Abstract
Heavy metals enter river basins through industrial effluents, agricultural wastes, surface run-offs, and other human activities, negatively impacting aquatic and terrestrial life by bioaccumulating in the food chain. This problem is on a continuous rise in under-developed and developing countries, such as in Pakistan. Therefore, the current study was aimed to determine concentrations of heavy metals, essential trace elements, and macrominerals (Zn, Pb, Ni, Mn, Mg, Fe, Cu, Cr, Co, Cd, Ca, and As) in the water, sediments, and tissues (gills, liver, and muscles) of Bagarius bagarius and Bagre marinus in the Jhelum River, Pakistan. The hematological and biochemical profiles of these fish across two sampling sites (Jhelum Bridge Khushab, upstream, and Langarwala Pull—downstream) were also evaluated. Results showed greater bioaccumulation of heavy metals in fish downstream, correlating with higher concentrations of these metals in water and sediments downstream. In the case of B. marinus, the highest concentration observed was 16.59 mg/g (Ca), and the lowest concentration was 9.51 mg/g (Fe). In the case of B. bagarius, the highest concentration observed was 17.47 mg/g (Ca), and the lowest concentration was 7.95 mg/g (Mg). Increased activities of alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and lactate dehydrogenase (LDH) were observed downstream. Hematological changes included increased white blood cells (WBCs) and decreased red blood cells (RBCs), lymphocytes, hemoglobin (Hb), platelets (Plt), and hematocrit (Hct). A significant correlation was observed among heavy metals across the water, sediment, and different tissues of B. marinus and B. bagarius. Moreover, principal component analysis (PCA) for both species along both sampling sites illustrated the relationship between fish tissues and metals. The current study concluded that the fish accumulated a significantly higher concentration of heavy metals downstream, which might be linked with dumping of the domestic wastes and industrial and agricultural runoff, adversely affecting both fish and human health.
1. Introduction
Water is essential for sustaining life and the survival of biotic communities. One cannot imagine life without water as it is the fundamental building block. Unfortunately, our water sources are continuously contaminated due to industrialization, rapid urbanization, exponential growth of the population, and various anthropogenic activities [1,2]. This echoing and grave concern has led to a variety of serious problems. The environmental contamination is further escalated by various anthropogenic activities resulting in pollution, deforestation, habitat destruction, soil erosion, land degradation, and species extinction. These diverse human activities have worsened our biosphere, resulting in substantial changes throughout our ecosystems [3]. Due to human’s irresponsible and indiscriminate behavior, which are intensified by overpopulation, they share a significant amount of responsibility in fueling this mass deterioration of the environment [4]. These problems are even more pronounced in developing or under-developed countries.
Heavy metals (such as cadmium, lead, mercury, arsenic, cobalt, etc.), being one of the core categories of pollutants, are emitted from industries. Heavy metals are widely utilized in industrial processes, and their excess is detrimental to both fish and humans [5,6]. The inability of heavy metals to be broken down by living organisms leads to their bioaccumulation and biomagnification in the food chain [7]. Polluted water containing heavy metals poses a significant threat to aquatic life, making water pollution the most urgent of all these problems to address [6]. The overview of heavy metal bioaccumulation in several fish species underlines that both freshwater and marine species are affected, where the habitat and eating behaviors are the determinants of bioaccumulation [8]. Among fish species, Bagarius bagarius has been identified as one the suitable bioindicators of heavy metal pollution and comparably contain a higher metal load, reflecting the pollution levels in their aquatic habitats [9]. Micronutrients, such as copper, zinc, nickel, and chromium, are essential for redox reactions and various biochemical processes, impacting osmotic pressure, electrostatic interactions, and enzyme functions [10,11]. In contrast, non-essential toxic metals like cadmium, mercury, lead, arsenic, and silver have little biological significance but are highly detrimental to the environment [12,13]. A balance of metals are important in maintaining metabolic and physiological processes in aquatic organisms; where disruption affects both the health of these species and the ecological balance.
Research on heavy metals is echoing across the horizon of environmental sciences, bridging different fields of studies including zoology, environmental chemistry, limnology, ecology, etc. A gigantic amount of the literature indicates its importance in the recent past. Studies on the presence of heavy metals in water, their bioaccumulation in different aquatic organisms including fish, and acute or chronic impacts of heavy metals on animals and humans are widely established in well-documented research. Research has confirmed the presence of heavy metals in different water bodies across the globe such as in the US [14,15], China [16,17], India [18,19], Pakistan [20,21], etc. Similarly, heavy metals are also reported in fish [22], mammals [23], plants [24], soil [25], and air [26] in Pakistan. Studies revealed that heavy metal exposure led to toxic effects in animals such as fish [27,28] and birds [29,30] as well as in humans, including metabolism disturbance [31], enzymes’ malfunctioning [32], neurotoxicity [33], genotoxicity [34], carcinogenicity [35], etc. The wide range of effects of heavy metals on animals and humans make them one of the most researched environmental pollutants; however, studies concerning the presence of heavy metals in the Jhelum River and their accumulation in different tissues of fish is still scanty. Similarly, studies concerning hematological and biochemical profiling of fish are scarce. Therefore, the current study was carried out to fill the gap.
The Jhelum River is a significant tributary of the Indus River system and flows through the Kashmir Valley, Pakistan. It supports the local population through supplying potable and drinkable water for daily use and providing irrigation water for agriculture. Additionally, the river’s diverse fish species are of importance for its socioeconomic value [36]. The river travels 450 miles (725 km), starting from the district of Jhelum and flows through the district of Khushab, traversing the Salt Range along its route. The Jhelum River is the lifeline of the said areas; however, its continuous contamination is of grave concern over the past two decades. It is receiving effluents and pollutants from a number of sources (industrial effluents, domestic wastes, and agricultural runoffs), which subsequently impact the river’s ecosystem and water table [37,38]. The aquatic life in this region is increasingly threatened by industrial waste. Moreover, there is a lack of data regarding the health status of fish species from the Jhelum River, particularly their hematology and biochemistry. This study aims to gather information on the serum biochemical and hematological parameters of B. bagarius and B. marinus inhabiting the water bodies of this region. These two fish species were selected on account of their higher prevalence in the Jhelum River and higher consumption rate in the adjacent areas. Moreover, there are studies on other species from different parts of Punjab province; however, studies on Bagarius bagarius and Bagre marinus are still scanty. Furthermore, a single study on B. bagarius [34] from river Chenab (Punjab, Pakistan) indicated its assemblage sensitivity in response to environmental toxicants and change. Hence, as per this only previous study, their sensitivity to environmental pollutants makes them suitable model organisms for assessing water quality and heavy metal contamination. Keeping in view the feeding behavior of both species, they can bioaccumulate heavy metals in higher concentrations, which might make them more susceptible and can alter their hemato-biochemical profile. Furthermore, this study will be instrumental in devising strategies for managing and mitigating this continuously increasing issue to ensure a thriving population of fish, devising conservation strategies, and ensuring safer public health.
2. Materials and Methods
2.1. Sampling Site
Sampling was conducted both upstream and downstream to pinpoint the pollution sources. Upstream is expected to provide a baseline (based on its good water quality) serving as a reference point, while downstream would mainly reflect the cumulative impacts on aquatic ecosystems. The results of this study would be effectively used in the management and protection of the Jhelum River at Khushab. The two sampling sites, (1) the Jhelum Pull (also known as the Khushab Pull (upstream—32°17′59″ N 72°24′14″ E)) and (2) the Langar Wala Pull over the Jhelum River close to Girote (downstream—32°01′09″ N 72°15′19″ E) were selected as the sampling locations, shown in Figure S1. The reason for selecting these sampling locations was the contrasting characters of the sampling locations in terms of the ambient water quality as well as the deteriorated water quality. The upstream Jhelum River (Khushab Pull) has better water quality because of consistent monitoring and regular assessment; however, downstream (Langar Wala Pull) is quite contaminated because of different reasons such as the dumping of domestic wastes, agricultural runoffs, etc., downstream. Moreover, the waste of the poultry industry, hotel industry, and plastic market are dumped downstream. So, this comparative study was carried out to know the impact of these wastes on the concentration of heavy metals, their bioaccumulation in fish, and the hemato-biochemical health profile of the exposed fish species downstream in comparison to upstream.
2.2. Sample Collection
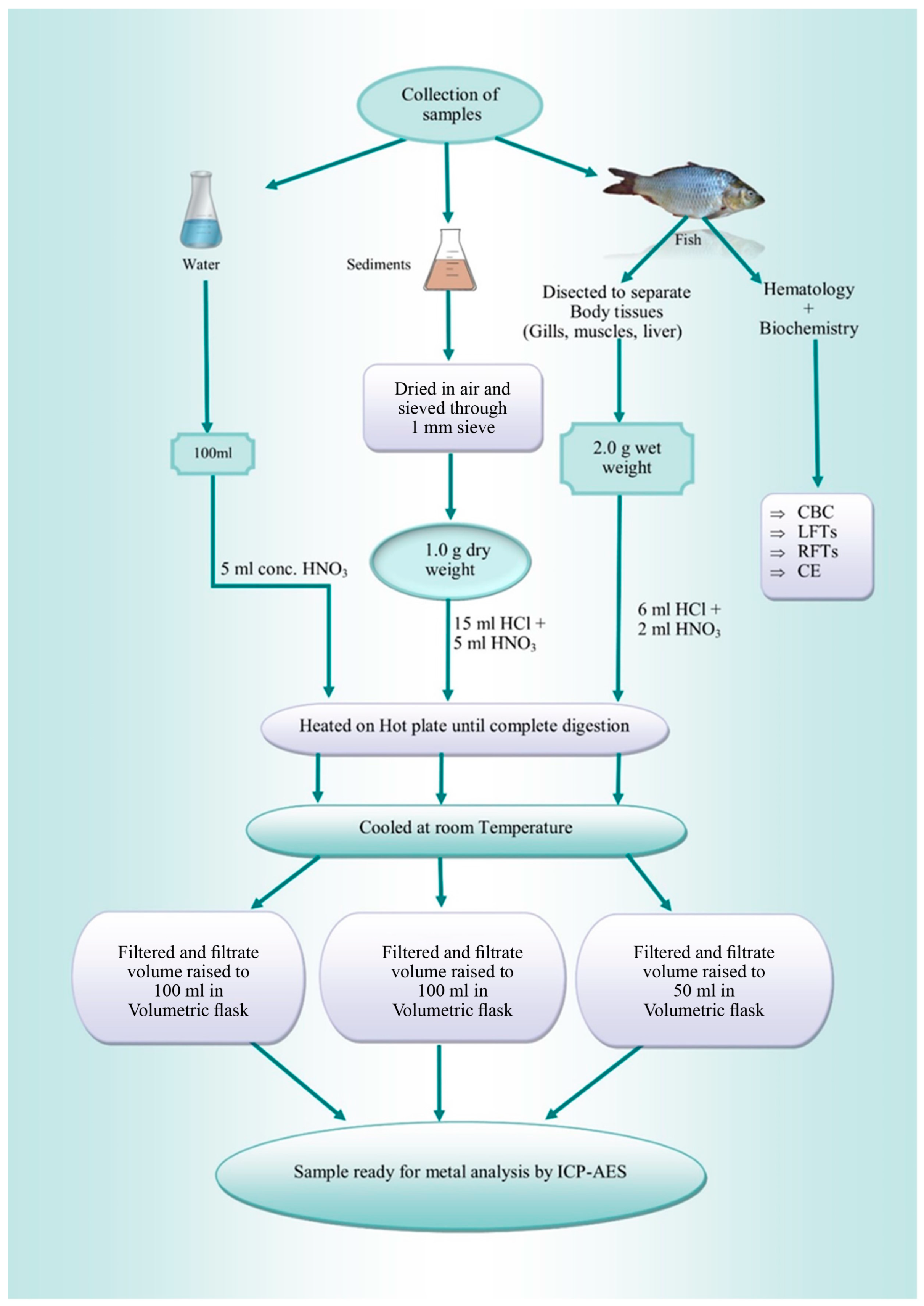
Two fish species [B. bagarius (30–37 cm, 210–265 g) and B. marinus (27–30 cm, 190–210 g)] were considered for sampling because of their consumption rate and presence/easy availability, irrespective of their sex and age. Triplicate samples of sediments, water, and fish were taken from pre-selected locations upstream and downstream of the Jhelum River. With the assistance of fishermen, a total of 12 fish were gathered from two distinct sites along the Jhelum River in the district of Khushab. Six samples from each site were then taken to the lab for heavy metal analysis. In the laboratory, each sample was cleaned and labelled. Portions of fish tissues (gills, liver, and muscles) were then taken out by dissecting the fish and were stored in zip lock bags and labelled. Tissues were stored at −20 °C until further analysis of the hematological and biochemical profile. The sampling was carried out in January and February of 2023. Figure 1 shows sample collection, preparation, and analysis in brief.
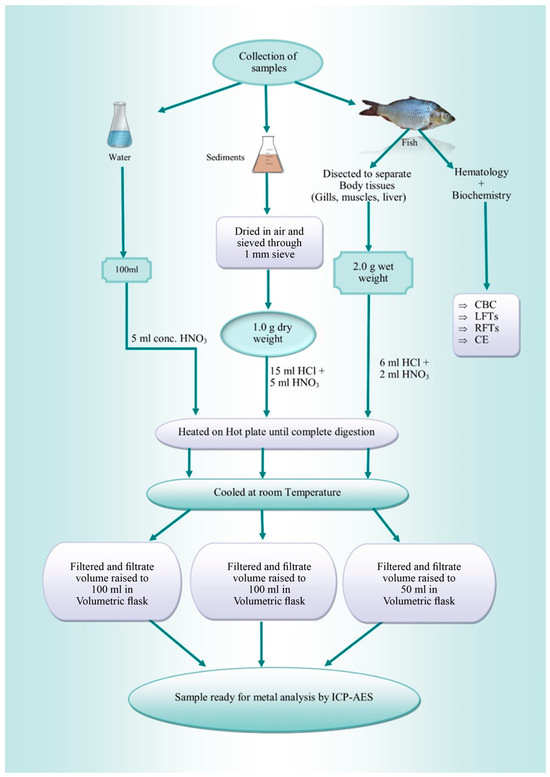
Figure 1.
Summary of the preparation of samples for analysis.
2.3. Water Sample Preparation
Three water samples each were taken in acid-washed polythene bottles from the upstream and then downstream site, separately. The samples were digested using nitric acid. The procedure involved the addition of 5 mL of concentrated HNO3 to a 100 mL water sample that was put in a beaker. In a fume hood, the beaker was heated on a hot plate, and every 20 min, the temperature was raised progressively until the solution turned clear. Following digestion, the sample was passed through Whatman No. 1 filter paper and put into a 250 mL volumetric flask. Once the sample had cooled, distilled water was added to bring the volume up to 100 mL to be further analyzed using Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP-AES).
2.4. Sediment Sample Preparation
Sediment samples were collected in triplicate using polyethylene containers from the sampling sites, 20 cm below the surface, and prepared according to the previously described method. For processing, 3 g of the homogenously mixed sample was air-dried for an hour. Mortar and pestle were used to crush the sample and then filtered the dried sample. The material was digested by adding 3:1 HCl and HNO3. The mixture was cooked on a hot plate in a fume hood for digestion. After every 20 min, the temperature was increased until all the sample dissolved and turned pale yellow. After digestion, Whatman No. 1 filter paper was used to filter the sample into a 250 mL volumetric flask. After chilling, distilled water was added to raise sample volume to 100 mL. For the remaining samples, the same method was followed to be further analyzed by ICP-AES.
2.5. Fish Tissue Sample Preparation
Liver, gill, and muscle tissues were extracted from selected fish species. Then, 2 g of each tissue was extracted following dissection and digested in HCl and HNO3 at a 3:1 ratio. The hot plate temperature was increased every 20 min to boil the mixture. The digested material was then filtered using Whatman No. 1 filter paper and allowed to cool. The filtrate was diluted to 50 mL with distilled water, and samples were subsequently analyzed using ICP-AES.
2.6. Hematological Parameters
A heparinized syringe (3 mL) was used to collect blood from caudal vein of fish. Samples were then transferred to the lab for hematological analysis. Fish blood was examined for hematocrit (Hct), lymphocytes (LYMs), red blood cells (RBCs), platelets, white blood cells (WBCs), and hemoglobin (Hb) as hematological parameters through an automatic hematology analyzer (Mindray BC-700 Series—Shenzhen, P.R. China).
2.7. Serum Biochemical Analysis
Caudal vein blood was collected for serum biochemical analysis where anticoagulants were not added to enable the blood to coagulate. To separate the serum, blood samples were centrifuged for 10 min at 3000 rpm. The centrifuged sample was stored at −80°C for subsequent analysis. Total proteins, bilirubin (total, direct, and indirect), globulin, albumin, creatinine, urea, L.D.H, ALT, AST, and ALP were measured using a biochemical analyzer (Tecno 786 Plus—Ireland).
2.8. Statistic
Data analysis was conducted using statistix (V.8), Microsoft Excel 365, and sigma plot (V. 16). Metal concentrations were expressed in mg/g for fish tissues while results were reported as Mean ± Standard Error (SE). ANOVA followed by Least Significant Difference (LSD) analysis was used for multiple comparison of heavy metal concentrations in fish tissues, and the significance level was set as p < 0.05. A two sample t-test was used for comparison of tissues between two sampling sites at p < 0.05. Principal component analysis (PCA) was carried out with an online sigma plot to visualize liver, gills, and muscle data in 2D. Pearson correlation analysis was carried out between heavy metal concentrations in water and sediments across sampling sites for the tested tissues and inter-tissue correlations.
3. Results
3.1. Bioaccumulation of Heavy Metals Across the Sampling Sites
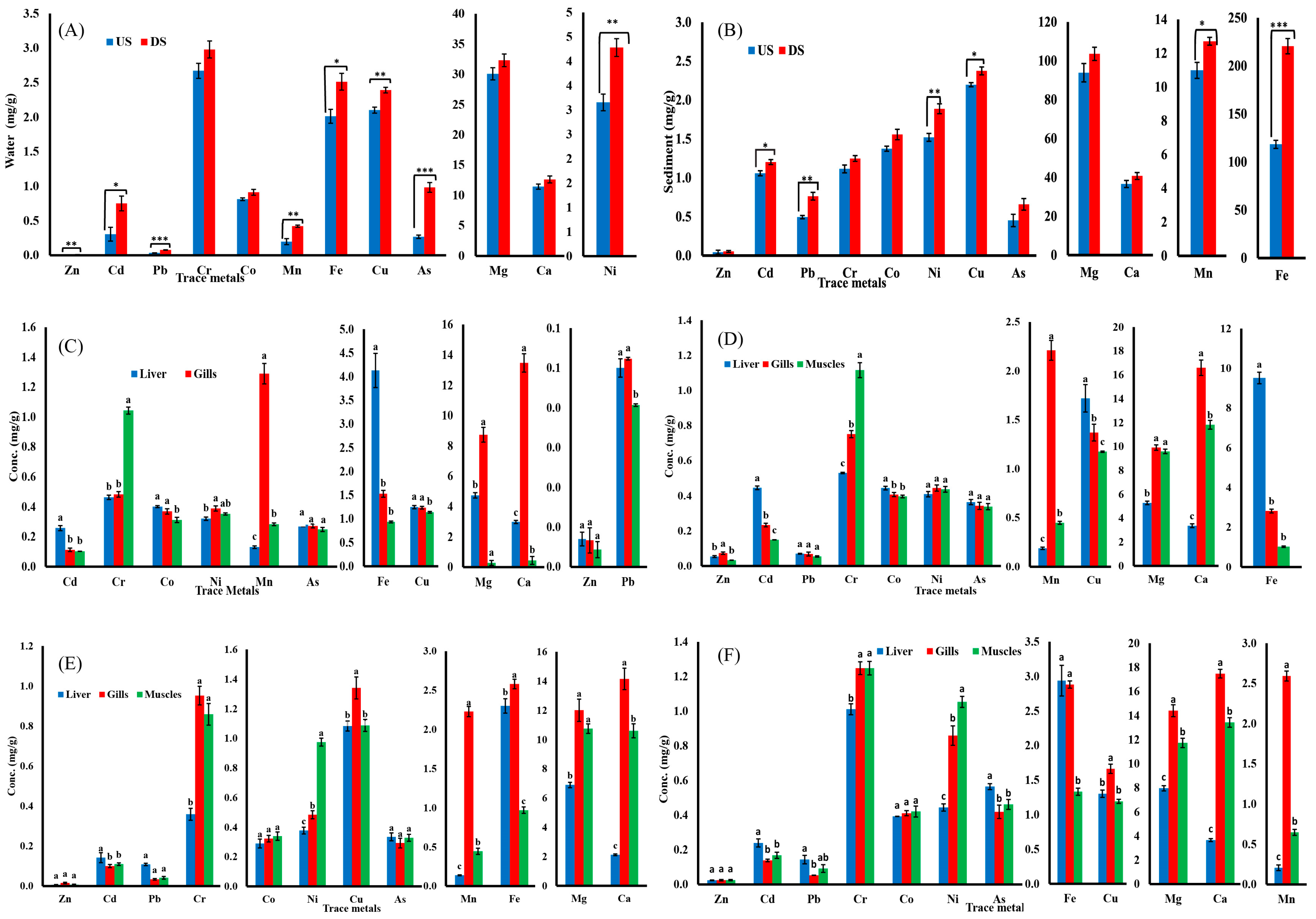
The concentration of all tested metals in water samples were higher downstream than upstream for both species (Table 1). Among the studied metals, Mg exhibits the highest accumulation, while Zn exhibits the least. Figure 2A presents a comparison of concentrations of metals in water samples collected from both the upstream and downstream sites. Regarding sediments, zinc accumulation was lowest while iron accumulation was highest along both sites (upstream and downstream). Figure 2B presents the comparison of quantities of metals in the sediment samples from upstream and downstream.

Table 1.
Concentration of heavy metals (HMs, mg/g, shown as Mean ± Standard Error) along two sampling sites for Bagre marinus and Bagarius bagarius.
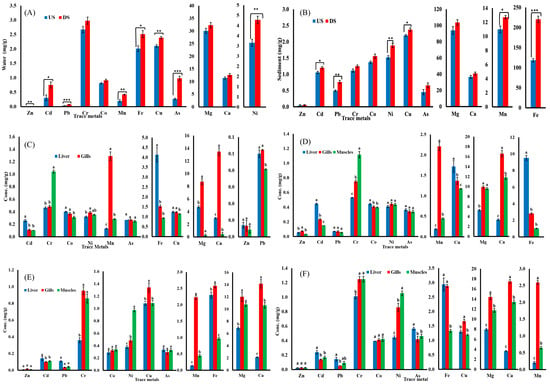
Figure 2.
Heavy metal concentrations in (A) water and (B) sediment; data presented as Mean ± SE; significant difference among sites is represented by asterisks (two-sample t-test, p < 0.05 = *, p < 0.01 = **, and p < 0.001 = ***). Heavy metal concentrations in tissues of (C) B. marinus (upstream), (D) B. marinus (downstream), (E). B. bagarius (upstream), and (F) B. bagarius (downstream); data are given as Mean ± SE and superscripted letters on bars show significance difference at p < 0.05 (ANOVA followed by LSD).
In the case of B. marinus in the upstream site, the concentrations of heavy metals in the liver, muscles, and gills were lower as compared to the downstream site. Ni, Mn, Cu, Mg, Ca, and Pb were observed to be the highest in the gills, whereas Cd, Co, As, Fe, and Zn were highest in the liver, and the muscles showed the highest concentrations of Cr upstream, as shown in Figure 2C. The liver, muscles, and gills had greater heavy metal contents downstream as compared to the upstream site. The gills of B. marinus showed greater Ca, Mg, Ni, Mn, and Zn concentrations, while the liver has higher Co, Pb, Cu, As, and Fe concentration, and the muscles have the highest Cr concentration, as shown in Figure 2D.
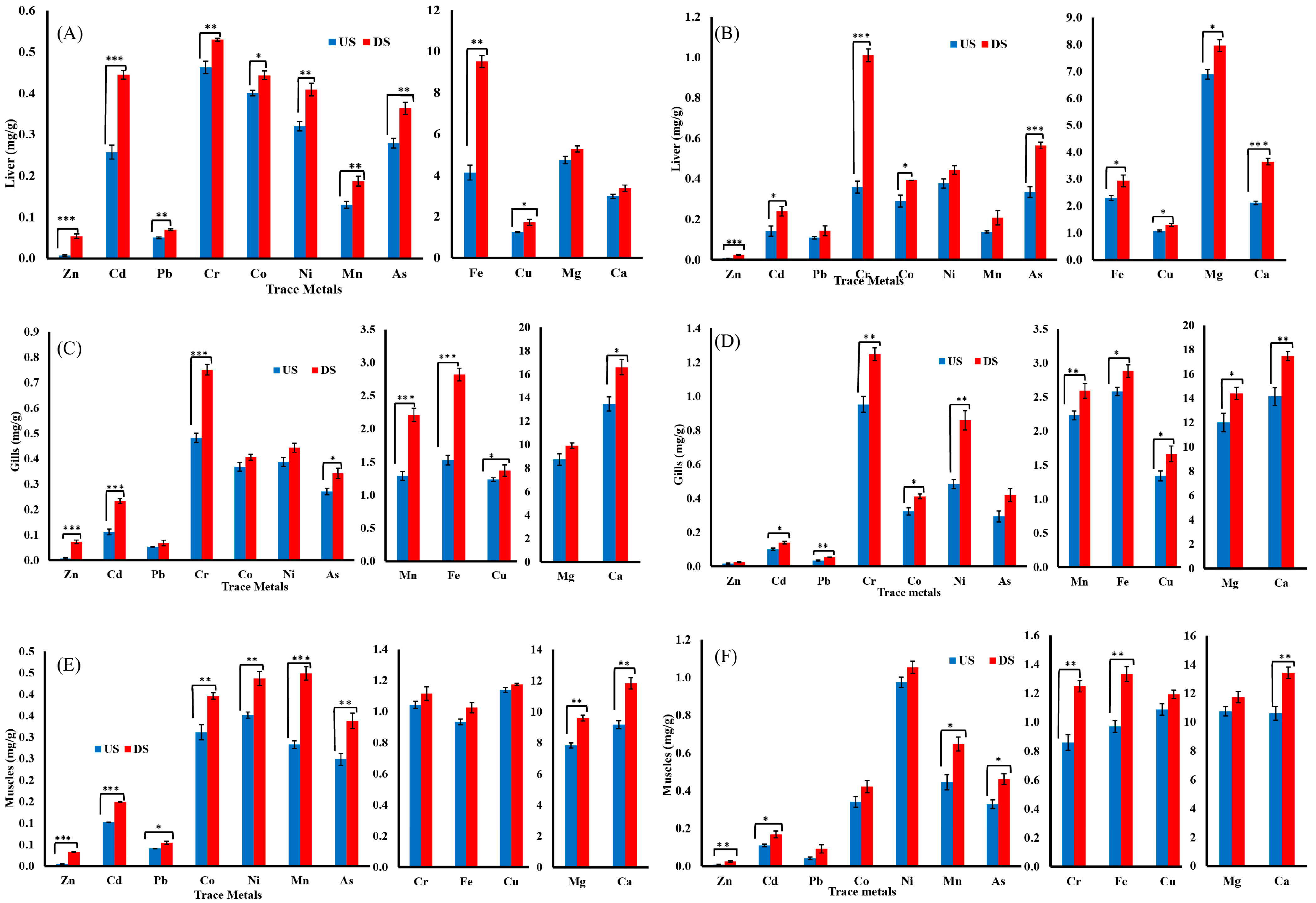
Similar to B. marinus, concentrations of heavy metals were higher in downstream specimens of B. bagarius as compared to upstream. In the case of B. bagarius, the upstream data follow the same pattern of accumulation as downstream for the liver, gills, and muscles, except for Fe and Cr—they are highly accumulated in gills downstream, as shown in Figure 2E. For downstream data, the gills accumulated the highest concentration of Zn, Cu, Mg, Ca, and Mn, whereas the liver has a higher accumulation of Cd, Pb, As, and Fe, and the muscles have a higher accumulation of Co and Ni. Cr was accumulated equally in the gills and muscles, as shown in Figure 2F. A comparison was also made for the concentrations of heavy metals in tissues (liver, gills, and muscles) of both B. marinus and B. bagarius upstream and downstream, and data are presented in Figure 3.
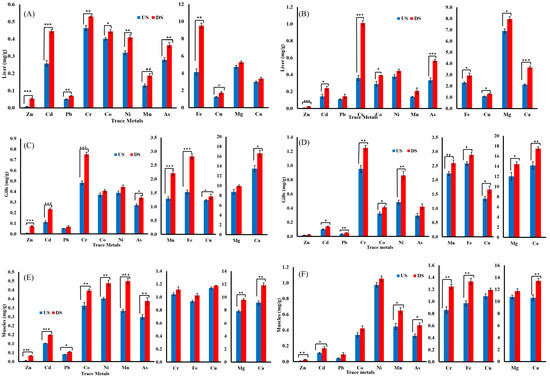
Figure 3.
Level of heavy metals in the tissues of Bagre marinus and Bagarius bagarius. (A) Liver of B. marinus, (B) liver of B. bagarius, (C) gills of B. marinus, (D) gills of B. bagarius, (E) muscles of B. marinus, and (F) muscles of B. bagarius. Data presented as Mean ± SE. Significant difference among sites is represented by an asterisks (two-sample t-test, p < 0.05 = *, p < 0.01 = **, and p < 0.001 = ***).
3.2. The Hematological and Biochemical Parameters
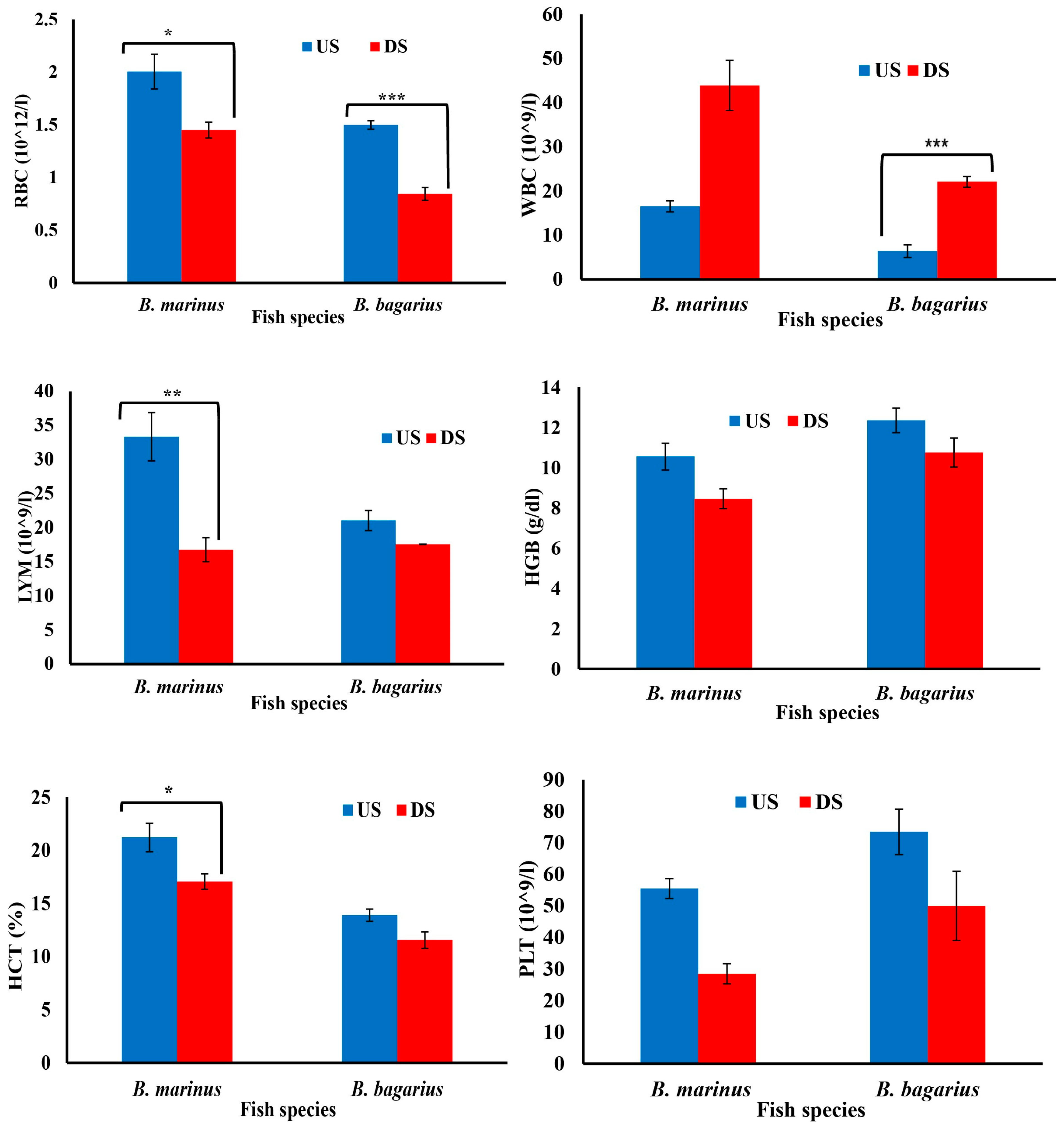
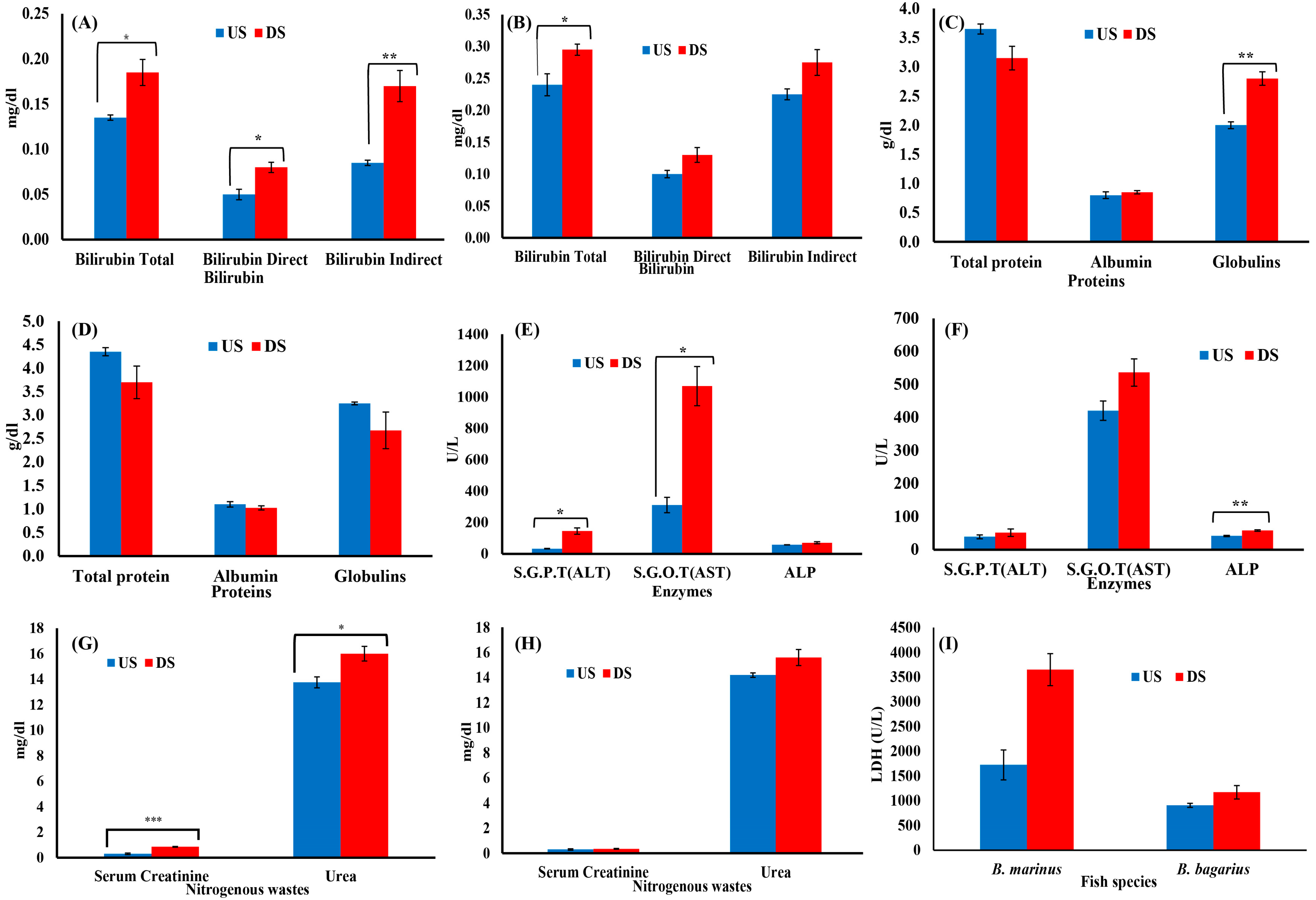
The hematological and biochemical parameters were observed to be disturbed downstream as compared to upstream. An increase was observed in white blood cells, whereas red blood cells, platelets, Hct, Hgb, and lymphocytes decreased downstream (Figure 4). In the case of serum biochemistry, the protein profile, bilirubin profile, serum creatinine, urea profile, and enzymes profiles were monitored. Figure 5A,B show the bilirubin profile for B. marinus and B. bagarius, showing a significant increase in bilirubin (total, direct, and indirect) along the downstream site, which is an indication that the liver was badly affected downstream. Figure 5C,D demonstrate a significant decrease in total protein content, while globulin and albumin levels increased in B. marinus and decreased in B. bagarius towards the downstream site. An increase in liver enzymes (ALT, AST, ALP, and LDH) and a significant increase in serum creatinine and urea levels were observed as shown in Figure 5E,F. Similarly, a comparison was drawn between the upstream and downstream specimen for nitrogenous wastes and LDH, which also indicated a disturbed profile of these two parameters downstream, as shown in Figure 5G,I.
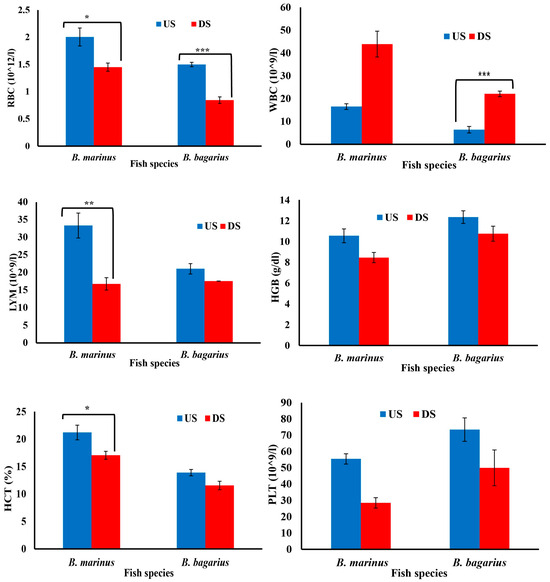
Figure 4.
Hematological profile of Bagre marinus and Bagarius Bagarius from upstream (US) and downstream (DS) of Jhelum River in Khushab District. Data represented as Mean ± SE. Significant difference among sites is represented by asterisks (two-sample t-test, p < 0.05 = *, p < 0.01 = **, and p < 0.001 = ***).
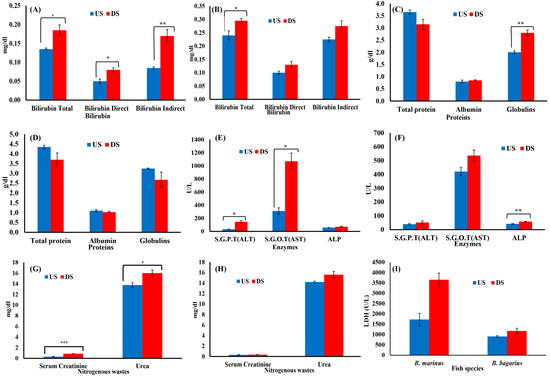
Figure 5.
Serum biochemical profile of Bagre marinus and Bagarius bagarius from upstream (US) and downstream (DS) of Jhelum River in Khushab. (A) Bilirubin for B. marinus, (B) bilirubin for B. bagarius, (C) protein for B. marinus, (D) protein for B. bagarius, (E) enzymes for B. marinus, (F) enzymes for B. bagarius, (G) nitrogenous wastes for B. marinus, (H) nitrogenous wastes for B. bagarius, and (I) LDH for both species. Data presented as Mean ± SE. Significant difference among sites is represented by asterisks (two-sample t-test, p < 0.05 = *, p < 0.01 = ** and p < 0.001 = ***).
3.3. Tissue Specific Profile
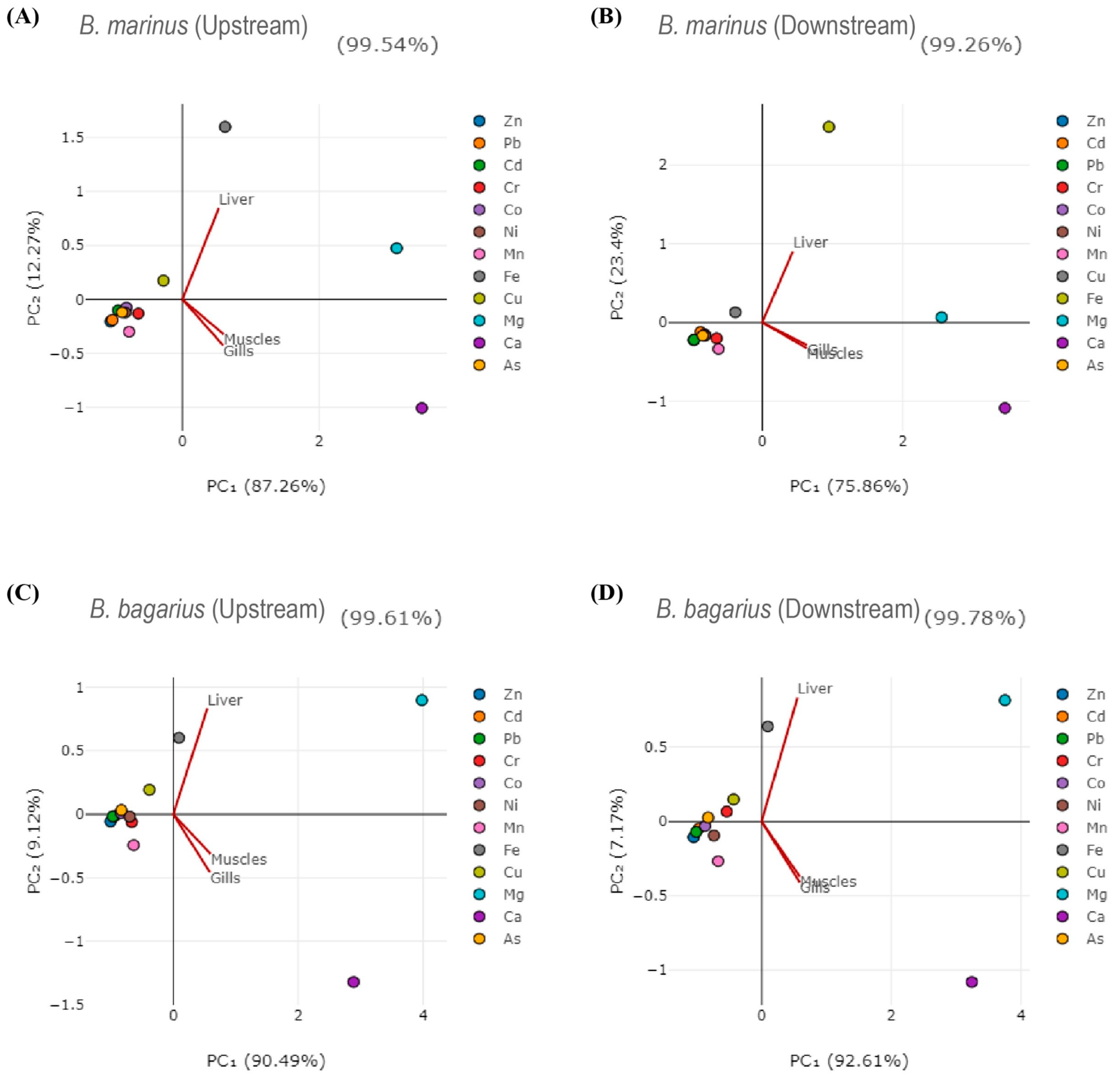
Correlations were established among heavy metals in water, sediment, and fish tissues. Table 2 shows the correlation of heavy metals in water with that of the liver of B. bagarius downstream. The correlation among heavy metals in fish tissues (liver, gills, and muscles) for B. marinus and B. bagarius, water, and sediment was also observed (shown in Tables S1–S29). Principal component analysis was carried out as well. Figure 6 shows the principal component analysis (PCA diagrams) for both species along both sampling sites—upstream and downstream. Figure 6A,B present PCA for B. marinus whereas Figure 6C,D show PCA for B. bagarius, illustrating the relationship between fish tissues and metals in a 2D space.

Table 2.
Pearson correlation between heavy metals (HMs) in water and liver of Bagarius bagarius downstream of Jhelum River. Bold r values > 0.500 are significant at p < 0.05 showing positive correlation.
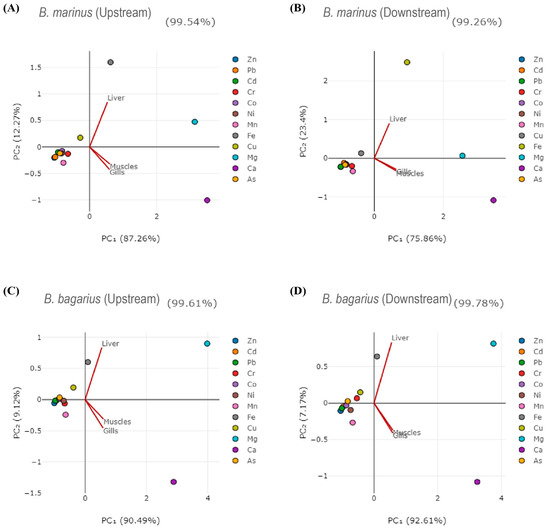
Figure 6.
PCA diagrams for Bagre marinus and Bagarius bagarius along upstream and downstream sites of Jhelum River in Khushab District. (A) shows Bagre marinus at upstream, (B) shows Bagre marinus downstream, (C) shows Bagarius bagarius upstream and (D) shows Bagarius bagarius downstream.
4. Discussion
4.1. Bioaccumulation of Heavy Metals Across Sampling Sites and Studied Tissues
Metal pollution of the aquatic environment creates a source of stress for aquatic animals. Due to the severe impact of these toxicants and the sensitivity of fish, exposure to these toxicants lead to a wide range of physiological dysfunctions [39]. Assessing the physiological condition of fish holds paramount importance, with hematological and biochemical markers playing a key role in this evaluation. The alterations observed in these indicators and their characteristics are contingent upon the metal concentration and their exposure time for the fish, as shown in different lab-based studies [6,40]. Analyzing these changes in potentially contaminated environments is crucial for the effective management and protection of aquatic species and their habitats. The Jhelum River in Khushab in Pakistan serves as the basis for this study, contaminated with urban, agricultural, and industrial effluents and wastes. These activities along with natural geochemical processes are shown to contribute to higher concentrations of pollutants downstream in rivers as compared to upstream [41]. The current study also confirmed higher mineral and heavy metal concentrations in the downstream sampling site. Moreover, the bioaccumulation of heavy metals in a higher concentration downstream might be attributed to several reasons such as agricultural runoffs, domestic waste discharge, and dumping of wastes, trashes, remains, and effluents from the nearby markets.
The bioaccumulation of heavy metals was observed to differ across the studied sampling sites. Similarly, different metals were observed to be accumulating in a species-specific and tissue-specific manner. As the liver is a primary organ for detoxifying various substances, including heavy metals and the presence of metal-binding proteins such as metallothioneins, therefore the accumulation of these metals in liver tissues was expected [42]. In the current study, higher concentrations were mainly present in the liver followed by gill tissue. As observed in previous studies, exposure to higher concentrations of heavy metals led to an increase in the activities of hepatic enzymes [43]; this study also observed higher activities of liver enzymes including ALT, ALP, AST, and LDH in both species. The observed increase in the bioaccumulation of heavy metals in the liver is consistent with previous studies, which reported similar patterns of elevated heavy metal accumulation in the livers of various fish species [44,45,46,47].
Although the fish take in heavy metals in a similar fashion, their presence and persistence within a specific tissue might be associated with other metal or metals as well. A significant correlation was seen between the concentration of heavy metals in fish tissues and the surrounding water and sediments. This relation clearly indicates that heavy metals accumulate into fish tissues from water and sediments. Similar biogeochemical behavior is shown by the abundance of highly significant correlations between element pairs. When one element shows a high concentration in a particular fish, this study helps determine which counterpart elements should be examined [47], as observed in the current study (tables showing inter-metal correlation for liver of Bagarius bagarius and water along the downstream site of Jhelum River in the Khushab region and similar correlations as shown in Supplementary Tables S1–S29).
In Pakistan, the maximum residue limits (MRLs) of heavy metals in fish and fishery products are regulated by the Pakistan Standards and Quality Control Authority (PSQCA) under the Pakistan Food Standards and Quality Control Act, 1961. The recommended MRL for zinc is 0.5 mg/g to 0.1 mg/g, for lead 0.0003 mg/g, for nickel 0.0005 mg/g, for manganese 0.01 mg/g, for iron 100 mg/g, for copper 0.01 mg/g, for cobalt 0.0001 to 0.0005 mg/g, for cadmium 0.00005 mg/g, and for arsenic 0.0001 mg/g in fish or fish-based products and live animals. As compared to the MRLs, the findings of the current study reveal that the levels of lead, cadmium, and arsenic in the fish samples exceed the permissible limits established for safe consumption. Meanwhile, the analysis of heavy metal concentrations in different tissues of fish reveals a clear variation in the accumulation of these metals across the liver, muscles, and gills. For instance, the concentration of lead in the liver of Bagre marinus from the downstream site is significantly higher (0.445 mg/g), exceeding the MRL compared to its concentration in muscles (0.149 mg/g) and gills (0.233 mg/g). Therefore, the differential accumulation of heavy metals in various fish tissues, as observed in the liver, muscles, and gills, has significant implications when compared to the maximum residue limits (MRLs) established for safe consumption. The excessive levels of heavy metals, exceeding MRLs, such as lead, cadmium, and arsenic found in fish tissues, can pose severe health risks to humans, including neurological damage, kidney dysfunction, and increased cancer risk due to prolonged exposure to these toxic elements [48].
Well-documented research revealed that the accumulation of heavy metals over the permissible limit or above the MRL leads to different human health complications. These include renal dysfunction after exposure to cadmium; abdominal pain, constipation, irritability, memory loss, tiredness, headache, appetite loss, pain or tingling in hands and feet, and general weakness after exposure to lead; and pigmentation change, hard patches on palms, hyperkeratosis, and skin lesions after exposure to arsenic; however, exposure to arsenic for a prolonged period can lead to cancer such as skin, kidney, bladder, and lung cancer; chromium VI is carcinogenic, whereas chromium can have chronic effects including breathing problems (including asthma, cough, wheezing, and shortening of breath), nose ulcers, and allergic reactions; higher concentrations of serum iron are linked with colorectal cancer, while its accumulation in the liver leads to hepatocellular carcinoma; cobalt is a carcinogen in acute concentrations and can lead to allergy (skin allergy, eyes, throat, and nose) in sub-acute concentrations; and nickel incudes different toxic effects in humans after exposure and accumulation such as contact dermatitis, gastrointestinal manifestation, nasal cancer, liver fibrosis, respiratory manifestation, headache, lung cancer, and cardiovascular diseases [49,50,51,52,53,54].
4.2. The Hematological Profile
Severe hematological changes were observed downstream as compared to upstream. These changes might be associated with the higher concentration of heavy metals downstream, as indicated by lab-based studies showing that exposure to heavy metals disturbed the hematological profile in several fish species [28]. In the current study, red blood cells decreased by 27.68% (B. marinus) and 43.67% (B. bagarius) downstream as compared to upstream. The decrease in RBCs can be attributed to the inhibition of hematopoiesis leading to a decreased count of RBCs or suppression of bone marrow leading to the reduced ability of marrow to produce new blood cells including RBCs. The decreased count of RBCs might also be associated with higher production of reactive oxygen species, which leads to hemolysis (destruction of RBCs). The disruption of erythropoietin production can lead to lower production of both red blood cells and hemoglobin. Previous research revealed that accelerated eryptosis in the hematopoietic tissues, osmoregulatory disturbance, and impaired erythropoietic activity led to a decreased level of RBCs [55].
The current study revealed that hemoglobin levels also differ across the sampling sites, as the hemoglobin level decreased by 19.905% (B. marinus) and 12.96% (B. bagarius) downstream as compared to upstream. The decrease in HGB might be associated with the inhibition of erythropoiesis [56] or different enzymes involved in hemoglobin synthesis, such as delta-aminolevulinic acid dehydratase (ALAD) and ferrochelatase. The inhibition of these enzymes might lead to the prevention of heme production, which is a critical component of hemoglobin synthesis. Similarly, heavy metals are capable of disrupting iron metabolism; therefore, its absorption, availability, and utilization might reduce heme synthesis, leading to a lower production of HGB [57].
The increase observed in WBCs by 62.301% (B. marinus) and 70.909% (B. bagarius) downstream in the current study might be associated with the activation of the immune system. The activated immune system releases inflammatory mediators (i.e., cytokines), which subsequently leads to the production/release of WBCs [54]. Similarly, WBCs’ release or production might have been enhanced on account of chronic inflammation in the studied species on account of exposure to increased heavy metals downstream, as revealed in previous lab-based experiments [58]. Hematopoietic stimulation might lead to increased production of white blood cells as well. The analysis of the blood of the sampled species revealed an increase in the WBC concentration, which is consistent with earlier research findings [59,60].
Since the contaminants obstruct the erythropoiesis process, diminished level of hematocrit downstream might be caused by the inhibition of erythropoiesis [47]. The decrease in hematocrit is accompanied by a decrease in red blood cells as well as hemoglobin. So, additionally, it might be connected to hypoxia, which consequently prevented or reduced red blood cell genesis. Production of red blood cells is also affected by low levels of hemoglobin [61]. Similarly, the production of hematocrit is dependent on the production of red blood cells, i.e., an increase in RBCs will lead to increased production of hematocrit and vice versa. However, as observed in the current study, the RBC count decreased downstream, which might subsequently lead to decreased hematocrit. Hence, the decrease in hematocrit corresponds to the decrease in RBCs. Previous research revealed a decrease in the concentration of both hemoglobin and hematocrit in the same studies, confirming the finding of the current study [62,63].
The current study revealed that the number of platelets decreased by 48.18% (B. marinus) and 31.973% (B. bagarius) downstream, which might be associated with the exposure to a mix of heavy metals. The thrombocytopenia observed in the current study might be associated with several mechanisms, such as the suppression of megakaryocytes in the bone marrow, which are responsible for the production of platelets, and damage to these cells lead to reduced platelets production. The increase in the concentration of heavy metals downstream might lead to enhanced ROS production, which subsequently resulted in the destruction of platelets at their premature stage after rupturing their membrane. Furthermore, the decrease in platelets might also be associated with an autoimmune response, where the immune system mistakenly targets and destroys platelets. Similar kinds of findings are reported in earlier research where heavy metal exposure of Garra gotyla gotyla resulted in a decline in platelet count [64]. Studies conducted on the catfish species Clarias batrachus [65] and Channa punctatus [66] revealed the same results after exposure to arsenic trioxide and cadmium chloride, respectively, in a time reliant manner, as they also observed a decrease in the number of platelets. Both studies demonstrate that exposure to heavy metals like arsenic trioxide and cadmium chloride can significantly reduce platelet counts in fish species. This decrease was noted alongside reductions in other red blood cell indices, such as Hb and packed cell volume (PCV) [60]. The reduction in platelets, as observed in these studies, is likely due to the toxic impact on the hematopoietic system, which is responsible for producing blood cells. The decrease in platelets of the fish downstream might be associated with a damaged liver as well. The reduction in platelet count observed in this study aligns with the increase in liver damage biomarkers (enzymes) that were also investigated in the current study.
A decrease in lymphocytes was observed downstream as compared to upstream. The decrease in lymphocytes might be associated with impaired lymphocyte proliferation and function. Apoptosis of lymphocytes might also be the reason for the lower levels, which might be associated with impairment of the antioxidant defense system or ROS-mediated apoptosis of lymphocytes [67]. The development and maturation of lymphocytes might be affected by impaired lymphopoiesis, as heavy metals might interrupt the production and regulation of lymphocytes. The current study’s observed decrease in lymphocytes is in congruence with previous studies [58].
A plethora of well-document research revealed that exposure to different heavy metals leads to hematological toxicity. These studies are focused on the exposure of fish to single as well as multiple heavy metals. Similarly, different heavy metals can pose similar effects hematologically; therefore, it is difficult to elucidate which metals lead to this kind of effect if employed in a mixture. The effects can be synergistic or antagonistic, which makes the observation even more difficult. Zinc exposure led to different hematological changes in Oreochromis niloticus such as decreased RBCs, increased hemoglobin, mean corpuscular hemoglobin, and a decrease in WBCs [68]. Lead exposure led to disturbed counts of RBCs, WCs, MCV, MCHC, MCH, and WBCs in Hypophthalmycthys nobilis [69]. Arsenic exposure led to a decrease in hemoglobin, hematocrit, RBCs, and WBCs in Channa punctatus [70]. Arsenic exposure led to anemia in Clarias batrachus [71]. Arsenic exposure led to a significant increase in cholesterol, triglyceride, and free amino acid, whereas a significant decrease in glucose, protein, and bilirubin in Mystus vittatus was reported [72]. The results of the current study are well aligned with these studies.
Interpolating the lab-based studies with the current study, the hematological changes might be associated with the increasing concentrations of heavy metals downstream. Overall, the observed increase in WBC count is indicative of an immune response triggered by the presence of toxicants, as the body attempts to respond to the damage caused by toxicants associated with heavy metals in the current study. Conversely, the reduction in RBCs, platelets, Hb, and Hct levels suggests that heavy metals are impairing the hematopoietic system, leading to anemia and a diminished capacity for oxygen transport and blood clotting. The impact on liver function is further corroborated by elevated levels of bilirubin and liver enzymes (ALT, AST, ALP, and LDH), which point to hepatic stress and damage. The significant increase in serum creatinine and urea levels indicates kidney dysfunction, likely due to the bioaccumulation of heavy metals, which impairs the kidneys’ ability to filter waste. These physiological changes collectively highlight the toxic effects of the exposed heavy metals, which disrupt homeostasis, possibly leading to compromised health and increased mortality to be confirmed with further studies.
4.3. The Biochemical Profile
Serum biochemistry is widely used in ecological, chemical risk assessment, toxicological, and safety research due to being a sensitive biomarker of toxicant-induced stress [73,74]. Coupled with a disturbed hematological profile, the studied fish species (B. bagarius and B. marinus) were also observed to exhibit a devastated biochemical profile downstream. The urea level in the serum of the fish blood is studied as a key metabolic health indicator. The increase in the level of urea in the current study downstream might be attributed to a rise in heavy metal contamination, which ultimately led to the malfunctioning of the gills, kidney, and liver [45,75]. Gills manage gas exchange, waste removal, and water–ion balance, whereas heavy metal exposure disrupts these functions. Calcium also builds up in the gills, with structural changes like epithelial swelling and oedema, reducing gas exchange and oxygen intake [76]. These changes also affect pH and electrolyte balance. Oxidative stress from heavy metals further damages gills, leading to ineffective urea excretion and its accumulation, indicating gill dysfunction [47]. Among tested minerals and metals, magnesium and calcium levels were found to be the highest, and zinc and lead were the lowest, which is congruent with a previous study on B. bagarius from the Chenab River [77]. Similarly, kidneys are damaged by heavy metals, inducing nephrotoxicity and subsequently leading to an inability of the kidneys to filter and excrete urea which leads to their accumulation or increase in blood serum. Similarly, this might be the case in the current study as exposure to higher concentrations of heavy metals might damage the liver of the studied fish species, which resulted in increased serum urea downstream due to a disrupted urea cycle. Heavy metals might interfere with enzymes involved in the urea cycle such as carbamoyl phosphate synthetase and ornithine transcarbamylase.
Indicators for the integrity of the liver and general health state include the serum total protein level in fish [78]. In fish exposed to heavy metal pollution, this level typically drops (hypoproteinemia) because of severe liver injury and stressful circumstances [79]. The proteins in fish serve many purposes, including providing energy and facilitating the metabolism of nitrogen. Because of the buildup of heavy metals downstream, the total protein concentration might reduce considerably downstream as indicated by both species in the current study. The reduced total protein concentration may be due to lower oxygen levels in the fish blood, which in turn hindered the oxidative metabolism. To meet the rising energy demands, the fish switched from an aerobic to an anaerobic mode of respiration because of the decreasing oxygen levels. Total protein contents may decline due to their increased consumption for meeting energy demands as well [47]. Protein serves as an alternate energy source for fish under stressful conditions. The decline in serum protein contents in fish aligns with earlier studies, where researchers observed similar effects of heavy metals on the protein profile of Lethrinus harak in the Red Sea [45]. This may also be related to protein production being weak, impaired, or reduced downstream, which might be associated with heavy metal stress.
The accumulation of heavy metals was higher downstream as compared to upstream, with magnesium and calcium showing the highest bioaccumulation. Different organs have different affinities for different heavy metals; therefore, their accumulation falls within varying ranges across the sampling sites and species. However, the current study observed that the concentration of heavy metals was quite high in the muscles of the studied fish species downstream. It is assumed that this might have led to various behavioral inconsistencies in these fish species in the wild; they would have difficulty in swimming, reduced agility, and hindered hunting and prey capturing. Similarly, keeping in view the current study, it has been projected that these muscles are eaten, and hence, it can pose serious public health concerns to the consumers as well. Moreover, heavy metal exposure leads to stress, which leads to a reduction in glycogen in the liver and muscles of the fish [80]. It renders the fish weak, having less energy (glucose), and hence, they start consuming their protein (protein sparing effect). We observed a decline in total protein content. Hence, it can be assumed that the increase in heavy metal concentrations in the muscles was congruent with the decrease in total protein content. Fish with low muscle glycogen stores reportedly have reduced physical stamina. In addition, it may impede the healing and regeneration process following stress and traumas.
The changes observed in the biochemical profile confirmed damage to various tissues as declining albumin and protein levels may indicate liver dysfunction, while increased urea levels suggest gill dysfunction [47,81]. The serum biochemistry assessment also consisted of various proteins and enzymes. Higher bilirubin levels, primarily produced in the liver and eliminated in bile, signal liver damage. Elevated bilirubin can lead to additional health issues like inflammation and anemia. The current investigation found higher bilirubin levels downstream, likely due to heavy metals contamination downstream, impairing the liver’s ability to remove bilirubin. Further, the increase in bilirubin concentration downstream might be associated with any of these toxic effects of heavy metals exposure including hepatotoxicity, disrupted bilirubin conjugation, hemolysis, cholestasis, bile duct damage, or disruption of liver enzymes involved in bilirubin metabolism such as UDP-glucuronosyltransferase.
Different enzymes, such as AST, ALT, LDH, and ALP, in the current study served as valuable biomarkers, indicated various physiological disturbances—often associated with organ dysfunction or tissue damage. For instance, AST and ALT are primarily associated with liver function and their elevated levels indicate liver damage (linked with different factors such as contaminated water in the current study). LDH is associated with various tissues including the liver, heart, and muscles. The elevated level of LDH signal tissue damage or stress due to various factors including physical injury to fish, exposure to pollution, and low oxygen levels (hypoxia). Similarly, ALP is primarily found in the liver, intestine, and bone, and its elevated levels indicate liver damage and bone disorders. The elevation of ALP levels is linked with exposure to environmental stressors or pollutants.
A significant increase in the enzymatic profile of both fish species downstream as compared to upstream specimens might be associated with the accumulation of heavy metals in higher concentrations. The increased level of these enzymes downstream might be associated with several reasons. The alterations observed in LDH in the current study might be associated with some of these changes including cellular damage in the liver, heart, muscles, and kidneys; membrane disruption; excessive production of ROS leading to damage of cellular components such as DNA, lipids, and proteins; oxidative stress causing necrosis or apoptosis; and tissue hypoxia involving LDH activity for anaerobic metabolism. The alteration observed in AST elevation might be associated with liver damage, muscle damage, oxidative stress, and inflammation. The alteration observed in ALP might be associated with hepatobiliary dysfunction (indicating cholestasis), myotoxicity (muscle and bone damage), and systemic inflammation. Elevated enzyme levels serve as biomarkers indicating the severity of the toxic effects and the specific organs or tissues that are affected.
An increase was observed in lactate dehydrogenase (LDH) levels downstream. LDH is crucial for fish metabolism, converting glucose to energy (ATP) and aiding in anaerobic respiration during oxygen scarcity. The highest concentration of LDH was observed in Bagre marinus. Pollutants and toxins alter cells and tissues, causing LDH to leak into the bloodstream. Elevated LDH levels might be associated with higher concentrations of heavy metals downstream as indicated by a previous study [82]. Elevated LDH demonstrated liver damage as well an urge for energy under stress. Apart from LDH, the level of creatinine and urea was higher downstream as compared to upstream, which indicated impaired kidney function. The disturbed balance of nutrients and electrolytes hinder fish growth and increase susceptibility of the fish to illness or infection, leading to higher mortality [83]. An increase in these parameters also indicated osmotic stress, electrolyte imbalance, and dehydration downstream [45]. This study indicates that poor water quality is a significant factor in the disrupted biochemical profile of the studied fish species. A higher increase and higher values of LDH were observed in B. marinus, which indicated that B. marinus is more vulnerable as compared to B. bagarius.
A significant increase in the level of AST was observed downstream. It indicated that the liver (hepatotoxicity or liver inflammation), muscle (myopathy or muscle trauma), kidney (renal injury), and heart (cardiac injury) of fish residing downstream are damaged. It might also be due to general cellular damage or an inflammatory response. However, keeping in view the other parameters such as an increase in ALT and ALP, it might be predominantly associated with liver injury in the current study. A slight but significant increase was observed in ALT and ALP downstream. An increase in these enzymes (ALT and ALP) along with an increase in AST indicates liver damage (hepatotoxicity or inflammation) or liver diseases (liver cirrhosis, fibrosis, or steatosis).
On account of the exposure to a mixture of heavy metals and other pollutants, it is difficult to clarify which pollutant or metal leads to a specific change in serum biochemistry in the real-world environment. However, it is easier to observe metal-specific effects in the laboratory. Our research is in conformity with previous studies on the effects of heavy metals on serum biochemistry. Exposure of Oreochromis niloticus to a mixture of metals (Ag, Cd, Cr, Cu, and Zn) led to subtle effects on different biochemical parameters including ALP, ALT, AST, total protein, triglyceride, urea, sodium, and glucose [84]. Lead exposure led to altered total cholesterol, glucose, total protein, and albumin in Hypophthalmychthys nobilis [69]. Cadmium exposure led to a significant dose-dependent increase in cholesterol, ALP, ALT, AST, and glucose and a decrease in triglyceride and total protein in Clarias gariepinus [85].
5. Conclusions, Recommendations, and Future Perspective
The present study concluded that B. marinus and B. bagarius bioaccumulated higher concentrations of heavy metals downstream as compared to upstream. The hematological profiles were observed to be significantly disturbed downstream as compared to upstream, such as WBC increases, whereas RBCs and platelets decreased downstream. The biochemical analysis revealed varying degree of disruption downstream as well; for instance, increased AST, LDH, ALP, and ALT levels downstream indicated liver injury; increased LDH and creatinine indicated affected gills and muscles; decreased total protein and increased bilirubin indicated severe stress to fish downstream. Pearson correlation analysis showed varying degrees of significant correlations among the studied metals. Principal component analysis illustrated the relationship between heavy metals and fish tissues (liver, gills, and muscles). The current study affirmed hematology and serum biochemistry as valuable biomarkers for assessing the health of aquatic animals and conducting research on non-invasive pollution.
The current study recommends the proper disposal of heavy metals, pesticides, industrial effluents, domestic pollutants, market trash, and agricultural wastes, which is crucial to protect freshwater ecosystems. Moreover, the water bodies should be monitored regularly to ensure ambient water quality and mitigate health concerns of both fish and humans from hazardous chemicals and heavy metals. Assessing geographical and seasonal variability of heavy metals and other pollutants might ease addressing this issue of grave concern. A mass awareness campaign should be a part of the stakeholder’s agenda.
The current study was focused on the bioaccumulation of heavy metals in the tissue of two fish species and profiling their hemato-biochemical health. Therefore, future research studies should involve molecular aspects of fish health along with these conventional biomarkers. The parameters that might be focused on in future research are genotoxicity, endocrine disruption, developmental deformities, reproductive failure, etc. In addition to these parameters, future work should be extended to other species and should examine them at population and community levels combined with biodiversity-based findings.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w16243603/s1. Figure S1. Sampling sites of the study; Tables S1–S29. Pearson correlation coefficient matrices for water, sediment, and fish tissues (liver, gills, and muscles) for both the species.
Author Contributions
Conceptualization, S.U. and A.M.; methodology, S.U., M.B. and A.M.; validation, S.U. and B.Y.-D.; formal analysis, M.S. and S.U.; investigation, M.S., M.B. and S.U.; data curation, M.B. and M.S.; writing—original draft preparation, S.U., M.S. and B.Y.-D.; writing—review and editing, B.Y.-D. and C.F.; visualization, S.U. and B.Y.-D.; supervision, S.U. and C.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by EIRB and notified by OGS under No. UE/OGS/2022/67. All the standard ethical procedures at the institutional, governmental, and international levels were followed during this study.
Data Availability Statement
The data used in this paper are contained within it.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kanwal, H.; Raza, A.; Zaheer, M.S.; Nadeem, M.; Ali, H.H.; Manoharadas, S.; Rizwan, M.; Kashif, M.S.; Ahmad, U.; Ikram, K. Transformation of Heavy Metals from Contaminated Water to Soil, Fodder and Animals. Sci. Rep. 2024, 14, 11705. [Google Scholar] [CrossRef]
- Yurdakok-Dikmen, B.; Kuzukiran, O.; Filazi, A.; Kara, E. Measurement of Selected Polychlorinated Biphenyls (PCBs) in Water via Ultrasound Assisted Emulsification–Microextraction (USAEME) Using Low-Density Organic Solvents. J. Water Health 2015, 14, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Ansari, Z.; Matondkar, S. Anthropogenic Activities Including Pollution and Contamination of Coastal Marine Environment. J. Ecophysiol. Occup. Health 2014, 14, 71–78. [Google Scholar] [CrossRef]
- Prakash, S.; Verma, A. Anthropogenic Activities and Biodiversity Threats. Int. J. Biol. Innov. IJBI 2022, 4, 94–103. [Google Scholar] [CrossRef]
- Shiry, N.; Derakhshesh, N.; Gholamhosseini, A.; Pouladi, M.; Faggio, C. Heavy Metal Concentrations in Cynoglossus arel (Bloch & Schneider, 1801) and Sediment in the Chabahar Bay, Iran. Int. J. Environ. Res. 2021, 15, 773–784. [Google Scholar]
- Shahjahan, M.; Taslima, K.; Rahman, M.S.; Al-Emran, M.D.; Alam, S.I.; Faggio, C. Effects of Heavy Metals on Fish Physiology–a Review. Chemosphere 2022, 300, 134519. [Google Scholar] [CrossRef] [PubMed]
- Masindi, V.; Muedi, K.L. Environmental Contamination by Heavy Metals. In Heavy Metals; Saleh, H.E.-D.M., Aglan, R.F., Eds.; IntechOpen: London, UK, 2018; pp. 10, 115–132. [Google Scholar]
- Jamil Emon, F.; Rohani, M.F.; Sumaiya, N.; Tuj Jannat, M.F.; Akter, Y.; Shahjahan, M.; Goh, K.W. Bioaccumulation and Bioremediation of Heavy Metals in Fishes—A Review. Toxics 2023, 11, 510. [Google Scholar] [CrossRef]
- Mahamood, M.; Khan, F.R.; Zahir, F.; Javed, M.; Alhewairini, S.S. Bagarius Bagarius, and Eichhornia Crassipes Are Suitable Bioindicators of Heavy Metal Pollution, Toxicity, and Risk Assessment. Sci. Rep. 2023, 13, 1824. [Google Scholar] [CrossRef]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy Metals, Occurrence and Toxicity for Plants: A Review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Festa, R.A.; Thiele, D.J. Copper: An Essential Metal in Biology. Curr. Biol. 2011, 21, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Altowayti, W.A.H.; Almoalemi, H.; Shahir, S.; Othman, N. Comparison of Culture-Independent and Dependent Approaches for Identification of Native Arsenic-Resistant Bacteria and Their Potential Use for Arsenic Bioremediation. Ecotoxicol. Environ. Saf. 2020, 205, 111267. [Google Scholar] [CrossRef] [PubMed]
- Margesin, R.; Płaza, G.A.; Kasenbacher, S. Characterization of Bacterial Communities at Heavy-Metal-Contaminated Sites. Chemosphere 2011, 82, 1583–1588. [Google Scholar] [CrossRef] [PubMed]
- Flores, C.M.; Del Angel, E.; Frías, D.M.; Gómez, A.L. Evaluación de Parámetros Fisicoquímicos y Metales Pesados En Agua y Sedimento Superficial de La Laguna de Las Ilusiones, Tabasco, México. Tecnol. Cienc. Agua 2018, 9, 39–57. [Google Scholar] [CrossRef]
- Rocha, C.H.B.; Costa, H.F.; Azevedo, L.P. Heavy Metals in the São Mateus Stream Basin, Peixe River Basin, Paraiba Do Sul River Basin, Brazil. Ambient. Agua 2019, 14, e2329. [Google Scholar] [CrossRef][Green Version]
- Jia, Z.; Li, S.; Liu, Q.; Jiang, F.; Hu, J. Distribution and Partitioning of Heavy Metals in Water and Sediments of a Typical Estuary (Modaomen, South China): The Effect of Water Density Stratification Associated with Salinity. Environ. Pollut. 2021, 287, 117277. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Xu, C.; Kang, S.; Huo, A.; Lyu, J.; Zhou, M.; Nover, D. Heavy Metals in Water and Surface Sediments of the Fenghe River Basin, China: Assessment and Source Analysis. Water Sci. Technol. 2021, 84, 3072–3090. [Google Scholar] [CrossRef]
- Prasad, S.; Saluja, R.; Joshi, V.; Garg, J.K. Heavy Metal Pollution in Surface Water of the Upper Ganga River, India: Human Health Risk Assessment. Environ. Monit. Assess. 2020, 192, 742. [Google Scholar] [CrossRef]
- Khan, R.; Saxena, A.; Shukla, S.; Sekar, S.; Senapathi, V.; Wu, J. Environmental Contamination by Heavy Metals and Associated Human Health Risk Assessment: A Case Study of Surface Water in Gomti River Basin, India. Environ. Sci. Pollut. Res. 2021, 28, 56105–56116. [Google Scholar] [CrossRef]
- Ahmed, J.; Wong, L.P.; Chua, Y.P.; Channa, N.; Memon, U.-R.; Garn, J.V.; Yasmin, A.; VanDerslice, J.A. Heavy Metals Drinking Water Contamination and Health Risk Assessment among Primary School Children of Pakistan. J. Environ. Sci. Heal. Part A 2021, 56, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Afzaal, M.; Hameed, S.; Liaqat, I.; Ali Khan, A.A.; Abdul Manan, H.; Shahid, R.; Altaf, M. Heavy Metals Contamination in Water, Sediments and Fish of Freshwater Ecosystems in Pakistan. Water Pract. Technol. 2022, 17, 1253–1272. [Google Scholar] [CrossRef]
- Muhammad, S.; Ahmad, K. Heavy Metal Contamination in Water and Fish of the Hunza River and Its Tributaries in Gilgit–Baltistan: Evaluation of Potential Risks and Provenance. Environ. Technol. Innov. 2020, 20, 101159. [Google Scholar] [CrossRef]
- Sharaf, S.; Khan, M.-R.; Aslam, A.; Rabbani, M. Comparative Study of Heavy Metals Residues and Histopathological Alterations in Large Ruminants from Selected Areas around Industrial Waste Drain. Pak. Vet. J. 2020, 40, 55–60. [Google Scholar]
- Iqbal, Z.; Abbas, F.; Ibrahim, M.; Qureshi, T.I.; Gul, M.; Mahmood, A. Assessment of Heavy Metal Pollution in Brassica Plants and Their Impact on Animal Health in Punjab, Pakistan. Environ. Sci. Pollut. Res. 2021, 28, 22768–22778. [Google Scholar] [CrossRef] [PubMed]
- Ullah, R.; Muhammad, S. Heavy Metals Contamination in Soils and Plants along with the Mafic–Ultramafic Complex (Ophiolites), Baluchistan, Pakistan: Evaluation for the Risk and Phytoremediation Potential. Environ. Technol. Innov. 2020, 19, 100931. [Google Scholar] [CrossRef]
- Khan, S.A.; Muhammad, S.; Nazir, S.; Shah, F.A. Heavy Metals Bounded to Particulate Matter in the Residential and Industrial Sites of Islamabad, Pakistan: Implications for Non-Cancer and Cancer Risks. Environ. Technol. Innov. 2020, 19, 100822. [Google Scholar] [CrossRef]
- Rakib, M.R.J.; Jolly, Y.N.; Enyoh, C.E.; Khandaker, M.U.; Hossain, M.B.; Akther, S.; Alsubaie, A.; Almalki, A.S.A.; Bradley, D.A. Levels and Health Risk Assessment of Heavy Metals in Dried Fish Consumed in Bangladesh. Sci. Rep. 2021, 11, 14642. [Google Scholar] [CrossRef]
- Naz, S.; Hussain, R.; Ullah, Q.; Chatha, A.M.M.; Shaheen, A.; Khan, R.U. Toxic Effect of Some Heavy Metals on Hematology and Histopathology of Major Carp (Catla catla). Environ. Sci. Pollut. Res. 2021, 28, 6533–6539. [Google Scholar] [CrossRef] [PubMed]
- Bauerová, P.; Krajzingrová, T.; Těšický, M.; Velová, H.; Hraníček, J.; Musil, S.; Svobodová, J.; Albrecht, T.; Vinkler, M. Longitudinally Monitored Lifetime Changes in Blood Heavy Metal Concentrations and Their Health Effects in Urban Birds. Sci. Total Environ. 2020, 723, 138002. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Ma, L.; Yi, Y.; Lin, T. Assessment of Heavy Metal Pollution and Exposure Risk for Migratory Birds—A Case Study of Caohai Wetland in Guizhou Plateau (China). Environ. Pollut. 2021, 275, 116564. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Xi, S. The Effects of Heavy Metals on Human Metabolism. Toxicol. Mech. Methods 2019, 30, 167–176. [Google Scholar] [CrossRef]
- Witkowska, D.; Słowik, J.; Chilicka, K. Heavy Metals and Human Health: Possible Exposure Pathways and the Competition for Protein Binding Sites. Molecules 2021, 26, 6060. [Google Scholar] [CrossRef] [PubMed]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy Metal Pollution in the Environment and Their Toxicological Effects on Humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- Shams, M.; Tavakkoli Nezhad, N.; Dehghan, A.; Alidadi, H.; Paydar, M.; Mohammadi, A.A.; Zarei, A. Heavy Metals Exposure, Carcinogenic and Non-Carcinogenic Human Health Risks Assessment of Groundwater around Mines in Joghatai, Iran. Int. J. Environ. Anal. Chem. 2020, 102, 1884–1899. [Google Scholar] [CrossRef]
- Rather, M.I.; Rashid, I.; Shahi, N.; Murtaza, K.O.; Hassan, K.; Yousuf, A.R.; Romsho, S.A.; Shah, I.Y. Massive Land System Changes Impact Water Quality of the Jhelum River in Kashmir Himalaya. Environ. Monit. Assess. 2016, 188, 185. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, H.H.; Shahid, N.; Qadir, A.; Ahmad, S.R.; Sarwar, S.; Ashraf, M.R.; Masood, N. Hydrological and Ichthyological Impact Assessment of Rasul Barrage, River Jhelum, Pakistan. Pol. J. Environ. Stud. 2017, 26, 107–114. [Google Scholar] [CrossRef]
- Mehmood, M.A.; Shafiq-ur-Rehman, A.R.; Ganie, S.A. Spatio-Temporal Changes in Water Quality of Jhelum River, Kashmir Himalaya. Int. J. Environ. Bioenergy 2017, 12, 1–29. [Google Scholar]
- Shokr, E.-S.A. Effect of Ammonia Stress on Blood Constitutes in Nile Tilapia. Egypt. Acad. J. Biol. Sci. B Zool. 2015, 7, 37–44. [Google Scholar] [CrossRef]
- Vosylienė, M.Z. The Effect of Heavy Metals on Haematological Indices of Fish (Survey). Acta Zool. Litu. 1999, 9, 76–82. [Google Scholar] [CrossRef]
- Liao, J.; Chen, J.; Ru, X.; Chen, J.; Wu, H.; Wei, C. Heavy Metals in River Surface Sediments Affected with Multiple Pollution Sources, South China: Distribution, Enrichment and Source Apportionment. J. Geochem. Explor. 2017, 176, 9–19. [Google Scholar] [CrossRef]
- Vali, S.; Majidiyan, N.; Azadikhah, D.; Varcheh, M.; Tresnakova, N.; Faggio, C. Effects of Diazinon on the Survival, Blood Parameters, Gills, and Liver of Grass Carp (Ctenopharyngodon idella Valenciennes, 1844; Teleostei: Cyprinidae). Water 2022, 14, 1357. [Google Scholar] [CrossRef]
- Ishaq, S.; Jabeen, G.; Arshad, M.; Kanwal, Z.; Nisa, F.U.; Zahra, R.; Shafiq, Z.; Ali, H.; Samreen, K.B.; Manzoor, F. Heavy metal toxicity arising from the industrial effluents repercussions on oxidative stress, liver enzymes and antioxidant activity in brain homogenates of Oreochromis niloticus. Sci. Rep. 2023, 13, 19936. [Google Scholar] [CrossRef]
- Ahmad, K.; Azizullah, A.; Shama, S.; Khan Khattak, M.N. Determination of Heavy Metal Contents in Water, Sediments, and Fish Tissues of Shizothorax plagiostomus in River Panjkora at Lower Dir, Khyber Pakhtunkhwa, Pakistan. Environ. Monit. Assess. 2014, 186, 7357–7366. [Google Scholar] [CrossRef] [PubMed]
- Al-Hasawi, Z.; Hassanine, R. Effect of Heavy Metal Pollution on the Blood Biochemical Parameters and Liver Histology of the Lethrinid Fish, Lethrinus harak from the Red Sea. Pak. J. Zool. 2022, 55, 1–8. [Google Scholar] [CrossRef]
- Staniskiene, B.; Matusevicius, P.; Budreckiene, R.; Skibniewska, K.A. Distribution of Heavy Metals in Tissues of Freshwater Fish in Lithuania. Polish J. Environ. Stud. 2006, 15, 585–591. [Google Scholar]
- Ullah, S.; Li, Z.; Hassan, S.; Ahmad, S.; Guo, X.; Wanghe, K.; Nabi, G. Heavy Metals Bioaccumulation and Subsequent Multiple Biomarkers Based Appraisal of Toxicity in the Critically Endangered Tor putitora. Ecotoxicol. Environ. Saf. 2021, 228, 113032. [Google Scholar] [CrossRef] [PubMed]
- Pelić, M.; Mihaljev, Ž.; Živkov Baloš, M.; Popov, N.; Gavrilović, A.; Jug-Dujaković, J.; Ljubojević Pelić, D. Health Risks Associated with the Concentration of Heavy Metals in Sediment, Water, and Carp Reared in Treated Wastewater from a Slaughterhouse. Water 2023, 16, 94. [Google Scholar] [CrossRef]
- NJDHSS New Jersey Department of Health and Senior Services. Hazardous Substances Fact Sheet: Cobalt; NJDHSS New Jersey Department of Health and Senior Services: Trenton, NJ, USA, 2005.
- Singh, N.; Kumar, D.; Sahu, A.P. Arsenic in the Environment: Effects on Human Health and Possible Prevention. J. Environ. Biol. 2007, 28, 359. [Google Scholar]
- Martin, S.; Grisworld, W. Human Health Effects of Heavy Metals. Environ. Sci. Technol. Briefs Citiz. 2009, 15, 1–6. [Google Scholar]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human Health and Environmental Toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. Review of Human Carcinogens: Metals, Arsenic, Dusts and Fibres; World Health Organization: Geneva, Switzerland, 2012; ISBN 9283213203. [Google Scholar]
- Goyer, R.; Golu, M.; Choudhury, H.; Hughes, M.; Kenyon, E.; Stifelman, M. Issue Paper on the Human Health Effects of Metals; U.S. Environmental Protection Agency: Washington, DC, USA, 2004.
- Witeska, M.; Kondera, E.; Bojarski, B. Hematological and Hematopoietic Analysis in Fish Toxicology—A Review. Animals 2023, 13, 2625. [Google Scholar] [CrossRef] [PubMed]
- Javed, M.; Ahmad, I.; Ahmad, A.; Usmani, N.; Ahmad, M. Studies on the Alterations in Haematological Indices, Micronuclei Induction and Pathological Marker Enzyme Activities in Channa punctatus (Spotted Snakehead) Perciformes, Channidae Exposed to Thermal Power Plant Effluent. SpringerPlus 2016, 5, 761. [Google Scholar] [CrossRef]
- Joshp, P.K.; Bose, M.; Harish, D. Changes in Certain Haematological Parameters in a Siluroid Cat Fish Clarias batrachus (Linn) Exposed to Cadmium Chloride. Pollut. Res. 2002, 21, 129–131. [Google Scholar]
- Suchana, S.A.; Ahmed, M.S.; Islam, S.M.M.; Rahman, M.L.; Rohani, M.F.; Ferdusi, T.; Ahmmad, A.K.S.; Fatema, M.K.; Badruzzaman, M.; Shahjahan, M. Chromium Exposure Causes Structural Aberrations of Erythrocytes, Gills, Liver, Kidney, and Genetic Damage in Striped Catfish Pangasianodon hypophthalmus. Biol. Trace Elem. Res. 2021, 199, 3869–3885. [Google Scholar] [CrossRef]
- Barathinivas, A.; Ramya, S.; Neethirajan, K.; Jayakumararaj, R.; Pothiraj, C.; Balaji, P.; Faggio, C. Ecotoxicological Effects of Pesticides on Hematological Parameters and Oxidative Enzymes in Freshwater Catfish, Mystus keletius. Sustainability 2022, 14, 9529. [Google Scholar] [CrossRef]
- Saha, S.; Dhara, K.; Chukwuka, A.V.; Pal, P.; Saha, N.C.; Faggio, C. Sub-Lethal Acute Effects of Environmental Concentrations of Inorganic Mercury on Hematological and Biochemical Parameters in Walking Catfish, Clarias batrachus. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 264, 109511. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Tawwab, M.; Monier, M.N.; Hoseinifar, S.H.; Faggio, C. Fish Response to Hypoxia Stress: Growth, Physiological, and Immunological Biomarkers. Fish Physiol. Biochem. 2019, 45, 997–1013. [Google Scholar] [CrossRef] [PubMed]
- Parekh, H.M.; Tank, S.K. Studies of Haematological Parameters of Oreochromis niloticus Exposed to Cadmium Chloride (CdCl2, 2H2O). Int. J. Environ. 2015, 4, 116–127. [Google Scholar] [CrossRef]
- Dos Santos, C.R.; Cavalcante, A.L.M.; Hauser-Davis, R.A.; Lopes, R.M.; Da Costa Mattos, R.D.C.O. Effects of Sub-Lethal and Chronic Lead Concentrations on Blood and Liver ALA-D Activity and Hematological Parameters in Nile Tilapia. Ecotoxicol. Environ. Saf. 2016, 129, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Langer, S. Effect of Manganese on Haematological Parameters of Fish, Garra gotyla gotyla. J. Entomol. Zool. Stud. 2014, 2, 77–81. [Google Scholar]
- Pichhode, M.; Gaherwal, S. Toxicological Effects of Arsenic Trioxide Exposure on Haematolical Profile in Catfish, Clarias batrachus. Int. J. Curr. Res. Rev. 2019, 11, 9–12. [Google Scholar] [CrossRef]
- Ramesh, P.; Ramachandra Mohan, M. Effects of Cadmium Chloride on Hematological Profiles in Freshwater Fish Channa punctatus (Bloch). Environ. Ecol. 2021, 39, 1096–1101. [Google Scholar]
- Yaghoobi, Z.; Safahieh, A.; Ronagh, M.T.; Movahedinia, A.; Mousavi, S.M. Hematological Changes in Yellowfin Seabream (Acanthopagrus latus) Following Chronic Exposure to Bisphenol A. Comp. Clin. Path. 2017, 26, 1305–1313. [Google Scholar] [CrossRef]
- Çelik, E.Ş.; Kaya, H.; Yilmaz, S.; Akbulut, M.; Tulgar, A. Effects of Zinc Exposure on the Accumulation, Haematology and Immunology of Mozambique Tilapia, Oreochromis mossambicus. Afr. J. Biotechnol. 2013, 12, 744–753. [Google Scholar]
- Shahzadi, K. Effect of Lead on Hematological Parameters and Serum Biochemistry of Bighead Carp (Hypophthalmycthys nobilis). Adv. Soc. Sci. Manag. 2023, 1, 73–79. [Google Scholar]
- Jha, D.K.; Mishra, B.B.; Thakur, K.R.; Kumar, V.; Verma, P.; Khan, P.K. Toxicological Effects of Arsenic Exposure on Haematology of Fresh Water Fish Channa punctatus. Der Pharma Chem. 2017, 9, 1–5. [Google Scholar]
- Kumar, R.; Banerjee, T.K. Arsenic Induced Hematological and Biochemical Responses in Nutritionally Important Catfish Clarias batrachus (L.). Toxicol. Rep. 2016, 3, 148–152. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Prakash, S.; Verma, A.K. Effect of Arsenic on Serum Biochemical Parameters of a Fresh Water Cat Fish, Mystus vittatus. Int. J. Biol. Innov. 2020, 2, 11–19. [Google Scholar] [CrossRef]
- Schuijt, L.M.; Peng, F.-J.; van den Berg, S.J.P.; Dingemans, M.M.L.; Van den Brink, P.J. (Eco)Toxicological Tests for Assessing Impacts of Chemical Stress to Aquatic Ecosystems: Facts, Challenges, and Future. Sci. Total Environ. 2021, 795, 148776. [Google Scholar] [CrossRef] [PubMed]
- Janz, D.M. Biomarkers in Fish Ecotoxicology BT—Encyclopedia of Aquatic Ecotoxicology; Férard, J.-F., Blaise, C., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 211–220. ISBN 978-94-007-5704-2. [Google Scholar]
- Alkaladi, A.; El-Deen, N.A.M.N.; Afifi, M.; Zinadah, O.A.A. Hematological and Biochemical Investigations on the Effect of Vitamin E and C on Oreochromis niloticus Exposed to Zinc Oxide Nanoparticles. Saudi J. Biol. Sci. 2015, 22, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Demeke, A.; Tassew, A. A Review on Water Quality and Its Impact on Fish Health. Int. J. Fauna Biol. Stud. 2016, 3, 21–31. [Google Scholar]
- Kausar, F.; Aiman, U.; Qadir, A.; Ahmad, S.R. Assessment of Fish Assemblage in Highly Human Managed Reservoirs Located on River Chenab. J. Biodivers. Endanger Species 2018, 6, 2. [Google Scholar] [CrossRef]
- Sandre, L.C.G.; Buzollo, H.; Neira, L.M.; Nascimento, T.M.T.; Jomori, R.K.; Carneiro, D.J. Growth and Energy Metabolism of Tambaqui (Colossoma macropomum) Fed Diets with Different Levels of Carbohydrates and Lipids. Fish. Aquac. J. 2017, 8, 1–8. [Google Scholar] [CrossRef]
- Shaheen, T.; Akhtar, T. Assessment of Chromium Toxicity in Cyprinus carpio through Hematological and Biochemical Blood Markers. Turk. J. Zool. 2012, 36, 682–690. [Google Scholar] [CrossRef]
- Bakshi, A.; Panigrahi, A.K. A Comprehensive Review on Chromium Induced Alterations in Fresh Water Fishes. Toxicol. Rep. 2018, 5, 440–447. [Google Scholar] [CrossRef]
- Osman, H.A.M.; Ismaiel, M.M.; Abbas, T.W.; Ibrahim, T.B. An Approach to the Interaction between Trichodiniasis and Pollution with Benzo-Apyrene in Catfish (Clarias gariepinus). World J. Fish Mar. Sci. 2009, 1, 283–289. [Google Scholar]
- Perumalsamy, N.; Arumugam, K. Enzymes Activity in Fish Exposed to Heavy Metals and the Electro-Plating Effluent at Sub-Lethal Concentrations. Water Qual. Expo. Health 2013, 5, 93–101. [Google Scholar] [CrossRef]
- Hadi, A.; Shokr, A.; Alwan, S. Effects of Aluminum on the Biochemical Parameters of Fresh Waterfish Tilapia Zillii. J. Sci. Appl 2009, 3, 33–41. [Google Scholar]
- Öner, M.; Atli, G.; Canli, M. Changes in Serum Biochemical Parameters of Freshwater Fish Oreochromis niloticus Following Prolonged Metal (Ag, Cd, Cr, Cu, Zn) Exposures. Environ. Toxicol. Chem. 2008, 27, 360–366. [Google Scholar] [CrossRef]
- Tabat, J.L.; Jehu, A.; Kogi, E.; Habila, J.D. Sublethal Toxicity Effects of Cadmium (Cd2+) on Serum Biochemistry in Fingerlings and Juveniles of Fresh Water Catfish, Clarias gariepinus (Burchell, 1822). Int. J. Fish. Aquat. Stud 2021, 9, 1–6. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).