Comparison of UV/PAA and VUV/PAA Processes for Eliminating Diethyl Phthalate in Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedures
3. Results and Discussion
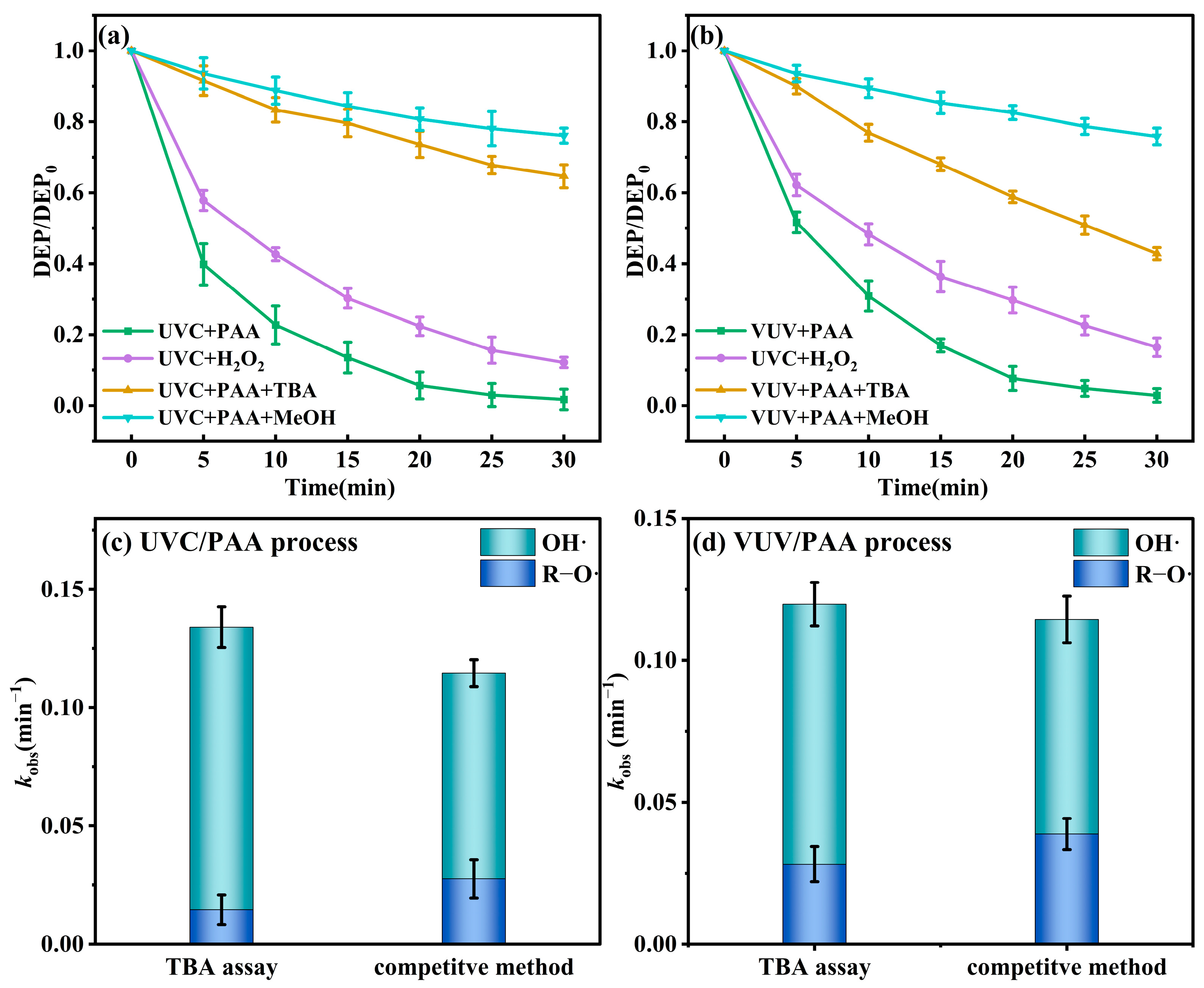
3.1. Effect of UVA, UVC and VUV on the Removal of DEP
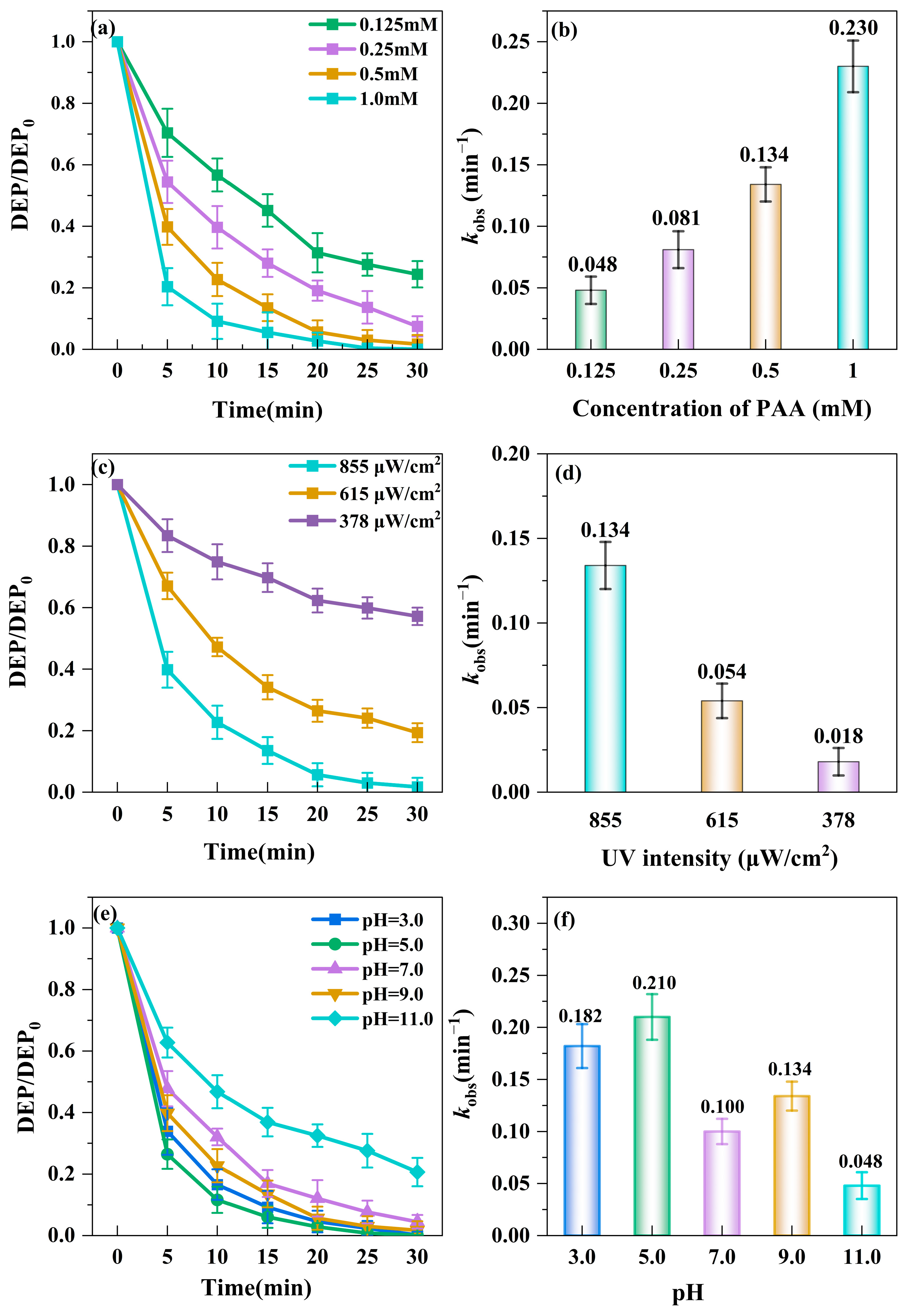
3.2. Factors Affecting DEP Degradation
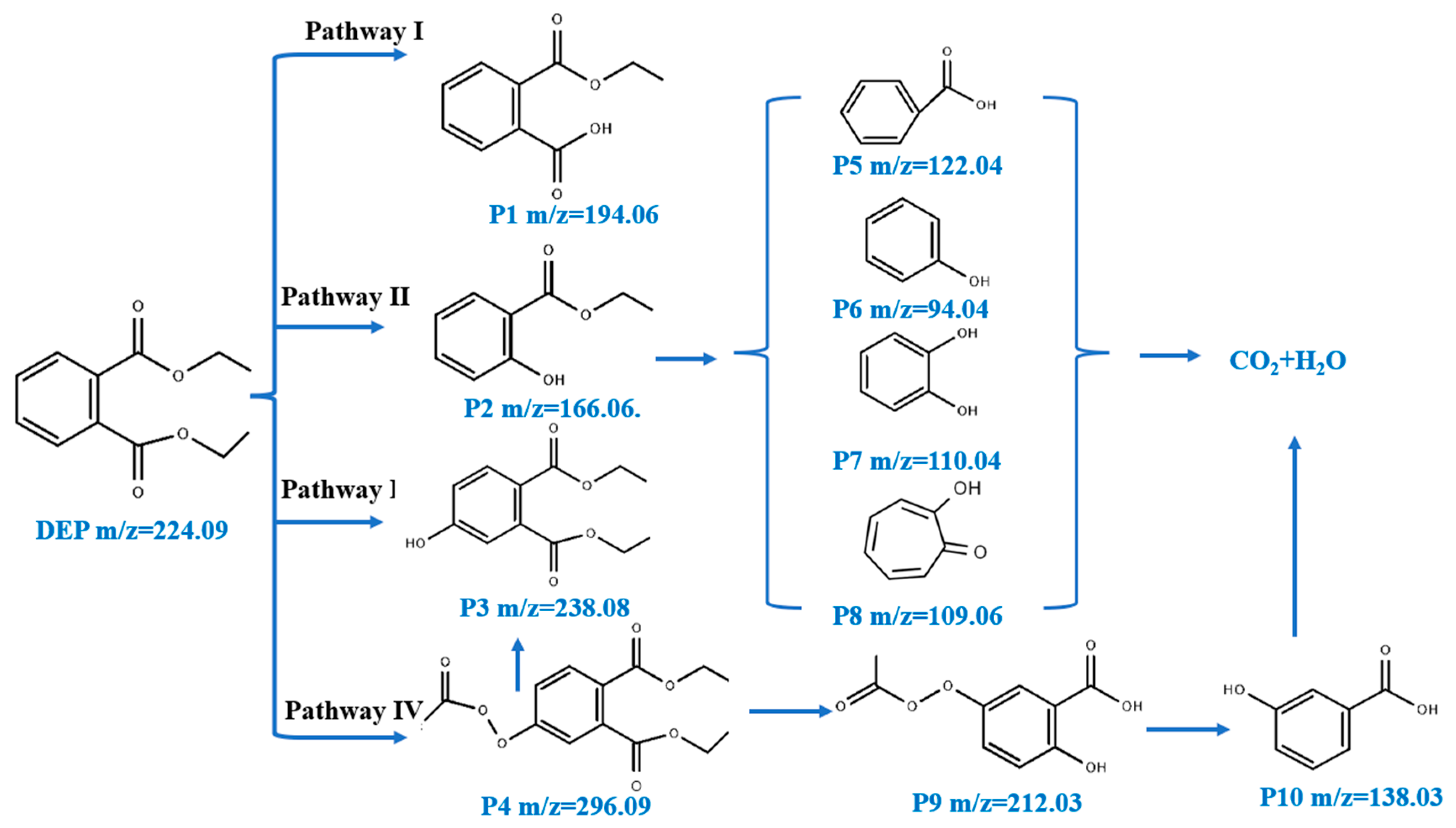
3.3. Possible Degradation Pathways
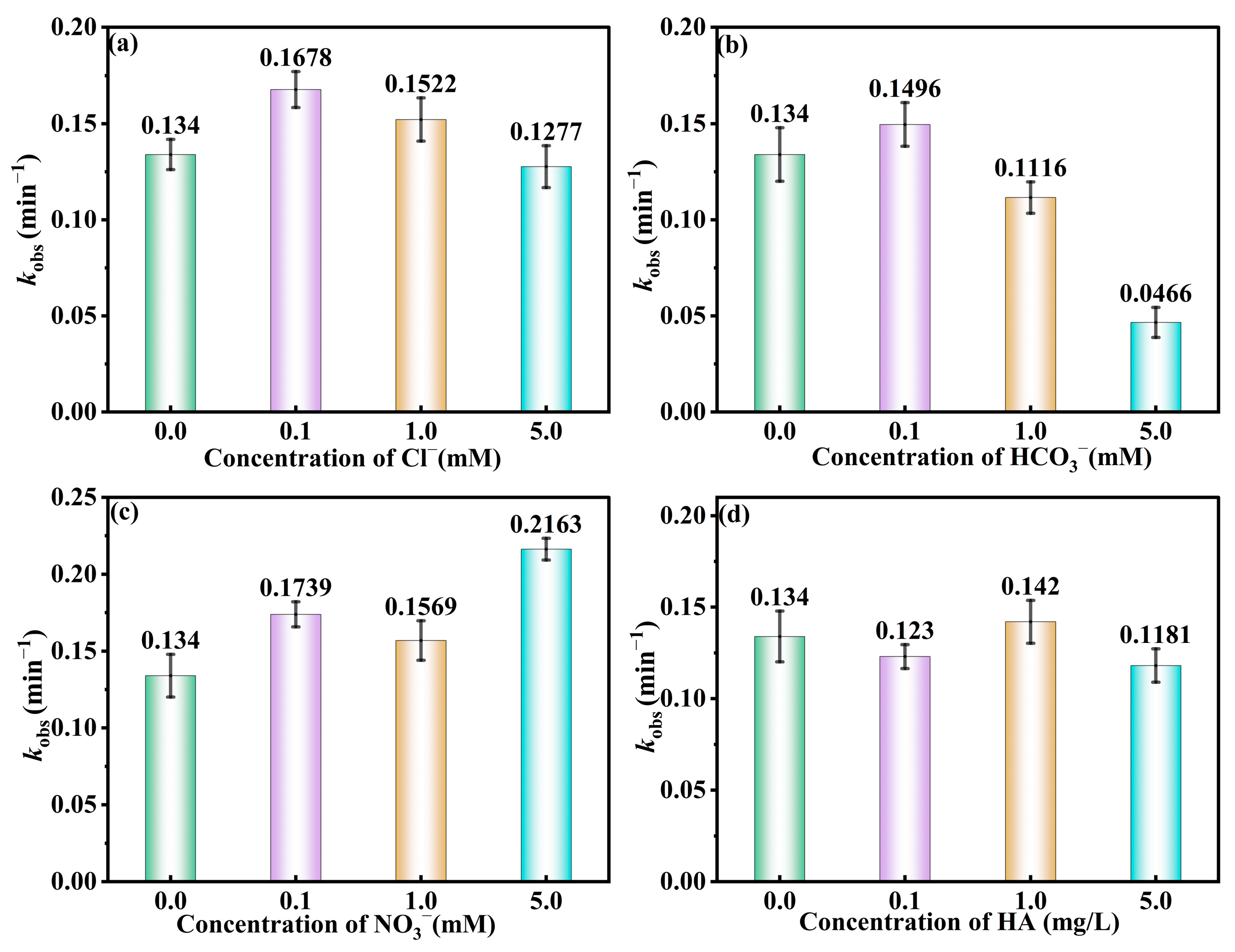
3.4. Effect of Anions on DEP Degradation
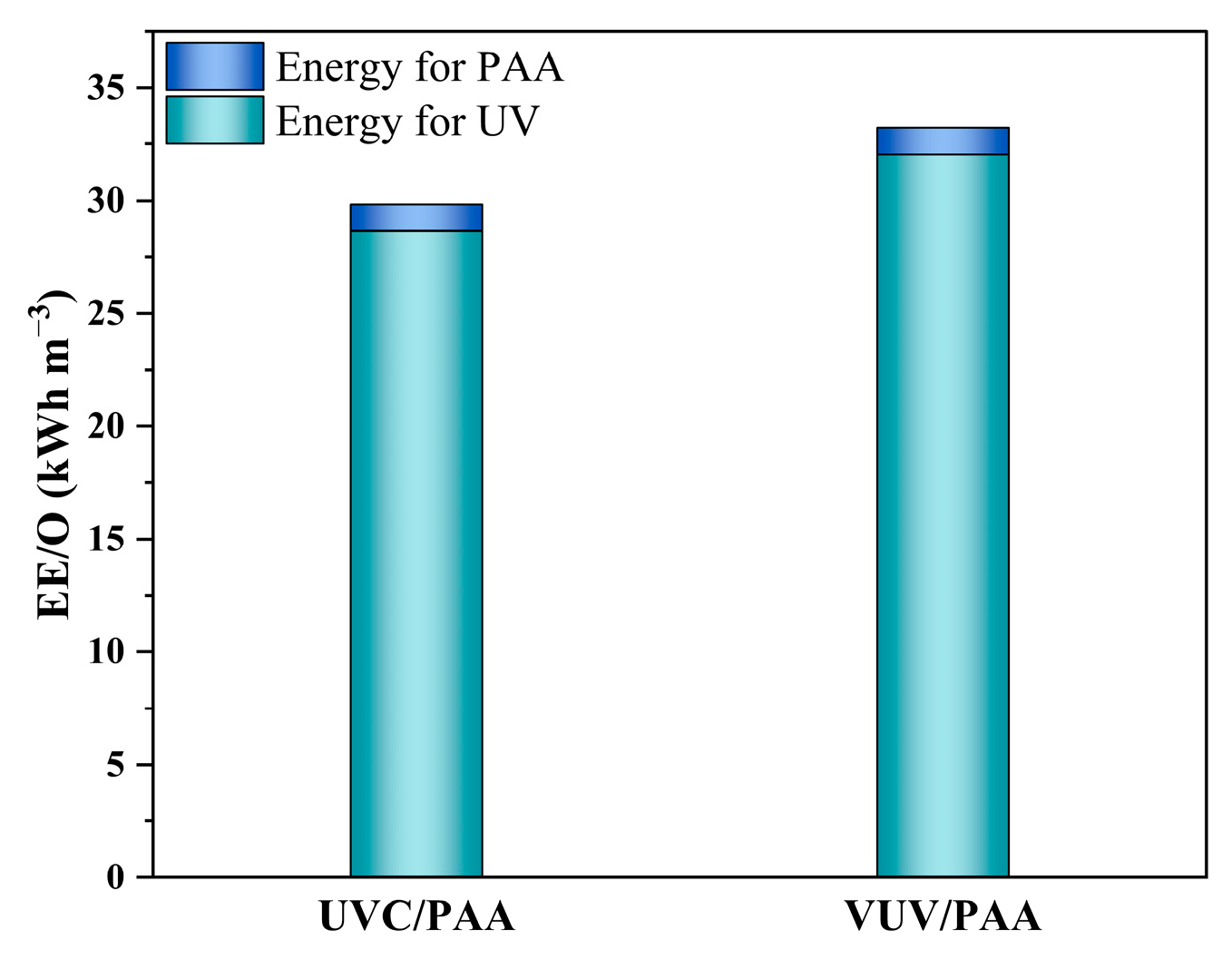
3.5. Energy Consumption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, N.; Shuai, W.; Hao, X.; Zhang, H.; Zhou, D.; Gao, J. Contamination of Phthalate Esters in Vegetable Agriculture and Human Cumulative Risk Assessment. Pedosphere 2017, 27, 439–451. [Google Scholar] [CrossRef]
- Dargnat, C.; Teil, M.-J.; Chevreuil, M.; Blanchard, M. Phthalate Removal throughout Wastewater Treatment Plant: Case Study of Marne Aval Station (France). Sci. Total Environ. 2009, 407, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Kotowska, U.; Kapelewska, J.; Sawczuk, R. Occurrence, Removal, and Environmental Risk of Phthalates in Wastewaters, Landfill Leachates, and Groundwater in Poland. Environ. Pollut. 2020, 267, 115643. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Gong, S.; Cao, Y.; Liu, M.; Zhang, W.; Zhang, X.; Fan, L.; Li, L.; Du, H.; Tysklind, M.; et al. Characterization and Sources of Childhood PAEs Exposure from Residential Airborne Dust in China Cities. J. Environ. Sci. 2025, 152, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Yang, J.; Zhu, C.; Lu, L.; Fang, Q.; Xu, C.; He, Z.; Song, S.; Shen, Y. Unveiling the Metallic Size Effect on O2 Adsorption and Activation for Enhanced Electro-Fenton Degradation of Aromatic Compounds. J. Hazard. Mater. 2024, 462, 132739. [Google Scholar] [CrossRef]
- Ma, T.T.; Wu, L.H.; Chen, L.; Zhang, H.B.; Teng, Y.; Luo, Y.M. Phthalate Esters Contamination in Soils and Vegetables of Plastic Film Greenhouses of Suburb Nanjing, China and the Potential Human Health Risk. Environ. Sci. Pollut. Res. 2015, 22, 12018–12028. [Google Scholar] [CrossRef]
- He, Y.; Wang, Q.; He, W.; Xu, F. The Occurrence, Composition and Partitioning of Phthalate Esters (PAEs) in the Water-Suspended Particulate Matter (SPM) System of Lake Chaohu, China. Sci. Total Environ. 2019, 661, 285–293. [Google Scholar] [CrossRef]
- Liu, B.; Jiang, T.; Li, Z.; Ge, W.; Wu, J.; Song, N.; Chai, C. Phthalate Esters in Surface Sediments from Fishing Ports in Circum-Bohai-Sea Region, China. Mar. Pollut. Bull. 2021, 171, 112782. [Google Scholar] [CrossRef]
- Horn, O.; Nalli, S.; Cooper, D.; Nicell, J. Plasticizer Metabolites in the Environment. Water Res. 2004, 38, 3693–3698. [Google Scholar] [CrossRef]
- Pogribny, I.P.; Tryndyak, V.P.; Boureiko, A.; Melnyk, S.; Bagnyukova, T.V.; Montgomery, B.; Rusyn, I. Mechanisms of Peroxisome Proliferator-Induced DNA Hypomethylation in Rat Liver. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2008, 644, 17–23. [Google Scholar] [CrossRef]
- Tang, J.; Zhu, J.; Liu, L.; Xia, L.; He, Z.; Wang, D.; Xu, X.; Song, S. Coupling Urchin-like TiO2 Nanospheres with Nitrogen and Sulfur Co-Doped Graphene Quantum Dots for Visible-Light-Induced Degradation of Toluene. Chem. Eng. J. 2024, 482, 148813. [Google Scholar] [CrossRef]
- Dong, F.; Zhu, J.; Li, J.; Fu, C.; He, G.; Lin, Q.; Li, C.; Song, S. The Occurrence, Formation and Transformation of Disinfection Byproducts in the Water Distribution System: A Review. Sci. Total Environ. 2023, 867, 161497. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Qiu, L.; Xing, X.; Zhu, J.; Lu, M.; Dong, F.; Yu, Y.; Yu, W. Coupling Fe-Co Atomic Pair to Promote the Selective Reduction of Nitroaromatics under Mild Conditions. Sci. Total Environ. 2024, 912, 169161. [Google Scholar] [CrossRef] [PubMed]
- Fromme, H.; Küchler, T.; Otto, T.; Pilz, K.; Müller, J.; Wenzel, A. Occurrence of Phthalates and Bisphenol A and F in the Environment. Water Res. 2002, 36, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
- Bensalah, N.; Dbira, S.; Bedoui, A. Mechanistic and Kinetic Studies of the Degradation of Diethyl Phthalate (DEP) by Homogeneous and Heterogeneous Fenton Oxidation. Environ. Nanotechnol. Monit. Manag. 2019, 11, 100224. [Google Scholar] [CrossRef]
- Dong, Z.; Li, S.; Rene, E.R.; Tan, X.; Ma, W. Enhanced Removal of Dimethyl Phthalate Using Heterogeneous UVC/VUV-Fenton Amendment with Fe3O4@CM-β-CD/rGO Catalyst: Efficiency, Degradation Mechanism and Toxicity. Chemosphere 2024, 352, 141343. [Google Scholar] [CrossRef]
- Kumar, S.; Kaushik, R.D.; Upadhyay, G.K.; Purohit, L.P. rGO-ZnO Nanocomposites as Efficient Photocatalyst for Degradation of 4-BP and DEP Using High Temperature Refluxing Method in in-Situ Condition. J. Hazard. Mater. 2021, 406, 124300. [Google Scholar] [CrossRef]
- Na, S.; Jinhua, C.; Cui, M.; Khim, J. Sonophotolytic Diethyl Phthalate (DEP) Degradation with UVC or VUV Irradiation. Ultrason. Sonochemistry 2012, 19, 1094–1098. [Google Scholar] [CrossRef]
- Yu, Y.; Qi, Y.; Li, C.; Cao, W.; Chen, J.; Qu, R.; Zhou, D.; Wang, Z. Ferrate (VI)-Mediated Transformation of Diethyl Phthalate (DEP) in Soil: Kinetics, Degradation Mechanisms and Theoretical Calculation. Environ. Pollut. 2021, 290, 118053. [Google Scholar] [CrossRef]
- Zhang, S.; Wei, J.; Wu, N.; Allam, A.A.; Ajarem, J.S.; Maodaa, S.; Huo, Z.; Zhu, F.; Qu, R. Assessment of the UV/DCCNa and UV/NaClO Oxidation Process for the Removal of Diethyl Phthalate (DEP) in the Aqueous System. Environ. Pollut. 2024, 341, 122915. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, C.; Xu, J.; Lu, L.; Fang, Q.; Xu, C.; Zheng, Y.; Song, S.; Shen, Y. Efficient Dual-Pathway H2O2 Production Promoted by Covalent Triazine Frameworks with Integrated Dual Active Sites. Appl. Catal. B Environ. Energy 2024, 344, 123629. [Google Scholar] [CrossRef]
- Chen, T.; Cevallos, D.; Hurtado, A.; Mackey, E.; Wang, C.; Hofmann, R. Predicting Chlorine Demand by Peracetic Acid in Drinking Water Treatment. Water Res. 2023, 243, 120361. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, T.R.; das Neves, A.P.N.; Castro, D.A.; Cavallini, G.S. Pre-Oxidation with Peracetic Acid to Degradation of Chlorophyll-a from Drinking Water: A Comparative Study with Calcium Hypochlorite. J. Water Process Eng. 2020, 38, 101643. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Zhan, Y.; Zhang, Y.; Zhao, X.; Yang, M.; Ruan, W.; Zhang, Z.; Liang, X.; Ma, J. Peracetic Acid-Induced Nanoengineering of Fe-Based Metallic Glass Ribbon in Application of Efficient Drinking Water Treatment. Appl. Catal. B Environ. Energy 2024, 355, 124161. [Google Scholar] [CrossRef]
- Domínguez Henao, L.; Turolla, A.; Antonelli, M. Disinfection By-Products Formation and Ecotoxicological Effects of Effluents Treated with Peracetic Acid: A Review. Chemosphere 2018, 213, 25–40. [Google Scholar] [CrossRef]
- Kibbee, R.; Örmeci, B. Peracetic Acid (PAA) and Low-Pressure Ultraviolet (LP-UV) Inactivation of Coxsackievirus B3 (CVB3) in Municipal Wastewater Individually and Concurrently. Water Res. 2020, 183, 116048. [Google Scholar] [CrossRef]
- Zhang, T.; Huang, C.-H. Modeling the Kinetics of UV/Peracetic Acid Advanced Oxidation Process. Environ. Sci. Technol. 2020, 54, 7579–7590. [Google Scholar] [CrossRef]
- Rokhina, E.V.; Makarova, K.; Golovina, E.A.; Van As, H.; Virkutyte, J. Free Radical Reaction Pathway, Thermochemistry of Peracetic Acid Homolysis, and Its Application for Phenol Degradation: Spectroscopic Study and Quantum Chemistry Calculations. Environ. Sci. Technol. 2010, 44, 6815–6821. [Google Scholar] [CrossRef]
- Li, X.; Zhu, W.; Sun, S.-P. Peracetic Acid-Based UVA Photo-Fenton Reaction: Dominant Role of High-Valent Iron Species toward Efficient Selective Degradation of Emerging Micropollutants. J. Hazard. Mater. 2023, 454, 131448. [Google Scholar] [CrossRef]
- Moussavi, G.; Bakhtiarynasab, M.; Shekoohiyan, S.; Mohammadi, S.; Giannakis, S.; Li, M. High Potential of the Vacuum UV-Activated Peracetic Acid (VUV/PAA) Process in Eliminating Recalcitrant Contaminants and Waterborne Pathogens: Assessing the Efficacy of Annular and Helical Reactor Configurations. J. Water Process Eng. 2024, 60, 105143. [Google Scholar] [CrossRef]
- Li Puma, G.; Puddu, V.; Tsang, H.K.; Gora, A.; Toepfer, B. Photocatalytic Oxidation of Multicomponent Mixtures of Estrogens (Estrone (E1), 17β-Estradiol (E2), 17α-Ethynylestradiol (EE2) and Estriol (E3)) under UVA and UVC Radiation: Photon Absorption, Quantum Yields and Rate Constants Independent of Photon Absorption. Appl. Catal. B Environ. 2010, 99, 388–397. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, J.; Cheng, N.; Gan, P.; Li, Y.; Liu, W.; Ye, J.; Tong, M.; Liang, J. The Overlooked Role of UV185 Induced High-Energy Excited States in the Dephosphorization of Organophosphorus Pesticide by VUV/Persulfate. Chemosphere 2023, 334, 138993. [Google Scholar] [CrossRef] [PubMed]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of Rate Constants for Reactions of Hydrated Electrons, Hydrogen Atoms and Hydroxyl Radicals (⋅OH/⋅O− in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Wang, Z.; Shao, Y.; Gao, N.; Lu, X.; An, N. Degradation of Diethyl Phthalate (DEP) by UV/Persulfate: An Experiment and Simulation Study of Contributions by Hydroxyl and Sulfate Radicals. Chemosphere 2018, 193, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Behnajady, M.A.; Vahid, B.; Modirshahla, N.; Shokri, M. Evaluation of Electrical Energy per Order (E) with Kinetic Modeling on the Removal of Malachite Green by US/UV/HO processEO22. Desalination 2009, 249, 99–103. [Google Scholar] [CrossRef]
- Chen, Y.; Ye, J.; Chen, Y.; Hu, H.; Zhang, H.; Ou, H. Degradation Kinetics, Mechanism and Toxicology of Tris(2-Chloroethyl) Phosphate with 185 nm Vacuum Ultraviolet. Chem. Eng. J. 2019, 356, 98–106. [Google Scholar] [CrossRef]
- Orlando, J.J.; Tyndall, G.S. Gas Phase UV Absorption Spectra for Peracetic Acid, and for Acetic Acid Monomers and Dimers. J. Photochem. Photobiol. A Chem. 2003, 157, 161–166. [Google Scholar] [CrossRef]
- Na, S.; Ahn, Y.-G.; Cui, M.; Khim, J. Significant Diethyl Phthalate (DEP) Degradation by Combined Advanced Oxidation Process in Aqueous Solution. J. Environ. Manag. 2012, 101, 104–110. [Google Scholar] [CrossRef]
- Chen, S.; Cai, M.; Liu, Y.; Zhang, L.; Feng, L. Effects of Water Matrices on the Degradation of Naproxen by Reactive Radicals in the UV/Peracetic Acid Process. Water Res. 2019, 150, 153–161. [Google Scholar] [CrossRef]
- Guo, Y.; Long, J.; Huang, J.; Yu, G.; Wang, Y. Can the Commonly Used Quenching Method Really Evaluate the Role of Reactive Oxygen Species in Pollutant Abatement during Catalytic Ozonation? Water Res. 2022, 215, 118275. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, J.; Ma, J.; Yang, Y.; Luo, C.; Huangfu, X.; Guo, Z. Role of the Propagation Reactions on the Hydroxyl Radical Formation in Ozonation and Peroxone (Ozone/Hydrogen Peroxide) Processes. Water Res. 2015, 68, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Han, Q.; Dong, Z.; Zhu, J.; Jiang, C.; Qi, J. Enhanced Degradation and Deiodination of Diatrizoate in a Sulfite Mediated Photolytic System: Performance, Products and Possible Pathways. J. Hazard. Mater. Adv. 2022, 8, 100144. [Google Scholar] [CrossRef]
- Cai, M.; Sun, P.; Zhang, L.; Huang, C.-H. UV/Peracetic Acid for Degradation of Pharmaceuticals and Reactive Species Evaluation. Environ. Sci. Technol. 2017, 51, 14217–14224. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, P.; Koba-Ucun, O.; Arslan-Alaton, I. UV-C Activation of Peroxides for Bisphenol A Removal from a Real Water Sample. CLEAN—Soil Air Water 2021, 49, 2100050. [Google Scholar] [CrossRef]
- Lau, T.K.; Chu, W.; Graham, N. The Degradation of Endocrine Disruptor Di-n-Butyl Phthalate by UV Irradiation: A Photolysis and Product Study. Chemosphere 2005, 60, 1045–1053. [Google Scholar] [CrossRef]
- Muneer, M.; Theurich, J.; Bahnemann, D. Titanium Dioxide Mediated Photocatalytic Degradation of 1,2-Diethyl Phthalate. J. Photochem. Photobiol. A Chem. 2001, 143, 213–219. [Google Scholar] [CrossRef]
- Hu, J.; Li, T.; Zhang, X.; Ren, H.; Huang, H. Degradation of Steroid Estrogens by UV/Peracetic Acid: Influencing Factors, Free Radical Contribution and Toxicity Analysis. Chemosphere 2022, 287, 132261. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Fu, Y. Degradation Kinetics and Mechanism of Diclofenac by UV/Peracetic Acid. RSC Adv. 2020, 10, 9907–9916. [Google Scholar] [CrossRef]
- Sun, P.; Meng, T.; Wang, Z.; Zhang, R.; Yao, H.; Yang, Y.; Zhao, L. Degradation of Organic Micropollutants in UV/NH2Cl Advanced Oxidation Process. Environ. Sci. Technol. 2019, 53, 9024–9033. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, F.; Cheng, J.; Cheng, Y.; Ma, X. Comparison of UV/PAA and VUV/PAA Processes for Eliminating Diethyl Phthalate in Water. Water 2024, 16, 3533. https://doi.org/10.3390/w16233533
Dong F, Cheng J, Cheng Y, Ma X. Comparison of UV/PAA and VUV/PAA Processes for Eliminating Diethyl Phthalate in Water. Water. 2024; 16(23):3533. https://doi.org/10.3390/w16233533
Chicago/Turabian StyleDong, Feilong, Jiayi Cheng, Yifeng Cheng, and Xiaoyan Ma. 2024. "Comparison of UV/PAA and VUV/PAA Processes for Eliminating Diethyl Phthalate in Water" Water 16, no. 23: 3533. https://doi.org/10.3390/w16233533
APA StyleDong, F., Cheng, J., Cheng, Y., & Ma, X. (2024). Comparison of UV/PAA and VUV/PAA Processes for Eliminating Diethyl Phthalate in Water. Water, 16(23), 3533. https://doi.org/10.3390/w16233533







