A Comprehensive Review of Advanced Treatment Technologies for the Enhanced Reuse of Produced Water
Abstract
:1. Introduction
2. Characteristics of PW
3. Usage of PW for Irrigation
4. Conventional Treatment Process
5. Chemical Treatments Enabling Reuse of Produced Water
5.1. Advanced Oxidation Processes (AOPs)
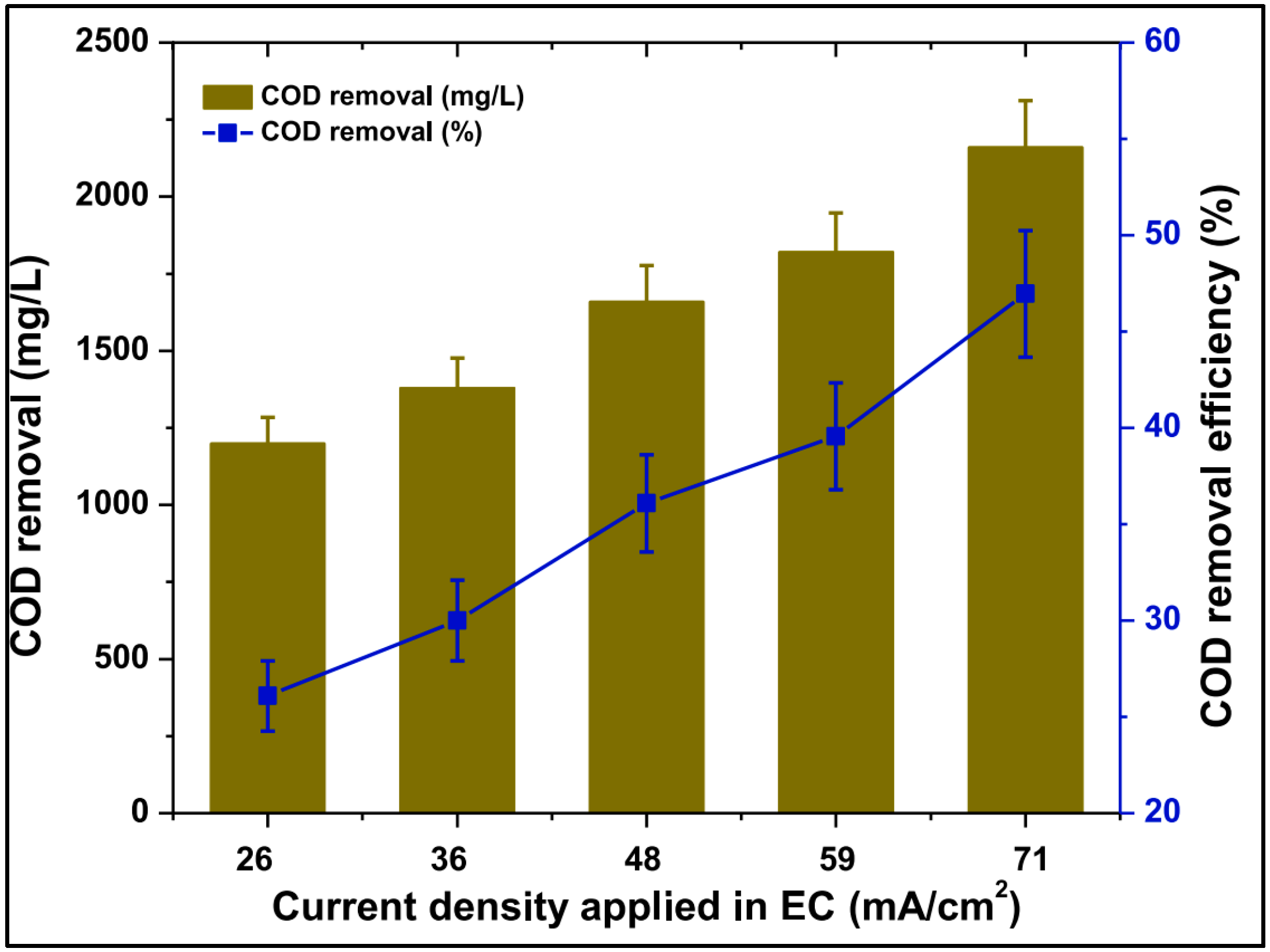
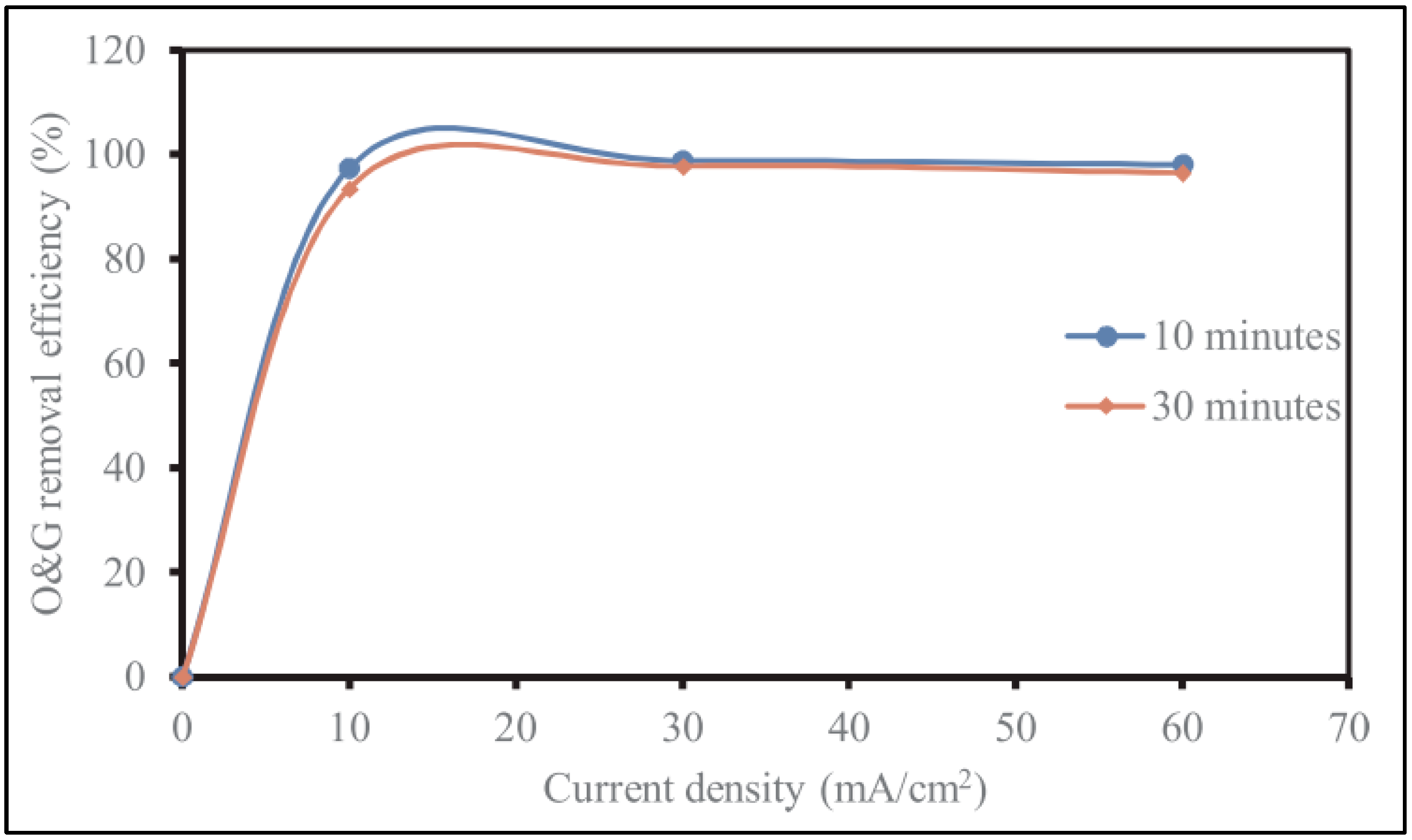
5.2. Electrocoagulation
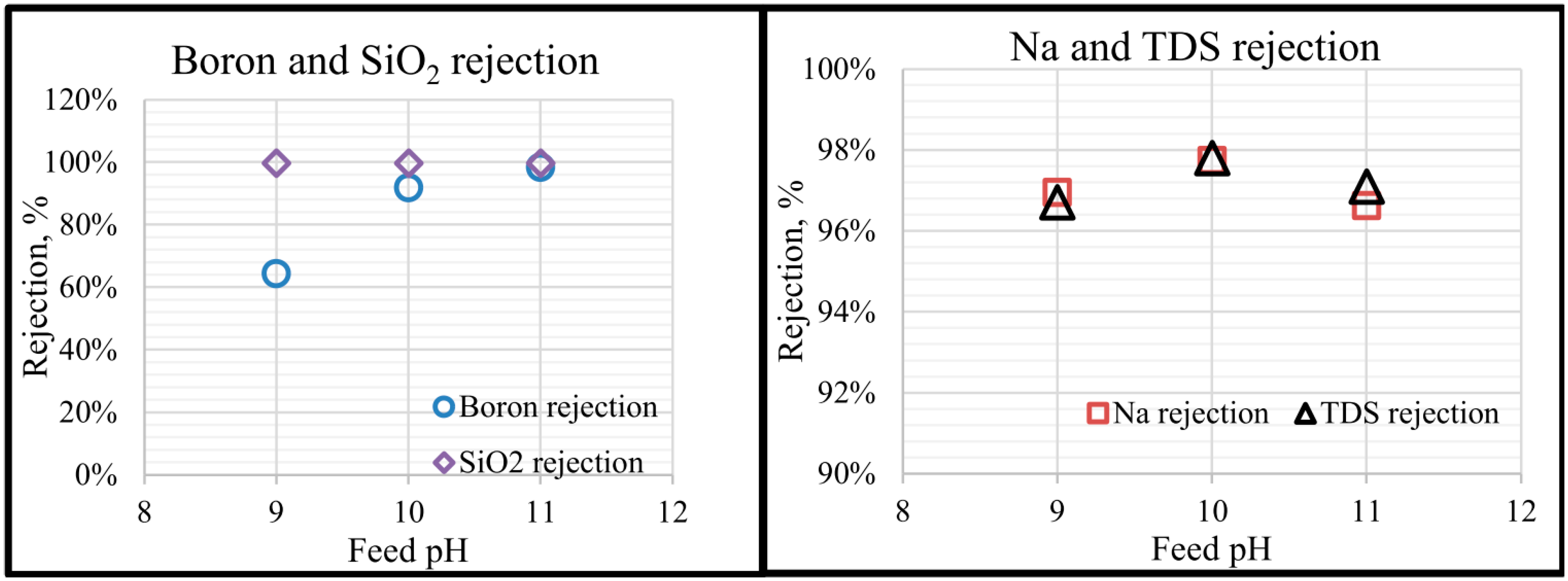
5.3. Desalination
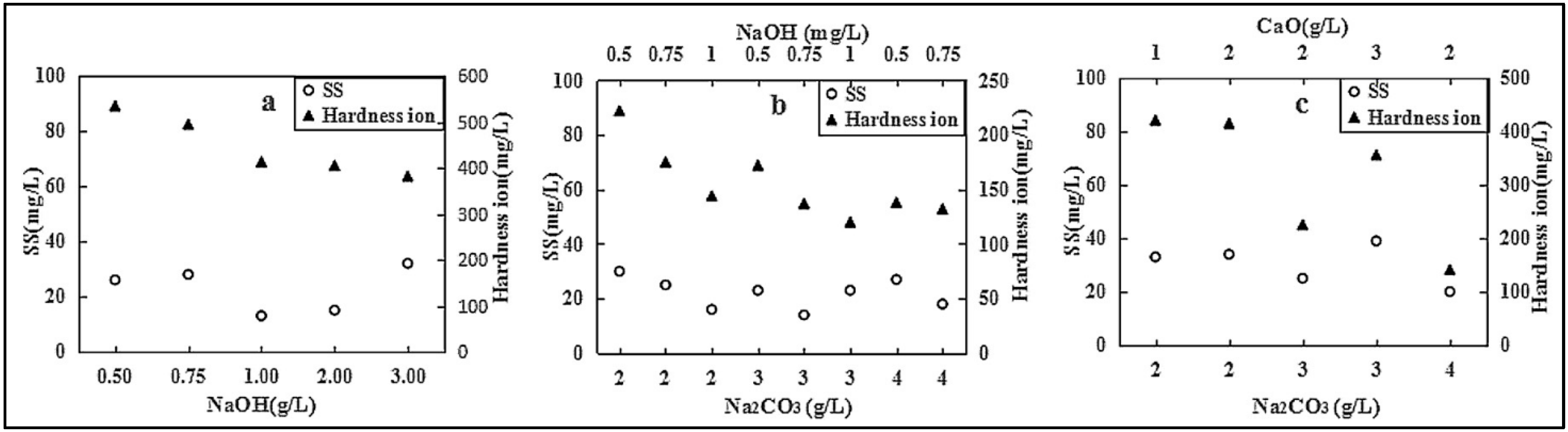
5.4. Alkali Precipitation
5.5. Microemulsion
5.6. Liquid–Liquid Extraction
5.7. PdAu-Catalyzed Oxidation
5.8. Bioelectrochemical Reactor
5.9. Ferrate (VI) Oxidation
5.10. Phytoremediation
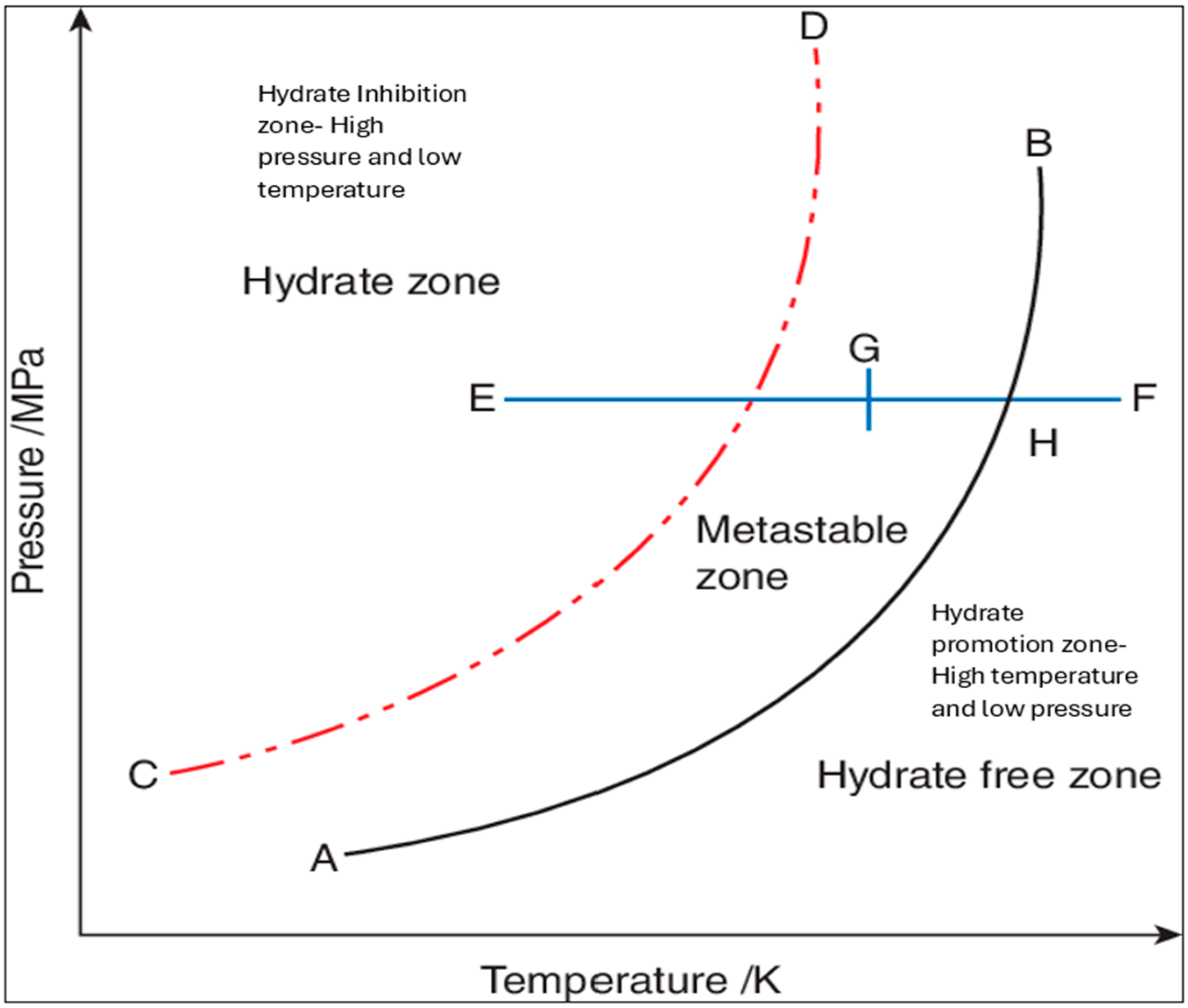
5.11. Gas Hydrate
5.12. Fibrous Coalescence Technique
6. Membrane Technologies for Physical Treatment
6.1. Membrane Bioreactors (MBRs)
6.2. Hydrophilic Polyvinylidene Fluoride (PVDF) Membranes
6.3. Fenton and Modified Fenton Oxidation Coupled with Membrane Distillation
6.4. Membrane Distillation (MD)
6.5. Ceramic Membranes
6.6. Pressure-Retarded Osmosis
6.7. Nanofiber Membrane
6.8. High-Temperature Membranes
6.9. Inclined Forward Osmosis
6.10. Membrane Technologies Challenges and Limitations
6.11. Membrane Fouling Mitigation
7. Biological Treatment
7.1. Biological Effects of Elevated Major Ions
7.2. Halotolerant Bacteria for Organic Compound Treatment
7.3. Anaerobic Treatment
8. Hybrid Treatment Process
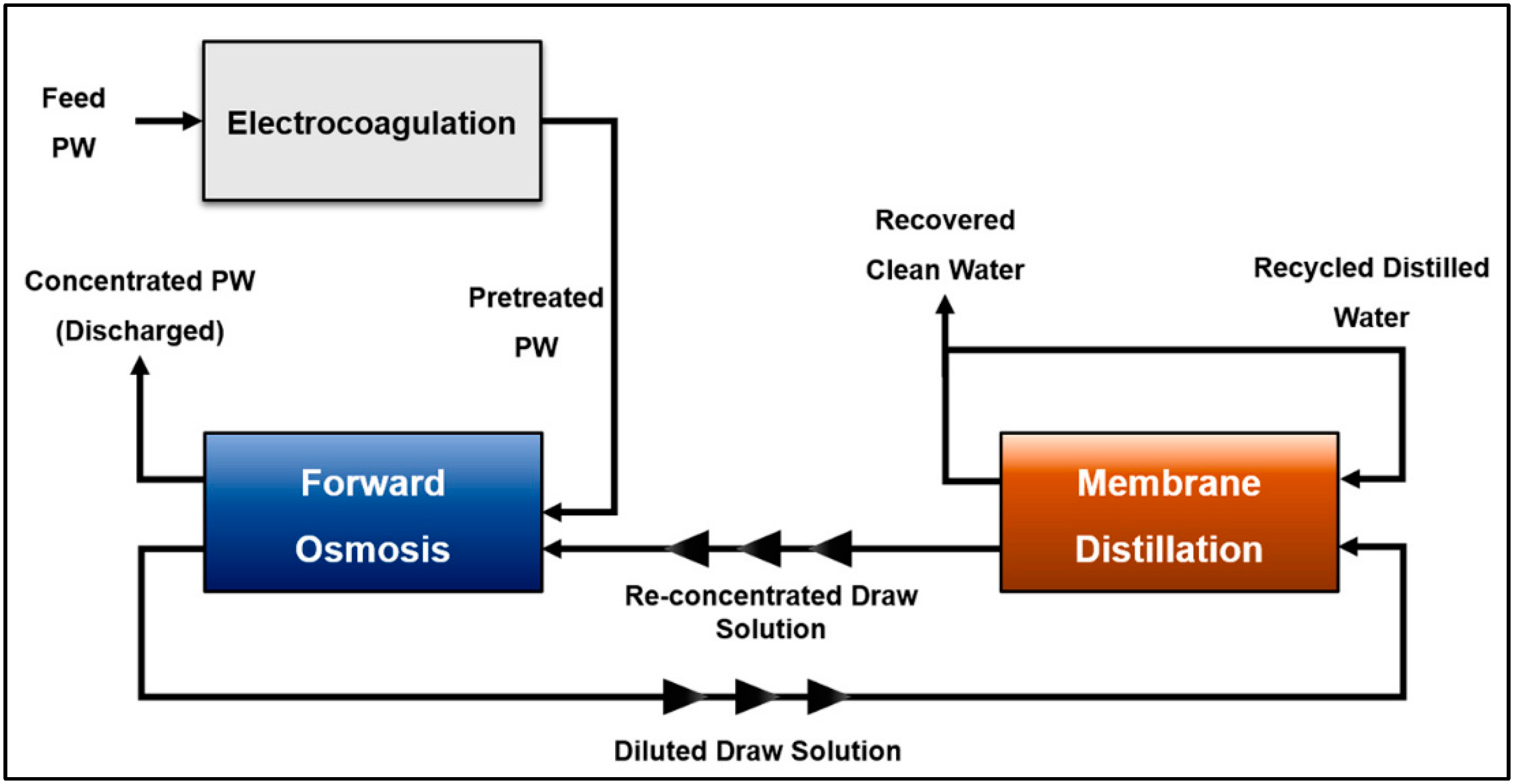
8.1. Integrated Electrocoagulation–Forward Osmosis–Membrane Distillation for PW
8.2. Electrocoagulation Process Through Hybrid Processes
8.3. Combined EC-MF-MD for PW Treatment
8.4. Electrochemical and Bioelectrochemical Systems
8.5. Electro-Coagulation/Forward Osmosis System
8.6. Organophosphonate Draw Solution
8.7. Bioelectrochemical Systems
9. Future Outlook and Challenges
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Al-Kaabi, M.A.; Zouari, N.; Da’na, D.A.; Al-Ghouti, M.A. Adsorptive batch and biological treatments of produced water: Recent progresses, challenges, and potentials. J. Environ. Manag. 2021, 290, 112527. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, H.; Li, Y.; Xu, H.; Pan, Z.; Dai, P.; Wang, H.; Yang, Q. A review of treatment technologies for produced water in offshore oil and gas fields. Sci. Total Environ. 2021, 775, 145485. [Google Scholar] [CrossRef] [PubMed]
- Olajire, A.A. Recent advances on the treatment technology of oil and gas produced water for sustainable energy industry-mechanistic aspects and process chemistry perspectives. Chem. Eng. J. Adv. 2020, 4, 100049. [Google Scholar] [CrossRef]
- Al-Kaabi, M.A.; Al-Ghouti, M.A.; Ashfaq, M.Y.M.; Ahmed, T.; Zouari, N. An integrated approach for produced water treatment using microemulsions modified activated carbon. J. Water Process Eng. 2019, 31, 100830. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Al-Kaabi, M.A.; Ashfaq, M.Y.; Da’na, D.A. Produced water characteristics, treatment and reuse: A review. J. Water Process Eng. 2019, 28, 222–239. [Google Scholar] [CrossRef]
- Nallakukkala, S.; Lal, B. Seawater and produced water treatment via gas hydrate: Review. J. Environ. Chem. Eng. 2021, 9, 105053. [Google Scholar] [CrossRef]
- El-badawy, T.; Othman, M.H.D.; Matsuura, T.; Bilad, M.R.; Adam, M.R.; Tai, Z.S.; Ravi, J.; Ismail, A.F.; Rahman, M.A.; Jaafar, J.; et al. Progress in treatment of oilfield produced water using membrane distillation and potentials for beneficial re-use. Sep. Purif. Technol. 2022, 278, 119494. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Z.; Yang, K.; Wei, J.; Li, Z.; Ma, C.; Yang, X.; Wang, T.; Zeng, G.; Yu, G.; et al. Removal of chloride from water and wastewater: Removal mechanisms and recent trends. Sci. Total Environ. 2022, 821, 153174. [Google Scholar] [CrossRef]
- Echchelh, A.; Hess, T.; Sakrabani, R. Agro-environmental sustainability and financial cost of reusing gasfield-produced water for agricultural irrigation. Agric. Water Manag. 2020, 227, 105860. [Google Scholar] [CrossRef]
- Elkholy, M.M. Environmental Impact of Irrigation Development. J. Soil Sci. Agric. Eng. 2014, 5, 1191–1205. [Google Scholar] [CrossRef]
- Scanlon, B.R.; Reedy, R.C.; Xu, P.; Engle, M.; Nicot, J.P.; Yoxtheimer, D.; Yang, Q.; Ikonnikova, S. Can we beneficially reuse produced water from oil and gas extraction in the U.S.? Sci. Total Environ. 2020, 717, 137085. [Google Scholar] [CrossRef] [PubMed]
- Miller, H.; Dias, K.; Hare, H.; Borton, M.A.; Blotevogel, J.; Danforth, C.; Wrighton, K.C.; Ippolito, J.A.; Borch, T. Reusing oil and gas produced water for agricultural irrigation: Effects on soil health and the soil microbiome. Sci. Total Environ. 2020, 722, 137888. [Google Scholar] [CrossRef] [PubMed]
- Clay, L.; Pichtel, J. Treatment of Simulated Oil and Gas Produced Water via Pilot-Scale Rhizofiltration and Constructed Wetlands. Int. J. Environ. Res. 2019, 13, 185–198. [Google Scholar] [CrossRef]
- McLaughlin, M.C.; Borch, T.; McDevitt, B.; Warner, N.R.; Blotevogel, J. Water quality assessment downstream of oil and gas produced water discharges intended for beneficial reuse in arid regions. Sci. Total Environ. 2020, 713, 136607. [Google Scholar] [CrossRef] [PubMed]
- Eldos, H.I.; Khan, M.; Zouari, N.; Saeed, S.; Al-Ghouti, M.A. Characterization and assessment of process water from oil and gas production: A case study of process wastewater in Qatar. Case Stud. Chem. Environ. Eng. 2022, 6, 100210. [Google Scholar] [CrossRef]
- Saleh, I.A.; Zouari, N.; Al-Ghouti, M.A. Removal of pesticides from water and wastewater: Chemical, physical and biological treatment approaches. Environ. Technol. Innov. 2020, 19, 101026. [Google Scholar] [CrossRef]
- Amakiri, K.T.; Canon, A.R.; Molinari, M.; Angelis-Dimakis, A. Review of oilfield produced water treatment technologies. Chemosphere 2022, 298, 134064. [Google Scholar] [CrossRef]
- Santos, M.A.; Capponi, F.; Ataíde, C.H.; Barrozo, M.A.S. Wastewater treatment using DAF for process water reuse in apatite flotation. J. Clean. Prod. 2021, 308, 127285. [Google Scholar] [CrossRef]
- Daverey, A.; Pandey, D.; Verma, P.; Verma, S.; Shah, V.; Dutta, K.; Arunachalam, K. Recent advances in energy efficient biological treatment of municipal wastewater. Bioresour. Technol. Rep. 2019, 7, 100252. [Google Scholar] [CrossRef]
- Skouteris, G.; Rodriguez-Garcia, G.; Reinecke, S.F.; Hampel, U. The use of pure oxygen for aeration in aerobic wastewater treatment: A review of its potential and limitations. Bioresour. Technol. 2020, 312, 123595. [Google Scholar] [CrossRef]
- Aziz, A.; Basheer, F.; Sengar, A.; Irfanullah; Khan, S.U.; Farooqi, I.H. Biological wastewater treatment (anaerobic-aerobic) technologies for safe discharge of treated slaughterhouse and meat processing wastewater. Sci. Total Environ. 2019, 686, 681–708. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.X.; Zhang, X.; Fan, W.Y.; Sheng, G.P. Molecular insight into the variation of dissolved organic phosphorus in a wastewater treatment plant. Water Res. 2021, 203, 117529. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Lu, H.; Pan, Z.; Dai, P.; Sun, G.; Yang, Q. A full-scale process for produced water treatment on offshore oilfield: Reduction of organic pollutants dominated by hydrocarbons. J. Clean. Prod. 2021, 296, 126511. [Google Scholar] [CrossRef]
- Al-Kaabi, M. Enhancing Produced Water Quality Using Modified Activated Carbon. Master’s Thesis, College of Arts and Sciences, Qatar University, Doha, Qatar, 2016. Volume 16, p. ii. [Google Scholar] [CrossRef]
- Jiménez, S.; Andreozzi, M.; Micó, M.M.; Álvarez, M.G.; Contreras, S. Produced water treatment by advanced oxidation processes. Sci. Total Environ. 2019, 666, 12–21. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Reactive species in advanced oxidation processes: Formation, identification and reaction mechanism. Chem. Eng. J. 2020, 401, 126158. [Google Scholar] [CrossRef]
- Sannino, D.; Vaiano, V.; Ciambelli, P.; Isupova, L.A. Structured catalysts for photo-Fenton oxidation of acetic acid. Catal. Today 2011, 161, 255–259. [Google Scholar] [CrossRef]
- Coha, M.; Farinelli, G.; Tiraferri, A.; Minella, M.; Vione, D. Advanced oxidation processes in the removal of organic substances from produced water: Potential, configurations, and research needs. Chem. Eng. J. 2021, 414, 128668. [Google Scholar] [CrossRef]
- Silva, P.C.; Ferraz, N.P.; Perpetuo, E.A.; Asencios, Y.J.O. Oil Produced Water Treatment Using Advanced Oxidative Processes: Heterogeneous-Photocatalysis and Photo-Fenton. J. Sediment. Environ. 2019, 4, 99–107. [Google Scholar] [CrossRef]
- Dhamorikar, R.S.; Lade, V.G.; Kewalramani, P.V.; Bindwal, A.B. Review on integrated advanced oxidation processes for water and wastewater treatment. J. Ind. Eng. Chem. 2024, 138, 104–122. [Google Scholar] [CrossRef]
- Priyadarshini, M.; Das, I.; Ghangrekar, M.M.; Blaney, L. Advanced oxidation processes: Performance, advantages, and scale-up of emerging technologies. J. Environ. Manag. 2022, 316, 115295. [Google Scholar] [CrossRef]
- Li, Y.; Dong, H.; Xiao, J.; Li, L.; Chu, D.; Hou, X.; Xiang, S.; Dong, Q.; Zhang, H. Advanced oxidation processes for water purification using percarbonate: Insights into oxidation mechanisms, challenges, and enhancing strategies. J. Hazard. Mater. 2023, 442, 130014. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, M.A.; Antony, S.P. Effect of polarity shift on the performance of electrocoagulation process for the treatment of produced water. Chemosphere 2021, 263, 128052. [Google Scholar] [CrossRef] [PubMed]
- Gholami, M.; Abbasi Souraki, B.; Pendashteh, A. Electro-activated persulfate oxidation (EC/PS) for the treatment of real oilfield produced water: Optimization, developed numerical kinetic model, and comparison with thermal/EC/PS and EC systems. Process Saf. Environ. Prot. 2021, 153, 384–402. [Google Scholar] [CrossRef]
- Ezechi, E.H.; Isa, M.H.; Muda, K.; Kutty, S.R.M. A comparative evaluation of two electrode systems on continuous electrocoagulation of boron from produced water and mass transfer resistance. J. Water Process Eng. 2020, 34, 101133. [Google Scholar] [CrossRef]
- Khajouei, G.; Park, H.I.; Finklea, H.O.; Ziemkiewicz, P.F.; Peltier, E.F.; Lin, L.S. Produced water softening using high-pH catholyte from brine electrolysis: Reducing chemical transportation and environmental footprints. J. Water Process Eng. 2021, 40, 101911. [Google Scholar] [CrossRef]
- Khor, C.M.; Wang, J.; Li, M.; Oettel, B.A.; Kaner, R.B.; Jassby, D.; Hoek, E.M.V. Performance, energy and cost of produced water treatment by chemical and electrochemical coagulation. Water 2020, 12, 3426. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Scaria, J.; Babu, D.S.; Kumar, M.S. An overview on combined electrocoagulation-degradation processes for the effective treatment of water and wastewater. Chemosphere 2021, 263, 127907. [Google Scholar] [CrossRef] [PubMed]
- Mehri, M.; Fallah, N.; Nasernejad, B. Mechanisms of heavy metal and oil removal from synthetic saline oilfield produced water by electrocoagulation. npj Clean Water 2021, 4, 45. [Google Scholar] [CrossRef]
- Shah, M.T.; Parmar, H.B.; Rhyne, L.D.; Kalli, C.; Utikar, R.P.; Pareek, V.K. A novel settling tank for produced water treatment: CFD simulations and PIV experiments. J. Pet. Sci. Eng. 2019, 182, 106352. [Google Scholar] [CrossRef]
- Babu, P.; Bollineni, C.; Daraboina, N. Energy Analysis of Methane-Hydrate-Based Produced Water Desalination. Energy Fuels 2021, 35, 2514–2519. [Google Scholar] [CrossRef]
- Wang, M.; Wang, M.; Chen, D.; Gong, Q.; Yao, S.; Jiang, W.; Chen, Y. Evaluation of pre-treatment techniques for shale gas produced water to facilitate subsequent treatment stages. J. Environ. Chem. Eng. 2019, 7, 102878. [Google Scholar] [CrossRef]
- Costa, T.C.; Hendges, L.T.; Temochko, B.; Mazur, L.P.; Marinho, B.A.; Weschenfelder, S.E.; Florido, P.L.; da Silva, A.; Ulson de Souza, A.A.; Guelli Ulson de Souza, S.M.A. Evaluation of the technical and environmental feasibility of adsorption process to remove water soluble organics from produced water: A review. J. Pet. Sci. Eng. 2022, 208, 109360. [Google Scholar] [CrossRef]
- Mansour, M.S.M.; Abdel-Shafy, H.I.; El Azab, W.I.M. Innovative reuse of drinking water sludge for the treatment of petroleum produced water to enhance oil recovery. Egypt. J. Pet. 2020, 29, 163–169. [Google Scholar] [CrossRef]
- Hisham, S.; Ayub Khan, F.; Aljlil, S.A.; Ghasemi, M. Investigating new techniques for the treatment of oil field produced water and energy production. SN Appl. Sci. 2019, 1, 646. [Google Scholar] [CrossRef]
- Da Silva, D.C.; dos Santos Lucas, C.R.; de Moraes Juviniano, H.B.; de Alencar Moura, M.C.P.; Dantas Neto, A.A.; de Castro Dantas, T.N. Novel produced water treatment using microemulsion systems to remove oil contents. J. Water Process Eng. 2020, 33, 101006. [Google Scholar] [CrossRef]
- Hollanda, L.R.; Brito, S.; Santos, F.; Gabriela, J.; Arruda, A. Oil field—produced water treatment: Characterization, photochemical systems, and combined processes. Environ. Sci. Pollut. Res. 2021, 28, 52744–52763. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.S.B.; Ferreira Júnior, J.M.; Simonelli, G.; Souza, J.R.; Góis, L.M.N.; Santos, L.C.L. Removal of oil contents and salinity from produced water using microemulsion. J. Water Process Eng. 2020, 38, 101548. [Google Scholar] [CrossRef]
- Klemz, A.C.; Weschenfelder, S.E.; Lima de Carvalho Neto, S.; Pascoal Damas, M.S.; Toledo Viviani, J.C.; Mazur, L.P.; Marinho, B.A.; dos Santos Pereira, L.; da Silva, A.; Borges Valle, J.A.; et al. Oilfield produced water treatment by liquid-liquid extraction: A review. J. Pet. Sci. Eng. 2021, 199, 108282. [Google Scholar] [CrossRef]
- Yin, Y.B.; Heck, K.N.; Coonrod, C.L.; Powell, C.D.; Guo, S.; Reynolds, M.A.; Wong, M.S. PdAu-catalyzed oxidation through in situ generated H2O2 in simulated produced water. Catal. Today 2020, 339, 362–370. [Google Scholar] [CrossRef]
- Mohanakrishna, G.; Al-Raoush, R.I.; Abu-Reesh, I.M.; Aljaml, K. Removal of petroleum hydrocarbons and sulfates from produced water using different bioelectrochemical reactor configurations. Sci. Total Environ. 2019, 665, 820–827. [Google Scholar] [CrossRef]
- Haneef, T.; Ul Mustafa, M.R.; Yusof, K.W.; Isa, M.H.; Bashir, M.J.K.; Ahmad, M.; Zafar, M. Removal of polycyclic aromatic hydrocarbons (PAHs) from produced water by Ferrate (VI) oxidation. Water 2020, 12, 3132. [Google Scholar] [CrossRef]
- Atia, F.A.M.; Al-Ghouti, M.A.; Al-Naimi, F.; Abu-Dieyeh, M.; Ahmed, T.; Al-Meer, S.H. Removal of toxic pollutants from produced water by phytoremediation: Applications and mechanistic study. J. Water Process Eng. 2019, 32, 100990. [Google Scholar] [CrossRef]
- Wenzlick, M.; Siefert, N. Techno-economic analysis of converting oil & gas produced water into valuable resources. Desalination 2020, 481, 114381. [Google Scholar] [CrossRef]
- Lu, H.; Liu, Y.-Q.; Cai, J.-B.; Xu, X.; Xie, L.-S.; Yang, Q.; Li, Y.-X.; Zhu, K. Treatment of offshore oily produced water: Research and application of a novel fibrous coalescence technique. J. Pet. Sci. Eng. 2019, 178, 602–608. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, H.; Liu, P.; Li, Y.; Wu, S.; Dai, P.; Yang, Q. Treatment of the complex liquid phase that contains produced water, condensate oil, and floccule from an offshore gas field: A pilot system for the South China Sea. J. Nat. Gas Sci. Eng. 2021, 94, 104125. [Google Scholar] [CrossRef]
- Fulazzaky, M.; Setiadi, T.; Fulazzaky, M.A. An evaluation of the oilfield-produced water treatment by the membrane bioreactor. J. Environ. Chem. Eng. 2020, 8, 104417. [Google Scholar] [CrossRef]
- Al-Asheh, S.; Bagheri, M.; Aidan, A. Membrane bioreactor for wastewater treatment: A review. Case Stud. Chem. Environ. Eng. 2021, 4, 100109. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Q.; Miao, W.; Lu, P.; You, C.; Wang, Z. Hydrophilic PVDF membrane with versatile surface functions fabricated via cellulose molecular coating. J. Memb. Sci. 2021, 640, 119817. [Google Scholar] [CrossRef]
- Nawi, N.I.M.; Chean, H.M.; Shamsuddin, N.; Bilad, M.R.; Narkkun, T.; Faungnawakij, K.; Khan, A.L. Development of hydrophilic PVDF membrane using vapour induced phase separation method for produced water treatment. Membranes 2020, 10, 121. [Google Scholar] [CrossRef]
- Farinelli, G.; Coha, M.; Minella, M.; Fabbri, D.; Pazzi, M.; Vione, D.; Tiraferri, A. Evaluation of Fenton and modified Fenton oxidation coupled with membrane distillation for produced water treatment: Benefits, challenges, and effluent toxicity. Sci. Total Environ. 2021, 796, 148953. [Google Scholar] [CrossRef]
- Thakur, A.K.; Hsieh, I.M.; Islam, M.R.; Lin, B.; Chen, C.C.; Malmali, M. Performance of sweeping gas membrane distillation for treating produced water: Modeling and experiments. Desalination 2020, 492, 114597. [Google Scholar] [CrossRef]
- Wang, J.; Tanuwidjaja, D.; Bhattacharjee, S.; Edalat, A.; Jassby, D.; Hoek, E.M.V. Produced water desalination via pervaporative distillation. Water 2020, 12, 3560. [Google Scholar] [CrossRef]
- Marzouk, S.S.; Naddeo, V.; Banat, F.; Hasan, S.W. Preparation of TiO2/SiO2 ceramic membranes via dip coating for the treatment of produced water. Chemosphere 2021, 273, 129684. [Google Scholar] [CrossRef]
- Weschenfelder, S.E.; Fonseca, M.J.C.; Costa, B.R.S.; Borges, C.P. Influence of the use of surfactants in the treatment of produced water by ceramic membranes. J. Water Process Eng. 2019, 32, 100955. [Google Scholar] [CrossRef]
- Zoubeik, M.; Salama, A.; Henni, A. A comprehensive experimental and artificial network investigation of the performance of an ultrafiltration titanium dioxide ceramic membrane: Application in produced water treatment. Water Environ. J. 2019, 33, 459–475. [Google Scholar] [CrossRef]
- Dardor, D.; Al Maas, M.; Minier-Matar, J.; Janson, A.; Abdel-Wahab, A.; Shon, H.K.; Adham, S. Evaluation of pretreatment and membrane configuration for pressure-retarded osmosis application to produced water from the petroleum industry. Desalination 2021, 516, 115219. [Google Scholar] [CrossRef]
- Abd Halim, N.S.; Wirzal, M.D.H.; Bilad, M.R.; Md Nordin, N.A.H.; Adi Putra, Z.; Sambudi, N.S.; Mohd Yusoff, A.R. Improving Performance of Electrospun Nylon 6,6 Nanofiber Membrane for Produced Water Filtration via Solvent Vapor Treatment. Polymers 2019, 11, 2117. [Google Scholar] [CrossRef]
- Chen, C.; Huang, X.; Prakash, P.; Chilekar, S.; Franks, R. Produced water desalination using high temperature membranes. Desalination 2021, 513, 115144. [Google Scholar] [CrossRef]
- Hizam, S.M.; Bilad, M.R.; Nordin, N.A.H.; Sambudi, N.S.; Wirzal, M.D.H.; Yusof, N.; Klaysom, C.; Jaafar, J. Inclined forward osmosis module system for fouling control in sustainable produced water treatment using seawater as draw solution. J. Water Process Eng. 2021, 40, 101752. [Google Scholar] [CrossRef]
- Abdelrazeq, H.; Khraisheh, M.; Ashraf, H.M.; Ebrahimi, P.; Kunju, A. Sustainable innovation in membrane technologies for produced water treatment: Challenges and limitations. Sustainability 2021, 13, 6759. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Y.; Dudek, M.; Qin, J.; Oye, G.; Osterhus, S.W. A multivariate study of backpulsing for membrane fouling mitigation in produced water treatment. J. Environ. Chem. Eng. 2021, 9, 104839. [Google Scholar] [CrossRef]
- Veil, J.A. Produced Water Management Options and Technologies; Lee, K., Neff, J., Eds.; Springer: New York, NY, USA, 2011. [Google Scholar]
- Wang, N.; Kunz, J.L.; Cleveland, D.; Steevens, J.A.; Cozzarelli, I.M. Biological Effects of Elevated Major Ions in Surface Water Contaminated by a Produced Water from Oil Production. Arch. Environ. Contam. Toxicol. 2019, 76, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Ojagh, S.M.A.; Fallah, N.; Nasernejad, B. Biological treatment of organic compounds in produced water with use of halotolerant bacteria. J. Environ. Chem. Eng. 2020, 8, 104412. [Google Scholar] [CrossRef]
- Ahmadi, M.; Ahmadmoazzam, M.; Saeedi, R.; Abtahi, M.; Ghafari, S.; Jorfi, S. Biological treatment of a saline and recalcitrant petrochemical wastewater by using a newly isolated halo-tolerant bacterial consortium in mbbr. Desalin. Water Treat. 2019, 167, 84–95. [Google Scholar] [CrossRef]
- Huang, Z.; He, X.; Nye, C.; Bagley, D.; Urynowicz, M.; Fan, M. Effective anaerobic treatment of produced water from petroleum production using an anaerobic digestion inoculum from a brewery wastewater treatment facility. J. Hazard. Mater. 2021, 407, 124348. [Google Scholar] [CrossRef]
- Fukuhara, S. A basis for the space of modular forms. Acta Arith. 2012, 151, 421–427. [Google Scholar] [CrossRef]
- Sardari, K.; Fyfe, P.; Ranil Wickramasinghe, S. Integrated electrocoagulation—Forward osmosis—Membrane distillation for sustainable water recovery from hydraulic fracturing produced water. J. Memb. Sci. 2019, 574, 325–337. [Google Scholar] [CrossRef]
- Almukdad, A.; Hafiz, M.A.; Yasir, A.T.; Alfahel, R.; Hawari, A.H. Unlocking the application potential of electrocoagulation process through hybrid processes. J. Water Process Eng. 2021, 40, 101956. [Google Scholar] [CrossRef]
- Wei, X.; Kazemi, M.; Zhang, S.; Wolfe, F.A. Petrochemical wastewater and produced water: Treatment technology and resource recovery. Water Environ. Res. 2020, 92, 1695–1700. [Google Scholar] [CrossRef]
- Jebur, M.; Chiao, Y.H.; Thomas, K.; Patra, T.; Cao, Y.; Lee, K.; Gleason, N.; Qian, X.; Hu, Y.; Malmali, M.; et al. Combined electrocoagulation-microfiltration-membrane distillation for treatment of hydraulic fracturing produced water. Desalination 2021, 500, 114886. [Google Scholar] [CrossRef]
- Mohanakrishna, G.; Al-Raoush, R.I.; Abu-Reesh, I.M. Integrating electrochemical and bioelectrochemical systems for energetically sustainable treatment of produced water. Fuel 2021, 285, 119104. [Google Scholar] [CrossRef]
- Al Hawli, B.; Benamor, A.; Hawari, A.A. A hybrid electro-coagulation/forward osmosis system for treatment of produced water. Chem. Eng. Process.—Process Intensif. 2019, 143, 107621. [Google Scholar] [CrossRef]
- Ding, C.; Zhang, X.; Xiong, S.; Shen, L.; Yi, M.; Liu, B.; Wang, Y. Organophosphonate draw solution for produced water treatment with effectively mitigated membrane fouling via forward osmosis. J. Memb. Sci. 2020, 593, 117429. [Google Scholar] [CrossRef]
- Cabrera, J.; Irfan, M.; Dai, Y.; Zhang, P.; Zong, Y.; Liu, X. Bioelectrochemical system as an innovative technology for treatment of produced water from oil and gas industry: A review. Chemosphere 2021, 285, 131428. [Google Scholar] [CrossRef]








| Elemental Component/Ion | Maximum Concentration in Seawater | Concentration Range in Produced Water |
|---|---|---|
| Salinity | 35,000 | 23,000–67,300 |
| Sodium | 10,565 | 46,100–141,000 |
| Chloride | 18,982 | 2530–25,800 |
| Calcium | 406 | 530–4300 |
| Magnesium | 1258 | 130–3100 |
| Potassium | 384 | 210–1170 |
| Sulfate | 2716 | 46–1200 |
| Bromide | 88 | 7–1000 |
| Ammonium Bicarbonate | 77–560 | |
| Iodide | 147 | 3–210 |
| Boron | 166 | 8–40 |
| Carbonate | 4.42 | 30–450 |
| Lithium | 0.17 | 3–50 |
| TDS | 35,490 | 463,000 |
| Strontium | 12 | 23–300 |
| Parameter | Oil Field | Gas Field |
|---|---|---|
| Total oil/grease (mg/L) | 2–565 | 2.3–60 |
| Total organic carbon (mg/L) | 500–2000 | 67–38,000 |
| Total suspended solids (mg/L) | 1.2–1000 | 8–5484 |
| Total dissolved solids (mg/L) | 247,000 | 2600–360,000 |
| Chemical oxygen demand (mg/L) | 1220 | 2600–120,000 |
| Sodium (mg/L) | 132–97,000 | 520–120,000 |
| Chloride (mg/L) | 80–200,000 | 1400–190,000 |
| pH | 4.3–10 | 3.1–7.0 |
| Parameter | Unit | Produced Water (Run-1) | Produced Water (Run-2) | Produced Water (Run-3) | Mean | SD |
|---|---|---|---|---|---|---|
| Test Parameter | ||||||
| PH | NA | 4.43 | 4.43 | 4.44 | 4.43 | 0.01 |
| COD | ppm | 10,370 | 10,440 | 10,680 | 10,496.67 | 162.58 |
| TOC | ppm | 2424 | 2401 | 2392 | 2405.67 | 16.50 |
| BOD | ppm | 1034 | 1076 | 992 | 1034.00 | 42.00 |
| Salinity | ppm | 4528 | 4460.8 | 4518.4 | 4502.40 | 36.35 |
| Conductivity | µs/cm | 7075 | 6970 | 7060 | 7035.00 | 56.79 |
| TSS | ppm | 25 | 21 | 18 | 21.33 | 3.51 |
| HEM | ppm | 36.4 | 40.4 | 44.8 | 40.53 | 4.20 |
| Ions and organics | ||||||
| Sulfide | ppm | 349 | 324 | 306 | 326.33 | 21.59 |
| Silica | ppm | 1.9 | 2.0 | 2.09 | 2.00 | 0.10 |
| Phosphate | ppm | 2.13 | 2.07 | 1.98 | 2.06 | 0.08 |
| Sulphate | ppm | 46.3 | 45.92 | 46.16 | 46.13 | 0.19 |
| Chloride | ppm | 2913 | 2933 | 2917 | 2921.00 | 10.58 |
| Formate | ppm | 0.39 | 0.32 | 0.33 | 0.35 | 0.04 |
| Acetate | ppm | 373 | 368 | 365 | 368.67 | 4.04 |
| Propionate | ppm | 18.2 | 16.2 | 17.7 | 17.37 | 1.04 |
| Phenol | ppm | 1.92 | 1.905 | 2.04 | 1.96 | 0.07 |
| Metals | ||||||
| Aluminum | ppb | 4.16 | 9.17 | 17.52 | 10.28 | 6.75 |
| Arsenic | ppb | 5.47 | 7.00 | 9.23 | 7.23 | 1.89 |
| Barium | ppb | 60.93 | 60.03 | 60.58 | 60.51 | 0.45 |
| Boron | ppb | 5665.38 | 5717.93 | 5850.66 | 5744.66 | 95.49 |
| Cadmium | ppb | 0.05 | 0.05 | 0.05 | 0.05 | 0.00 |
| Calcium | ppb | 283,547.66 | 285,227.87 | 287,920.52 | 285,565.35 | 2205.88 |
| Cobalt | ppb | 7.54 | 7.34 | 6.24 | 7.04 | 0.70 |
| Chromium | ppb | 30.46 | 29.89 | 30.59 | 30.31 | 0.37 |
| Copper | ppb | 0.66 | 0.57 | 0.64 | 0.62 | 0.05 |
| Iron | ppb | 4262.88 | 4035.3 | 4134.41 | 4144.20 | 114.11 |
| Manganese | ppb | 259.04 | 255.21 | 260.53 | 258.26 | 2.74 |
| Magnesium | ppb | 44,354.25 | 46,476.78 | 44,362.22 | 45,064.42 | 1223.15 |
| Molybdenum | ppb | 5.53 | 5.53 | 5.5 | 5.52 | 0.02 |
| Nickel | ppb | 7.35 | 7.09 | 6.8 | 7.08 | 0.28 |
| Potassium | ppb | 101,024.28 | 100,956.16 | 100,786.56 | 100,922.33 | 122.42 |
| Sodium | ppb | 121,5547.0 | 1,182,652.96 | 1,196,301.16 | 1,198,167.04 | 16,526.21 |
| Strontium | ppb | 13,128.02 | 13,103.48 | 13,313.63 | 13,181.71 | 114.90 |
| Vanadium | ppb | 2.58 | 2.52 | ND | 2.55 | 0.04 |
| Zinc | ppb | 5.25 | 4.98 | 4.7 | 4.98 | 0.28 |
| Glycol and inhibitors | ||||||
| Corrosion Inhibitor | ppm | 609.6 | 620.1 | 640.23 | 623.31 | 15.57 |
| KHI | % | 0.27 | 0.27 | 0.27 | 0.27 | 0.00 |
| MEG | % | 0.33 | 0.33 | 0.33 | 0.33 | 0.00 |
| BTEX and TN | ||||||
| Benzene | ppb | 8031 | 16,069 | 9410 | 11,170.00 | 4298.32 |
| Ethyl benzene | ppb | 4084 | 5415.5 | 4446.5 | 4648.67 | 688.39 |
| Toluene | ppb | 262 | 289.5 | 283 | 278.17 | 14.37 |
| Xylene | ppb | 1055.5 | 1201.5 | 1213.5 | 1156.83 | 87.96 |
| TN | ppm | 47.6 | 47.51 | 47.13 | 47.41 | 0.25 |
| Test Parameters | Unit | Sand Filter (Run-1) | Sand Filter (Run-2) | Sand Filter (Run-3) | Mean | SD | SF% |
|---|---|---|---|---|---|---|---|
| pH | NA | 7.54 | 7.79 | 7.43 | 7.59 | 0.18 | −71.13 |
| COD | ppm | 9400 | 9600 | 9300 | 9433.33 | 152.75 | 10.13 |
| TOC | ppm | 2383 | 2433 | 2456 | 2424.00 | 37.32 | −0.76 |
| Conductivity | µs/cm | 8500 | 8810 | 8820 | 8710.00 | 181.93 | −23.81 |
| TSS | ppm | 5 | 5 | 5 | 5.00 | 0.00 | 76.56 |
| HIM | ppm | 1.2 | 1.3 | 1.1 | 1.20 | 0.10 | 97.04 |
| Ions and organics | |||||||
| Sulfide | ppm | 0.03 | 0.03 | 0.03 | 0.03 | 0.00 | 99.99 |
| Silica | ppm | 0.848 | 0.848 | 0.94 | 0.88 | 0.05 | 55.99 |
| Phosphate | ppm | 2.5 | 1.7 | 1.7 | 1.97 | 0.46 | 4.53 |
| Sulphate | ppm | 379 | 312 | 419 | 370.00 | 54.06 | −702.14 |
| Chloride | ppm | 2582 | 2583 | 2587.7 | 2584.23 | 3.04 | 11.53 |
| Formate | ppm | 0.39 | 0.32 | 0.33 | 0.35 | 0.04 | 0.00 |
| Acetate | ppm | 313.298 | 323.98 | 382.215 | 339.83 | 37.09 | 7.82 |
| Propionate | ppm | 15.247 | 16.773 | 17.7 | 16.57 | 1.24 | 4.57 |
| Phenol | ppm | 1.392 | 1.416 | 1.368 | 1.39 | 0.02 | 28.80 |
| Metals | |||||||
| Cadmium | ppb | 0.15 | 0.23 | 0.03 | 0.14 | 0.10 | −173.33 |
| Aluminum | ppb | ND | ND | ND | ND | ND | ND |
| Barium | ppb | 655.41 | 415.6 | 495.73 | 522.25 | 122.08 | −763.04 |
| Boron | ppb | 4850 | 5002 | 4869 | 4907.00 | 82.82 | 14.58 |
| Arsenic | ppb | 5.95 | 6.98 | 5.12 | 6.02 | 0.93 | 16.82 |
| Calcium | ppb | 449,454 | 503,736 | 534,554 | 495,914.67 | 43,085.76 | −73.66 |
| Cobalt | ppb | 0.58 | 0.59 | 0.52 | 0.56 | 0.04 | 92.00 |
| Chromium | ppb | 0.43 | 1.34 | 0.48 | 0.75 | 0.51 | 97.53 |
| Copper | ppb | 132.37 | 124.74 | 84.65 | 113.92 | 25.63 | −18,175.94 |
| Iron | ppb | 17.46 | 28.14 | 21.45 | 22.35 | 5.40 | 99.46 |
| Manganese | ppb | 7.67 | 12.21 | 12.46 | 10.78 | 2.70 | 95.83 |
| Magnesium | ppb | 53,366 | 55,000 | 50,412 | 52,926.00 | 2325.43 | −17.45 |
| Molybdenum | ppb | 0.55 | 0.55 | 0.55 | 0.55 | 0.00 | 90.04 |
| Nickel | ppb | 10.49 | 12.53 | 8.93 | 10.65 | 1.81 | −50.42 |
| Potassium | ppb | 87,793 | 87,550 | 85,247 | 86,863.33 | 1405.05 | 13.93 |
| Sodium | ppb | 1,138,533 | 1,140,287 | 1,262,204 | 1,180,341.33 | 70,900.57 | 1.49 |
| Strontium | ppb | 13,854 | 14,257 | 14,378 | 14,163.00 | 274.36 | −7.44 |
| Vanadium | ppb | 1.62 | 2 | 2.21 | 1.94 | 0.30 | 23.79 |
| Zinc | ppb | 154.18 | 156.79 | 132.16 | 147.71 | 13.53 | −2868.05 |
| Glycol and inhibitors | |||||||
| Corrosion Inhibitor | ppm | 35.1 | 36.73 | 38.16 | 36.66 | 1.53 | 94.12 |
| KHI | % | 0.1 | 0.1 | 0.1 | 0.10 | 0.00 | 62.96 |
| MEG | % | 0.01 | 0.01 | 0.01 | 0.01 | 0.00 | 96.97 |
| BTEX and TN | |||||||
| Benzene | ppb | 1006 | 519 | 773 | 766.00 | 243.58 | 93.14 |
| Ethyl benzene | ppb | 7.66 | 4.3 | 7.43 | 6.46 | 1.88 | 99.86 |
| Toluene | ppb | 353 | 158.64 | 315 | 275.55 | 103.01 | 0.94 |
| Xylene | ppb | 43.04 | 24.56 | 46.95 | 38.18 | 11.96 | 96.70 |
| TN | ppm | 17.2 | 17.5 | 16.84 | 17.18 | 0.33 | 63.77 |
| Produced Water Treatment Methods | |||||
|---|---|---|---|---|---|
| Microfiltration | Ultrafiltration | Reverse Osmosis | Adsorption | Ion-Exchange | |
| Advantages |
|
|
|
|
|
| Drawbacks |
|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Ajmi, F.; Al-Marri, M.; Almomani, F.; AlNouss, A. A Comprehensive Review of Advanced Treatment Technologies for the Enhanced Reuse of Produced Water. Water 2024, 16, 3306. https://doi.org/10.3390/w16223306
Al-Ajmi F, Al-Marri M, Almomani F, AlNouss A. A Comprehensive Review of Advanced Treatment Technologies for the Enhanced Reuse of Produced Water. Water. 2024; 16(22):3306. https://doi.org/10.3390/w16223306
Chicago/Turabian StyleAl-Ajmi, Fahad, Mohammed Al-Marri, Fares Almomani, and Ahmed AlNouss. 2024. "A Comprehensive Review of Advanced Treatment Technologies for the Enhanced Reuse of Produced Water" Water 16, no. 22: 3306. https://doi.org/10.3390/w16223306
APA StyleAl-Ajmi, F., Al-Marri, M., Almomani, F., & AlNouss, A. (2024). A Comprehensive Review of Advanced Treatment Technologies for the Enhanced Reuse of Produced Water. Water, 16(22), 3306. https://doi.org/10.3390/w16223306







