Climate Change Impacts on Water Temperatures in Urban Lakes: Implications for the Growth of Blue Green Algae in Fairy Lake
Abstract
1. Introduction
2. Scope of Contributing Variables
3. Modelling Approach
3.1. Advantages and Needs of Modeling
3.2. MIKE-3 Modelling
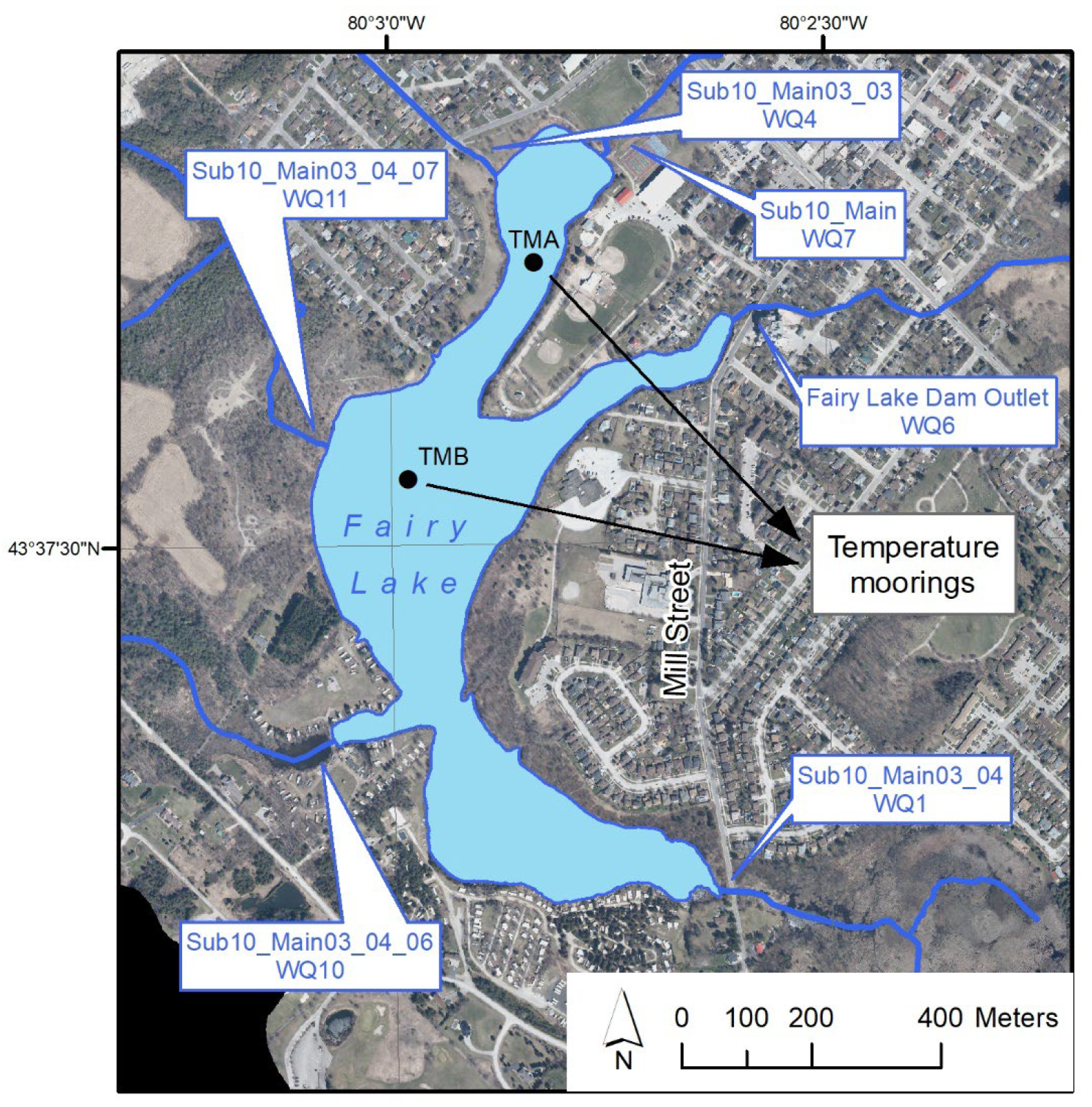
4. Description of Fairy Lake Site
4.1. Characteristics of Fairy Lake
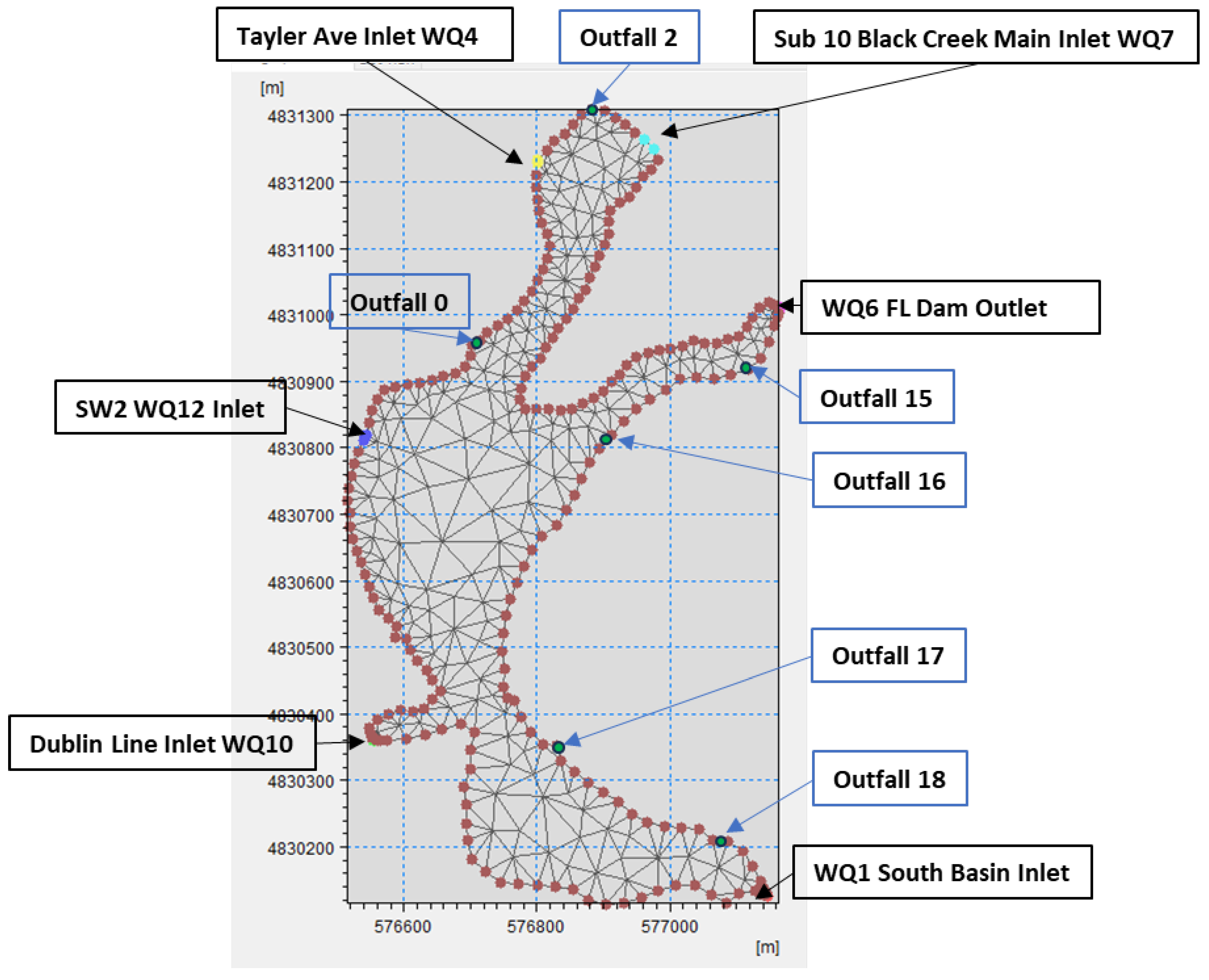
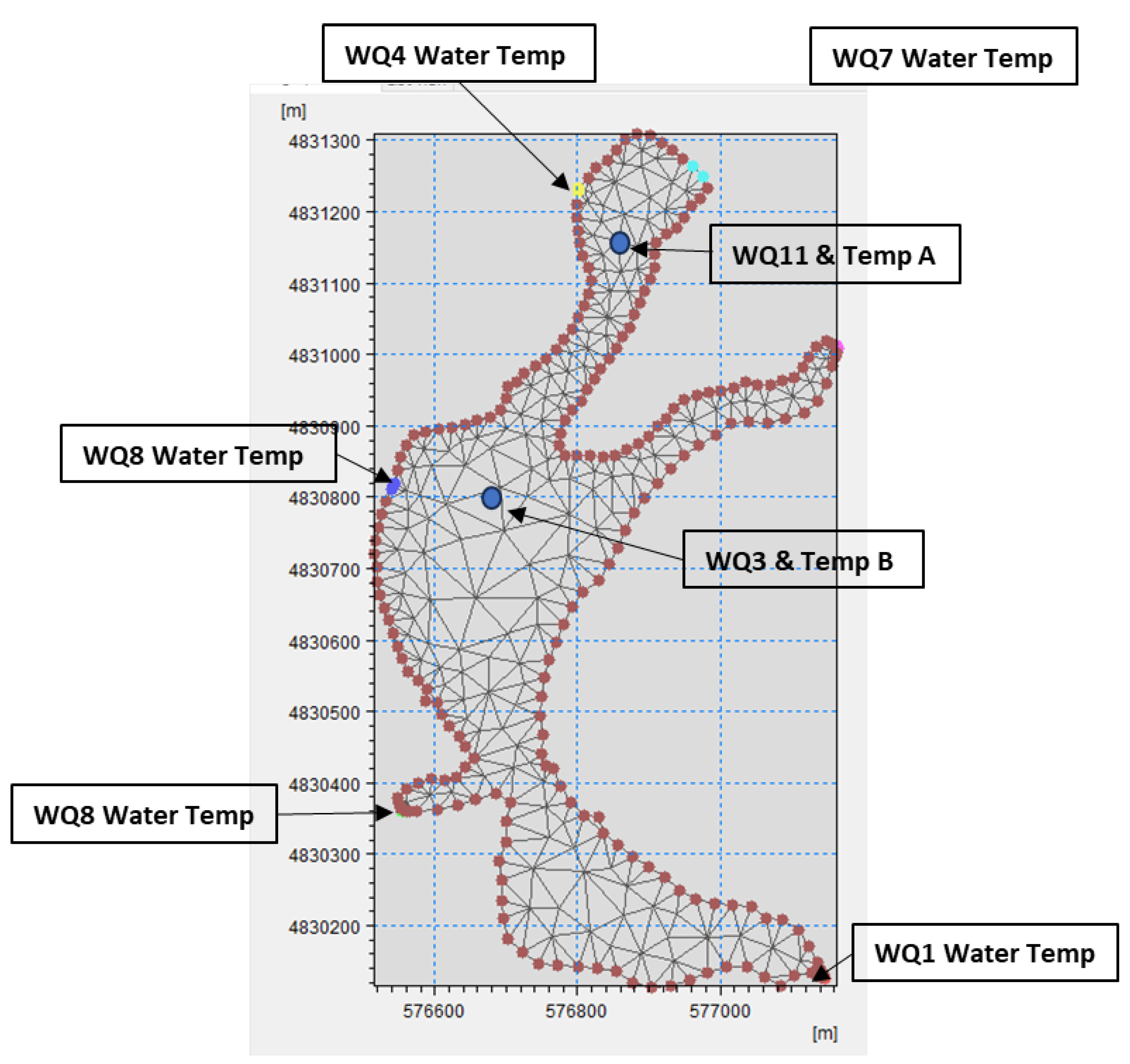
4.2. Hydrodynamic Model Setup on Fairy Lake
- (a)
- Bathymetry and Mesh Generation
- (b)
- Hydrodynamic Module Parameters
- (c)
- Model Boundaries
- (d)
- Temperature/Salinity Module Setup
- (e)
- Boundary Conditions and Sources for Temperature/Salinity Module
- (f)
- Turbulence Module Set Up
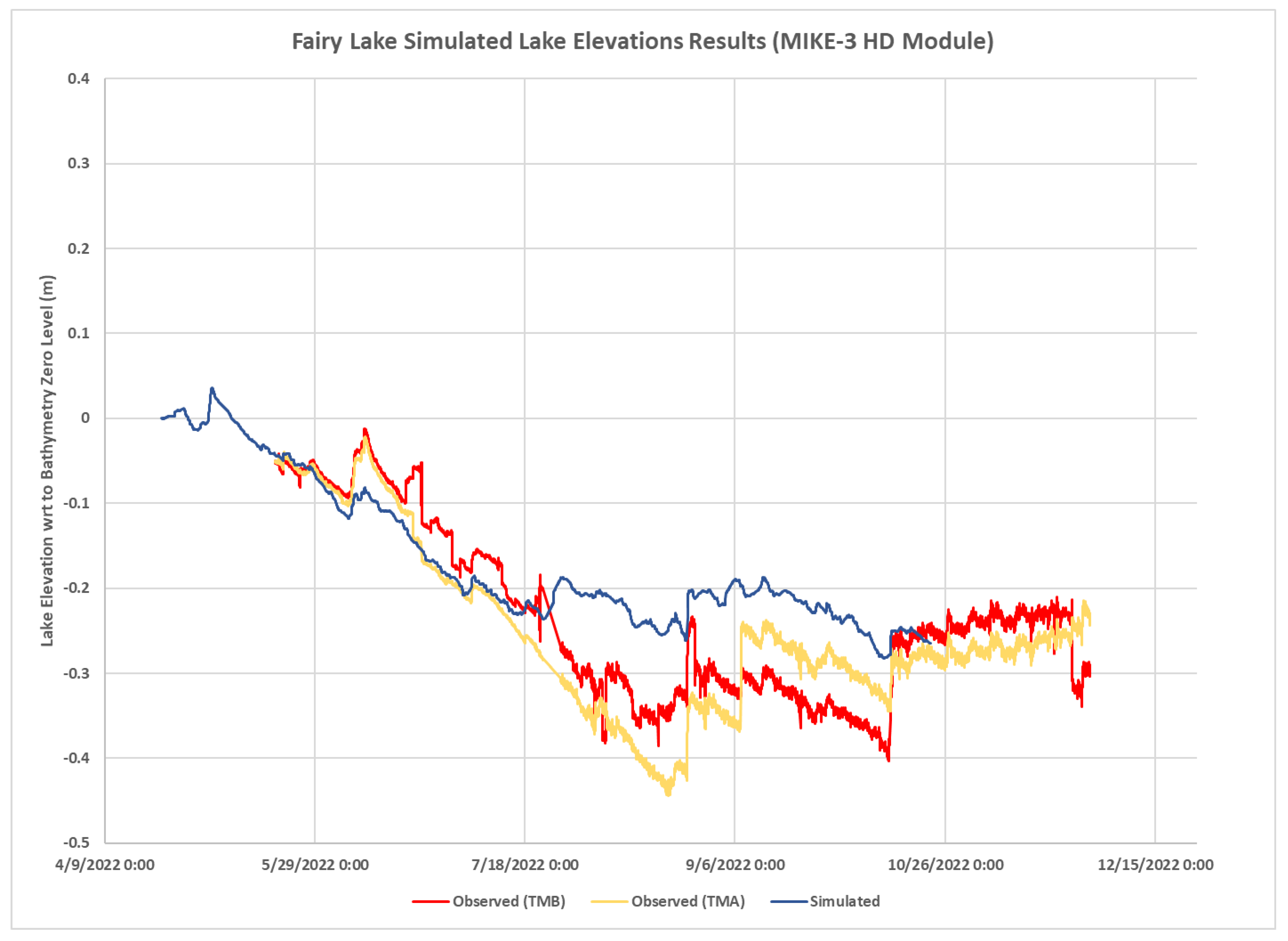
4.3. Modeling Results
- (a)
- Hydrodynamic Model Calibration
- (b)
- Calibration of the Temperature module
- (c)
- Statistics of simulated water temperatures
5. Climate Change Impact Assessment on Fairy Lake
5.1. Introduction
5.2. Number of Hours Temperature Increased or Decreased in Various Temperature Ranges
5.3. Increase in Duration of Water Temperature above Minimal Optimum Temperature for Cyanobacterial Growth
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kaloudis, T.; Hiskia, A.; Triantis, T.M. Cyanotoxins in Bloom: Ever-Increasing Occurrence and Global Distribution of Freshwater Cyanotoxins from Planktic and Benthic Cyanobacteria. Toxins 2022, 14, 264. [Google Scholar] [CrossRef] [PubMed]
- Health Canada. Guidelines for Recreational Water Quality Guideline Technical Document Cyanobacteria and Their Toxins; Health Canada: Ottawa, ON, Canada, 2022. [Google Scholar]
- Chorus, I. Cyanobacterial Toxin Research and Its Application in Germany: A Review of the Current Status. Environ. Toxicol. 2002, 17, 358–360. [Google Scholar] [CrossRef] [PubMed]
- Erratt, K.J.; Creed, I.F.; Trick, C.G. Harmonizing science and management options to reduce risks of Cyanobacteria. Harmful Algae 2022, 116, 102264. [Google Scholar] [CrossRef] [PubMed]
- Mitrovic, S.M.; Kobayashi, T.; Roelke, D.L. Cyanobacteria in inland waters: New monitoring, reporting, modelling and ecological research. Mar. Freshw. Res. 2020, 71, i–iv. [Google Scholar] [CrossRef]
- Schmale, D.G., III; Ault, A.P.; Saad, W.; Scott, D.T.; Westrick, J.A. Perspectives on Harmful Algal Blooms (HABs) and the Cyberbiosecurity of Freshwater Systems. Front. Bioeng. Biotechnol. 2019, 7, 128. [Google Scholar] [CrossRef] [PubMed]
- Health Canada. Guidelines for Canadian Drinking Water Quality: Guideline Technical Document—Cyanobacterial Toxins; Catalogue No H144-38/2017EPDF; Water and Air Quality Bureau, Healthy Environments and Consumer Safety Branch, Health Canada: Ottawa, ON, Canada, 2017. [Google Scholar]
- Abbot, M. Resource guide for public health response to harmful algal blooms in Florida: Based on recommendations of the Florida Harmful Algal Bloom Task Force Public Health Technical Panel. In B.C. Drinking Water and Recreational Water; 9 British Columbia, 2018, Decision Protocols for Cyanobacterial Toxins; Health Protection Branch: Victoria, BC, Canada, 2009. [Google Scholar]
- Chen, Q.; Rui, H.; Li, W.; Zhang, Y. Analysis of algal bloom risk with uncertainties in lakes by integrating self-organizing map and fuzzy information theory. Sci. Total Environ. 2014, 482–483, 318–324. [Google Scholar] [CrossRef]
- Uduma, U.A.; McBean, E.A.; Gharabaghi, B. Risk assessment of cyanobacteria-toxins for small drinking water treatment plants with lake water intakes. Int. J. Water Resour. Environ. Eng. 2017, 9, 121–126. [Google Scholar] [CrossRef]
- Dupont, A.; Botrel, M.; St-Gelais, N.F.; Timothée Poisot, T.; Maranger, R. A social–ecological geography of southern Canadian lakes. bioRxiv 2023. [Google Scholar] [CrossRef]
- Council of Canadians. Concerns about Blue-Green Algae on the Rise. Available online: https://canadians.org/analysis/concerns-about-blue-green-algae-rise (accessed on 4 January 2024).
- Favot, E.J.; Holeton, C.; DeSellas, A.M.; Paterson, A.M. Cyanobacterial blooms in Ontario, Canada: Continued increase in reports through the 21st century. Lake Reserv. Manag. 2023, 39, 1–20. [Google Scholar] [CrossRef]
- Nieto, P.J.G.; García-Gonzalo, E.; Lasheras, F.S.; Fernández, J.R.A.; Muñiz, C.M.; Juez, F.J.C. Cyanotoxin level prediction in a reservoir using gradient boosted regression trees: A case study. Environ. Sci. Pollut. Res. 2018, 25, 22658–22671. [Google Scholar] [CrossRef]
- Sorlini, S.; Gialdini, F.; Collivignarelli, C. Removal of Cyanobacterial cells and Microcystin-LR from drinking water using a hollow fiber microfiltration pilot plant. Desalination 2013, 309, 106–112. [Google Scholar] [CrossRef]
- Isenstein, E.M.; Kim, D.; Park, M.-H. Modeling for multi-temporal Cyanobacterial bloom dominance and distributions using landsat imagery. Ecol. Inform. 2020, 59, 101119. [Google Scholar] [CrossRef]
- Beal, M.R.W.; O’Reilly, B.; Hietpas, K.R.; Block, P. Development of a sub-seasonal Cyanobacteria prediction model by leveraging local and global scale predictors. Harmful Algae 2021, 108, 102100. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.R.; Beversdorf, L.J.; Weirich, C.A.; Bartlett, S.L. 201, Cyanobacterial Toxins of the Laurentian Great Lakes, Their Toxicological Effects, and Numerical Limits in Drinking Water. Mar. Drugs 2017, 15, 160. [Google Scholar] [CrossRef] [PubMed]
- Orihel, D.M.; Bird, D.F.; Brylinsky, M.; Chen, H.; Donald, D.B.; Huang, D.Y.; Giani, A.; Kinniburgh, D.; Kling, H.; Kotak, B.G.; et al. High microcystin concentrations occur only at low nitrogen-to-phosphorus ratios in nutrient-rich Canadian lakes. Can. J. Fish. Aquat. Sci. 2012, 69, 1457–1462. [Google Scholar] [CrossRef]
- WHO. Cyanobacterial Toxins: Microcystin-LR in Drinking-Water, Background Document for Development of WHO Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Mellios, N.; Papadimitriou, T.; Laspidou, C. Predictive modeling of microcystin concentrations in a hypertrophic lake by means of Adaptive Neuro Fuzzy Inference System (ANFIS). Eur. Water 2016, 55, 91–103. [Google Scholar]
- Reinl, K.L.; Brookes, J.D.; Carey, C.C.; Harris, T.D.; Ibelings, B.W.; Morales-Williams, A.M.; Domis, L.N.D.S.; Atkins, K.S.; Isles, P.D.F.; Mesman, J.P.; et al. Cyanobacterial blooms in oligotrophic lakes: Shifting the high-nutrient paradigm. Freshw. Biol. 2021, 66, 1846–1859. [Google Scholar] [CrossRef]
- O’Keeffe, J. Cyanobacteria and Drinking Water: Occurrence, Risks, Management and Knowledge Gaps for Public Health; Juliette O’Keeffe National Collaborating Centre for Environmental Health: Vancouver, Canada, 2019. [Google Scholar]
- Neilan, B.A.; Pearson, L.A.; Muenchhoff, J.; Moffitt, M.C.; Dittmann, E. Mini review Environmental conditions that influence toxin biosynthesis in Cyanobacteria. Environ. Microbiol. 2013, 15, 1239–1253. [Google Scholar] [CrossRef] [PubMed]
- Woolway, R.I.; Merchant, C.J.; Van Den Hoek, J.; Azorin-Molina, C.; Nõges, P.; Laas, A.; Mackay, E.B.; Jones, I.D. Northern hemisphere atmospheric stilling accelerates lake thermal responses to a warming world. Geophys. Res Lett. 2019, 46, 11983–11992. [Google Scholar] [CrossRef]
- Jane, S.F.; Hansen, G.J.A.; Kraemer, B.M.; Leavitt, P.R.; Mincer, J.L.; North, R.L.; Pilla, R.M.; Stetler, J.T.; Williamson, C.E.; Woolway, R.I.; et al. Widespread deoxygenation of temperate lakes. Nature 2021, 594, 66–70. [Google Scholar] [CrossRef]
- Pick, F.R. Blooming algae: A Canadian perspective on the rise of toxic Cyanobacteria Can. J. Fish. Aquat. Sci. 2016, 73, 1149–1158. [Google Scholar] [CrossRef]
- Lürling, M.; Mendes e Mello, M.; van Oosterhout, F.; de Senerpont Domis, L.; Marinho, M.M. Response of natural Cyanobacteria and algae assemblages to a nutrient pulse and elevated temperature. Front. Microbiol. 2018, 9, 1851. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.S.; Shao, N.F.; Yang, S.T.; Ren, H.; Ge, Y.R.; Feng, P.; Dong, B.E.; Zhao, Y. Predicting Cyanobacteria bloom occurrence in lakes and reservoirs before blooms occur. Sci. Total Environ. 2019, 670, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Huang, Y.; Liu, X.; Zhang, J.; Gin, K.Y.-H. A feature reconstruction-based multi-task regression model for Cyanobacterial distribution forecasting along the water column. J. Clean. Prod. 2021, 292, 126025. [Google Scholar] [CrossRef]
- Li, H.; Qin, C.; He, W.; Sun, F.; Du, P. Improved predictive performance of Cyanobacterial blooms using a hybrid statistical and deep-learning method, Environ. Res. Lett. 2021, 16, 124045. [Google Scholar] [CrossRef]
- Guimarães, A.; da Silva, P.H.; Carneiro, F.M.; Silva, D.P. Using distribution models to estimate blooms of phytosanitary Cyanobacteria in Brazil. Biota Neotrop. 2020, 20, e20190756. [Google Scholar] [CrossRef]
- Iiames, J.S.; Salls, W.B.; Mehaffey, M.H.; Nash, M.S.; Christensen, J.R.; Schaeffer, B.A. Modeling anthropogenic and environmental influences on freshwater harmful algal bloom development detected by MERIS over the central United States. Water Resour. Res. 2021, 57, e2020WR028946. [Google Scholar] [CrossRef]
- Rousso, B.Z.; Bertone, E.; Stewart, R.; Hamilton, D.P. A systematic literature review of forecasting and predictive models for Cyanobacteria blooms in freshwater lakes. Water Res. 2020, 182, 115959. [Google Scholar] [CrossRef]
- Pyo, J.C.; Park, L.J.; Pachepsky, Y.; Baek, S.-S.; Kim, K.; Cho, K.H. Using convolutional neural network for predicting Cyanobacteria concentrations in river water. Water Res. 2020, 186, 116349. [Google Scholar] [CrossRef]
- Inchio, L.; Zhengchao, X.; Ung, W.K.; Mok, K.M. Integrating Support Vector Regression with Particle Swarm Optimization for Numerical Modeling for Algal Blooms of Freshwater. In Advances in Monitoring and Modelling Algal Blooms in Freshwater Reservoirs: General Principles and a Case Study of Macau; Inchio, L., Boping, H., Weiying, Z., Eds.; Imprint Springer: Dordrecht, The Netherlands, 2017; Chapter 8; ISBN 9789402409338. [Google Scholar]
- Zamani, B.; Koch, M. Comparison Between Two Hydrodynamic Models in Simulating Physical Processes of a Reservoir with Complex Morphology: Maroon Reservoir. Water 2020, 12, 814. [Google Scholar] [CrossRef]
- Burn, D.H.; McBean, E.A. Optimization modeling of water quality in an uncertain environment. Water Resour. Res. 1985, 21, 934–940. [Google Scholar] [CrossRef]
- Manivanan, D.R. Water Quality Modelling for Coastal Engineering Projects—A Review. Oceanogr. Fish. Open Access J. 2017, 3, 113–117. [Google Scholar]
- DHI. MIKE-3 Flow Model FM, Hydrodynamic Module User Guide, MIKE 2022. Available online: https://manuals.mikepoweredbydhi.help/2017/Coast_and_Sea/MIKE_FM_HD_3D.pdf (accessed on 24 March 2023).
- DHI. MIKE-3 Flow Model FM ECO Lab/Oil Spill Module User Guide, MIKE 2022; DHI: Hørsholm, Denmark, 2022. [Google Scholar]
- Regional Municipality of Halton. Prospect Park Well Field Re-Rating and Water Purification, Plant Expansion, Class Environmental Assessment; Environmental Study Report; Region of Halton: Oakville, ON, Canada, 2015. [Google Scholar]
- Town of Halton Hills. 2021, Fairy Lake Water Quality Study Update—Project Charter and Terms of Reference; Report NO.: RP-2021-0003, RP-2021-0003, Town of Halton Hills, 2021; Available online: https://pub-haltonhills.escribemeetings.com/filestream.ashx?DocumentId=9545, (accessed on 24 March 2023).
- Town of Halton Hills. Fairy Lake Water Quality Study, Prepared by: AECOM, Project Number: 107983 Date: December 2009. Available online: https://www.haltonhills.ca/en/explore-and-play/resources/Documents/FairLakeWaterQualityStudy.pdf (accessed on 10 January 2024).
- Town of Halton Hills. Personal communication, 2023.
- Town of Halton Hills, 2020, Fairy Lake Water Quality Study Update—Project Terms of Reference, V1—Feb 2020. Available online: https://pub-haltonhills.escribemeetings.com/filestream.ashx?DocumentId=9548 (accessed on 10 January 2024).
- Regional Municipality of Halton. Fairy Lake Mitigation Strategy: Water Balance Analysis, Final Report; XCG File No.: 3-595-55-01; XCG Consultants Ltd.: Kitchener, ON, Canada; Dance Environmental Inc.: Drumbo, ON, Canada, 2016. [Google Scholar]
- McBean, E.; Bhatti, M.; Singh, A.; Mattern, L.; Murison, L.; Delaney, P. Temperature Modeling, a Key to Assessing Impact on Rivers Due to Urbanization and Climate Change. Water 2022, 14, 1994. [Google Scholar] [CrossRef]
- Berg, M.; Sutula, M. Factors Affecting the Growth of Cyanobacteria with Special Emphasis on the Sacramento-San Joaquin Delta; Southern California Coastal Water Research Project Technical Report 869; The Central Valley Regional Water Quality Control Board and The California Environmental Protection Agency State Water Resources Control Board: Sacramento, CA, USA, 2015. [Google Scholar]
- Hu, L.; Wang, H.; Cui, J.; Zou, W.; Li, J.; Shan, K. Temperature-Dependent Growth Characteristics and Competition of Pseudanabaena and Microcystis. Water 2023, 15, 2404. [Google Scholar] [CrossRef]
- Lurling, M.; Eshetu, F.; Faassen, E.J.; Kosten, S.; Huszar, V.L.M. Comparison of Cyanobacterial and green algal growth rates at different temperatures. Freshw. Biol. 2013, 58, 552–559. [Google Scholar] [CrossRef]
- Health Canada. Guidelines for Canadian Drinking Water Quality: Guideline Technical Document—Cyanobacterial Toxins. 2021. Available online: https://www.canada.ca/en/health-canada/services/publications/healthy-living/guidelines-canadian-drinking-water-quality-guideline-technical-document-Cyanobacterial-toxins-document.html (accessed on 24 March 2023).
- Auld, H.; Switzman, H.; Comer, N.; Eng, S.; Hazen, S.; Milner, G. Region of Peel. Climate Trends and Future Projections in Region of Peel; Technical Report; Ontario Climate Consortium: Toronto, ON, Canada, 2016. [Google Scholar]


| Average | Standard Deviation | Statistics | ||||
|---|---|---|---|---|---|---|
| Measured | Simulated | Measured | Simulated | R2 | NSE | |
| Lake Relative Level with TMA | −0.25 | −0.19 | 0.11 | 0.06 | 0.77 | 0.40 |
| Lake Relative Level With TMB | −0.23 | −0.19 | 0.11 | 0.06 | 0.75 | 0.50 |
| Average | Standard Deviation | Statistics | ||||
|---|---|---|---|---|---|---|
| Measured | Simulated | Measured | Simulated | R2 | NSE | |
| Hourly temp at the Surface | 21.14 | 22.68 | 1.80 | 1.97 | 0.90 | 0.98 |
| Hourly temp at 1 m Depth | 21.79 | 22.76 | 1.88 | 1.96 | 0.78 | 0.88 |
| Hourly temp at 2 m Depth | 21.45 | 22.57 | 1.84 | 1.93 | 0.82 | 0.90 |
| Hourly temp at 3 m Depth | 20.81 | 22.38 | 2.18 | 1.91 | 0.77 | 0.87 |
| Hourly temp at 4 m Depth | 20.81 | 19.94 | 2.18 | 3.47 | 0.86 | 0.51 |
| Hourly temp at Deep | 16.43 | 17.29 | 3.35 | 3.17 | 0.98 | 0.82 |
| Average | Standard Deviation | Statistics | ||||
|---|---|---|---|---|---|---|
| Measured | Simulated | Measured | Simulated | R2 | NSE | |
| Hourly temp at the Surface | 21.71 | 22.86 | 1.86 | 1.91 | 0.76 | 0.89 |
| Hourly temp at 1 m Depth | 21.58 | 23.01 | 1.84 | 1.90 | 0.78 | 0.92 |
| Hourly temp at 2 m Depth | 21.71 | 22.67 | 1.86 | 1.82 | 0.90 | 0.96 |
| Hourly temp at 3 m Depth | 20.7 | 22.40 | 2.3 | 1.9 | 0.80 | 0.88 |
| Hourly temp at 4 m Depth | 18.97 | 22.18 | 3.49 | 1.92 | 0.70 | 0.42 |
| Hourly temp at Deep | 16.63 | 14.88 | 3.60 | 2.45 | 0.97 | 0.59 |
| Depth | Temperature Range in °C | Frequency | No. of Hours Temperature Increased or Decreased with CC4.5 | |
|---|---|---|---|---|
| Base | With CC 4.5 | |||
| Hours | Hours | |||
| 0 m | >20 | 2721 | 2995 | 274 |
| 1 m | >20 | 2743 | 3001 | 258 |
| 2 m | >20 | 2681 | 2958 | 277 |
| 3 m | >20 | 2622 | 2910 | 288 |
| 4 m | >20 | 2553 | 2889 | 336 |
| 5 m | >20 | 2419 | 2848 | 429 |
| 0 m | 15–20 | 693 | 504 | −189 |
| 1 m | 15–20 | 653 | 492 | −161 |
| 2 m | 15–20 | 710 | 533 | −177 |
| 3 m | 15–20 | 752 | 567 | −185 |
| 4 m | 15–20 | 813 | 575 | −238 |
| 5 m | 15–20 | 937 | 588 | −349 |
| 0 m | 10–15 | 625 | 678 | 53 |
| 1 m | 10–15 | 644 | 682 | 38 |
| 2 m | 10–15 | 653 | 671 | 18 |
| 3 m | 10–15 | 669 | 676 | 7 |
| 4 m | 10–15 | 662 | 672 | 10 |
| 5 m | 10–15 | 661 | 693 | 32 |
| 0 m | 5–10 | 353 | 215 | −138 |
| 1 m | 5–10 | 352 | 217 | −135 |
| 2 m | 5–10 | 348 | 230 | −118 |
| 3 m | 5–10 | 349 | 239 | −110 |
| 4 m | 5–10 | 364 | 256 | −108 |
| 5 m | 5–10 | 375 | 263 | −112 |
| Depth | Temperature Range in °C | Frequency | No. of Hours Temperature Increased or Decreased with RCP4.5 | |
|---|---|---|---|---|
| Base | With RCP 4.5 | |||
| Hours | Hours | |||
| 0 m | >20 | 2796 | 3029 | 233 |
| 1 m | >20 | 2829 | 3045 | 216 |
| 2 m | >20 | 2738 | 2969 | 231 |
| 3 m | >20 | 2632 | 2926 | 294 |
| 4 m | >20 | 2568 | 2882 | 314 |
| 5 m | >20 | 2386 | 2721 | 335 |
| 0 m | 15–20 | 627 | 489 | −138 |
| 1 m | 15–20 | 599 | 493 | −106 |
| 2 m | 15–20 | 667 | 545 | −122 |
| 3 m | 15–20 | 752 | 557 | −195 |
| 4 m | 15–20 | 803 | 578 | −225 |
| 5 m | 15–20 | 967 | 743 | −224 |
| 0 m | 10–15 | 646 | 667 | 21 |
| 1 m | 10–15 | 649 | 657 | 8 |
| 2 m | 10–15 | 654 | 643 | −11 |
| 3 m | 10–15 | 665 | 662 | −3 |
| 4 m | 10–15 | 668 | 668 | 0 |
| 5 m | 10–15 | 712 | 651 | −61 |
| 0 m | 5–10 | 323 | 207 | −116 |
| 1 m | 5–10 | 315 | 197 | −118 |
| 2 m | 5–10 | 333 | 235 | −98 |
| 3 m | 5–10 | 343 | 247 | −96 |
| 4 m | 5–10 | 353 | 264 | −89 |
| 5 m | 5–10 | 327 | 277 | −50 |
| Depth | No. of Hours | Percentage Increase |
|---|---|---|
| Surface Temperature | 647 | 39.7% |
| 1 m Depth Temperature | 194 | 9.3% |
| 2 m Depth Temperature | 195 | 9.4% |
| 3 m Depth Temperature | 197 | 9.5% |
| Depth | No. of Hours | Percentage Increase |
|---|---|---|
| Surface Temperature | 221 | 10.7% |
| 1 m Depth Temperature | 670 | 29.9% |
| 2 m Depth Temperature | 45 | 2.0% |
| 3 m Depth Temperature | 196 | 9.4% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhatti, M.; Singh, A.; McBean, E.; Vijayakumar, S.; Fitzgerald, A.; Siwierski, J.; Murison, L. Climate Change Impacts on Water Temperatures in Urban Lakes: Implications for the Growth of Blue Green Algae in Fairy Lake. Water 2024, 16, 587. https://doi.org/10.3390/w16040587
Bhatti M, Singh A, McBean E, Vijayakumar S, Fitzgerald A, Siwierski J, Murison L. Climate Change Impacts on Water Temperatures in Urban Lakes: Implications for the Growth of Blue Green Algae in Fairy Lake. Water. 2024; 16(4):587. https://doi.org/10.3390/w16040587
Chicago/Turabian StyleBhatti, Munir, Amanjot Singh, Edward McBean, Sadharsh Vijayakumar, Alex Fitzgerald, Jan Siwierski, and Lorna Murison. 2024. "Climate Change Impacts on Water Temperatures in Urban Lakes: Implications for the Growth of Blue Green Algae in Fairy Lake" Water 16, no. 4: 587. https://doi.org/10.3390/w16040587
APA StyleBhatti, M., Singh, A., McBean, E., Vijayakumar, S., Fitzgerald, A., Siwierski, J., & Murison, L. (2024). Climate Change Impacts on Water Temperatures in Urban Lakes: Implications for the Growth of Blue Green Algae in Fairy Lake. Water, 16(4), 587. https://doi.org/10.3390/w16040587







