, W.; Kim, J.; Choi, H. Evaluation of steel slag for organic and inorganic removals in soil aquifer treatment. Water Res. 2006, 40, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Qian, C.; Wang, R. The properties and mechanism of microbial mineralized steel slag bricks. Constr. Build. Mater. 2016, 113, 815–823. [Google Scholar] [CrossRef]
- Shi, C.; Wang, X.; Zhou, S.; Zuo, X.; Wang, C. Mechanism, application, influencing factors and environmental benefit as-sessment of steel slag in removing pollutants from water: A review. J. Water Process Eng. 2022, 47, 102666. [Google Scholar] [CrossRef]
- Lu, H.; Xiao, L.; Wang, T.; Lu, S.; Wang, H.; Guo, X.; Li, J. The application of steel slag in a multistage pond constructed wetland to purify low-phosphorus polluted river water. J. Environ. Manag. 2021, 292, 112578. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wen, T.; Wang, L.; Miki, T.; Bai, H.; Lu, X.; Yu, H.; Nagasaka, T. The stability of the compounds formed in the process of removal Pb(II), Cu (II) and Cd(II) by steelmaking slag in an acidic aqueous solution. J. Environ. Manag. 2019, 231, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Feng, Y.; Liu, N.; Zhao, Y.; Wang, X.; Yang, S.; Long, Y.; Qiu, L. Preparation of Sn/Mn loaded steel slag zeolite particle electrode and its removal effect on rhodamine B(RhB). J. Water Process. Eng. 2020, 37, 101417. [Google Scholar] [CrossRef]
- Shao, Q.; Yi, Y.; Xie, Y.; Yang, H.; Guo, J.; Liu, Z.; Chen, Y.; Wan, J. Selective sorption of organic phosphonate HEDP by steel slag: Efficiency and mechanism. Process. Saf. Environ. Prot. 2024, 186, 645–655. [Google Scholar] [CrossRef]
- GB 5085.6-2007; Identification Standards for Hazardous Wastes Identification for Toxic Substance Content. China Environmental Science Press: Beijing, China, 2017.
- Wang, S.; Yao, S.; Du, K.; Yuan, R.; Chen, H.; Wang, F.; Zhou, B. The mechanisms of conventional pollutants adsorption by modified granular steel slag. Environ. Eng. Res. 2021, 26, 190352. [Google Scholar] [CrossRef]
- Okoye, P.U.; Abdullah, A.Z.; Hameed, B.H. Stabilized ladle furnace steel slag for glycerol carbonate synthesis via glycerol transesterification reaction with dimethyl carbonate. Energy Conv. Manag. 2017, 133, 477–485. [Google Scholar] [CrossRef]
- Gagliano, E.; Falciglia, P.P.; Zaker, Y.; Karanfil, T.; Roccaro, P. Microwave regeneration of granular activated carbon saturated with PFAS. Water Res. 2021, 198, 117121. [Google Scholar] [CrossRef]
- Nahm, S.W.; Shim, W.G.; Park, Y.-K.; Kim, S.C. Thermal and chemical regeneration of spent activated carbon and its adsorption property for toluene. Chem. Eng. J. 2012, 210, 500–509. [Google Scholar] [CrossRef]
- Vanvliet, B.; Venter, L. Infrared Thermal Regeneration of Spent Activated Carbon from Water Reclamation. Water Sci. Technol. 1985, 17, 1029–1042. [Google Scholar] [CrossRef]
- Sun, S.; Shan, C.; Yang, Z.; Wang, S.; Pan, B. Self-Enhanced Selective Oxidation of Phosphonate into Phosphate by Cu(II)/H2O2: Performance, Mechanism, and Validation. Environ. Sci. Technol. 2022, 56, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, S.; Shan, C.; Pan, B. Analysis of trace phosphonates in authentic water samples by pre-methylation and LC-Orbitrap MS/MS. Water Res. 2019, 161, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Li, Y.; Xu, X.; Shang, Y.; Gao, B.; Yue, Q. Selective removal of phosphate by dual Zr and La hydroxide/cellulose-based bio-composites. J. Colloid Interface Sci. 2019, 533, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Shen, W.; Huang, J.; Yang, Y.; Zhang, D.; Huang, X.; Lv, Z.; Ji, X. Process to utilize crushed steel slag in cement industry directly: Multi-phased clinker sintering technology. J. Clean Prod. 2019, 217, 520–529. [Google Scholar] [CrossRef]
- Gonzalez, P.L.L.; Novais, R.M.; Labrincha, J.A.; Blanpain, B.; Pontikes, Y. Modifications of basic-oxygen-furnace slag micro-structure and their effect on the rheology and the strength of alkali-activated binders. Cem. Concr. Compos. 2019, 97, 143–153. [Google Scholar] [CrossRef]
- Yu, J.; Liang, W.; Wang, L.; Li, F.; Zou, Y.; Wang, H. Phosphate removal from domestic wastewater using thermally modified steel slag. J. Environ. Sci. 2015, 31, 81–88. [Google Scholar] [CrossRef]
- Rasee, A.I.; Awual, E.; Rehan, A.I.; Hossain, M.S.; Waliullah, R.M.; Kubra, K.T.; Sheikh, M.C.; Salman, M.S.; Hasan, M.N.; Hasan, M.M.; et al. Efficient separation, adsorption, and recovery of Samarium(III) ions using novel ligand-based composite adsorbent. Surf. Interfaces 2023, 41, 103276. [Google Scholar] [CrossRef]
- Bulyarskii, S.V.; Basaev, A.S. Thermodynamics and kinetics of adsorption of atoms and molecules by carbon nanotubes. J. Exp. Theor. Phys. 2009, 108, 688–698. [Google Scholar] [CrossRef]
- Zhou, Z.; Xu, Q.; Wu, Z.; Fang, X.; Zhong, Q.; Yang, J.; Yan, J.; Li, Q. Preparation and characterization of clay-oyster shell composite adsorption material and its application in phosphorus removal from wastewater. Sustain. Chem. Pharm. 2023, 32, 101023. [Google Scholar] [CrossRef]
- Li, X.; Wen, B.; Li, Y. Adsorption of the Malachite Green by Magnetic Clam Shell Powder. Pol. J. Environ. Stud. 2021, 30, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yang, Q.; Liu, D.; Nie, H.; Liu, Y. Removal of organic phosphonate HEDP by Eu-MOF/GO composite membrane. J. Environ. Chem. Eng. 2021, 9, 106895. [Google Scholar] [CrossRef]
- Li, C.; Yang, Q.; Nie, H.; Liu, D.; Liu, Y. Adsorption removal of organic phosphonate HEDP by magnetic composite doped with different rare earth elements. Chem. Eng. J. Adv. 2022, 9, 100221. [Google Scholar] [CrossRef]
- Jiang, H.; Li, Q.; Sun, J.; Huang, Y.; Zhang, P.; Mao, Y.; Qu, Y.; Liu, X. Studies on competitive adsorption characteristics of bisphenol A and 17α-ethinylestradiol on thermoplastic polyurethane by site energy distribution theory. Environ. Geochem. Health 2023, 45, 5181–5194. [Google Scholar] [CrossRef] [PubMed]
- Alhujaily, A.; Mao, Y.; Zhang, J.; Ifthikar, J.; Zhang, X.; Ma, F. Facile fabrication of Mg-Fe-biochar adsorbent derived from spent mushroom waste for phosphate removal. J. Taiwan Inst. Chem. Eng. 2020, 117, 75–85. [Google Scholar] [CrossRef]
- Li, C.; Yang, Q.; Lu, S.; Liu, Y. Adsorption and mechanism study for phosphonate antiscalant HEDP removal from reverse osmosis concentrates by magnetic La/Zn/Fe3O4@PAC composite. Colloids Surf. A Physicochem. Eng. Asp. 2021, 613, 126056. [Google Scholar] [CrossRef]
- Liu, X.; Tian, J.; Li, Y.; Sun, N.; Mi, S.; Xie, Y.; Chen, Z. Enhanced dyes adsorption from wastewater via Fe3O4 nanoparticles functionalized activated carbon. J. Hazard. Mater. 2019, 373, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Azeem, M.; Li, R.; Xing, L.; Li, Y.; Zhang, Y.; Guo, Z.; Wang, Q.; Ngo, H.H.; Qu, G.; et al. Zirconium hydroxide nanoparticle encapsulated magnetic biochar composite derived from rice residue: Application for As(III) and As(V) polluted water purification. J. Hazard. Mater. 2022, 423, 127081. [Google Scholar] [CrossRef]
- Wu, B.; Fang, L.; Fortner, J.D.; Guan, X.; Lo, I.M.C. Highly efficient and selective phosphate removal from wastewater by magnetically recoverable La(OH)3/Fe3O4 nanocomposites. Water Res. 2017, 126, 179–188. [Google Scholar] [CrossRef]





| Adsorbent | Specific Surface/(m2·g−1) | Pore Volume/(cm3·g−1) | Pore Size/(nm) |
|---|---|---|---|
| Unmodified steel slag | 2.048 | 0.008994 | 19.6616 |
| 700 °C modified steel slag | 2.3379 | 0.108621 | 15.5464 |
| Kinetic Model | Adsorbent | Parameter | Result |
|---|---|---|---|
| The pseudo-first order | Unmodified steel slag | Qe/(mg·g−1) | 7.1091 |
| k1/min−1 | 0.5547 | ||
| R2 | 0.9949 | ||
| 700 °C modified steel slag | Qe/(mg·g−1) | 9.8893 | |
| k1/min−1 | 0.5012 | ||
| R2 | 0.9986 | ||
| The pseudo-second order | Unmodified steel slag | Qe/(mg·g−1) | 8.2616 |
| k2/(g·mg−1·min−1) | 0.07841 | ||
| R2 | 0.9701 | ||
| 700 °C modified steel slag | Qe/(mg·g−1) | 12.5881 | |
| k2/(g·mg−1·min−1) | 0.0495 | ||
| R2 | 0.9917 | ||
| Weber–Morris fitting | Unmodified steel slag | C1 | 3.9205 |
| R2 | 0.9963 | ||
| C2 | 0.01701 | ||
| R2 | / | ||
| 700 °C modified steel slag | C1 | 5.1569 | |
| R2 | 0.9957 | ||
| C2 | 0.0512 | ||
| R2 | / |
| Adsorbent | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| Qm/(mg·g−1) | KL/(L·mg−1) | R2 | KF/(mg·g−1) | 1/n | R2 | |
| Unmodified steel slag | 22.085 | 13.639 | 0.990 | 12.389 | 0.241 | 0.901 |
| 700 °C modified steel slag | 34.965 | 0.083 | 0.973 | 3.311 | 0.510 | 0.929 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, W.; Nie, Y.; Wang, Z.; Huang, T.; Xu, X.; Liu, H.; Li, P.; Wu, B. Adsorption Removal of Organophosphates from Water by Steel Slag: Modification, Performance, and Energy Site Analysis. Water 2024, 16, 3145. https://doi.org/10.3390/w16213145
Wu W, Nie Y, Wang Z, Huang T, Xu X, Liu H, Li P, Wu B. Adsorption Removal of Organophosphates from Water by Steel Slag: Modification, Performance, and Energy Site Analysis. Water. 2024; 16(21):3145. https://doi.org/10.3390/w16213145
Chicago/Turabian StyleWu, Wei, Yiming Nie, Zhixin Wang, Tianyin Huang, Xiaoyi Xu, Hanhan Liu, Peirong Li, and Bingdang Wu. 2024. "Adsorption Removal of Organophosphates from Water by Steel Slag: Modification, Performance, and Energy Site Analysis" Water 16, no. 21: 3145. https://doi.org/10.3390/w16213145
APA StyleWu, W., Nie, Y., Wang, Z., Huang, T., Xu, X., Liu, H., Li, P., & Wu, B. (2024). Adsorption Removal of Organophosphates from Water by Steel Slag: Modification, Performance, and Energy Site Analysis. Water, 16(21), 3145. https://doi.org/10.3390/w16213145









