Overlooked Risk of Microplastic from Kitchen Waste Short Stacking Phase
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Material
2.2. Experimental Design
2.3. Analytical Testing
2.4. Quality Assurance and Quality Control
2.5. Statistical Analysis
3. Results and Discussions
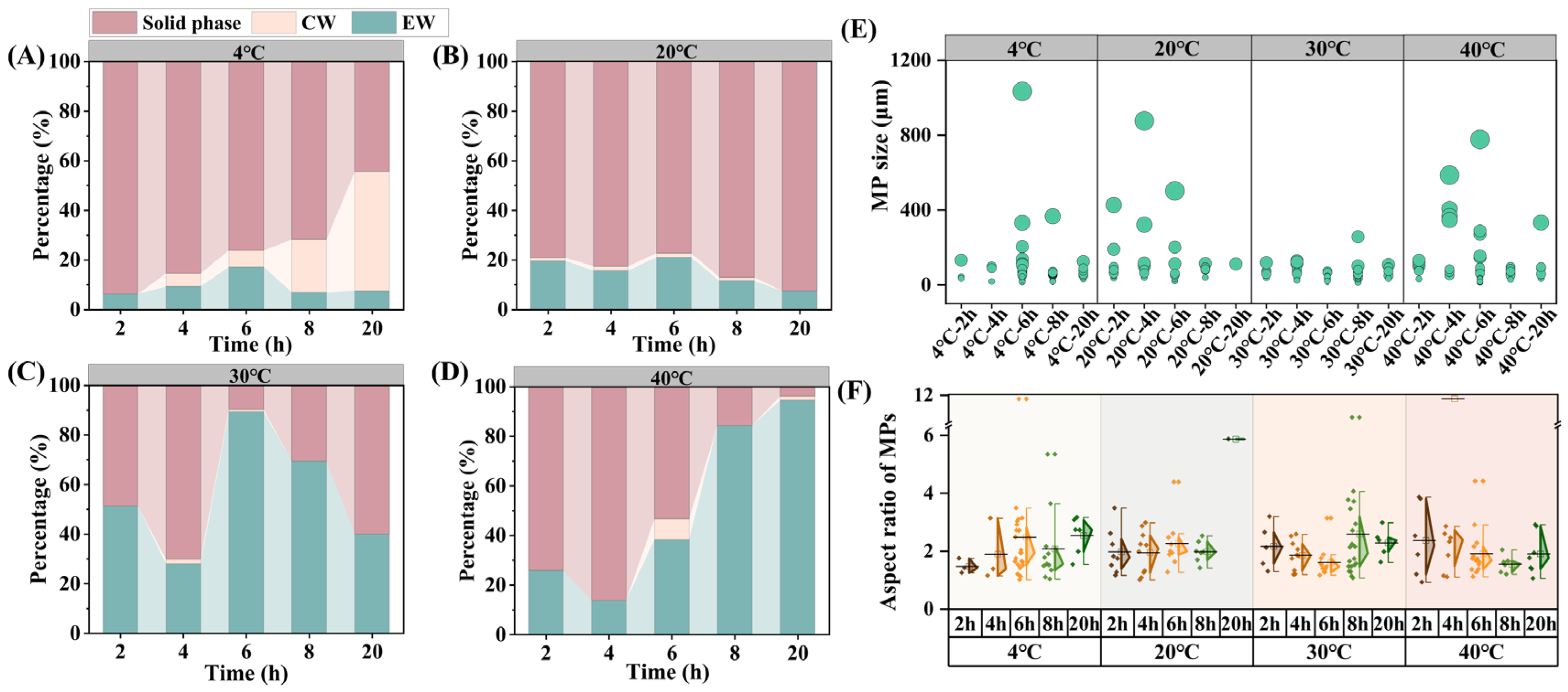
3.1. Release Characteristics of Microplastics During Kitchen Waste Stacking
3.2. Physical and Chemical Properties of Entrapped Water During Kitchen Waste Stacking
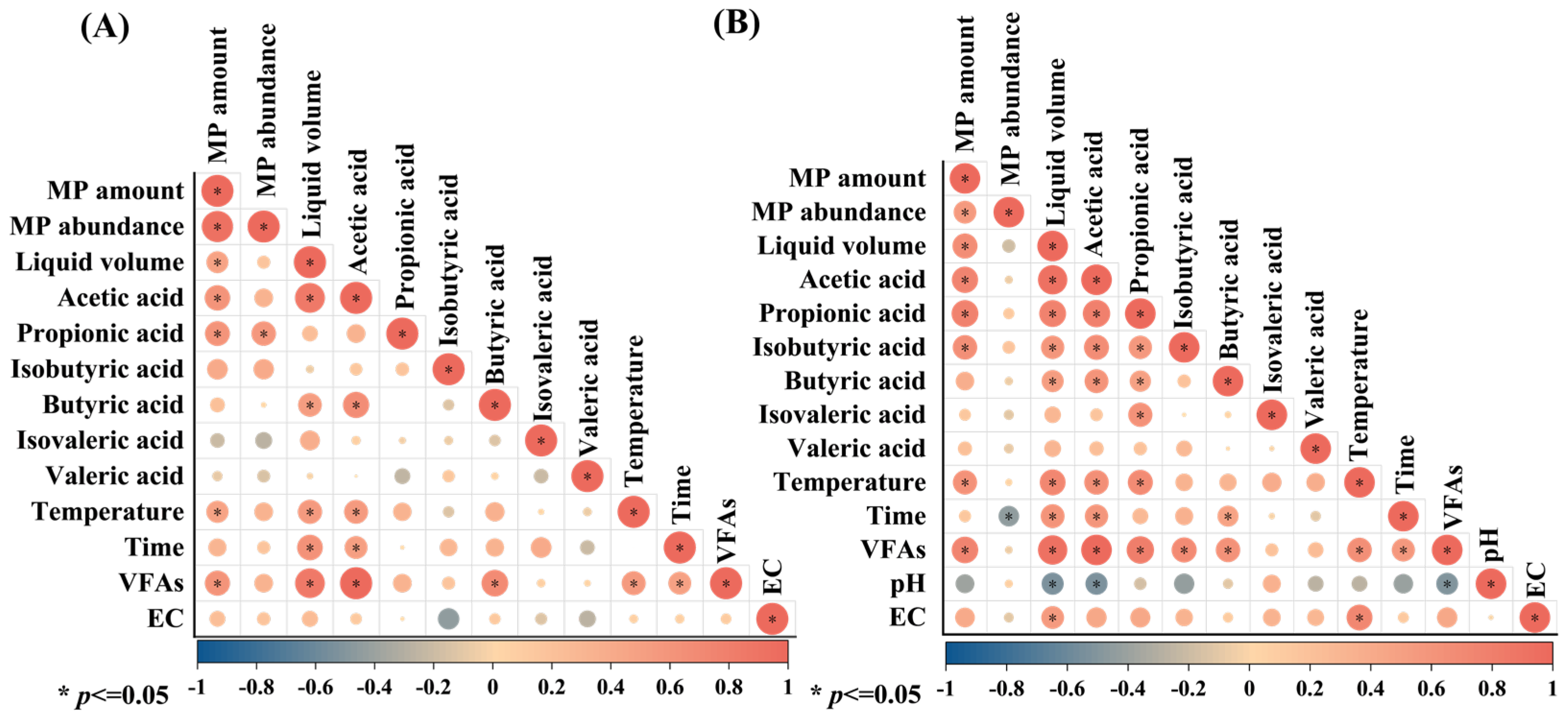
3.3. Relationship Between Microplastics and Liquid Phase Properties in Kitchen Waste Stacking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Revell, L.E.; Kuma, P.; Le Ru, E.C.; Somerville, W.R.C.; Gaw, S. Direct radiative effects of airborne microplastics. Nature 2021, 598, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Pan, J.; Xiao, S.; Wang, J.; Gong, X.; Yin, G.; Hou, L.; Liu, M.; Zheng, Y. Microplastics alter nitrous oxide production and pathways through affecting microbiome in estuarine sediments. Water Res. 2022, 221, 118733–118743. [Google Scholar] [CrossRef] [PubMed]
- Sajjad, M.; Huang, Q.; Khan, S.; Khan, M.A.; Liu, Y.; Wang, J.; Lian, F.; Wang, Q.; Guo, G. Microplastics in the soil environment: A critical review. Environ. Technol. Innov. 2022, 27, 102408–102432. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, Z.; Wang, T.; Hu, H.; Qin, G.; Lu, T.; Hong, W.; Hu, J.; Penuelas, J.; Qian, H. Global distribution of marine microplastics and potential for biodegradation. J. Hazard. Mater. 2023, 451, 131198–131206. [Google Scholar] [CrossRef]
- Wang, Y.; Okochi, H.; Tani, Y.; Hayami, H.; Minami, Y.; Katsumi, N.; Takeuchi, M.; Sorimachi, A.; Fujii, Y.; Kajino, M.; et al. Airborne hydrophilic microplastics in cloud water at high altitudes and their role in cloud formation. Environ. Chem. Lett. 2023, 21, 3055–3062. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, T.; Kang, S.; Allen, S.; Luo, X.; Allen, D. Microplastics in glaciers of the Tibetan Plateau: Evidence for the long-range transport of microplastics. Sci. Total Environ. 2021, 758, 143634–143638. [Google Scholar] [CrossRef]
- Tan, M.Y.; Sun, Y.; Gui, J.X.; Wang, J.L.; Chen, X.; Song, W.; Wu, D.L. Distribution characteristics of microplastics in typical organic solid wastes and their biologically treated products. Sci. Total Environ. 2022, 852, 158440–158449. [Google Scholar] [CrossRef]
- Zafiu, C.; Binner, E.; Beigl, P.; Vay, B.; Ebmer, J.; Huber-Humer, M. The dynamics of macro- and microplastic quantity and size changes during the composting process. Waste Manag. 2023, 162, 18–26. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, N.; Ding, W.; Han, B.; Zhao, M.; Wang, X.; Wang, J.; Cao, B.; Zou, G.; Chen, Y. Microplastic pollution and the related ecological risks of organic composts from different raw materials. J. Hazard. Mater. 2023, 458, 131911–131922. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Y.; Luo, D.; Chong, Z.; Li, E.; Kong, X. A review of municipal solid waste in China: Characteristics, compositions, influential factors and treatment technologies. Environ. Dev. Sustain. 2021, 23, 6603–6622. [Google Scholar] [CrossRef]
- Sholokhova, A.; Ceponkus, J.; Sablinskas, V.; Denafas, G. Abundance and characteristics of microplastics in treated organic wastes of Kaunas and Alytus regional waste management centres, Lithuania. Environ. Sci. Pollut. Res. 2021, 29, 20665–20674. [Google Scholar] [CrossRef] [PubMed]
- Danopoulos, E.; Jenner, L.C.; Twiddy, M.; Rotchell, J.M. Microplastic Contamination of Seafood Intended for Human Consumption: A Systematic Review and Meta-Analysis. Environ. Health Perspect. 2020, 128, 126002–126033. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Luo, Y.; Li, R.; Zhou, Q.; Peijnenburg, W.J.G.M.; Yin, N.; Yang, J.; Tu, C.; Zhang, Y. Effective uptake of submicrometre plastics by crop plants via a crack-entry mode. Nat. Sustain. 2020, 3, 929–937. [Google Scholar] [CrossRef]
- Makhdoumi, P.; Pirsaheb, M.; Amin, A.A.; Kianpour, S.; Hossini, H. Microplastic pollution in table salt and sugar: Occurrence, qualification and quantification and risk assessment. J. Food Compos. Anal. 2023, 119, 105261–105271. [Google Scholar] [CrossRef]
- Luo, Y.; Gibson, C.T.; Chuah, C.; Tang, Y.; Naidu, R.; Fang, C. Raman imaging for the identification of Teflon microplastics and nanoplastics released from non-stick cookware. Sci. Total Environ. 2022, 851, 158293–158302. [Google Scholar] [CrossRef]
- Luo, Y.; Awoyemi, O.S.; Naidu, R.; Fang, C. Detection of microplastics and nanoplastics released from a kitchen blender using Raman imaging. J. Hazard. Mater. 2023, 453, 131403–131411. [Google Scholar] [CrossRef]
- Yadav, H.; Khan, M.R.H.; Quadir, M.; Rusch, K.a.A.; Mondal, P.P.; Orr, M.; Xu, E.G.; Iskander, S.M. Cutting Boards: An Overlooked Source of Microplastics in Human Food? Environ. Sci. Technol. 2023, 57, 8225–8235. [Google Scholar] [CrossRef]
- Kadac-Czapska, K.; Knez, E.; Gierszewska, M.; Olewnik-Kruszkowska, E.; Grembecka, M. Microplastics Derived from Food Packaging Waste—Their Origin and Health Risks. Materials 2023, 16, 674. [Google Scholar] [CrossRef]
- Ali, S.S.; Elsamahy, T.; Koutra, E.; Kornaros, M.; El-Sheekh, M.; Abdelkarim, E.A.; Zhu, D.; Sun, J. Degradation of conventional plastic wastes in the environment: A review on current status of knowledge and future perspectives of disposal. Sci. Total Environ. 2021, 771, 144719–144740. [Google Scholar] [CrossRef]
- Yang, Z.; Lü, F.; Hu, T.; Xu, X.; Zhang, H.; Shao, L.; Ye, J.; He, P. Occurrence of macroplastics and microplastics in biogenic waste digestate: Effects of depackaging at source and dewatering process. Waste Manag. 2022, 154, 252–259. [Google Scholar] [CrossRef]
- Wohlleben, W.; Rückel, M.; Meyer, L.; Pfohl, P.; Battagliarin, G.; Hüffer, T.; Zumstein, M.; Hofmann, T. Fragmentation and Mineralization of a Compostable Aromatic—Aliphatic Polyester during Industrial Composting. Environ. Sci. Technol. Lett. 2023, 10, 698–704. [Google Scholar] [CrossRef]
- Hussain, K.A.; Romanova, S.; Okur, I.; Zhang, D.; Kuebler, J.; Huang, X.; Wang, B.; Fernandez-Ballester, L.; Lu, Y.; Schubert, M.; et al. Assessing the Release of Microplastics and Nanoplastics from Plastic Containers and Reusable Food Pouches: Implications for Human Health. Environ. Sci. Technol. 2023, 57, 9782–9792. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Jin, T.; Zou, T.; Xu, L.; Xi, B.; Xu, D.; He, J.; Xiong, L.; Tang, C.; Peng, J.; et al. Current progress on plastic/microplastic degradation: Fact influences and mechanism. Environ. Pollut. 2022, 304, 119159–119169. [Google Scholar] [CrossRef] [PubMed]
- Lü, F.; Xu, X.; Shao, L.; He, P. Importance of storage time in mesophilic anaerobic digestion of food waste. J. Environ. Sci. 2016, 45, 76–83. [Google Scholar] [CrossRef]
- Nilsson Påledal, S.; Hellman, E.; Moestedt, J. The effect of temperature, storage time and collection method on biomethane potential of source separated household food waste. Waste Manag. 2018, 71, 636–643. [Google Scholar] [CrossRef]
- Nie, E.; He, P.; Zou, J.; Zhang, H.; Lü, F. Neglected effect of transportation on the property of municipal biowaste and the subsequent biomethane potential. J. Clean. Prod. 2022, 352, 131603–131611. [Google Scholar] [CrossRef]
- Khan, M.I.H.; Wellard, R.M.; Nagy, S.A.; Joardder, M.U.H.; Karim, M.A. Investigation of bound and free water in plant-based food material using NMR T 2 relaxometry. Innov. Food Sci. Emerg. Technol. 2016, 38, 252–261. [Google Scholar] [CrossRef]
- Kocbek, E.; Garcia, H.A.; Hooijmans, C.M.; Mijatović, I.; Kržišnik, D.; Humar, M.; Brdjanovic, D. Effects of the sludge physical-chemical properties on its microwave drying performance. Sci. Total Environ. 2022, 828, 154142. [Google Scholar] [CrossRef]
- Shen, D.-S.; Yang, Y.-Q.; Huang, H.-L.; Hu, L.-F.; Long, Y.-Y. Water state changes during the composting of kitchen waste. Waste Manag. 2015, 38, 381–387. [Google Scholar] [CrossRef]
- Sikorski, Z.E. (Ed.) Chemical and Functional Properties of Food Components, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2006; pp. 44–45. [Google Scholar]
- Li, Y.; Li, X.A.; Wang, P.L.; Su, Y.L.; Xie, B. Size-dependent effects of polystyrene microplastics on anaerobic digestion performance of food waste: Focusing on oxidative stress, microbial community, key metabolic functions. J. Hazard. Mater. 2022, 438, 129493–129503. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Zhao, Y.; Zhang, L.; Zhang, X. Inhibition of aged microplastics and leachates on methane production from anaerobic digestion of sludge and identification of key components. J. Hazard. Mater. 2023, 446, 130717–130728. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Han, Z.; Guo, N.; Zhou, Z.; Liu, Y.; Tang, Q. Microplastics spatiotemporal distribution and plastic-degrading bacteria identification in the sanitary and non-sanitary municipal solid waste landfills. J. Hazard. Mater. 2022, 438, 129452–129462. [Google Scholar] [CrossRef]
- Claessens, M.; Van Cauwenberghe, L.; Vandegehuchte, M.B.; Janssen, C.R. New techniques for the detection of microplastics in sediments and field collected organisms. Mar. Pollut. Bull. 2013, 70, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Erni-Cassola, G.; Gibson, M.I.; Thompson, R.C.; Christie-Oleza, J.A. Lost, but Found with Nile Red: A Novel Method for Detecting and Quantifying Small Microplastics (1 mm to 20 μm) in Environmental Samples. Environ. Sci. Technol 2017, 51, 13641–13648. [Google Scholar] [CrossRef]
- Guo, S.; Wu, Z.; Li, X.; Shen, D.; Shentu, J.; Lu, L.; Qi, S.; Zhu, M.; Long, Y. Microplastic, a possible trigger of landfill sulfate reduction process. Sci. Total Environ. 2024, 906, 167662–167669. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, S.; Shen, D.; Shentu, J.; Lv, L.; Qi, S.; Zhu, M.; Long, Y. Microplastic release and sulfate reduction response in the early stage of a simulated landfill. Waste Manag. 2024, 175, 22–29. [Google Scholar] [CrossRef]
- Öling-Wärnå, V.; Åkerback, N.; Engblom, S. Digestate from Biowaste and Sewage Sludge as Carriers of Microplastic into the Environment: Case Study of a Thermophilic Biogas Plant in Ostrobothnia, Finland. Water Air Soil Pollut. 2023, 234, 432–443. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Zhang, J.; Chen, F.; Khalid, M.; Ye, J.; Romantschuk, M.; Hui, N. Hydrolase and plastic-degrading microbiota explain degradation of polyethylene terephthalate microplastics during high-temperature composting. Bioresour. Technol. 2024, 393, 130108–130118. [Google Scholar] [CrossRef]
- Tan, H.W.; Yue, T.T.; Xu, Y.; Zhao, J.; Xing, B.S. Microplastics Reduce Lipid Digestion in Simulated Human Gastrointestinal System. Environ. Sci. Technol. 2020, 54, 12285–12294. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Q.; Maa, J.P.Y.; Shen, X.; Liang, J.; Yu, L.; Ge, L.; Wang, G. Effects of organic matter on interaction forces between polystyrene microplastics: An experimental study. Sci. Total Environ. 2022, 844, 157186–157198. [Google Scholar] [CrossRef]
- Kaseke, T.; Lujic, T.; Velickovic, T.C. Nano- and Microplastics Migration from Plastic Food Packaging into Dairy Products: Impact on Nutrient Digestion, Absorption, and Metabolism. Foods 2023, 12, 3043. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhao, Z.-Y.; Xiong, X.-B.; Wang, N.; Zhou, R.; Zhang, Z.-M.; Ding, F.; Hao, M.; Wang, S.; Ma, Y.; et al. Microplastics affect soil bacterial community assembly more by their shapes rather than the concentrations. Water Res. 2023, 245, 120581–120593. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Feng, Q.; Chen, J.; Yan, J.; Li, X.; Guo, L. Quantification analysis of microplastics released from disposable polystyrene tableware with fluorescent polymer staining. Sci. Total Environ. 2023, 864, 161155–161163. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, J.; Zhou, F.; Shen, D.; Shentu, J.; Lu, L.; Qi, S.; Zhu, M.; Long, Y. Overlooked Risk of Microplastic from Kitchen Waste Short Stacking Phase. Water 2024, 16, 3190. https://doi.org/10.3390/w16223190
Qian J, Zhou F, Shen D, Shentu J, Lu L, Qi S, Zhu M, Long Y. Overlooked Risk of Microplastic from Kitchen Waste Short Stacking Phase. Water. 2024; 16(22):3190. https://doi.org/10.3390/w16223190
Chicago/Turabian StyleQian, Jialu, Fanping Zhou, Dongsheng Shen, Jiali Shentu, Li Lu, Shengqi Qi, Min Zhu, and Yuyang Long. 2024. "Overlooked Risk of Microplastic from Kitchen Waste Short Stacking Phase" Water 16, no. 22: 3190. https://doi.org/10.3390/w16223190
APA StyleQian, J., Zhou, F., Shen, D., Shentu, J., Lu, L., Qi, S., Zhu, M., & Long, Y. (2024). Overlooked Risk of Microplastic from Kitchen Waste Short Stacking Phase. Water, 16(22), 3190. https://doi.org/10.3390/w16223190





