Microbial Community Dynamics in Groundwater of a Petrochemical Refinery: Influence of BTEX and Dichloroethane Contamination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Description and Sampling Procedure
2.2. Analysis of the Groundwater Physiochemical Parameters
2.3. Analysis of Microbial Communities
2.4. Statistical Analysis
3. Results
3.1. Groundwater Physicochemical Parameters and Main Contaminants in Study’s Wells
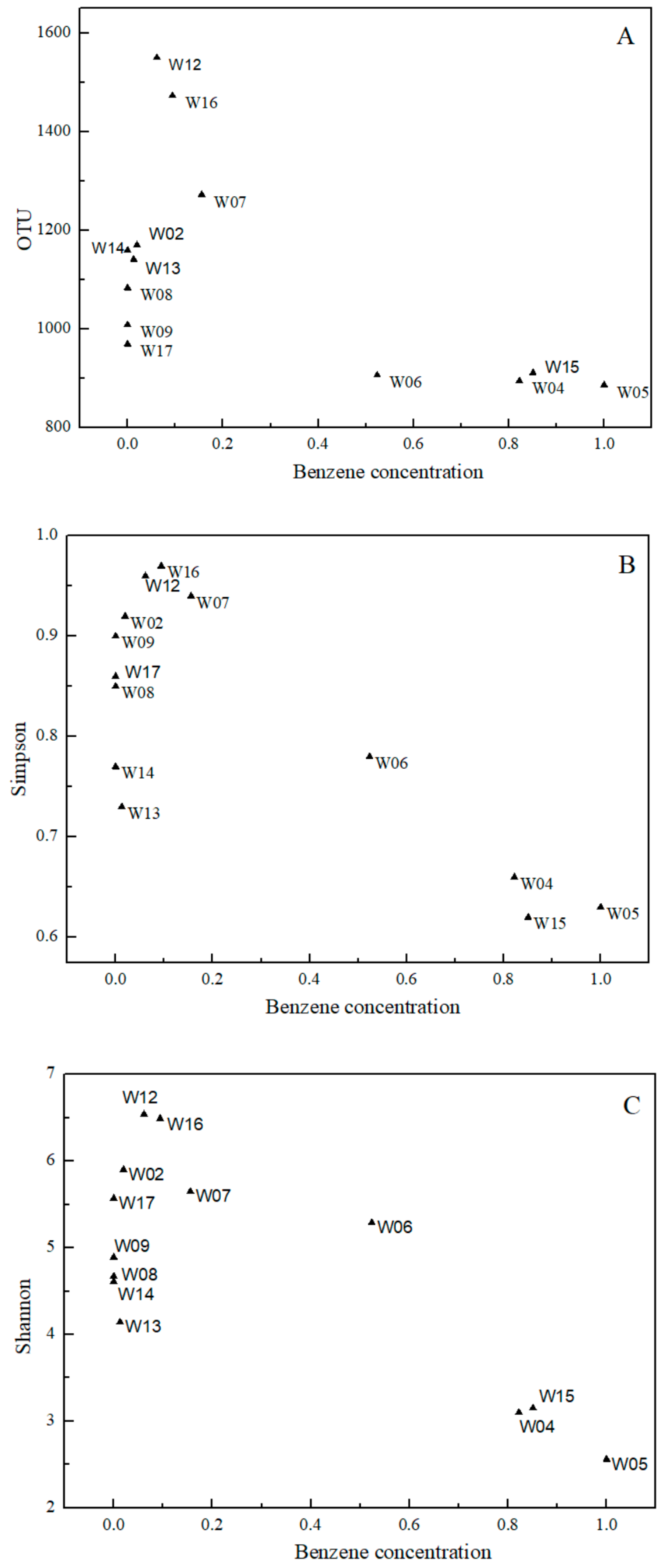
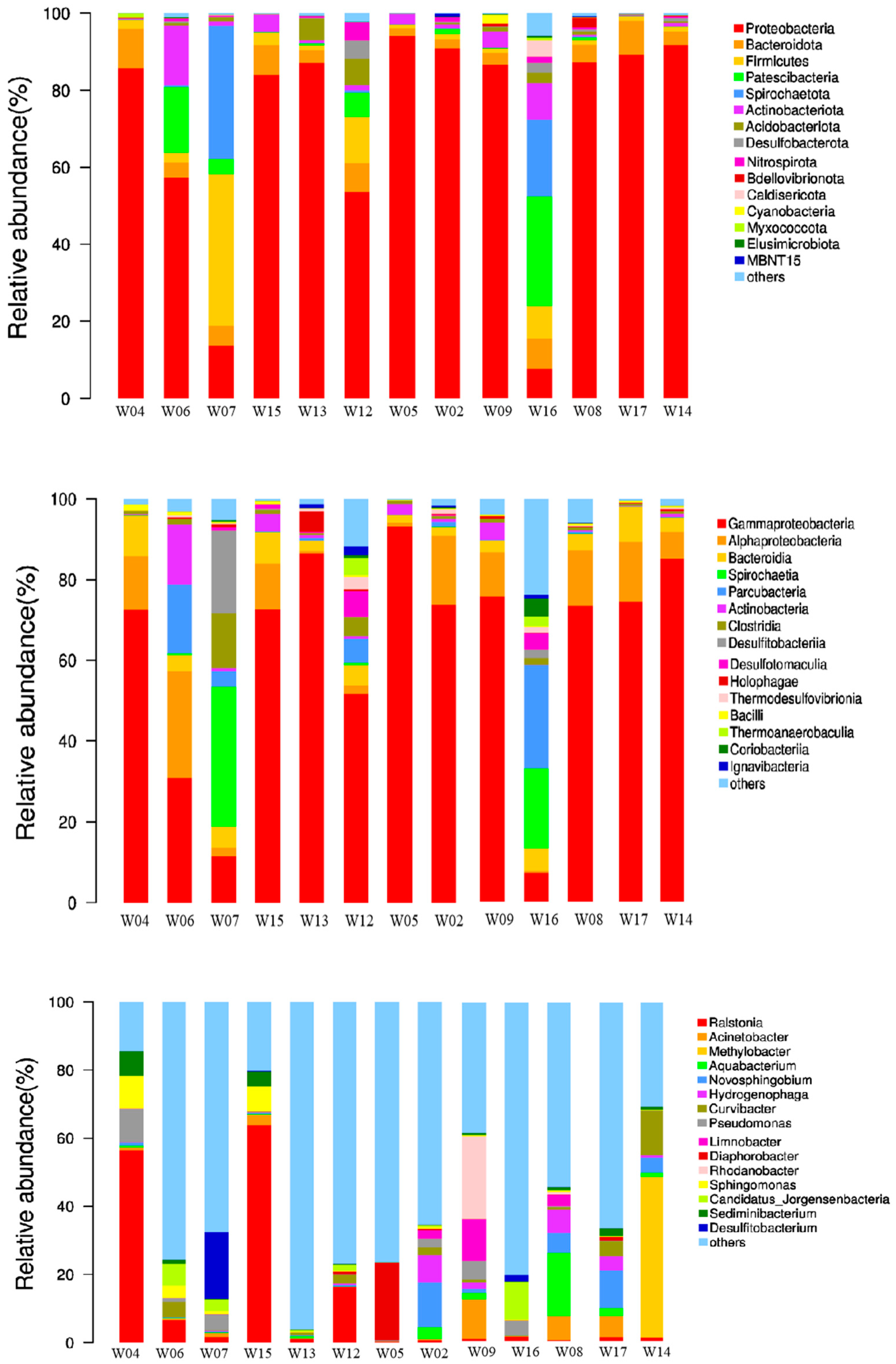
3.2. Characteristics of Microbial Community Structure in Study’s Wells
3.3. Relationships Between Microbial Community Characteristics and Groundwater Physiochemical Parameters
- There is a positive correlation between the presence of Fe2⁺ and the abundance of Acidobacteriota, Desulfobacterota, and Nitrospirota. This suggests that these microbial phyla might thrive in iron-rich environments, possibly involving iron-reducing or -oxidizing processes.
- An intriguing finding is the positive correlation between DCA and both Firmicutes and Spirochaetota. This implies that DCA could potentially act as a growth promoter for these two phyla, enhancing their numbers or activity in the groundwater.
- The presence of M,P-xylene shows a positive correlation with Actinobacteriota. This indicates that Actinobacteria might be involved in the degradation or metabolism of this particular contaminant.
- Proteobacteria showed a negative correlation with Patescibacteria, Firmicutes, and Spirochaetota. This suggests that the proliferation of Proteobacteria might be hindered when these other phyla are dominant, possibly due to competition for resources or differing tolerances to contaminant type.
- Fe2⁺ shows a positive correlation with Thermodesulfovibrionia, Thermoanaerobaculia, and Ignavibacteria, indicating that these classes may preferentially thrive in iron-rich environments, potentially participating in iron-related metabolic processes.
- A positive correlation was found between total alkalinity and the classes Spirochaetia, Clostridia, and Desulfitobacteriia in Table S4. This suggests that more alkaline conditions might encourage the growth and activity of these microbial classes in the groundwater.
- A strong positive link between phenol concentration and the class Holophagae points towards the ability of these bacteria to adapt and survive in environments with high phenol content, perhaps even utilizing phenolic compounds as a carbon source.
- Actinobacteria is positively associated with ethylbenzene and M,P-xylene, suggesting a role in the degradation of these aromatic hydrocarbons. Studies by Balachandran et al. [46] and Baoune et al. [47] confirm the capacity of Actinobacteria to degrade various hydrocarbons including naphthalene, phenanthrene, diesel, gasoline, kerosene, benzene, toluene, xylene, and cyclohexane, supporting their involvement in hydrocarbon degradation in contaminated soils and potentially in groundwater as well.
- pH had a positive correlation with Acinetobacter, Limnobacter, and Rhodanobacter. This indicates that a higher-pH environment may be more conducive to the proliferation of these microbial genera, potentially making them better suited for bioremediation.
- DO was positively correlated with Novosphingobium and Hydrogenophaga, both of which are known aerobic microbes. Wells W02 and W17, with the highest DO levels, had the highest proportions of these genera.
- Ralstonia, Sphingomonas, and Sediminibacterium exhibited a positive correlation with TOC, benzene, and ortho-xylene. This suggests that these microbial genera may play a role in the degradation of BTEX contaminants in the groundwater. The positive correlation among these genera hints at a cooperative or synergistic mechanism in breaking down these contaminants.
- DCA concentration showed a strong positive link with Desulfitobacterium. Intriguingly, Desulfitobacterium was only detected in the phreatic groundwaters of wells W7 and W16, which are contaminated with DCA. Desulfitobacterium has been recognized as a specific microbial species capable of degrading chlorinated hydrocarbons [24]. This points to the potential of Desulfitobacterium in metabolizing or degrading DCA, providing insight into how the presence of this contaminant influences the composition and functional potential of the microbial community in these specific wells.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, H.; Jiang, Y.; Zhao, J.; Li, S.; Schulz, S.; Deng, L. BTEX biodegradation is linked to bacterial community assembly patterns in contaminated groundwater ecosystem. J. Hazard. Mater. 2021, 419, 126205. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-F.; Liu, S.-H.; Su, Y.-M.; Chen, Y.-R.; Lin, C.-W.; Lin, K.-L. Bioremediation capability evaluation of benzene and sulfolane contaminated groundwater: Determination of bioremediation parameters. Sci. Total. Environ. 2018, 648, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Daghio, M.; Tatangelo, V.; Franzetti, A.; Gandolfi, I.; Papacchini, M.; Careghini, A.; Sezenna, E.; Saponaro, S.; Bestetti, G. Hydrocarbon degrading microbial communities in bench scale aerobic biobarriers for gasoline contaminated groundwater treatment. Chemosphere 2015, 130, 34–39. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, J.A.; Gerritse, J.; Hartog, N.; Ertl, S.; Parsons, J.R.; Hassanizadeh, S.M. Anaerobic degradation of benzene and other aromatic hydrocarbons in a tar-derived plume: Nitrate versus iron reducing conditions. J. Contam. Hydrol. 2022, 248, 104006. [Google Scholar] [CrossRef]
- Mosmeri, H.; Gholami, F.; Shavandi, M.; Dastgheib, S.M.M.; Alaie, E. Bioremediation of benzene-contaminated groundwater by calcium peroxide (CaO2) nanoparticles: Continuous-flow and biodiversity studies. J. Hazard. Mater. 2019, 371, 183–190. [Google Scholar] [CrossRef]
- Rodríguez-Uribe, M.L.; Peña-Cabriales, J.J.; Rivera-Cruz, M.d.C.; Délano-Frier, J.P. Native bacteria isolated from weathered petroleum oil-contaminated soils in Tabasco, Mexico, accelerate the degradation petroleum hydrocarbons in saline soil microcosms. Environ. Technol. Innov. 2021, 23, 101781. [Google Scholar] [CrossRef]
- Ossai, I.C.; Ahmed, A.; Hassan, A.; Hamid, F.S. Remediation of soil and water contaminated with petroleum hydrocarbon: A review. Environ. Technol. Innov. 2019, 17, 100526. [Google Scholar] [CrossRef]
- da Silva, M.L.B.; Corseuil, H.X. Groundwater microbial analysis to assess enhanced BTEX biodegradation by nitrate injection at a gasohol-contaminated site. Int. Biodeterior. Biodegrad. 2011, 67, 21–27. [Google Scholar] [CrossRef]
- Dou, J.; Ding, A.; Liu, X.; Du, Y.; Deng, D.; Wang, J. Anaerobic benzene biodegradation by a pure microbial culture of Bacillus cereus under nitrate reducing conditions. J. Environ. Sci. 2010, 22, 709–715. [Google Scholar] [CrossRef]
- Sperfeld, M.; Rauschenbach, C.; Diekert, G.; Studenik, S. Microbial community of a gasworks aquifer and identification of nitrate-reducing Azoarcus and Georgfuchsia as key players in BTEX degradation. Water Res. 2018, 132, 146–157. [Google Scholar] [CrossRef]
- Guo, L.; Wang, G.; Sheng, Y.; Shi, Z.; Sun, X. Groundwater microbial communities and their connection to hydrochemical environment in Golmud, Northwest China. Sci. Total. Environ. 2019, 695, 133848. [Google Scholar] [CrossRef] [PubMed]
- Varjani, S.J.; Upasani, V.N. A new look on factors affecting microbial degradation of petroleum hydrocarbon pollutants. Int. Biodeterior. Biodegrad. 2017, 120, 71–83. [Google Scholar] [CrossRef]
- Franklin, R.B.; Morrissey, E.M.; Morina, J.C. Changes in abundance and community structure of nitrate-reducing bacteria along a salinity gradient in tidal wetlands. Pedobiologia 2017, 60, 21–26. [Google Scholar] [CrossRef]
- Dell’Anno, A.; Mei, M.; Ianni, C.; Danovaro, R. Impact of bioavailable heavy metals on microbial activities in coastal marine sediments. World J. Microbiol. Biotechnol. 2003, 19, 93–100. [Google Scholar] [CrossRef]
- Jordaan, K.; Bezuidenhout, C.C. MicrobialMicrobial community composition of an urban river in the north west province, South Africa, in relation to physico-chemical water quality. Environ. Sci. Pollut. Res. 2015, 23, 5868–5880. [Google Scholar] [CrossRef]
- Brooijmans, R.J.W.; Pastink, M.I.; Siezen, R.J. Hydrocarbon-degrading bacteria: The oil-spill clean-up crew. Microb. Biotechnol. 2009, 2, 587–594. [Google Scholar] [CrossRef]
- Tremblay, J.; Yergeau, E.; Fortin, N.; Cobanli, S.; Elias, M.; King, T.L.; Lee, K.; Greer, C.W. Chemical dispersants enhance the activity of oil- and gas condensate-degrading marine bacteria. ISME J. 2017, 11, 2793–2808. [Google Scholar] [CrossRef]
- Cui, J.; Chen, H.; Sun, M.; Wen, J. Comparison of bacterial community structure and function under different petroleum hydrocarbon degradation conditions. Bioprocess Biosyst. Eng. 2019, 43, 303–313. [Google Scholar] [CrossRef]
- Wu, M.; Dick, W.A.; Li, W.; Wang, X.; Yang, Q.; Wang, T.; Xu, L.; Zhang, M.; Chen, L. Bioaugmentation and biostimulation of hydrocarbon degradation and the microbial community in a petroleum-contaminated soil. Int. Biodeterior. Biodegrad. 2016, 107, 158–164. [Google Scholar] [CrossRef]
- Zhou, Q. Quantitative analyses of relationships between ecotoxicological effects and combined pollution. Sci. China Life Sci. 2004, 47, 332–339. [Google Scholar] [CrossRef]
- Huang, W.-J.; Lin, Y.-H.; Chen, W.-Y.; Chen, H.-W.; Yu, R.-F. Causal relationships among biological toxicity, geochemical conditions and derived DBPs in groundwater. J. Hazard. Mater. 2015, 283, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Zuo, R.; Wang, J.-S.; Yang, J.; Li, Q.; Chen, M. The spatial variations of correlation between microbial diversity and groundwater quality derived from a riverbank filtration site, northeast China. Sci. Total. Environ. 2019, 706, 135855. [Google Scholar] [CrossRef] [PubMed]
- Kolehmainen, R.E.; Tiirola, M.; Puhakka, J.A. Spatial and temporal changes in Actinobacterial dominance in experimental artificial groundwater recharge. Water Res. 2008, 42, 4525–4537. [Google Scholar] [CrossRef] [PubMed]
- Leeson, A.; Becvar, E.; Henry, B.; Coyle, C. Principles and Practices of Enhanced Anaerobic Bioremediation of Chlorinated Solvents. TR-2250-ENV, Naval Facilities Engineering Command. 2004. Available online: https://www.researchgate.net/publication/235098519_Principles_and_Practices_of_Enhanced_Anaerobic_Bioremediation_of_Chlorinated_Solvents (accessed on 12 November 2024).
- Wang, A.; Fu, W.; Feng, Y.; Liu, Z.; Song, D. Synergetic effects of microbial-phytoremediation reshape microbial communities and improve degradation of petroleum contaminants. J. Hazard. Mater. 2022, 429, 128396. [Google Scholar] [CrossRef] [PubMed]
- van der Meer, J.R. Environmental pollution promotes selection of microbial degradation pathways. Front. Ecol. Environ. 2006, 4, 35–42. [Google Scholar] [CrossRef]
- Qiu, Z.; Li, M.; Song, L.; Wang, C.; Yang, S.; Yan, Z.; Wang, Y. Study on nitrogen-retaining microbial agent to reduce nitrogen loss during chicken manure composting and nitrogen transformation mechanism. J. Clean. Prod. 2020, 285, 124813. [Google Scholar] [CrossRef]
- Zhong, Z.; Wu, X.; Gao, L.; Lu, X.; Zhang, B. Efficient and microbial communities for pollutant removal in a distributed-inflow biological reactor (DBR) for treating piggery wastewater. RSC Adv. 2016, 6, 95987–95998. [Google Scholar] [CrossRef]
- Hou, G.; Yang, F.; Liu, S.; Gao, M.; Fan, Q.; Jia, C.; Liu, Y. The characteristics of saline submarine groundwater environment on a continental shelf: An example from the south of Laizhou Bay, China. Cont. Shelf Res. 2021, 229, 104557. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, C.; Li, W.; Weng, S.; Song, W.; Li, J.; Wang, Y. Functional diversity of microbial communities in inactive seafloor sulfide deposits. FEMS Microbiol. Ecol. 2021, 97, fiab108. [Google Scholar] [CrossRef]
- Gülay, A.; Musovic, S.; Albrechtsen, H.-J.; Abu Al-Soud, W.; Sørensen, S.J.; Smets, B.F. Ecological patterns, diversity and core taxa of microbial communities in groundwater-fed rapid gravity filters. ISME J. 2016, 10, 2209–2222. [Google Scholar] [CrossRef]
- Lan, P.T.N.; Sakamoto, M.; Sakata, S.; Benno, Y. Bacteroides barnesiae sp. nov., Bacteroides salanitronis sp. nov. and Bacteroides gallinarum sp. nov., isolated from chicken caecum. Int. J. Syst. Evol. Microbiol. 2006, 56, 2853–2859. [Google Scholar] [CrossRef] [PubMed]
- Yavari-Bafghi, M.; Shavandi, M.; Dastgheib, S.M.M.; Amoozegar, M.A. Simultaneous application of CaO2 nanoparticles and microbial consortium in Small Bioreactor Chambers (SBCs) for phenol removal from groundwater. Process Saf. Environ. Prot. 2022, 160, 465–477. [Google Scholar] [CrossRef]
- Vos, P.; Garrity, G.; Jones, D.; Krieg, N.R.; Ludwig, W.; Rainey, F.A.; Schleifer, K.H.; Whitman, W. (Eds.) Bergey’s Manual of Systematic Bacteriology, 2nd ed.; Volume 3: The Firmicutes; Springer: New York, NY, USA, 2009. [Google Scholar] [CrossRef]
- Heidari, A.; Asoodeh, A. A novel nitrile-degrading enzyme (nitrile hydratase) from Ralstonia sp. ZA96 isolated from oil-contaminated soils. Biocatal. Agric. Biotechnol. 2019, 21, 101285. [Google Scholar] [CrossRef]
- Biki, S.P.; Mahmud, S.; Akhter, S.; Rahman, J.; Rix, J.J.; Al Bachchu, A.; Ahmed, M. Polyethylene degradation by Ralstonia sp. strain SKM2 and Bacillus sp. strain SM1 isolated from land fill soil site. Environ. Technol. Innov. 2021, 22, 101495. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Ding, Y.; Song, H.; Liu, T. Analysis of microbial community resistance mechanisms in groundwater contaminated with SAs and high NH4+-Fe-Mn. Sci. Total. Environ. 2022, 817, 153036. [Google Scholar] [CrossRef]
- Al-Dhabaan, F.A. Morphological, biochemical and molecular identification of petroleum hydrocarbons biodegradation bacteria isolated from oil polluted soil in Dhahran, Saud Arabia. Saudi J. Biol. Sci. 2018, 26, 1247–1252. [Google Scholar] [CrossRef]
- Barman, S.R.; Banerjee, P.; Mukhopadhayay, A.; Das, P. Biodegradation of acenapthene and naphthalene by Pseudomonas mendocina: Process optimization, and toxicity evaluation. J. Environ. Chem. Eng. 2017, 5, 4803–4812. [Google Scholar] [CrossRef]
- Dutta, K.; Shityakov, S.; Khalifa, I.; Mal, A.; Moulik, S.P.; Panda, A.K.; Ghosh, C. Effects of secondary carbon supplement on biofilm-mediated biodegradation of naphthalene by mutated naphthalene 1, 2-dioxygenase encoded by Pseudomonas putida strain KD9. J. Hazard. Mater. 2018, 357, 187–197. [Google Scholar] [CrossRef]
- Martin, M.S.; Santos, I.C.; Carlton, D.D.; Stigler-Granados, P.; Hildenbrand, Z.L.; Schug, K.A. Characterization of Microbial Diversity in Contaminated Groundwater Using Matrix-assisted Laser Desorption/Ionization Time-of-flight Mass Spectrometry. Sci. Total. Environ. 2018, 622–623, 1562–1571. [Google Scholar] [CrossRef]
- Santos, I.C.; Martin, M.S.; Carlton, D.D.; Amorim, C.L.; Castro, P.M.L.; Hildenbrand, Z.L.; Schug, K.A. MALDI-TOF MS for the identification of cultivable organicdegrading bacteria in contaminated groundwater near unconventional natural gas extraction sites. Microorganisms 2017, 5, 47. [Google Scholar] [CrossRef]
- Allison, S.D.; Lu, Y.; Weihe, C.; Goulden, M.L.; Martiny, A.C.; Treseder, K.K.; Martiny, J.B.H. Microbial abundance and composition influence litter decomposition response to environmental change. Ecology 2013, 94, 714–725. [Google Scholar] [CrossRef] [PubMed]
- Blöthe, M.; Roden, E.E. Composition and activity of an autotrophic Fe (II)-oxidizing, nitrate-reducing enrichment culture. Appl. Environ. Microbiol. 2009, 75, 6937–6940. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Song, X.; Wei, C.; Jin, P.; Chen, X.; Tang, Z.; Li, K.; Ding, X.; Fu, H. In situ remediation of Cr(VI) contaminated groundwater by ZVI-PRB and the corresponding indigenous microbial community responses: A field-scale study. Sci. Total. Environ. 2022, 805, 150260. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, C.; Duraipandiyan, V.; Balakrishna, K.; Ignacimuthu, S. Petroleum and polycyclic aromatic hydrocarbons (PAHs) degradation and naphthalene metabolism in Streptomyces sp. (ERI-CPDA-1) isolated from oil contaminated soil. Bioresour. Technol. 2012, 112, 83–90. [Google Scholar] [CrossRef]
- Baoune, H.; Aparicio, J.D.; Acuña, A.; El Hadj-Khelil, A.O.; Sanchez, L.; Polti, M.A.; Alvarez, A. Effectiveness of the Zea mays-Streptomyces association for the phytoremediation of petroleum hydrocarbons impacted soils. Ecotoxicol. Environ. Saf. 2019, 184, 109591. [Google Scholar] [CrossRef]
| pH | ORP (mV) | Total Alkalinity (mg/L) | DO (mg/L) | NO3− (mg/L) | SO42− (mg/L) | Fe2+ (mg/L) | Mn2+ (mg/L) | TOC (mg/L) | Benzene | Toluene | Ethylbenzene | M,P-Xylene | O-Xylene | Phenol | Dichloroethane | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| W08 | 5.93 | 24 | 40.0 | 2.88 | 1.090 | 27.50 | 0.07 | 0.74 | 49.8 | ND | ND | ND | ND | ND | ND | ND |
| W07 | 6.08 | −105 | 332.0 | 1.20 | 0.058 | 5.60 | 1.02 | 1.48 | 163.0 | 0.15545 | 0.01318 | 0.00533 | ND | ND | 0.00062 | 0.021043 |
| W06 | 5.89 | −86 | 60.0 | 0.75 | 0.797 | 2.82 | 8.84 | 2.65 | 128.0 | 0.52370 | 0.02678 | 0.00796 | 0.00334 | 0.00045 | ND | ND |
| W05 | 6.12 | −102 | 44.8 | 0.39 | 0.042 | 1.03 | 1.64 | 11.80 | 573.0 | 1.00000 | 0.05356 | 0.00200 | 0.00092 | 0.00027 | 0.00039 | ND |
| W16 | 6.04 | −78 | 183.0 | 1.10 | 0.382 | 15.40 | 0.06 | 0.10 | 79.0 | 0.09431 | 6.87 × 10−5 | ND | ND | ND | ND | 7.58 × 10−5 |
| W04 | 6.13 | −95 | 61.3 | 0.83 | 0.134 | 2.29 | 1.58 | 0.08 | 682.0 | 0.82228 | 0.00156 | 0.00379 | 0.00094 | 0.00225 | 6.4 × 10−5 | ND |
| W15 | 6.02 | −132 | 68.8 | 0.97 | 0.252 | 4.84 | 0.84 | 0.18 | 707.0 | 0.85071 | 0.00483 | 0.00455 | 0.00034 | 0.00025 | 0.00027 | ND |
| W12 | 5.92 | −7 | 232.0 | 1.80 | 0.054 | 13.70 | 31.10 | 0.08 | 47.2 | 0.06137 | 0.00564 | ND | 0.00044 | 8.06 × 10−5 | 0.00015 | ND |
| W13 | 5.87 | 91 | 30.6 | 2.06 | 0.100 | 16.10 | 10.60 | 0.62 | 4.7 | 0.01265 | ND | ND | ND | ND | 0.00284 | ND |
| W02 | 6.23 | −35 | 121.0 | 2.49 | ND | 4.30 | 1.00 | 16.90 | 26.2 | 0.01957 | 0.00186 | 5.21 × 10−5 | ND | ND | 9.48 × 10−5 | ND |
| W09 | 6.49 | 38 | 75.2 | 2.40 | 0.702 | 21.60 | 8.08 | 3.04 | 6.3 | ND | 1.42 × 10−5 | 9.48 × 10−6 | 2.13 × 10−5 | ND | ND | ND |
| W17 | 6.15 | −30 | 204.0 | 2.97 | 0.041 | 24.55 | 1.66 | 16.80 | 8.4 | ND | ND | ND | ND | ND | ND | ND |
| W14 | 6.10 | 54 | 45.8 | 2.28 | 0.043 | 6.67 | 0.11 | 0.71 | 5.9 | ND | ND | ND | ND | ND | ND | ND |
| Sampling Point | Valid Tags | OTUs | ACE | Simpson | Shannon | Chao1 | Goods Coverage |
|---|---|---|---|---|---|---|---|
| W02 | 63,353 | 1170 | 1614 | 0.92 | 5.90 | 1547.44 | 0.9926 |
| W04 | 72,243 | 894 | 1335 | 0.66 | 3.10 | 1223.72 | 0.9934 |
| W05 | 69,684 | 886 | 1362 | 0.63 | 2.56 | 1400.74 | 0.9928 |
| W06 | 70,735 | 906 | 1211 | 0.78 | 5.29 | 1229.74 | 0.9941 |
| W07 | 67,803 | 1272 | 1515 | 0.94 | 5.65 | 1512.98 | 0.9931 |
| W08 | 55,684 | 1083 | 2017 | 0.85 | 4.67 | 1908.77 | 0.9914 |
| W09 | 55,644 | 1008 | 1416 | 0.90 | 4.89 | 1379.93 | 0.9933 |
| W12 | 59,492 | 1550 | 1933 | 0.96 | 6.54 | 1897.61 | 0.9913 |
| W13 | 64,999 | 1141 | 1517 | 0.73 | 4.14 | 1497.53 | 0.9926 |
| W14 | 57,903 | 1159 | 1720 | 0.77 | 4.61 | 1642.49 | 0.9921 |
| W15 | 72,553 | 911 | 1282 | 0.62 | 3.15 | 1303.91 | 0.9933 |
| W16 | 67,907 | 1473 | 1704 | 0.97 | 6.49 | 1712.61 | 0.9923 |
| W17 | 56,137 | 969 | 1378 | 0.86 | 5.57 | 1392.23 | 0.9931 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Lin, X.; Sun, M.; Ma, S.; Liu, J.; Zhang, S. Microbial Community Dynamics in Groundwater of a Petrochemical Refinery: Influence of BTEX and Dichloroethane Contamination. Water 2024, 16, 3275. https://doi.org/10.3390/w16223275
Liu Z, Lin X, Sun M, Ma S, Liu J, Zhang S. Microbial Community Dynamics in Groundwater of a Petrochemical Refinery: Influence of BTEX and Dichloroethane Contamination. Water. 2024; 16(22):3275. https://doi.org/10.3390/w16223275
Chicago/Turabian StyleLiu, Zhengwei, Xiaoyu Lin, Mingbo Sun, Shici Ma, Jingru Liu, and Shucai Zhang. 2024. "Microbial Community Dynamics in Groundwater of a Petrochemical Refinery: Influence of BTEX and Dichloroethane Contamination" Water 16, no. 22: 3275. https://doi.org/10.3390/w16223275
APA StyleLiu, Z., Lin, X., Sun, M., Ma, S., Liu, J., & Zhang, S. (2024). Microbial Community Dynamics in Groundwater of a Petrochemical Refinery: Influence of BTEX and Dichloroethane Contamination. Water, 16(22), 3275. https://doi.org/10.3390/w16223275






