Quick Groundwater Flow to Tropical Savanna Springs (Mataranka, Northern Territory, Australia)
Abstract
1. Introduction
2. Methods
2.1. Study Area
2.2. Sample Collection
2.3. Sample Analyses
2.4. Precipitation, River Height, and Groundwater Depth Data
3. Results
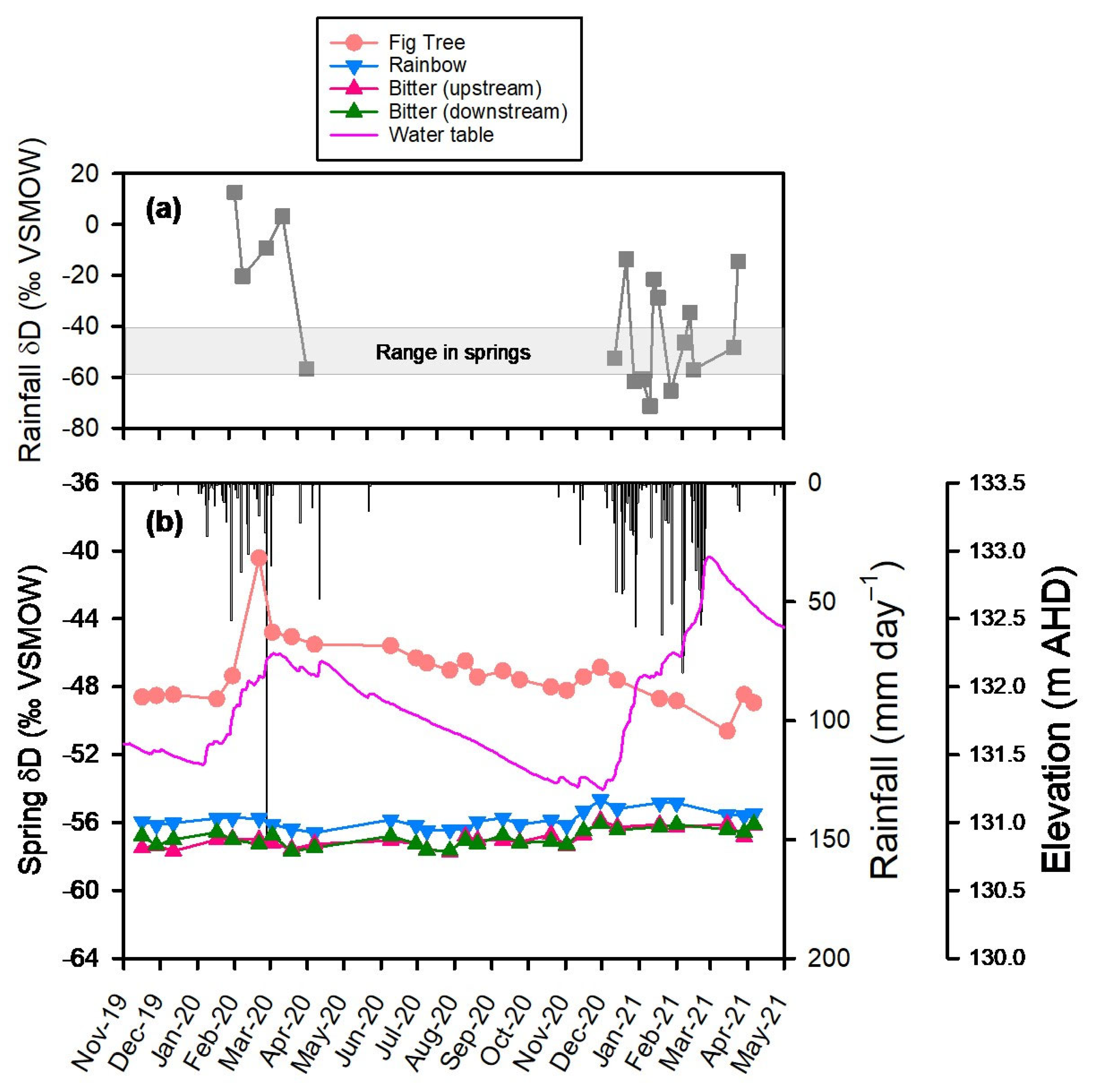
3.1. Rainfall, Groundwater and River Flow Dynamics
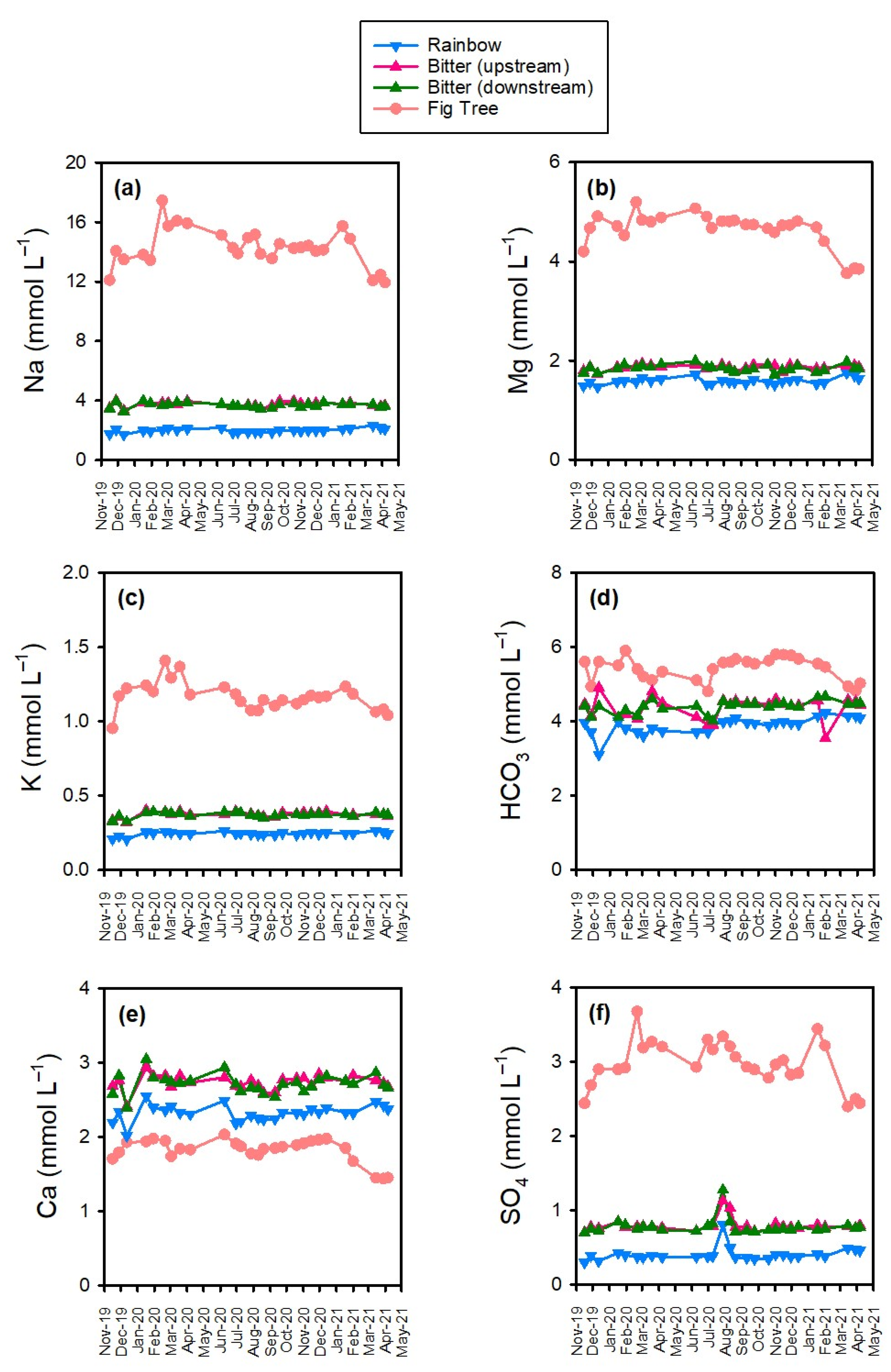
3.2. Major Ions
3.3. Trends in δD and δ18O over Time
3.4. Comparison of Spring Isotopic Composition to the Local Meteoric Water Lines
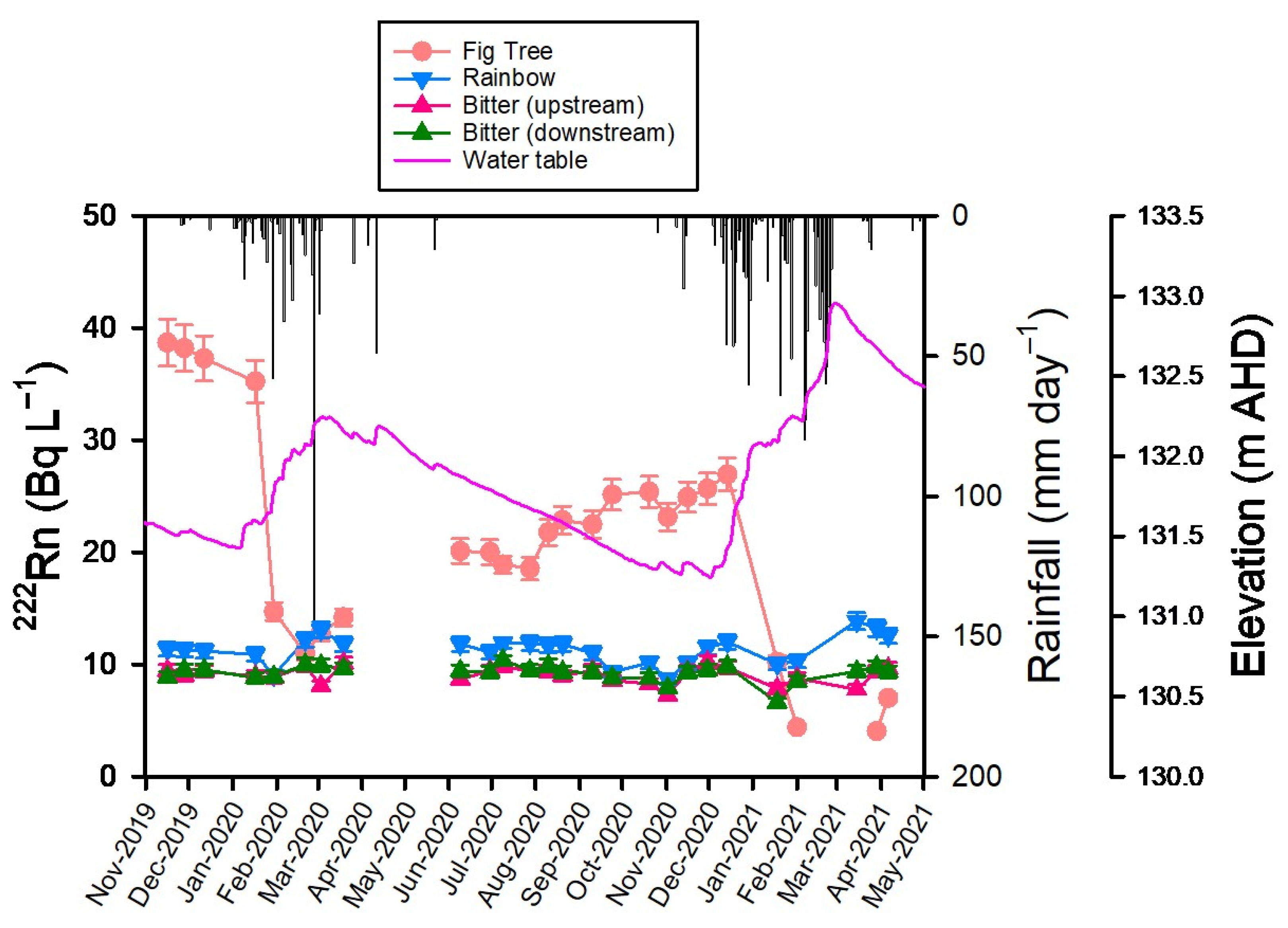
3.5. Radon-222
4. Discussion
4.1. Isotopic Response at Fig Tree Spring
4.2. Major Ions and Radon-222 at Fig Tree Spring
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cantonati, M.; Stevens, L.; Segadelli, S.; Springer, A.; Goldscheider, N.; Celico, F.; Filippini, M.; Ogata, K.; Gargini, A. Ecohydrogeology: The interdisciplinary convergence needed to improve the study and stewardship of springs and other groundwater-dependent habitats, biota, and ecosystems. Ecol. Indic. 2020, 110, 105803. [Google Scholar] [CrossRef]
- Cartwright, J.M.; Dwire, K.A.; Freed, Z.; Hammer, S.J.; McLaughlin, B.; Misztal, L.W.; Schenk, E.R.; Spence, J.R.; Springer, A.E.; Stevens, L.E. Oases of the future? Springs as potential hydrologic refugia in drying climates. Front. Ecol. Environ. 2020, 18, 245–253. [Google Scholar] [CrossRef]
- Currell, M.J.; Katz, B.G. Protecting springs in a changing world through sound science and policy. In Threats to Springs in a Changing World; Wiley: Hoboken, NJ, USA, 2022; pp. 1–5. [Google Scholar]
- Davis, J.A.; Kerezsy, A.; Nicol, S. Springs: Conserving perennial water is critical in arid landscapes. Biol. Conserv. 2017, 211, 30–35. [Google Scholar] [CrossRef]
- Lamontagne, S.; Suckow, A.; Gerber, C.; Deslandes, A.; Wilske, C.; Tickell, S. Groundwater sources for the Mataranka Springs (Northern Territory, Australia). Sci. Rep. 2021, 11, 24288. [Google Scholar] [CrossRef] [PubMed]
- Smerdon, B.D.; Payton Gardner, W.; Harrington, G.A.; Tickell, S.J. Identifying the contribution of regional groundwater to the baseflow of a tropical river (Daly River, Australia). J. Hydrol. 2012, 464–465, 107–115. [Google Scholar] [CrossRef]
- Toth, D.J.; Katz, B.G. Mixing of shallow and deep groundwater as indicated by the chemistry and age of karstic springs. Hydrogeol. J. 2006, 14, 1060–1080. [Google Scholar] [CrossRef]
- Suckow, A.; Gerber, C. Environmental tracers to study the origin and timescales of spring water. In Threats to Springs in a Changing World; Wiley: Hoboken, NJ, USA, 2022; pp. 85–109. [Google Scholar]
- Lerback, J.C.; Bowen, B.B.; Humphrey, C.E.; Fernandez, D.P.; Bernau, J.A.; Macfarlan, S.J.; Schniter, E.; Garcia, J.J. Geochemistry and Provenance of Springs in a Baja California Sur Mountain Catchment. Groundwater 2022, 60, 295–308. [Google Scholar] [CrossRef]
- Duvert, C.; Lim, H.-S.; Irvine, D.J.; Bird, M.I.; Bass, A.M.; Tweed, S.O.; Hutley, L.B.; Munksgaard, N.C. Hydrological processes in tropical Australia: Historical perspective and the need for a catchment observatory network to address future development. J. Hydrol. Reg. Stud. 2022, 43, 101194. [Google Scholar] [CrossRef]
- Karp, D. Surface and Groundwater Interaction in the Mataranka Area; Report No. Technical Report 17/2008D; NT Government: Palmerston, NT, Australia, 2008. [Google Scholar]
- Karp, D. Evaluation of Groundwater Flow by Dye Tracing Katherine Region; Report No. Report 22/2005D; NT Government: Palmerston, NT, Australia, 2005. [Google Scholar]
- Koeniger, P.; Toll, M.; Himmelsbach, T. Stable isotopes of precipitation and spring waters reveal an altitude effect in the Anti-Lebanon Mountains, Syria. Hydrol. Process. 2016, 30, 2851–2860. [Google Scholar] [CrossRef]
- Salas-Navarro, J.; Sánchez-Murillo, R.; Esquivel-Hernández, G.; Corrales-Salazar, J.L. Hydrogeological responses in tropical mountainous springs. Isot. Environ. Health Stud. 2019, 55, 25–40. [Google Scholar] [CrossRef]
- Tarafdar, S.; Bruijnzeel, L.A.; Kumar, B. Improved understanding of spring and stream water responses in headwaters of the Indian Lesser Himalaya using stable isotopes, conductivity and temperature as tracers. Hydrol. Sci. J. 2019, 64, 757–770. [Google Scholar] [CrossRef]
- Busenberg, E.; Plummer, L.N. A 17-Year Record of Environmental Tracers in Spring Discharge, Shenandoah National Park, Virginia, USA: Use of Climatic Data and Environmental Conditions to Interpret Discharge, Dissolved Solutes, and Tracer Concentrations. Aquat. Geochem. 2014, 20, 267–290. [Google Scholar] [CrossRef]
- Zwart, C.; Munksgaard, N.C.; Protat, A.; Kurita, N.; Lambrinidis, D.; Bird, M.I. The isotopic signature of monsoon conditions, cloud modes, and rainfall type. Hydrol. Process. 2018, 32, 2296–2303. [Google Scholar] [CrossRef]
- Zwart, C.; Munksgaard, N.C.; Kurita, N.; Bird, M.I. Stable isotopic signature of Australian monsoon controlled by regional convection. Quat. Sci. Rev. 2016, 151, 228–235. [Google Scholar] [CrossRef]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef]
- Taylor, A.; Crosbie, R.S.; Turnadge, C.; Lamontagne, S.; Deslandes, A.; Davies, P.J.; Barry, K.; Suckow, A.; Knapton, A.; Marshall, S.; et al. Hydrogeological Assessment of the Cambrian Limestone Aquifer and the Dook Creek Aquifer in the Roper Catchment, Northern Territory; CSIRO Technical Report; CSIRO: Canberra, Australia, 2024. [Google Scholar]
- Crosbie, R.S.; Rachakonda, P.K. Constraining probabilistic chloride mass-balance recharge estimates using baseflow and remotely sensed evapotranspiration: The Cambrian Limestone Aquifer in northern Australia. Hydrogeol. J. 2021, 29, 1399–1419. [Google Scholar] [CrossRef]
- Leaney, F.W.; Herczeg, A.L. A rapid field extraction method for determination of radon-222 in natural waters by liquid scintillation counting. Limnol. Oceanogr. Methods 2006, 4, 254–259. [Google Scholar] [CrossRef]
- Gröning, M.; Lutz, H.; Roller-Lutz, Z.; Kralik, M.; Gourcy, L.; Pöltenstein, L. A simple rain collector preventing water re-evaporation dedicated for δ18O and δ2H analysis of cumulative precipitation samples. J. Hydrol. 2012, 448–449, 195–200. [Google Scholar] [CrossRef]
- Munksgaard, N.C.; Wurster, C.M.; Bird, M.I. Continuous analysis of δ18O and δD values of water by diffusion sampling cavity ring-down spectrometry: A novel sampling device for unattended field monitoring of precipitation, ground and surface waters. Rapid Commun. Mass Spectrom. 2011, 25, 3706–3712. [Google Scholar] [CrossRef]
- Crosbie, R.; Morrow, P.; Cresswell, R.; Leaney, F.; Lamontagne, S.; Lefournour, M. New Insights into the Chemical and Isotopic Composition of Rainfall across Australia; CSIRO Water for a Healthy Country Flagship; CSIRO: Canberra, Australia, 2012. [Google Scholar]
- Małoszewski, P.; Rauert, W.; Stichler, W.; Herrmann, A. Application of flow models in an alpine catchment area using tritium and deuterium data. J. Hydrol. 1983, 66, 319–330. [Google Scholar] [CrossRef]
- Awaleh, M.O.; Boschetti, T.; Adaneh, A.E.; Chirdon, M.A.; Ahmed, M.M.; Dabar, O.A.; Soubaneh, Y.D.; Egueh, N.M.; Kawalieh, A.D.; Kadieh, I.H.; et al. Origin of nitrate and sulfate sources in volcano-sedimentary aquifers of the East Africa Rift System: An example of the Ali-Sabieh groundwater (Republic of Djibouti). Sci. Total Environ. 2022, 804, 150072. [Google Scholar] [CrossRef] [PubMed]
- Stadler, S.; Osenbrück, K.; Suckow, A.O.; Himmelsbach, T.; Hötzl, H. Groundwater flow regime, recharge and regional-scale solute transport in the semi-arid Kalahari of Botswana derived from isotope hydrology and hydrochemistry. J. Hydrol. 2010, 388, 291–303. [Google Scholar] [CrossRef]
- Stadler, S.; Osenbrück, K.; Knöller, K.; Suckow, A.; Sültenfuß, J.; Oster, H.; Himmelsbach, T.; Hötzl, H. Understanding the origin and fate of nitrate in groundwater of semi-arid environments. J. Arid Environ. 2008, 72, 1830–1842. [Google Scholar] [CrossRef]
- Rovan, L.; Zuliani, T.; Horvat, B.; Kanduč, T.; Vreča, P.; Jamil, Q.; Čermelj, B.; Bura-Nakić, E.; Cukrov, N.; Štrok, M.; et al. Uranium isotopes as a possible tracer of terrestrial authigenic carbonate. Sci. Total Environ. 2021, 797, 149103. [Google Scholar] [CrossRef]





| Spring/Bore | Longitude | Latitude | Screen Interval (m Below Ground Surface) | Ground Elevation (m AHD) | Geological Formation |
|---|---|---|---|---|---|
| Bitter Sp. (Up.) | 133.08997 | −14.91348 | |||
| Bitter Sp. (Dn.) | 133.08982 | −14.91253 | |||
| Rainbow Spring | 133.13702 | −14.92259 | |||
| Fig Tree Spring | 133.21535 | −14.95115 | |||
| RN034230 | 133.09292 | −14.93610 | 12–18 | 130.93 | Tindall Limestone |
| RN035796 | 133.13818 | −14.93191 | 20–26 | 121.58 | Tindall Limestone |
| RN034032 | 133.16431 | −14.93903 | 9.5–16 | 123.45 | Tindall L./tufa |
| RN035926 | 133.12994 | −14.97154 | 16–22 | 132.97 | Tindall Limestone |
| RN034031 | 133.23317 | −15.00226 | 35–41 | 136.61 | Tindall Limestone |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamontagne, S.; Duvert, C.; Suckow, A. Quick Groundwater Flow to Tropical Savanna Springs (Mataranka, Northern Territory, Australia). Water 2024, 16, 3395. https://doi.org/10.3390/w16233395
Lamontagne S, Duvert C, Suckow A. Quick Groundwater Flow to Tropical Savanna Springs (Mataranka, Northern Territory, Australia). Water. 2024; 16(23):3395. https://doi.org/10.3390/w16233395
Chicago/Turabian StyleLamontagne, Sébastien, Clément Duvert, and Axel Suckow. 2024. "Quick Groundwater Flow to Tropical Savanna Springs (Mataranka, Northern Territory, Australia)" Water 16, no. 23: 3395. https://doi.org/10.3390/w16233395
APA StyleLamontagne, S., Duvert, C., & Suckow, A. (2024). Quick Groundwater Flow to Tropical Savanna Springs (Mataranka, Northern Territory, Australia). Water, 16(23), 3395. https://doi.org/10.3390/w16233395







