The Pyrolysis of Biosolids in a Novel Closed Coupled Pyrolysis and Gasification Technology: Pilot Plant Trials, Aspen Plus Modelling, and a Techno-Economic Analysis
Abstract
1. Introduction
2. Materials and Methods
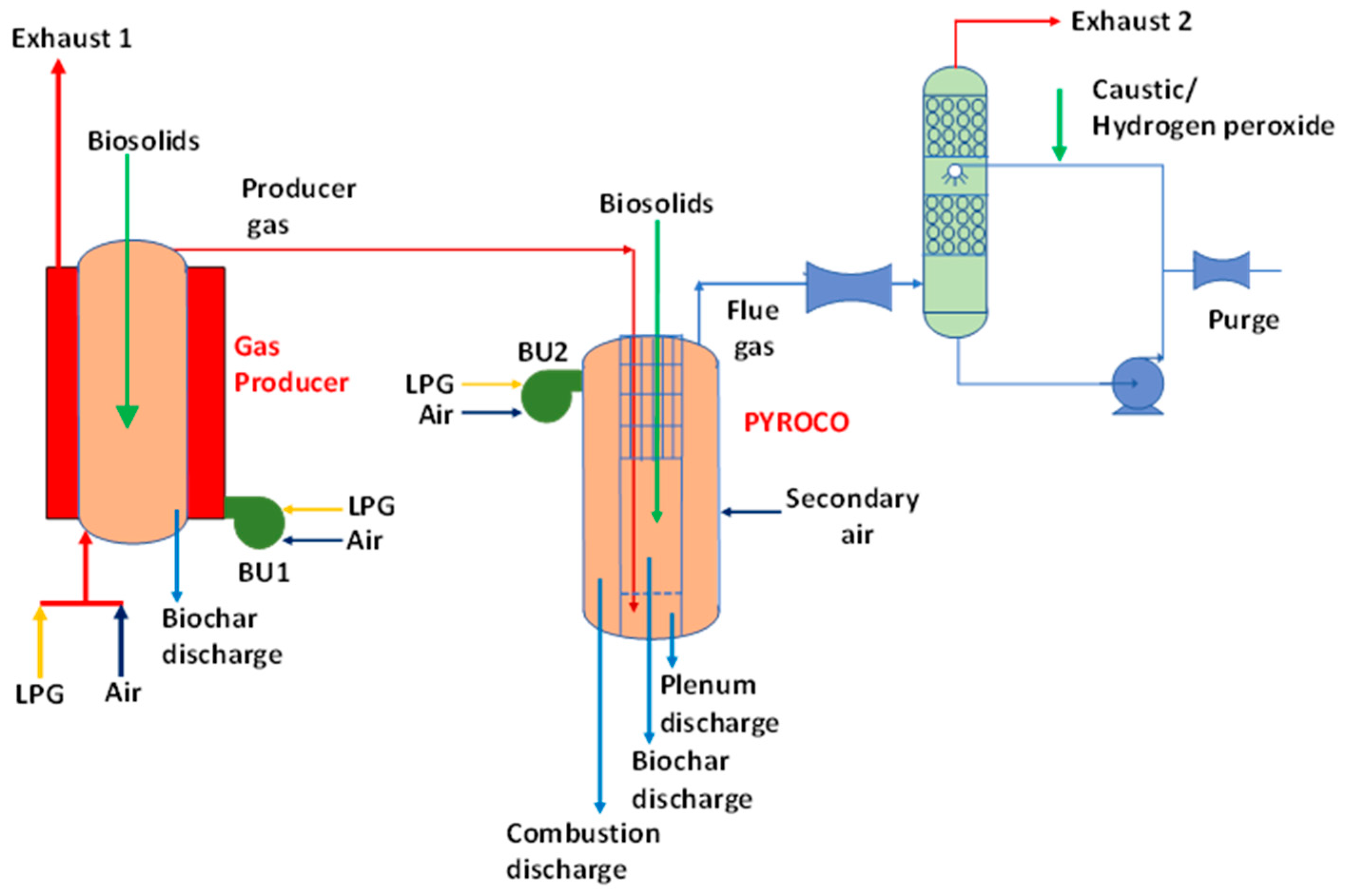
2.1. PYROCO™ Pilot Plant Trials
2.1.1. Sample and Preparation of Biosolids
2.1.2. Description of the Pilot Plant and Experimental Matrix
2.2. Aspen Plus Modelling
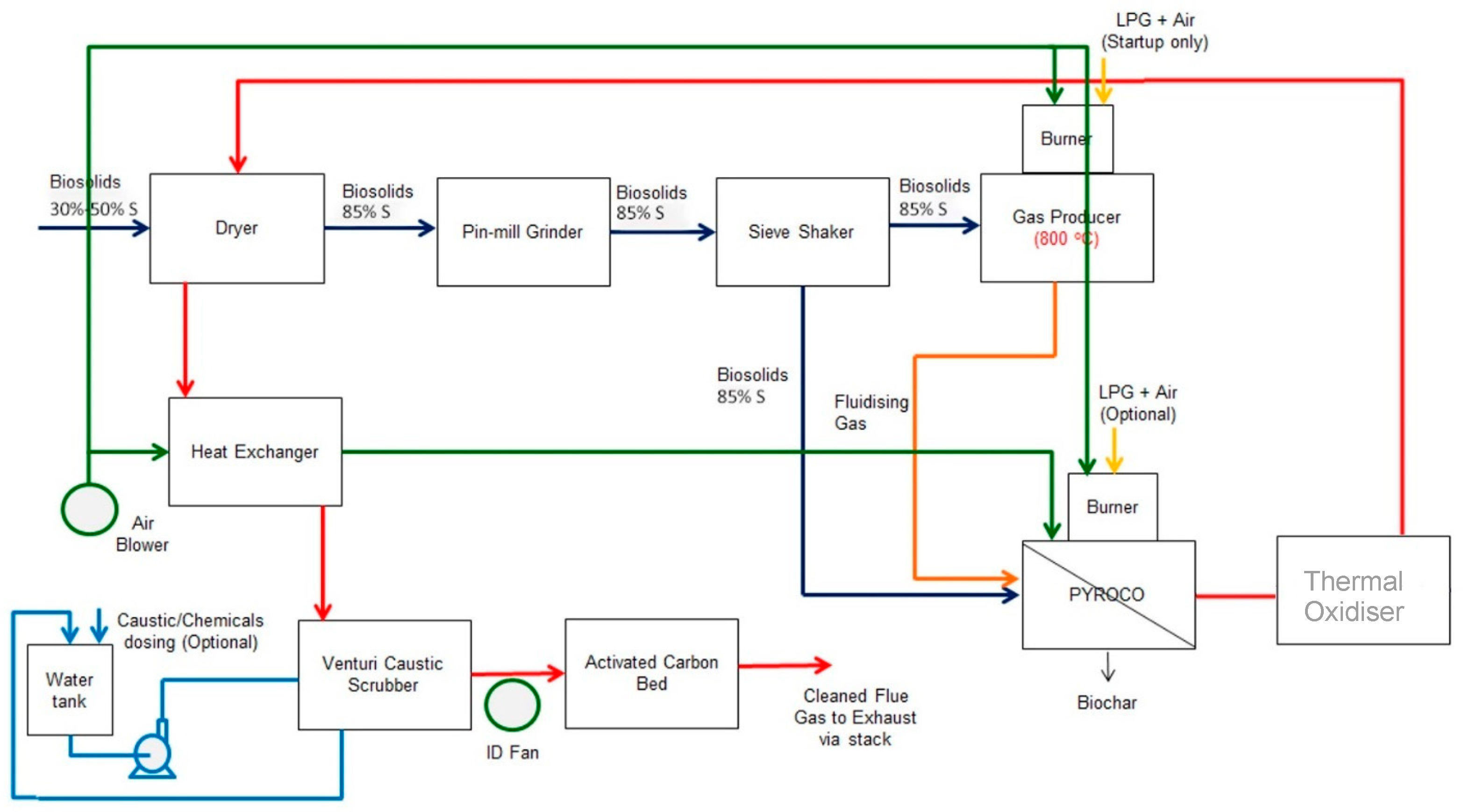
2.2.1. Process Flow Diagram of the Proposed Fully Integrated PYROCO™ Plant
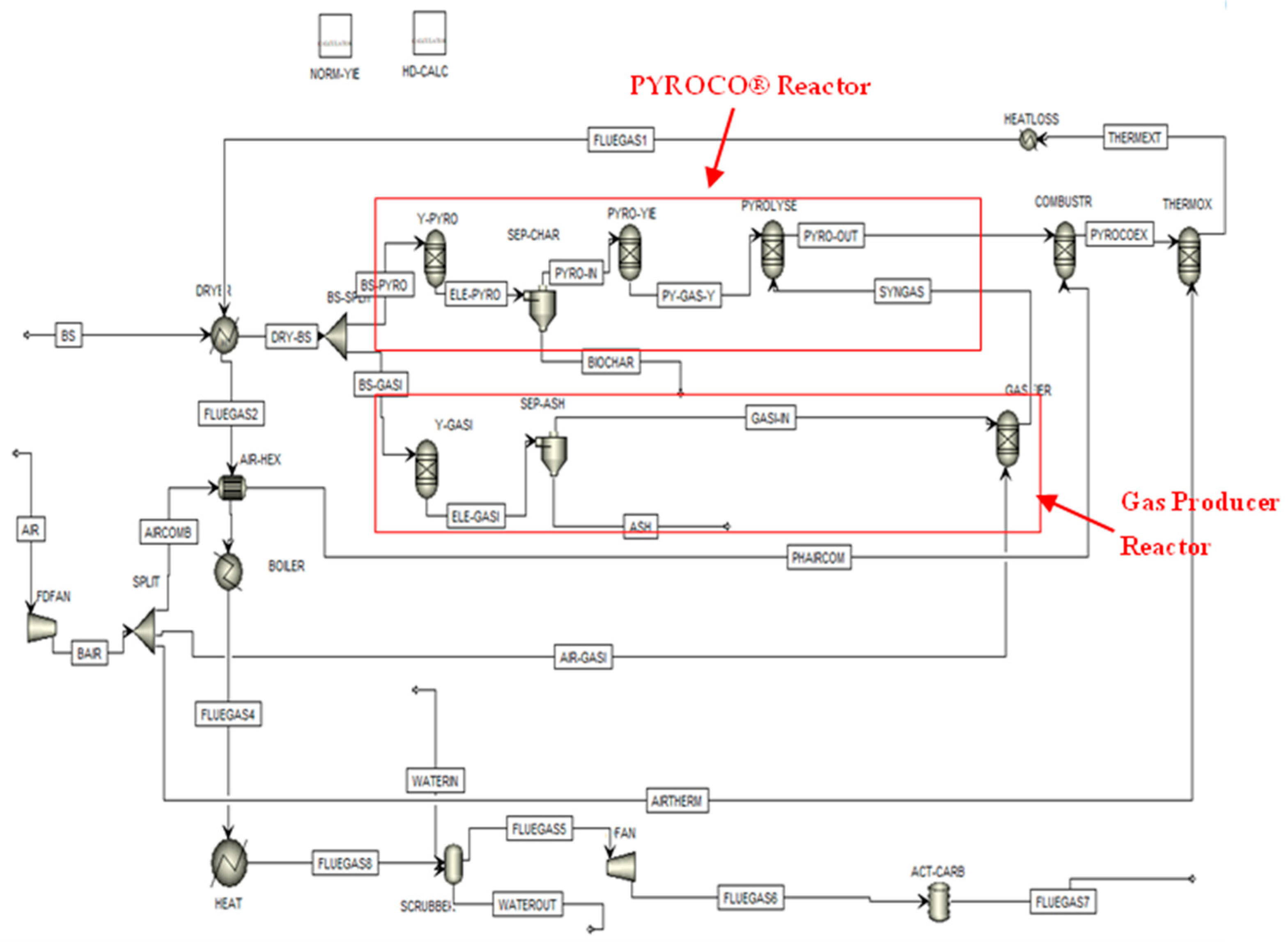
2.2.2. Aspen Flowsheet Development
- The biosolids were homogeneous, and the chemical composition was uniform throughout the material.
- The input data, including heat capacity, enthalpy, and entropy, represented the thermodynamic properties of the biosolids.
- The pyrolysis reactions followed thermodynamic (equilibrium) rate conversions based on experimental data, and the reaction rate was not defined in the pyrolysis reactor. However, it is assumed that close-to-equilibrium conversion values would be obtained due to high-temperature reactions.
- The pyrolysis process operated under steady-state conditions; the operating parameters, such as temperature, pressure, and feed rate, were constant throughout the reactor.
2.2.3. Model Validation
2.2.4. Energy Analysis
2.3. Economic Analysis
2.3.1. Capital Cost Estimation
2.3.2. Estimation of Operating Costs
2.3.3. Calculations of Net Present Value (NPV), Payback Period, and Benefit-to-Cost Ratio (BCR)
3. Results and Discussion
3.1. PYROCO™ Pilot Plant Trials
3.1.1. Mass and Energy Balance
3.1.2. Estimation of Biochar Yield
3.1.3. Properties of Biochar
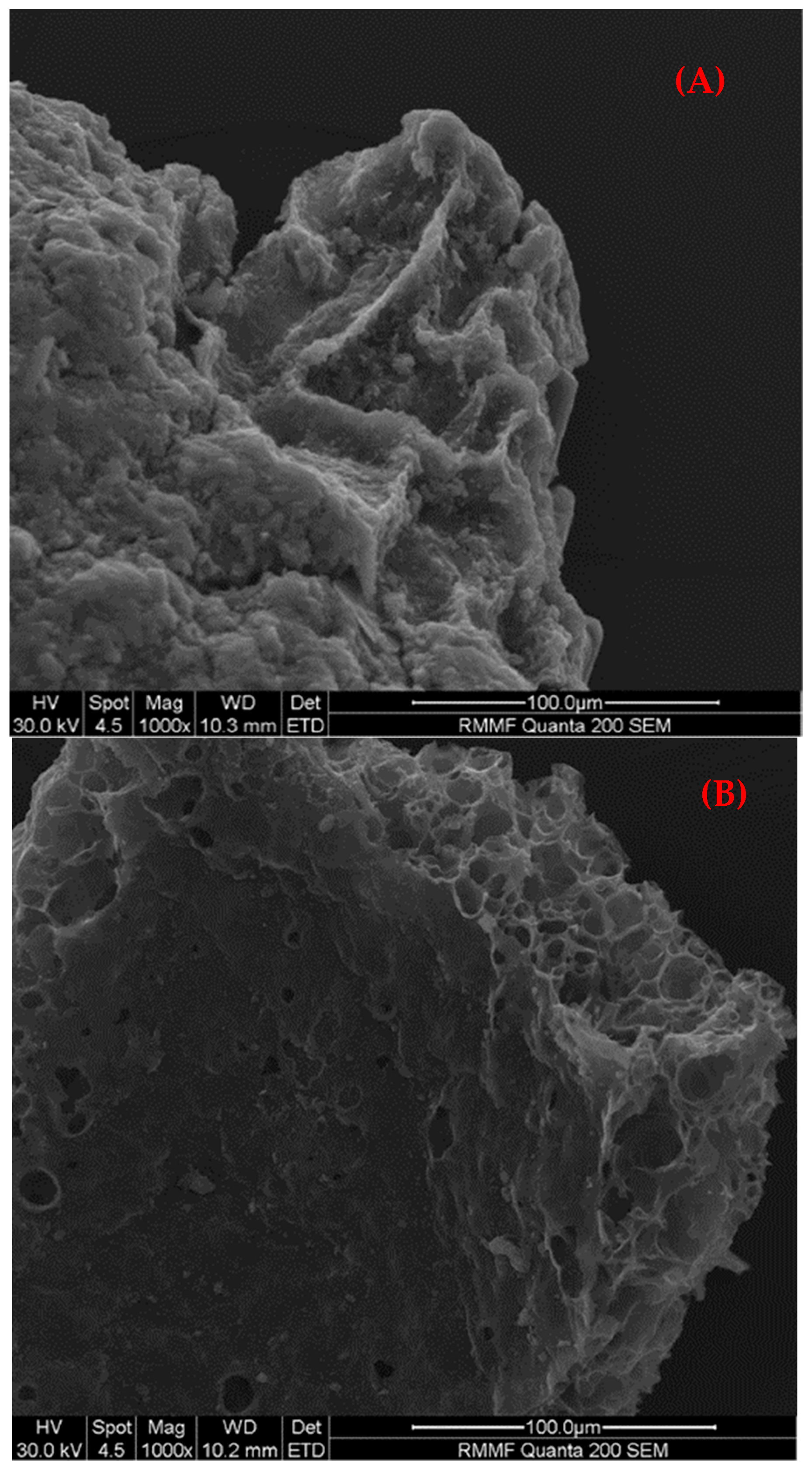
Physicochemical and Surface Properties
Compositions of Nutrients
Heavy Metal Contents
Contaminants of Emerging Concerns
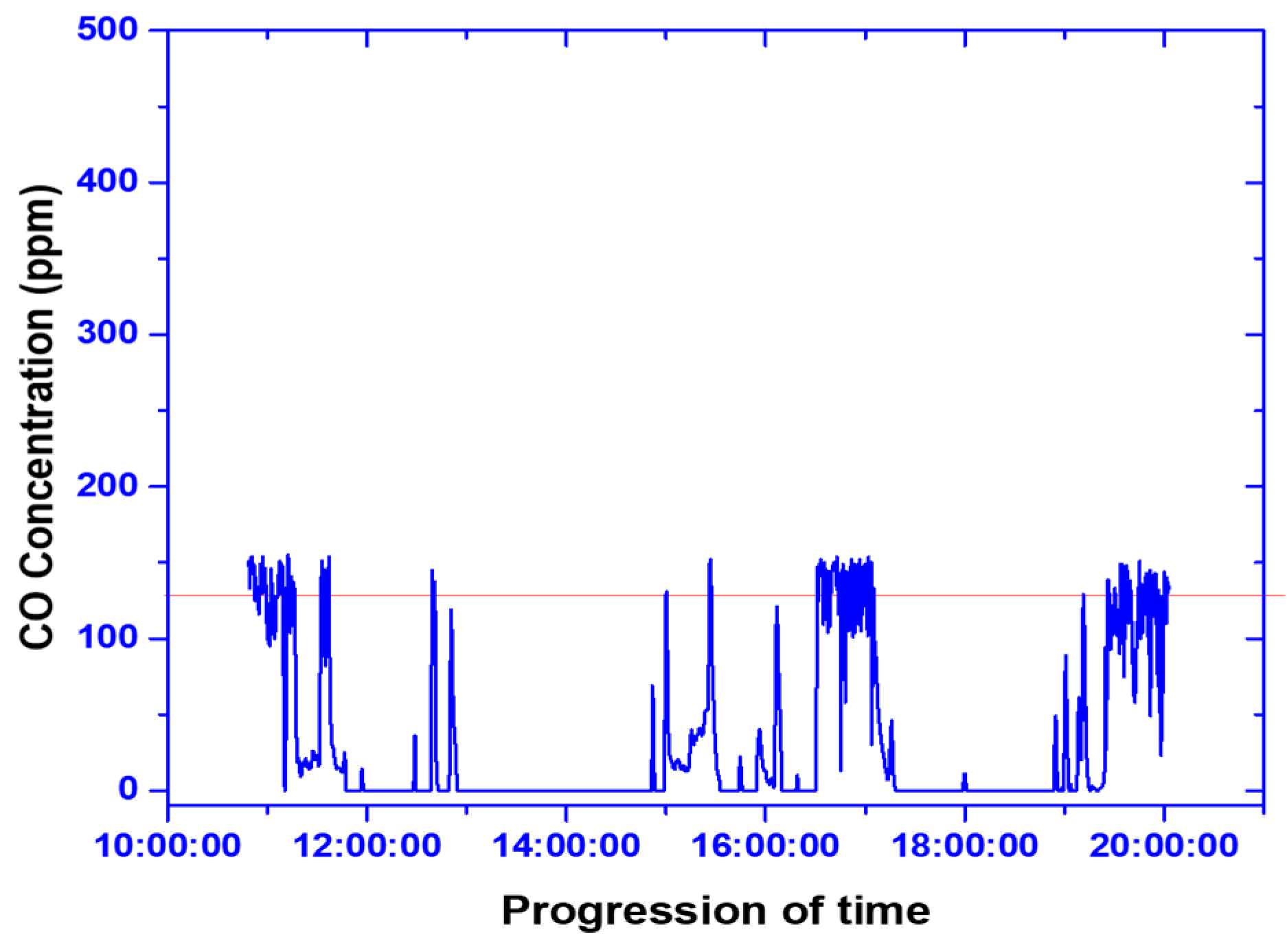
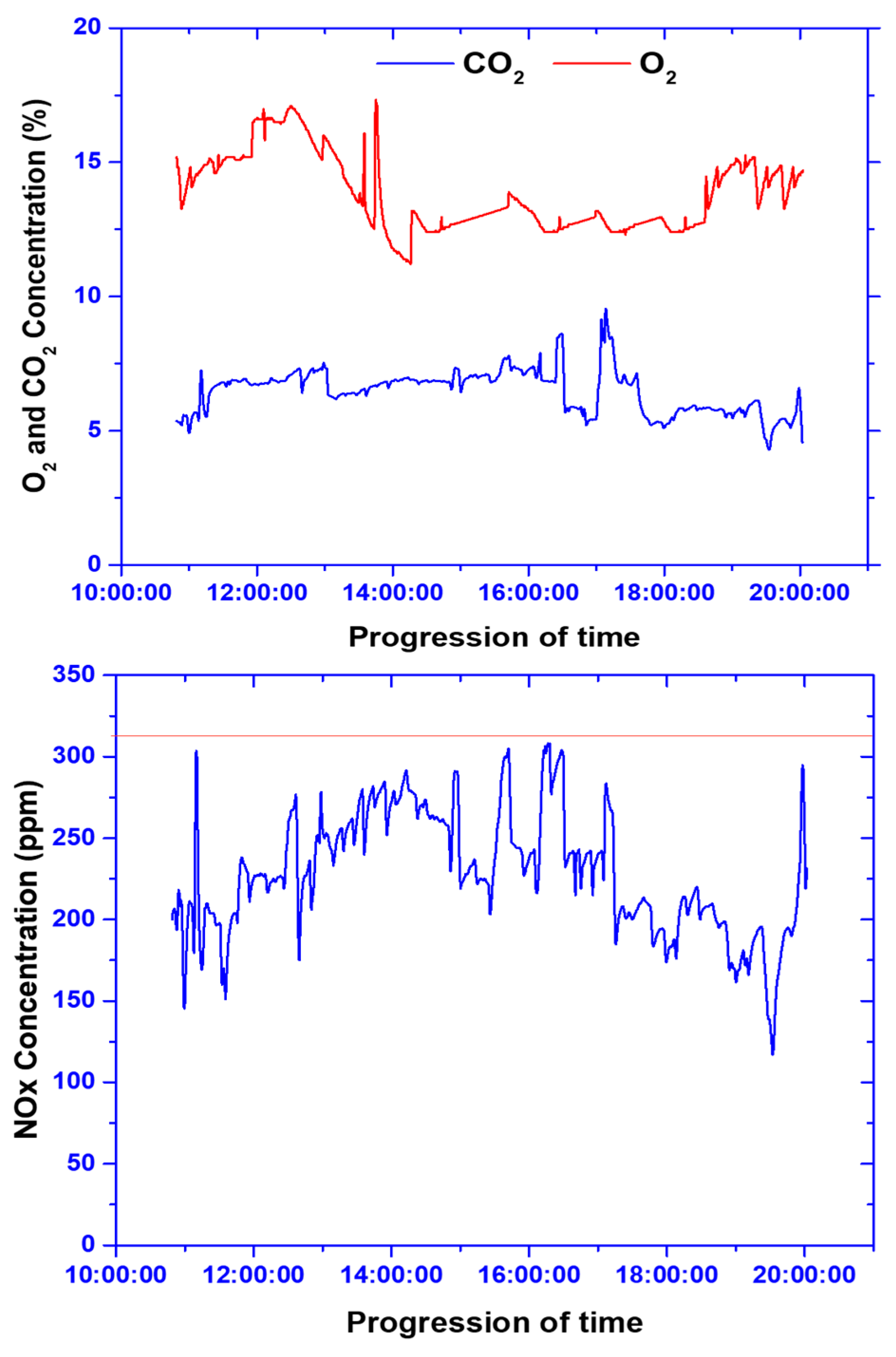
3.1.4. Stack Gas Emission Analysis
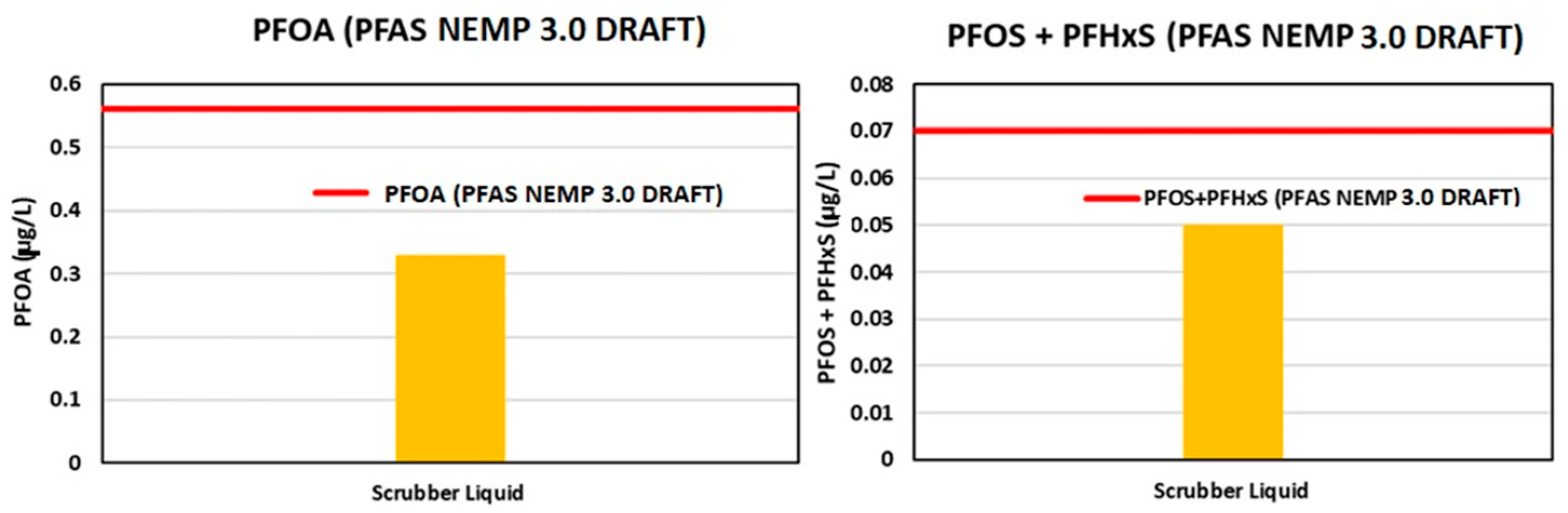
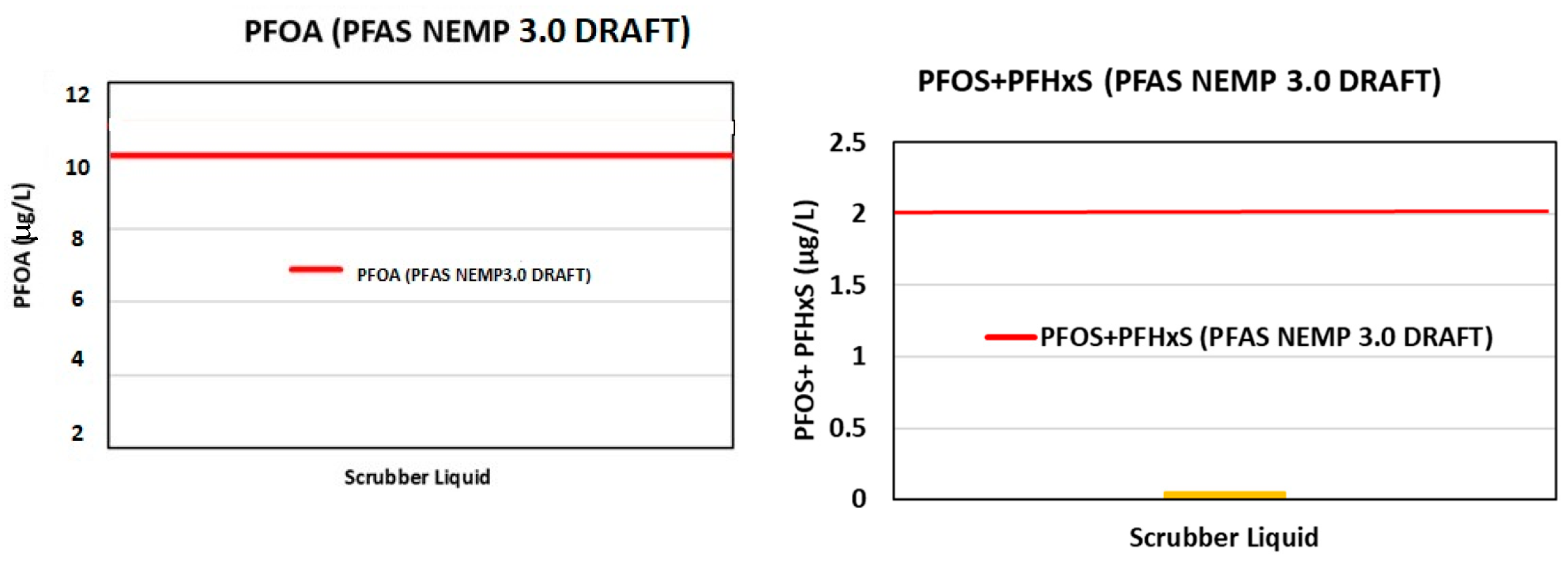
3.1.5. Scrubber Water Analysis
3.2. Validation and Results of ASPEN Process Model
3.2.1. Mass and Energy Balance
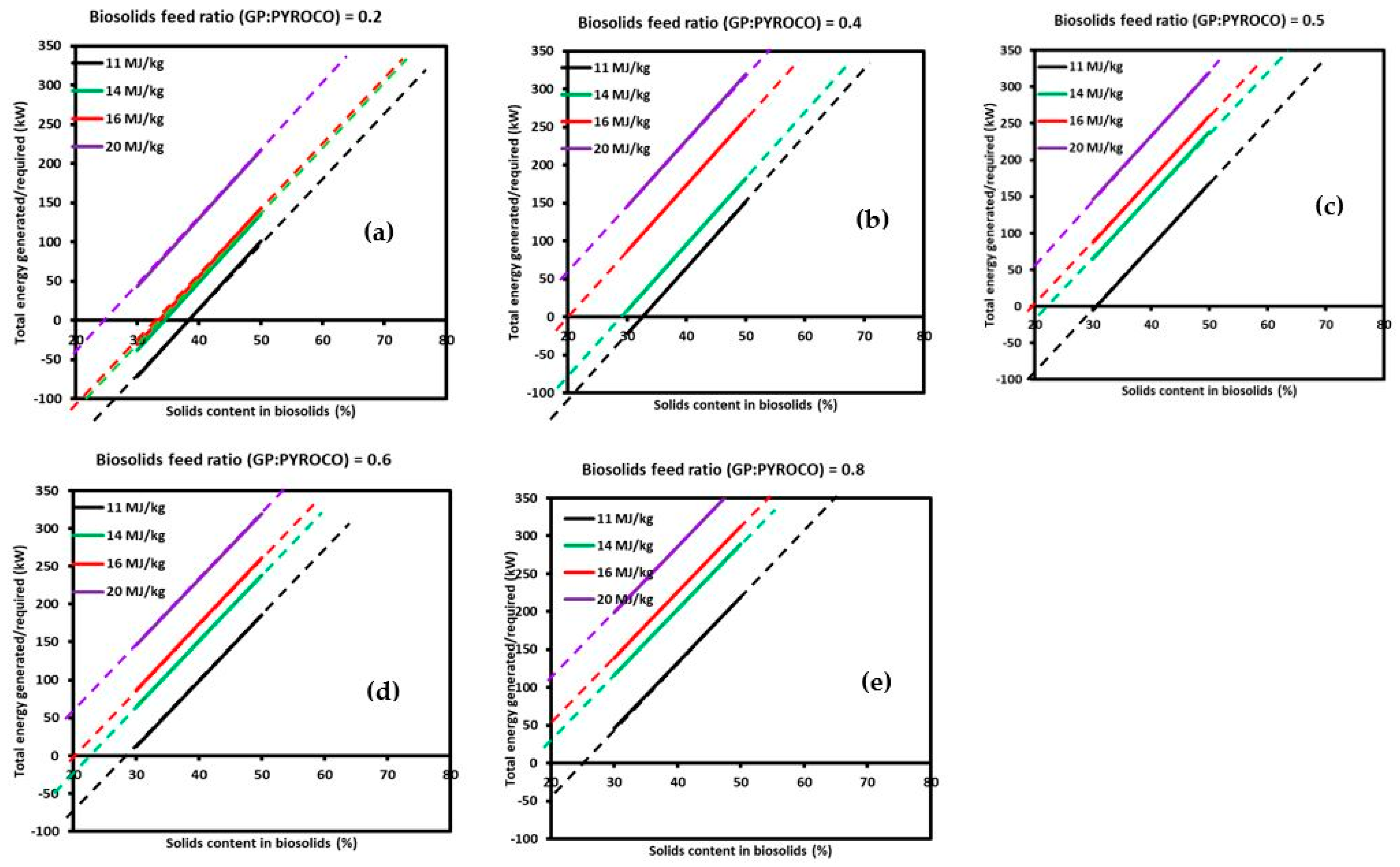
3.2.2. Extended Energy Analysis Using Validated ASPEN Process Model
3.3. Economic Analysis
3.3.1. Effect of Biochar Sale Price on NPV
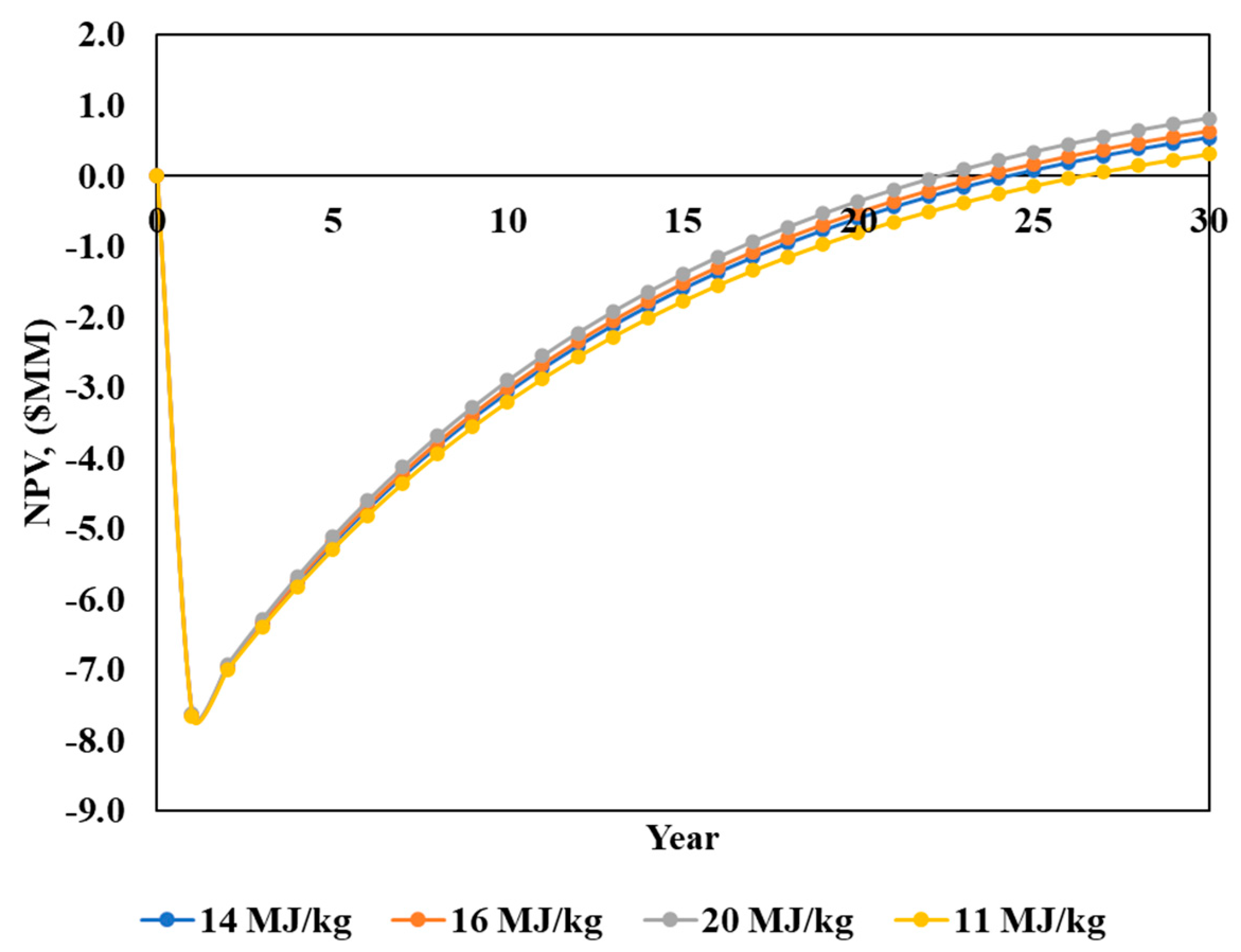
3.3.2. Effect of Biosolids’ Calorific Value on NPV
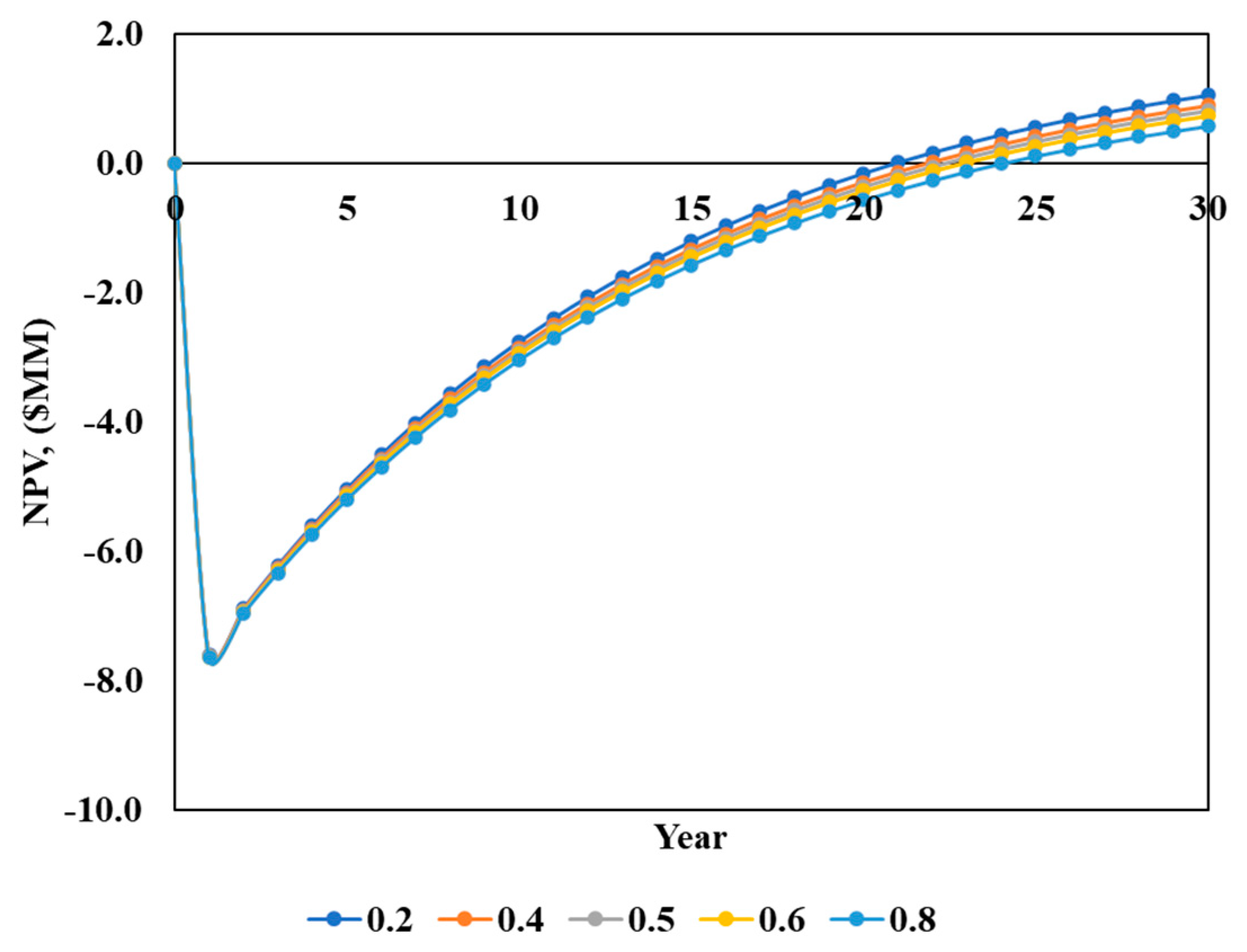
3.3.3. Effect of GP:PYROCO Ratio on NPV
3.3.4. Sensitivity Analysis of Different Economic Factors
4. Conclusions
- The PYROCO™ pilot trials successfully produced biochar with a 30% mass yield and desirable agronomic and physicochemical properties. The process produced biochar with high organic carbon and nutrient contents, the complete elimination of PFASs, and undetectable levels of PAHs, pathogens, and oestrogens. However, challenges in accurately analysing microplastics and siloxanes in biochar were identified, highlighting the need for enhanced analytical methods in subsequent trials.
- Mass and energy balances confirmed the PYROCO™ reactor’s capability for energy self-sufficiency with minimal reliance on external energy sources.
- The semi-empirical process modelling of a commercial-scale PYROCO™ plant further demonstrated that the process could operate in a thermal energy neutral mode with biosolids containing a solid content above 30%. For biosolids with a lower solid content, the system could still achieve energy self-sufficiency by adjusting the GP:PYROCO feed ratio, albeit at the cost of reduced biochar production.
- Higher calorific values of biosolids generated greater surplus energy, enhancing the process’s overall energy surplus. This finding underscores the importance of the biosolid feed quality in optimising this process’s performance.
- We conducted a preliminary economic analysis that focused on the potential revenue streams from biochar and electricity sales. This analysis suggested that while the current market prices for biochar do not offer substantial financial returns, exploring high-value applications for biochar could significantly improve the economic viability of the PYROCO™ process.
- The key economic drivers identified include the biochar sale price, the calorific value of biosolids, and the GP:PYROCO ratio. These factors influenced the NPV, indicating potential areas for strategic improvements to enhance economic outcomes.
- The results of the current techno-economic analysis should be used primarily for benchmarking purposes. For more definitive investment decisions, a more detailed study should be carried out involving technology providers to obtain detailed capital and operating cost estimates through a competitive tendering process.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Statistics, A.B. Australian Biosolids Statistics. Guidelines 2023. Available online: https://www.biosolids.com.au/guidelines/australian-biosolids-statistics/ (accessed on 27 October 2024).
- Tezel, U.; Tandukar, M.; Pavlostathis, S.G. Anaerobic Biotreatment of Municipal Sewage Sludge. In Comprehensive Biotechnology; Georgia Institute of Technology: Atlanta, GA, USA, 2011; pp. 447–461. [Google Scholar]
- Wang, H.; Brown, S.L.; Magesan, G.N.; Slade, A.H.; Quintern, M.; Clinton, P.W.; Payn, T.W. Technological options for the management of biosolids. Environ. Sci. Pollut. Res. Int. 2008, 15, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Agrafioti, E.; Bouras, G.; Kalderis, D.; Diamadopoulos, E. Biochar production by sewage sludge pyrolysis. J. Anal. Appl. Pyrolysis 2013, 101, 72–78. [Google Scholar] [CrossRef]
- Barry, D.; Barbiero, C.; Briens, C.; Berruti, F. Pyrolysis as an economical and ecological treatment option for municipal sewage sludge. Biomass Bioenergy 2019, 122, 472–480. [Google Scholar] [CrossRef]
- Liu, Z.; McNamara, P.; Zitomer, D. Autocatalytic Pyrolysis of Wastewater Biosolids for Product Upgrading. Environ. Sci. Technol. 2017, 51, 9808–9816. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhang, W.; Wang, S.; Zhuang, L.; Yang, Y.; Qiu, R. Characterisation of sewage sludge-derived biochars from different feedstocks and pyrolysis temperatures. J. Anal. Appl. Pyrolysis 2013, 102, 137–143. [Google Scholar] [CrossRef]
- Patel, S.; Kundu, S.; Paz-Ferreiro, J.; Surapaneni, A.; Fouche, L.; Halder, P.; Setiawan, A.; Shah, K. Transformation of biosolids to biochar: A case study. Environ. Prog. Sustain. Energy 2018, 38, 13113. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Nieto, A.; Méndez, A.; Askeland, M.P.J.; Gascó, G. Biochar from Biosolids Pyrolysis: A Review. Int. J. Environ. Res. Public Health 2018, 15, 956. [Google Scholar] [CrossRef]
- Sánchez, M.; Martínez, O.; Gómez, X.; Morán, A. Pyrolysis of mixtures of sewage sludge and manure: A comparison of the results obtained in the laboratory (semi-pilot) and in a pilot plant. Waste Manag. 2007, 27, 1328–1334. [Google Scholar] [CrossRef]
- Agarwal, M.; Tardio, J.; Mohan, S.V. Effect of pyrolysis parameters on yield and composition of gaseous products from activated sludge: Towards sustainable biorefinery. Biomass Convers. Biorefinery 2014, 5, 227–235. [Google Scholar] [CrossRef]
- Brown, J.N. Development of a lab-scale auger reactor forbiomass fast pyrolysis and process optimizationusing response surface methodology. In Mechanical Engineering; Biorenewable Resources and Technology; Iowa State University: Ames, IA, USA, 2009. [Google Scholar]
- Khanmohammadi, Z.; Afyuni, M.; Mosaddeghi, M.R. Effect of pyrolysis temperature on chemical and physical properties of sewage sludge biochar. Waste Manag. Res. 2015, 33, 275–283. [Google Scholar] [CrossRef]
- Ledakowicz, S.; Stolarek, P.; Malinowski, A.; Lepez, O. Thermochemical treatment of sewage sludge by integration of drying and pyrolysis/autogasification. Renew. Sustain. Energy Rev. 2019, 104, 319–327. [Google Scholar] [CrossRef]
- Gianico, A.; Braguglia, C.M.; Gallipoli, A.; Montecchio, D.; Mininni, G. Land Application of Biosolids in Europe: Possibilities, Con-Straints and Future Perspectives. Water 2021, 13, 103. [Google Scholar] [CrossRef]
- Australian and New Zeland Biosolids Partnership. Land Application of BiosolidsFact Sheet. Available online: https://www.biosolids.com.au/wp-content/uploads/Land-application-of-biosolids.pdf (accessed on 27 October 2024).
- Yang, Y.; Meehan, B.; Shah, K.; Surapaneni, A.; Hughes, J.; Fouché, L.; Paz-Ferreiro, J. Physicochemical Properties of Biochars Produced from Biosolids in Victoria, Australia. Int. J. Environ. Res. Public Health 2018, 15, 1459. [Google Scholar] [CrossRef]
- de Figueiredo, C.C.; Pinheiro, T.D.; de Oliveira, L.E.Z.; de Araujo, A.S.; Coser, T.R.; Paz-Ferreiro, J. Direct and residual effect of biochar derived from biosolids on soil phosphorus pools: A four-year field assessment. Sci. Total Environ. 2020, 739, 140013. [Google Scholar] [CrossRef] [PubMed]
- Nicomel, N.R.; Li, L.Y.; Du Laing, G. Biosolids-based activated carbon for enhanced copper removal from citric-acid-rich aqueous media. Environ. Sci. Pollut. Res. 2022, 29, 74742–74755. [Google Scholar] [CrossRef] [PubMed]
- Mong, G.R.; Chong, C.T.; Chong, W.W.F.; Ng, J.-H.; Ong, H.C.; Ashokkumar, V.; Tran, M.-V.; Karmakar, S.; Goh, B.H.H.; Yasin, M.F.M. Progress and challenges in sustainable pyrolysis technology: Reactors, feedstocks and products. Fuel 2022, 324, 124777. [Google Scholar] [CrossRef]
- Idris, J.; Shirai, Y.; Andou, Y.; Mohd Ali, A.A.; Othman, M.R.; Ibrahim, I.; Yamamoto, A.; Yasuda, N.; Hassan, M.A. Successful scaling-up of self-sustained pyrolysis of oil palm biomass under pool-type reactor. Waste Manag. Res. 2016, 34, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Thoma, E.D.; Wright, R.S.; George, I.; Krause, M.; Presezzi, D.; Villa, V.; Preston, W.; Deshmukh, P.; Kauppi, P.; Zemek, P.G. Pyrolysis processing of PFAS-impacted biosolids, a pilot study. J. Air Waste Manag. Assoc. 2022, 72, 309–318. [Google Scholar] [CrossRef]
- Kundu, S.; Patel, S.; Halder, P.; Patel, T.; Marzbali, M.H.; Pramanik, B.K.; Paz-Ferreiro, J.; de Figueiredo, C.C.; Bergmann, D.; Surapaneni, A.; et al. Removal of PFASs from biosolids using a semi-pilot scale pyrolysis reactor and the application of biosolids derived biochar for the removal of PFASs from contaminated water. Environ. Sci. Water Res. Technol. 2021, 7, 638–649. [Google Scholar] [CrossRef]
- Liu, Z.; Singer, S.; Zitomer, D.; McNamara, P. Sub-Pilot-Scale Autocatalytic Pyrolysis of Wastewater Biosolids for Enhanced Energy Recovery. Catalysts 2018, 8, 524. [Google Scholar] [CrossRef]
- Sarvi, M.; Kainulainen, A.; Malk, V.; Kaseva, J.; Rasa, K. Industrial pilot scale slow pyrolysis reduces the content of organic contaminants in sewage sludge. Waste Manag. 2023, 171, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Schlederer, F.; Martín-Hernández, E.; Vaneeckhaute, C. Ensuring safety standards in sewage sludge-derived biochar: Impact of pyrolysis process temperature and carrier gas on micropollutant removal. J. Environ. Manag. 2024, 352, 119964. [Google Scholar] [CrossRef] [PubMed]
- Campuzano, F.; Brown, R.C.; Martínez, J.D. Auger reactors for pyrolysis of biomass and wastes. Renew. Sustain. Energy Rev. 2019, 102, 372–409. [Google Scholar] [CrossRef]
- Chen, J.C.; Grace, J.R.; Golriz, M.R. Heat transfer in fluidised beds: Design methods. Powder Technol. 2005, 150, 123–132. [Google Scholar] [CrossRef]
- Flegkas, S.; Birkelbach, F.; Winter, F.; Groenewold, H.; Werner, A. Profitability Analysis and Capital Cost Estimation of a Thermochemical Energy Storage System Utilising Fluidised Bed Reactors and the Reaction System MgO/Mg(OH)2. Energies 2019, 12, 4788. [Google Scholar] [CrossRef]
- Onarheim, K.; Solantausta, Y.; Lehto, J. Process Simulation Development of Fast Pyrolysis of Wood Using Aspen Plus. Energy Fuels 2014, 29, 205–217. [Google Scholar] [CrossRef]
- Li, T.Y.; Xiang, H.; Yang, Y.; Wang, J.; Yildiz, G. Prediction of char production from slow pyrolysis of lignocellulosic biomass using multiple nonlinear regression and artificial neural network. J. Anal. Appl. Pyrolysis 2021, 159, 105286. [Google Scholar] [CrossRef]
- Craig, K.R.; Mann, M.K. Cost and Performance Analysis of Biomass-Based Integrated Gasification Combined-Cycle (BIGCC) Power Systems; National Renewable Energy Laboratory: Golden, CO, USA, 1996. [Google Scholar]
- PLANT COST INDEX. 2020. Available online: https://www.chemengonline.com/site/plant-cost-index/ (accessed on 27 October 2024).
- Nieder-Heitmann, M.; Savadkouhi, S.S.; Venderbosch, R.; Leijenhorst, E.; van der Pol, E.; Vleeming, H. Technoeconomic Feasibility of a Sunflower Husk Fast Pyrolysis Value Chain for the Production of Advanced Biofuels. Bioenergy Biofuels 2022, 36, 13084–13093. [Google Scholar] [CrossRef]
- Kazi, F.K.; Fortman, J.A.; Anex, R.P.; Hsu, D.D.; Aden, A.; Dutta, A.; Kothandaraman, G. Techno-economic comparison of process technologies for biochemical ethanol production from corn stover. Fuel 2010, 89, S20–S28. [Google Scholar] [CrossRef]
- Steve, T.W. Biosolids and Residuals Management. In Municipal Technology Branc; United States Environmental Protection Agency: Washington, DC, USA, 2000. [Google Scholar]
- Patel, S.; Kundu, S.; Halder, P.; Veluswamy, G.; Pramanik, B.; Paz-Ferreiro, J.; Surapaneni, A.; Shah, K. Slow pyrolysis of biosolids in a bubbling fluidised bed reactor using biochar, activated char and lime. J. Anal. Appl. Pyrolysis 2019, 144, 104697. [Google Scholar] [CrossRef]
- Isemin, R.L.; Kuzmin, S.N.; Viryasov, D.M. Co-Fluidization of Fine Particles and Straw Pellets at Room and Elevated Temperatures. Int. J. Chem. Eng. Appl. 2012, 3, 323–327. [Google Scholar] [CrossRef]
- Mian, I.; Li, X.; Jian, Y.; Dacres, O.D.; Zhong, M.; Liu, J.; Ma, F.; Rahman, N. Kinetic study of biomass pellet pyrolysis by using distributed activation energy model and Coats Redfern methods and their comparison. Bioresour. Technol. 2019, 294, 122099. [Google Scholar] [CrossRef] [PubMed]
- Paulionytė, J.; Vaiškūnaitė, R. Research on the physical and chemical properties of sewage treatment sludge biochar and its preparation for wastewater. Moksl.–Liet. Ateitis/Sci.–Future Lith. 2023, 15, 1–6. [Google Scholar] [CrossRef]
- Phillips, C.L.; Meyer, K.M.; Garcia-Jaramillo, M.; Weidman, C.S.; Stewart, C.E.; Wanzek, T.; Grusak, M.A.; Watts, D.W.; Novak, J.; Trippe, K.M. Towards predicting biochar impacts on plant-available soil nitrogen content. Biochar 2022, 4, 9. [Google Scholar] [CrossRef]
- Rathnayake, N.; Patel, S.; Halder, P.; Aktar, S.; Pazferreiro, J.; Sharma, A.; Surapaneni, A.; Shah, K. Co-pyrolysis of biosolids with alum sludge: Effect of temperature and mixing ratio on product properties. J. Anal. Appl. Pyrolysis 2022, 163, 105488. [Google Scholar] [CrossRef]
- Rathnayake, N.; Patel, S.; Hakeem, I.G.; Pazferreiro, J.; Sharma, A.; Gupta, R.; Rees, C.; Bergmann, D.; Blackbeard, J.; Surapaneni, A.; et al. Co-pyrolysis of biosolids with lignocellulosic biomass: Effect of feedstock on product yield and composition. Process Saf. Environ. Prot. 2023, 173, 75–87. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, J.; Xie, Y.; Xu, D.; Liu, Y.; Liu, J.; Hou, J. Biochar produced from the co-pyrolysis of sewage sludge and waste tire for cadmium and tetracycline adsorption from water. Water Sci. Technol. 2021, 83, 1429–1445. [Google Scholar] [CrossRef]
- Jin, J.; Wang, M.; Cao, Y.; Wu, S.; Liang, P.; Li, Y.; Zhang, J.; Zhang, J.; Wong, M.H.; Shan, S.; et al. Cumulative effects of bamboo sawdust addition on pyrolysis of sewage sludge: Biochar properties and environmental risk from metals. Bioresour. Technol. 2017, 228, 218–226. [Google Scholar] [CrossRef]
- Biosolids Land Application. In Guidelines for Environmental Management; EPA Victoria: Southbank, Victoria, Australia, 2004.
- HEPA. Draft PFAS National Environmental Management Plan, Version 3.0. 2022. Available online: https://consult.dcceew.gov.au/nemp-pfas (accessed on 27 October 2024).
- Alves, O.; Calado, L.; Panizio, R.M.; Gonçalves, M.; Monteiro, E.; Brito, P. Techno-economic study for a gasification plant processing residues of sewage sludge and solid recovered fuels. Waste Manag. 2021, 131, 148–162. [Google Scholar] [CrossRef]


| Proximate Analysis (wt%) c | Ultimate Analysis (wt%) c | |||||||
|---|---|---|---|---|---|---|---|---|
| Moisture a | Volatiles | Ash | Fixed Carbon | C | H | N | S | O b |
| 11.0 | 60.6 | 29.0 | 10.4 | 38.3 | 4.7 | 6.02 | 0.96 | 21.02 |
| Equipment | Temperature (°C) | Reaction Environment | Biosolids (kg/h) | LPG (kg/h) |
|---|---|---|---|---|
| PYROCO® | 600 and 700 | Producer gas | 12–16 | - |
| Gas producer | 800 | Partial air | - | 0.9 |
| Details | Mass Flow Rate (kg/h) | Energy (MJ/h) on a Dry Basis | |||
|---|---|---|---|---|---|
| In | Out | In | Loss/Consumed | Out | |
| Biosolids | 12 | 175 | |||
| LPG in gas producer | 0.9 | 42 | |||
| LPG in PYROCO™ pilot burner | 0.1 | 5 | |||
| Biochar from PYROCO® | 3.2 | 32 | |||
| Secondary air in PYROCO® | 5.7 | ||||
| Air in gas producer | 14.3 | ||||
| Air in PYROCO® | 104.6 | ||||
| PYROCO® | 62 | ||||
| Heat loss | 33 | ||||
| Exhaust | 134.4 | 95 | |||
| Total | 137.6 | 137.6 | 222 | 95 | 127 |
| Difference | 0 | 0 | |||
| Parameters | Units | Biosolids | Biochar (600 °C) | Biochar (700 °C) |
|---|---|---|---|---|
| Electrical conductivity (EC) | uS/cm | 6700 | 1100 | 1000 |
| pH | Units | 7.3 | 11.7 | 11.6 |
| Total solids | % w/wet | 81 | 100 | 100 |
| Volatile total solids | % | 64 | 20 | 20 |
| Total organic carbon | mg/kg | 240,000 | 220,000 | 210,000 |
| C:N ratio | 5.4 | 12.2 | 13.1 | |
| BET-specific surface area | m2/g | 2.3 | 11.7 | 25.1 |
| Nutrients | Units | Biosolids | Biochar (600 °C) | Biochar (700 °C) |
|---|---|---|---|---|
| Nitrogen (N) | ||||
| Total Kjeldahl nitrogen (TKN) | mg/kg | 43,000 | 18,000 | 16,000 |
| Ammonia (NH3) | mg N/kg | 7400 | 170 | 30 |
| Nitrite (NO2) | mg/kg | 1.3 | <0.2 | <0.2 |
| Nitrate (NO3) | mg/kg | 740 | <0.2 | <0.2 |
| NOx | mg N/kg | 740 | <0.1 | 0.1 |
| Total nitrogen | mg N/kg | 44,000 | 18,000 | 16,000 |
| Phosphorus (P) | ||||
| Total P | mg/kg | 25,000 | 36,000 | 42,000 |
| Olsen P | mg/kg | 1400 | 83 | 65 |
| Total potassium (K) | mg/kg | 4400 | 6600 | 8100 |
| Sulphur (S) | ||||
| OESEXTRA/S | mg/kg | 10,000 | 6700 | 7800 |
| Total sulfur | % | 0.4 | 0.95 | 1.05 |
| Total calcium (Ca) | mg/kg | 51,000 | 72,000 | 87,000 |
| Total magnesium (Mg) | mg/kg | 5800 | 6900 | 7400 |
| Total sodium (Na) | mg/kg | 3300 | 4400 | 5100 |
| Heavy Metals | Biosolids | Biochar (600 °C) | Biochar (700 °C) | VIC EPA [46] | International Biochar Initiative Guidelines [17] | |
|---|---|---|---|---|---|---|
| C1 Grade | C2 Grade | |||||
| Arsenic | <5 | <5 | 5 | 20 | 60 | 13–100 |
| Cadmium | 1.4 | 1.2 | 1.1 | 1 | 10 | 1.4–39 |
| Chromium | 28 | 49 | 45 | 400 | 3000 | 93–1200 |
| Copper | 720 | 900 | 1000 | 100 | 2000 | 143–6000 |
| Mercury | 0.94 | <0.05 | <0.05 | 1 | 5 | 1–17 |
| Nickel | 23 | 28 | 28 | 60 | 270 | 47–420 |
| Lead | 20 | 39 | 40 | 300 | 500 | 121–300 |
| Selenium | 6 | 6 | 7 | 3 | 50 | 2–200 |
| Zinc | 1100 | 1500 | 1700 | 200 | 2500 | 416–7400 |
| PFAS Compound Name | Limit of Reporting (LOR) | Biosolids | Biochar (600 °C) | Biochar (700 °C) |
|---|---|---|---|---|
| 10:2 Fluorotelomer Sulfonic Acid | <2.5 | <2.5 | <2.5 | <2.5 |
| 4:2 Fluorotelomer Sulfonic Acid | <2.5 | <2.5 | <2.5 | <2.5 |
| 6:2 Fluorotelomer Sulfonic Acid | <2.5 | <2.5 | <2.5 | <2.5 |
| 8:2 Fluorotelomer Sulfonic Acid | <2.5 | <2.5 | <2.5 | <2.5 |
| N-Ethyl Perfluorooctane Sulfonamido Acetic Acid (EtFOSAA) | <5 | <5 | <5 | <5 |
| N-Ethyl Perfluorooctane Sulfonamidoethanol (EtFOSE) | <5 | <5 | <5 | <5 |
| N-Methyl Perfluorooctane Sulfonamido Acetic Acid (MeFOSAA) | <10 | 12 | <10 | <10 |
| N-Methyl Perfluorooctane Sulfonamidoethanol (MeFOSE) | <5 | <5 | <5 | <5 |
| N-Ethyl Perfluorooctane Sulfonamide (EtFOSA) | <5 | <5 | <5 | <5 |
| N-Methyl Perfluorooctane Sulfonamide (MeFOSA) | <5 | <5 | <5 | <5 |
| Perfluorobutane Sulfonic Acid (PFBS) | <2.5 | 9.6 | <2.5 | <2.5 |
| Perfluorobutanoic Acid (PFBA) | <10 | <10 | <10 | <10 |
| Perfluorodecane Sulfonic Acid (PFDS) | <2.5 | <2.5 | <2.5 | <2.5 |
| Perfluorodecanoic Acid (PFDA) | <2.5 | 42 | <2.5 | <2.5 |
| Perfluorododecanoic Acid (PFDoDA) | <2.5 | 5 | <2.5 | <2.5 |
| Perfluoroheptane Sulfonic Acid (PFHpS) | <2.5 | <2.5 | <2.5 | <2.5 |
| Perfluoroheptanoic Acid (PFHpA) | <2.5 | <2.5 | <2.5 | <2.5 |
| Perfluorohexane Sulfonic Acid (PFHxS) | <2.5 | <2.5 | <2.5 | <2.5 |
| Perfluorohexanoic Acid (PFHxA) | <2.5 | 11 | <2.5 | <2.5 |
| Perfluorononanoic Acid (PFNA) | <2.5 | 2.8 | <2.5 | <2.5 |
| Perfluorooctane Sulfonamide (FOSA) | <2.5 | <2.5 | <2.5 | <2.5 |
| Perfluorooctane Sulfonic Acid (PFOS) | <2.5 | 26 | <2.5 | <2.5 |
| Perfluorooctanoic Acid (PFOA) | <2.5 | 21 | <2.5 | <2.5 |
| Perfluoropentane Sulfonic Acid (PFPeS) | <2.5 | <2.5 | <2.5 | <2.5 |
| Perfluoropentanoic Acid (PFPeA) | <2.5 | 10 | <2.5 | <2.5 |
| Perfluorotetradecanoic Acid (PFTeDA) | <5 | <5 | <5 | <5 |
| Perfluorotridecanoic Acid (PFTrDA) | <2.5 | <2.5 | <2.5 | <2.5 |
| Perfluoroundecanoic Acid (PFUnDA) | <2.5 | <2.5 | <2.5 | <2.5 |
| Sum of PFAS | <2.5 | 134 | <2.5 | <2.5 |
| GP Gas Profiles (%) | PYROCO™ Exit Gas Profile (%) | |||
|---|---|---|---|---|
| Pilot Plant Data | Simulation Data | Pilot Plant Data | Simulation Data | |
| CO2 | 9–11.5 | 11.2 | 11–17 | 12 |
| H2 | 4–5 | 5 | ||
| O2 | 0–1 | 5.5 × 10−12 | 5–10 | 7.0 |
| CO | 10–13 | 12.9 | 0–0.015 | 9.80 × 10−7 |
| NOx | 0.014–0.03 | 0.015 | ||
| Details | Mass Flow Rate (kg/h) | Energy (kW) | |||
|---|---|---|---|---|---|
| In | Out | In | Out | ||
| Loss | Gen. | ||||
| Biosolids | 417 | - | 943.6 | ||
| Dryer | - | 172 | 107.8 | ||
| Gas producer (autothermal) | 147 | 29 | |||
| Pyrolyzer+thermal oxidizer | 2145 | 69 | |||
| Heat loss in GP and PYROCO® | 19.6 | ||||
| Thermal energy in biochar | 192.4 | ||||
| Thermal energy in ash | 15.9 | ||||
| Low-grade heat recovery (LGHR) | 313.7 | ||||
| Exergy losses | 294.1 | ||||
| Exhaust | - | 2439 | |||
| Total | 2708 | 2708 | 943.6 | 629.9 | 313.7 |
| Difference (mass in–out) | 0 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rathnayake, N.; Patel, S.; Hakeem, I.G.; Veluswamy, G.; Al-Waili, I.; Agnihotri, S.; Vuppaladadiyam, A.K.; Surapaneni, A.; Bergmann, D.; Shah, K. The Pyrolysis of Biosolids in a Novel Closed Coupled Pyrolysis and Gasification Technology: Pilot Plant Trials, Aspen Plus Modelling, and a Techno-Economic Analysis. Water 2024, 16, 3399. https://doi.org/10.3390/w16233399
Rathnayake N, Patel S, Hakeem IG, Veluswamy G, Al-Waili I, Agnihotri S, Vuppaladadiyam AK, Surapaneni A, Bergmann D, Shah K. The Pyrolysis of Biosolids in a Novel Closed Coupled Pyrolysis and Gasification Technology: Pilot Plant Trials, Aspen Plus Modelling, and a Techno-Economic Analysis. Water. 2024; 16(23):3399. https://doi.org/10.3390/w16233399
Chicago/Turabian StyleRathnayake, Nimesha, Savankumar Patel, Ibrahim Gbolahan Hakeem, Ganesh Veluswamy, Ibrahim Al-Waili, Shivani Agnihotri, Arun Krishna Vuppaladadiyam, Aravind Surapaneni, David Bergmann, and Kalpit Shah. 2024. "The Pyrolysis of Biosolids in a Novel Closed Coupled Pyrolysis and Gasification Technology: Pilot Plant Trials, Aspen Plus Modelling, and a Techno-Economic Analysis" Water 16, no. 23: 3399. https://doi.org/10.3390/w16233399
APA StyleRathnayake, N., Patel, S., Hakeem, I. G., Veluswamy, G., Al-Waili, I., Agnihotri, S., Vuppaladadiyam, A. K., Surapaneni, A., Bergmann, D., & Shah, K. (2024). The Pyrolysis of Biosolids in a Novel Closed Coupled Pyrolysis and Gasification Technology: Pilot Plant Trials, Aspen Plus Modelling, and a Techno-Economic Analysis. Water, 16(23), 3399. https://doi.org/10.3390/w16233399










