Abstract
The textile industry is known for its excessive water consumption and environmental impact. One of the major challenges it faces is managing pollution generated by dyes, particularly bifunctional dyes, such as sumireact supra yellow s-hbu, with a red appearance and sumifix supra lemon-yellow e-xf, with a yellow appearance. This study aimed to investigate the decolorization kinetics of synthetic solutions of said bifunctional dyes, which comprise triazine and vinylsulfon. We conducted various tests, including modifications of pH, the addition of TiO2 P-50 nanoparticles, exposure to solar radiation, limited contact with oxygen, and eolic agitation. The initial solutions had a concentration of 1000 ppm of textile dye. The study showed that the reaction order for the “red” solutions in the R6 and R9 reactors and all the yellow solutions was ½. The concentration of nanoparticles and pH had a significant impact on the reaction rate. The yellow solutions with a concentration of 800 ppm and pH levels of 3.15, 4.13, and 2.25 demonstrated 100% color discoloration, followed by solutions with a concentration of 400 ppm and pH levels of 3.15, 2.25, and 4.13. The analysis of variance confirmed the reaction rate constants for the yellow solutions and emphasized the significance of pH in this process.
1. Introduction
In the development of this research, two reactive dyes were chosen with chemical structures that are protected by patents with the following commercial names: sumireact supra yellow s-hbu and sumifix supra lemon-yellow e-xf. These dyes, used for dyeing cotton, wool, and silk, exhibit a red and yellow appeance when dissolved in water, and are the materials of this study. The reactive groups of the dye react with the functional groups of the fiber, producing a covalent bond under the influence of temperature and pH [1]; its basic structure belongs to the triazine functional group. Wastewater generated by the textile industry contains refractory substances and undergoes a series of treatment methods to be mineralized. In these methods, combinations of oxidants, such as (O3/H2O2), a catalyst and an oxidant (Fe2+/H2O2), an oxidant and radiation (H2O2/UV), or radiation and a catalyst (UV/TiO2) are used. However, there are advanced treatments that combine all of the above chemicals with other processes [2]. One of the main disadvantages of these processes is their high energy consumption, limiting their applications. Consequently, there is a growing need to harness renewable energy sources, such as solar photocatalysis, which utilizes both UV solar radiation and visible radiation, intensified with titanium dioxide nanoparticles [3]. Solar energy, along with other renewable sources, could play a pivotal role in the mass production of chemicals and in addressing environmental problems. Recent advances, such as solar photocatalysis, have proven effective in treating contaminated water and air [4]. Heterogeneous photocatalysis is a process with high potential for reducing pollutants and treating waste [5]. Photocatalysis is the process in which the reaction rate changes due to UV, visible, or IR radiation in the presence of a catalyst that absorbs photons and participates in the chemical transformation of the reactants [6]. A suitable catalyst must be chemically and biologically stable, photoactive, harmless, and activated with sunlight.
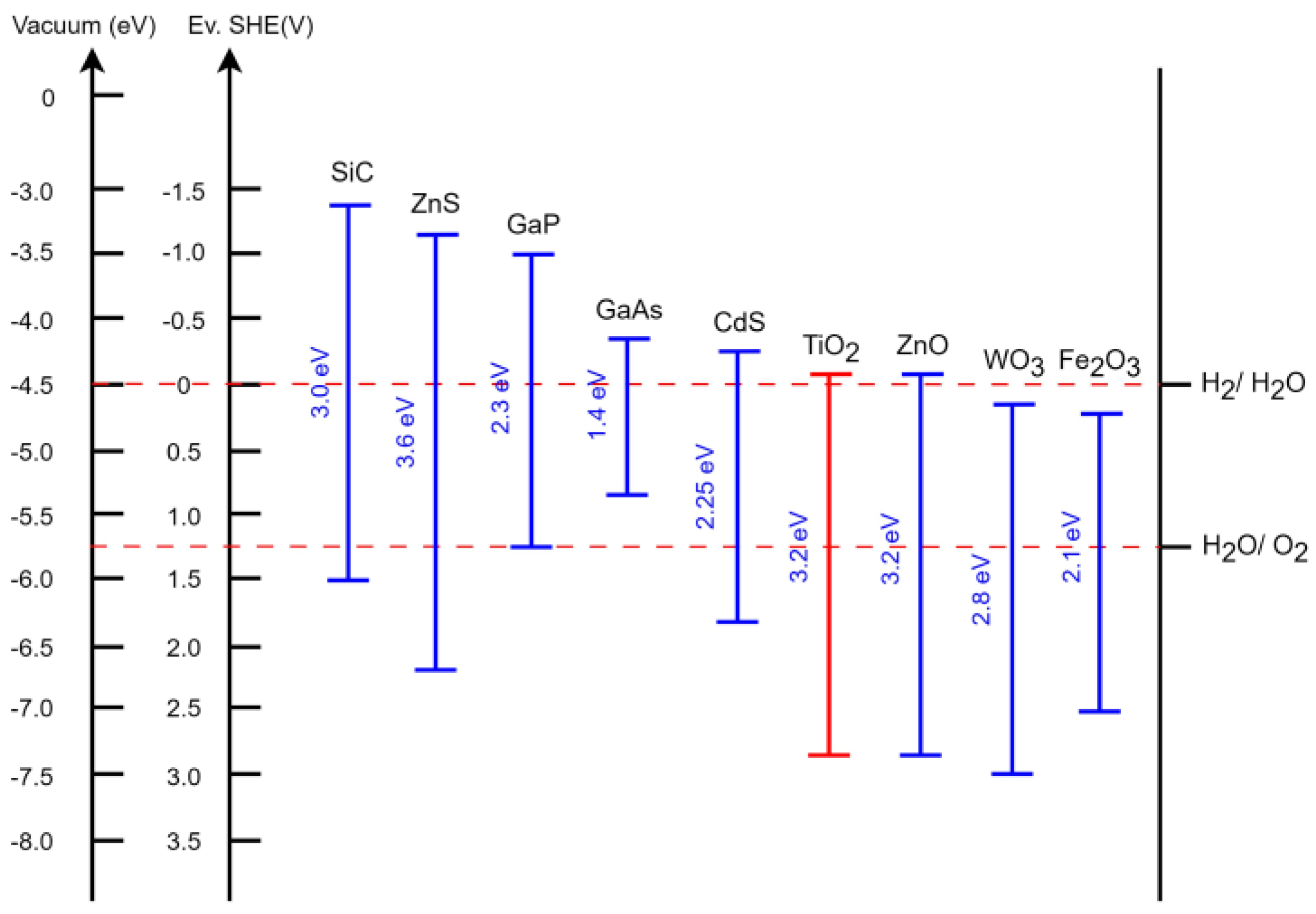
Heterogeneous photocatalysis employs various semiconductors [7,8,9], known for their characteristic transition from insulators at low temperatures to conductors under any form of energy input. When exposed to sufficient energy equal to or greater than the “gap”—the interval between the valence and conduction bands—electrons can transition from the valence band to the conduction band. This transition involves overcoming an energetic distance known as the forbidden band (Eg) and simultaneously creating an electronic vacancy or “hole” in the valence band [10]. Figure 1 illustrates the bandgap energies of different semiconductors, expressed in both electronvolts (eV) and volts relative to the potential of the normal hydrogen electrode ENHV [11,12].
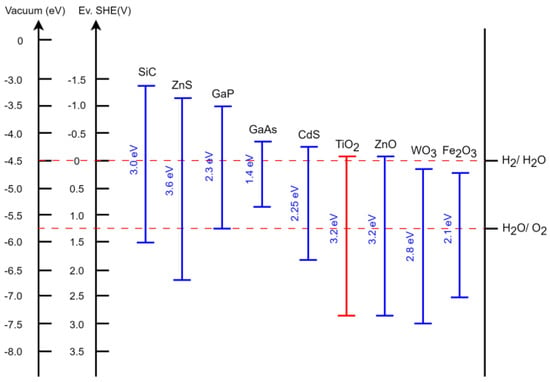
Figure 1.
Comparison of common semiconductors, their band gaps, and the oxidation/reduction potentials of their energy levels in contact with aqueous electrolyte at pH = 1 [11,12].
Most studies have focused on TiO2 [13,14,15] despite its limitations, such as its low efficiency and low response to light due to its bandwidth of 3.2 eV, which corresponds to light <400 nm [10,16]. The semiconductor TiO2 plays an important role as an active photocatalyst mainly due to its photoinduced effect and electron transfer properties. It is widely accepted that metastable anatase, in particular, has the highest photoactivity compared to rutile; however, the high electron recombination rate renders its application as a functional photocatalyst difficult. Therefore, it is crucial to further improve the photocatalytic activity of anatase TiO2, modifying its optical, morphological, and crystalline properties [17]. The main reactions that occur in the decontamination process of water containing organic substances in contact with a semiconductor in the presence of solar radiation UV [18,19,20,21] are shown through reactions (1)–(11).
The produced radical serves as a potent oxidizing agent, with a standard redox potential of +2.8 V, enabling the oxidation of a majority of azo dyes into mineral end products. The oxidation mechanism in the presence of visible solar radiation is different from oxidation with UV radiation. In the first case, the mechanism suggests that the excitation of the adsorbed dye takes place by visible light to the appropriate singlet or triplet states, subsequently followed by the injection of electrons from the excited dye to the molecule in the conduction band of the TiO2 particles, while the dye is converted into cationic dye radicals (Dye•+) that undergo degradation to generate the products provided in reactions (12)–(15) [22,23,24,25,26,27,28,29,30,31].
Cationic dye radicals readily react with hydroxyl ions, leading to oxidation, as elucidated with the following reactions (16) and (17), which effectively interact with the radicals , , or , generating intermediate species that finally lead to the formation of CO2 through the reactions (18)–(22) [31].
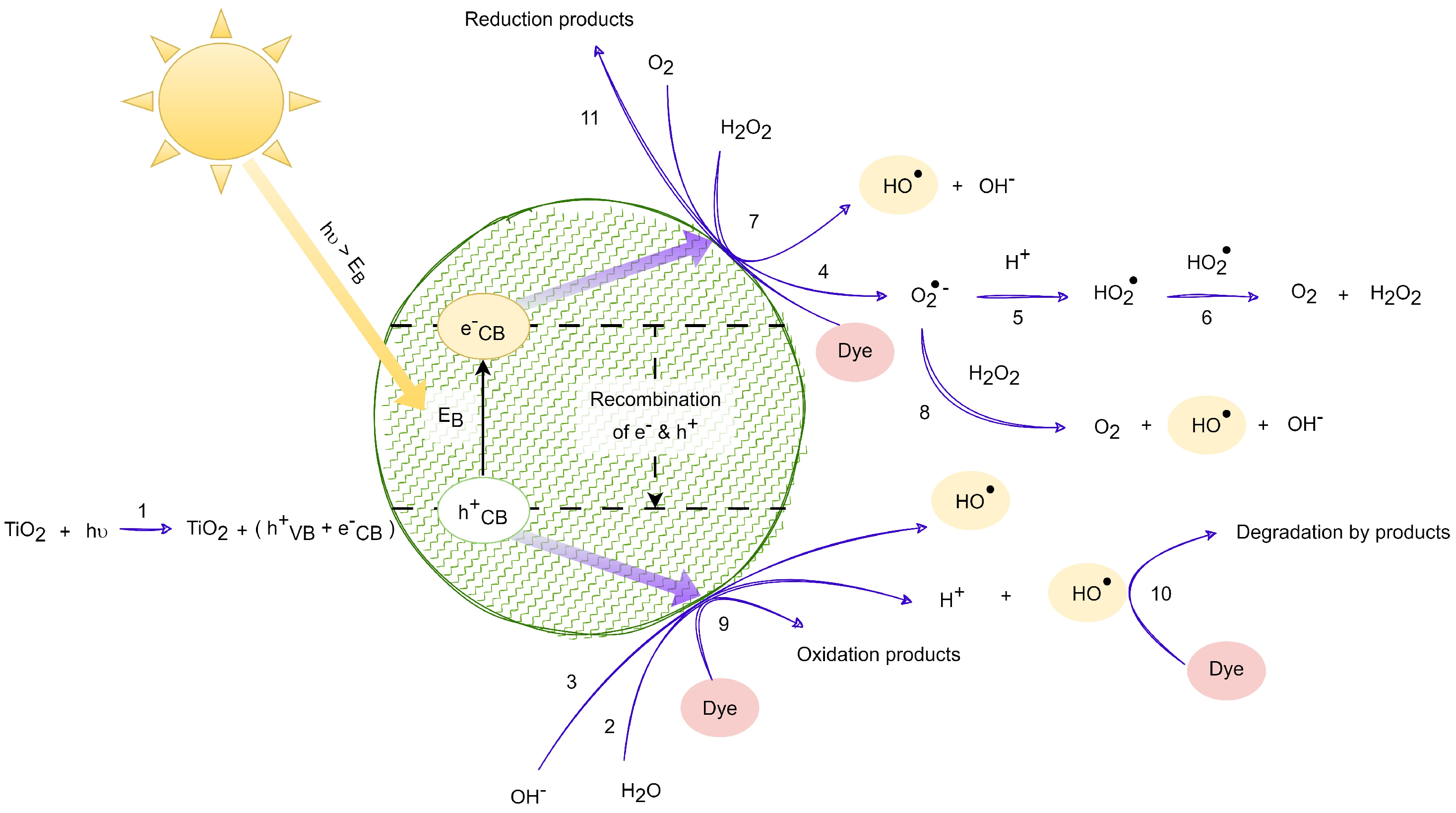
From Equations (1)–(22), it is inferred that the photocatalysis process encompasses a series of steps, including the generation of , y radicals. In Equation (20), the presence of hydrogen peroxide alongside the electron in the conduction band leads to the generation of the oxydryl radical and the hydroxyl anion, returning titanium dioxide to its ground state. As per Equation (21), the cationic radical derived from the dye undergoes oxidation in the presence of the superoxide radical, resulting in the formation of new degraded products. Equation (22) describes the oxidation of the cationic radical from the dye through the hydroperoxide or oxydryl radical, facilitating the creation of additional degraded products. These radicals exhibit potent oxidizing capabilities, contributing to the degradation of organic contaminants. Figure 2 illustrates the scheme of chemical and photochemical reactions facilitated by UV radiation, derived from Equations (1)–(11), while Figure 3 depicts the scheme of chemical and photochemical reactions induced with visible solar radiation, developed based on reactions (12)–(22).
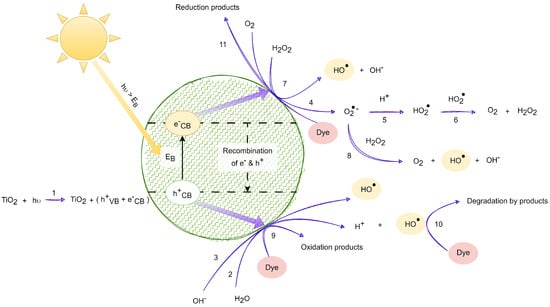
Figure 2.
Photocatalytic degradation induced with solar UV radiation. Adapted from [18,32,33].
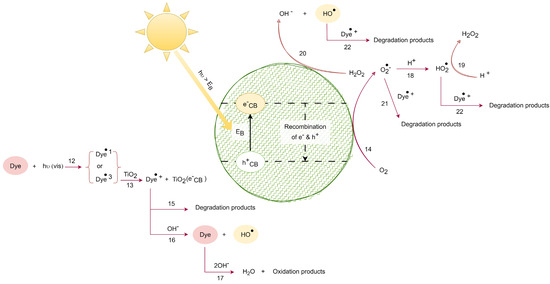
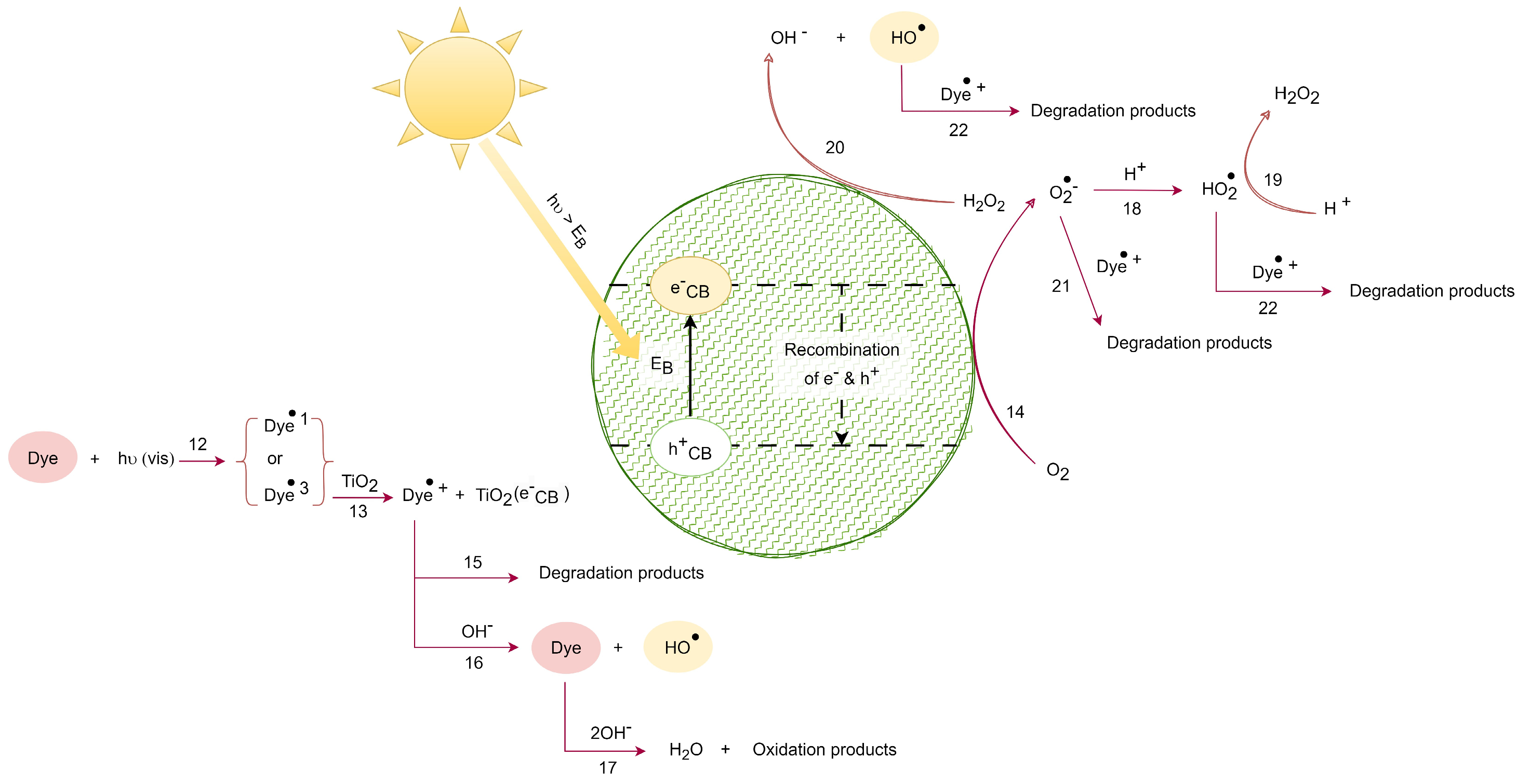
Figure 3.
VIS solar radiation-induced photocatalytic degradation. Adapted from [31].
Figure 2 shows the incidence of solar UV radiation on a titanium dioxide nanoparticle, shown in green, and the mechanism for the oxidation of dye compounds in textiles.
Figure 3 illustrates the photocatalytic degradation process induced with visible solar radiation. In the center, the TiO2 nanoparticle is represented by a green circle. The lines and arrows represent the reactions that take place to form the free radicals and degradation products.
The photogenerated electrons, in most cases, flow in the external circuit; however, some electrons can interact with oxygen through the catalytic and non-catalytic transformation of molecular oxygen and its different chemical bonds [34].
the applications of titanium dioxide, including its doped, supported, and composite forms, have been employed in various studies, primarily focusing on the removal of components found in wastewater from the textile industry.
In the degradation of naproxen, it is observed that as the concentration of TiO2 P-50 nanoparticles increases, both the degradation rate and the value of the reaction rate constant also increase [35]. Another study analyzed the activity of TiO2 in the form of anatase and rutile in the treatment of wastewater from the textile industry. It was found that the highest activity was that of anatase. A new method for the synthesis of TiO2 was presented from an extract of Thymus vulgaris and then doped with commercial Ag. The results offered a new, ecological, and simple method to prepare TiO2/Ag nanoparticles with high photocatalytic efficiency [36]. In another investigation, titanium dioxide in its anatase form was doped with vanadium in the form of crystallized vanadium pentoxide and used for the treatment of the dye “acid yellow” 36 (AY36) from textile wastewater with visible solar radiation, and the absorption band was reduced to 2.22 eV [37]. A nanocomposite of titanium dioxide and ferric oxide (TiO2@Fe2O3) was also synthesized as an effective photocatalyst for the degradation of p-nitrophenol (P-NP) as an organic compound in oilfield wastewater. In this case, the absorption band of the composite was 2.63 eV, which allowed the use of visible radiation for the respective treatment [38]. Another research study explored the preparation of nanocomposites of copper oxide and titanium dioxide (CuO@TiO2) with different proportions. The ratio of 1:5 of CuO to TiO2 reduced the absorption band to 1.88 eV (a value slightly higher than the absorption band of CuO but lower than the absorption band of TiO2), being useful for applications within visible radiation, as it was used to decolorize the textile dye AY36 from real textile wastewater [39]. Catalysts with a heterojunction based on BiVO4 and TiO2 were synthesized using various proportions of the former, resulting in a “gap” of 2.58 eV. These catalysts were employed in the degradation of hydrolyzed polyacrylamide in oilfields [40]. In an innovative study, TiO2-coated plastic sheets were employed to degrade wastewater from the textile industry under solar radiation exposure [41]. Another study utilized titanium dioxide supported on eggshells, enabling the decolorization of methylene blue with the assistance of a UV light lamp and magnetic stirring [42]. The investigation of the kinetics of textile dyes is a crucial technical aspect, providing insights into kinetic parameters used to estimate fading rates for substances of the same type but with varying concentrations. In heterogeneous reactions, the “Langmuir-Hinshelwood” mechanism is commonly considered, involving two limiting steps: adsorption and reaction [43]. This mechanism is used to calculate the reaction rate constant and the adsorption constant through a linearization process. In many heterogeneous photocatalysis reactions, the desorption process occurs rapidly, leading to a negligible surface concentration of adsorbed molecules. Consequently, the overall rate coincides with the adsorption rate of substrate molecules. In such cases, the overall rate is proportional to the concentration of the substrate in solution, indicating first-order kinetics. While many reactions exhibit first-order kinetics, others follow second-order kinetics [44]. However, there are reactions with other orders, such as the case of order ½. The ½ reaction order is directly linked to the intensity of solar radiation. The recombination of electrons in the conduction band and the holes generated in the valence band, which limit the use of available photons and the transition to a zero order, imply that the substrate cannot generate more pairs even with increased radiation intensity [45,46]. Since our study focuses on determining the kinetics of the discoloration of the following reactive textile dyes: sumireact supra yellow s-hbu and sumifix supra lemon-yellow e-xf, it was necessary to design and build 18 micro-agitation units that were functional for operation with wind energy, as required by the proposed design. Additionally, considering that the solar radiation had a relatively low index during the experimental period, a rotating plate operated using wind force was designed and constructed. The base and sides of this plate were composed of glass to enhance the distribution and utilization of solar radiation. Furthermore, only titanium dioxide nanoparticles were used with the modification of the pH of the solutions; no other additional reagents were incorporated.
With these experimental settings, the objective of the study was to determine the kinetics of the decolorization of Sumireact Supra Yellow S-HBU and Sumi-Fix Supra Lemon-Yellow E-XF reactive textile dyes through heterogeneous catalysis with titanium dioxide, solar radiation, and wind power agitation. The aim was to analyze the speed and efficiency of the process under an experimental design and determine the optimal treatment conditions.
2. Materials and Methods
2.1. Materials, Equipment, and Reagents
To carry out this study, 26 boro-silicate glass bottles of 500 mL capacity were used, each acting as a batch reactor, of which 18 were equipped with mini wind turbines to drive the agitation. A glass platform with rotation powered by wind energy was designed to improve the distribution and use of solar radiation. We used a UV-Visible spectrophotometer (UV1600PC, W&J Instrument Co., Ltd., Changzhou, China) imported from Mainland, China and a pyranometer (Hukseflux Thermal Sensor, Delft, The Netherlands) imported from the Netherlands to measure the solar radiation. Deionized water with a conductivity of 10 μS/cm was used. The reactive textile dyes sumireact supra yellow s-hbu and sumifix supra lemon-yellow e-xf were acquired from Comercial Química Masso Perú SAC. In addition, the titanium dioxide TiO2 nanoparticles (Anatase, 10 nm, 99.9% purity) were imported from Hongwu International Group LTD, and 98% sulfuric acid with a weight and density of 1.84 g/mL was provided by the Chemistry Laboratory of the National University of Callao.
2.2. Design of the Mini Eolic Turbins
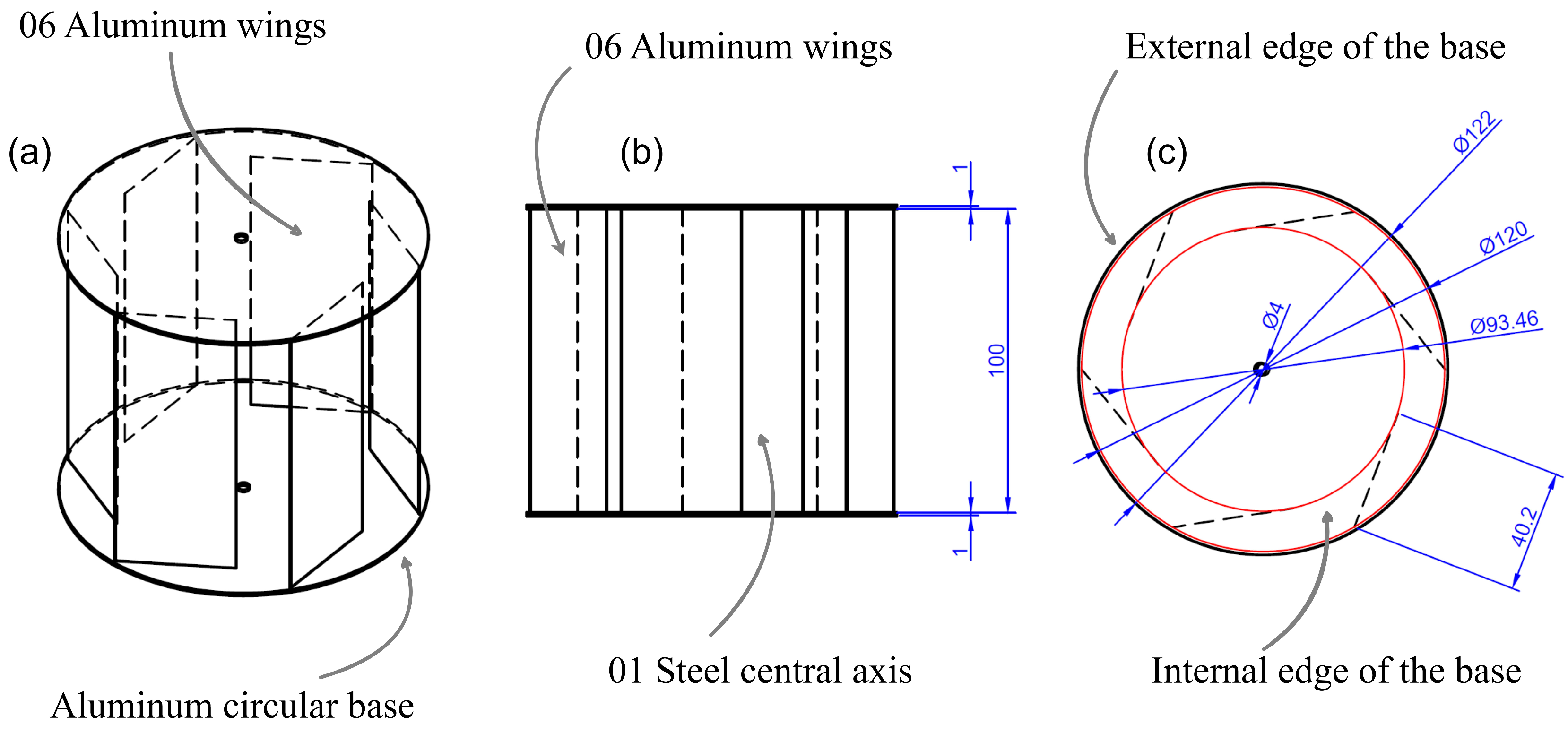
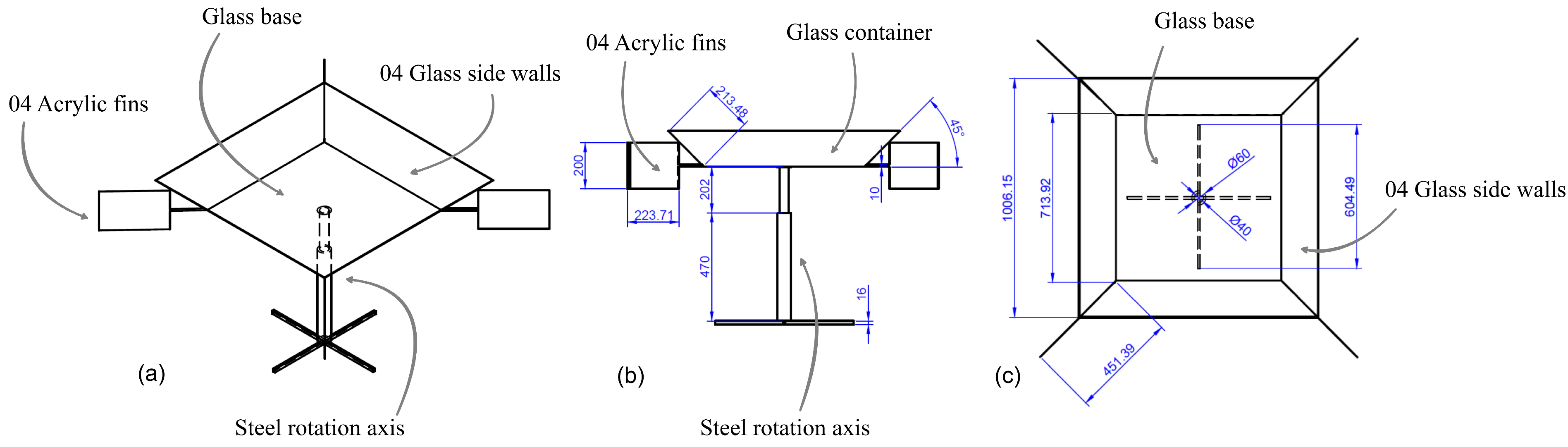
Each of the 18 reactors operated individually and required permanent agitation; in this sense, wind energy was used to achieve said agitation using an axial type of impeller with a diameter of 50 mm. The turbine design consisted of a 12-cm vertical axis device in diameter, equipped with 6 equally spaced ailerons, each 10 cm high and 402 cm wide. These were arranged in such a way that they take advantage of the energy of the wind to generate angular movement and, in turn, transmit energy to the axis of the agitator. The material used for construction was 1 mm-thick aluminum, as seen in Figure 4.
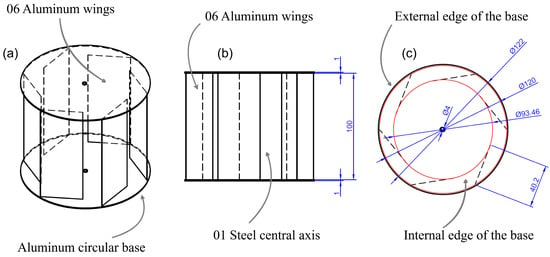
Figure 4.
Eolic turbine: (a) isometric view; (b) frontal view; (c) horizontal view.
2.3. Design of the Mobile Equipment for the Reactors
Given that the treatment was carried out using solar radiation, to optimize its utilization, a container was designed. It consisted of a rotating platform powered by wind energy, intending to uniformly distribute the solar radiation among all the reactors and harness the radiant energy reflected from the side walls of the container towards the reactors. Figure 5 shows the supports of the reactor with the samples along with their respective views.
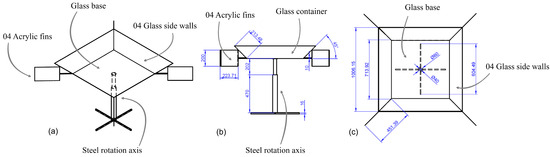
Figure 5.
Eolic equipment for the samples: (a) isometric view; (b) frontal view; (c) horizontal view.
2.4. Experimental Procedure
A 400 mL solution was prepared for each reactor with two types of reactive textile dye, sumireact supra yellow s-hbu (red) and sumifix supra lemon-yellow e-xf (yellow), with a concentration of 1000 ppm. The pH was modified, and varying amounts of titanium dioxide nanoparticles were added to each reactor as per the values specified in Table 1.

Table 1.
Dosing of TiO2 for the reactive textile dye samples (sumireact supra yellow s-hbu and sumifix supra lemon0yellow e-xf) in each reactor.
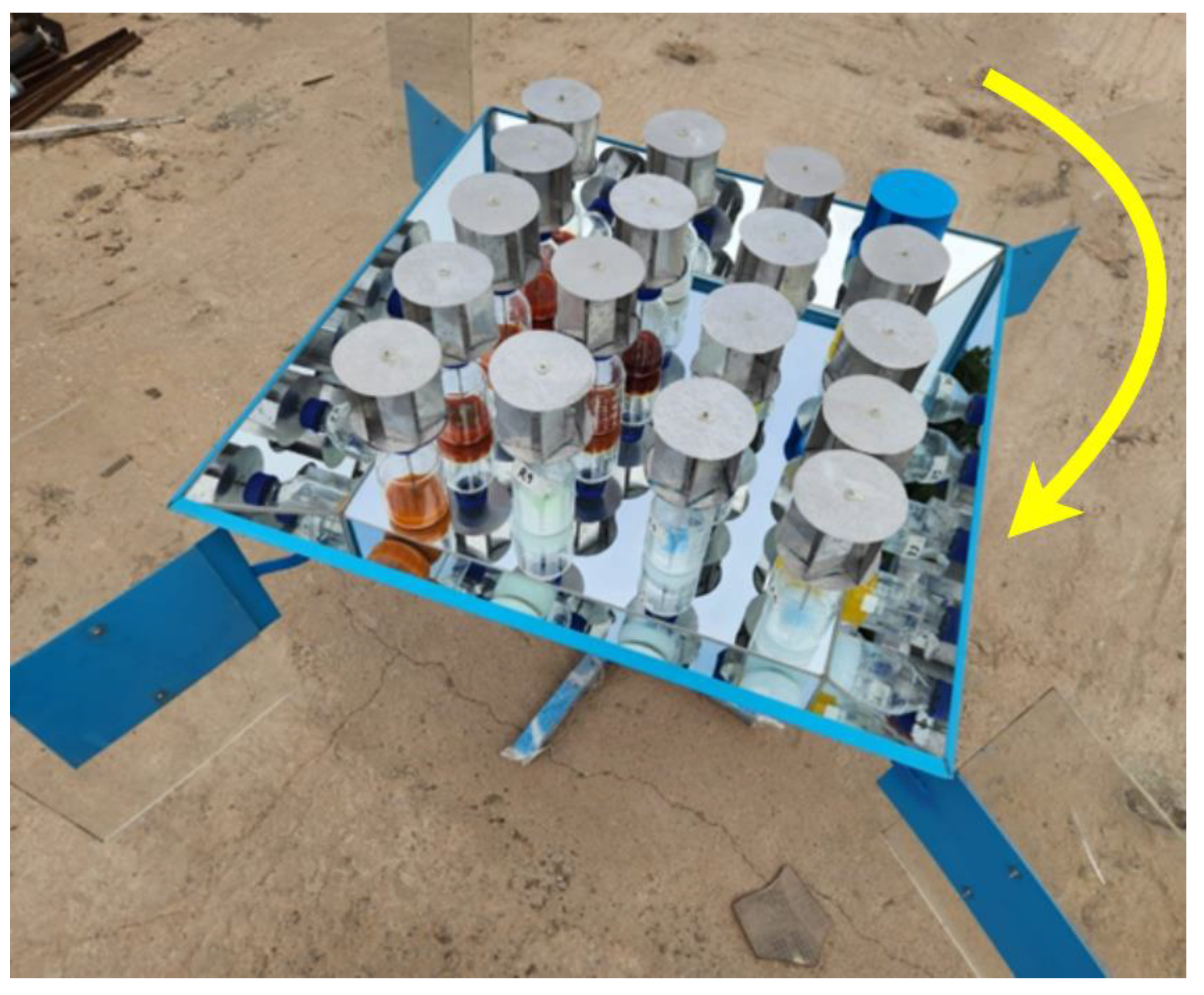
After dosing, the reactors were placed in a container and exposed to ambient solar radiation. The experimental phase took place between July and August 2023, during a period characterized by lower radiation indices on the Peruvian coast, specifically in the city of Lima. The radiation values were recorded using the Hukseflux Thermal Sensor pyranometer. Figure 6 illustrates the experimental setup, featuring 18 reactors equipped with agitators powered by a small wind turbine (Eolic mini turbine). Additionally, there were six samples that were only subjected to pH modification, and two samples consisted of a dye solution dissolved in water. The latter eight bottles did not require agitation, as no nanoparticles were added.
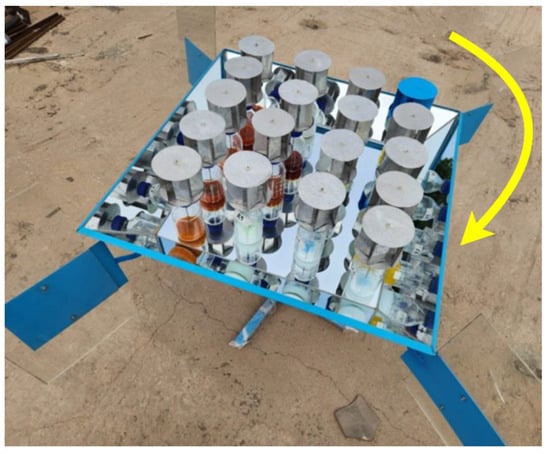
Figure 6.
Experimental sample treatment equipment.
The samples were continuously exposed for 65 days, but for the calculations, only half of the time, where solar radiation equivalent to 780 h, was considered. The samples were taken at variable periods due to the uncertainty and slowness of the observed changes.
To take the sample, the reactor lid was momentarily removed, and approximately 5 mL of the sample was taken while the nanoparticles were in suspension. Subsequently, the samples were centrifuged, and then a 2 mL aliquot was taken, which was diluted to 50 mL for its corresponding reading in the spectrophotometer and its transformation in terms of concentration through a standard curve.
The “red” dye samples were read at 426 nm, and the yellow samples were read at 427 nm after calibration in the spectrophotometer. The close wavelength spectra observed for both dyes were because sumireact supra yellow s-hbu dye has a red appearance; however, when it is subjected to the conditions of the process, it turns yellow.
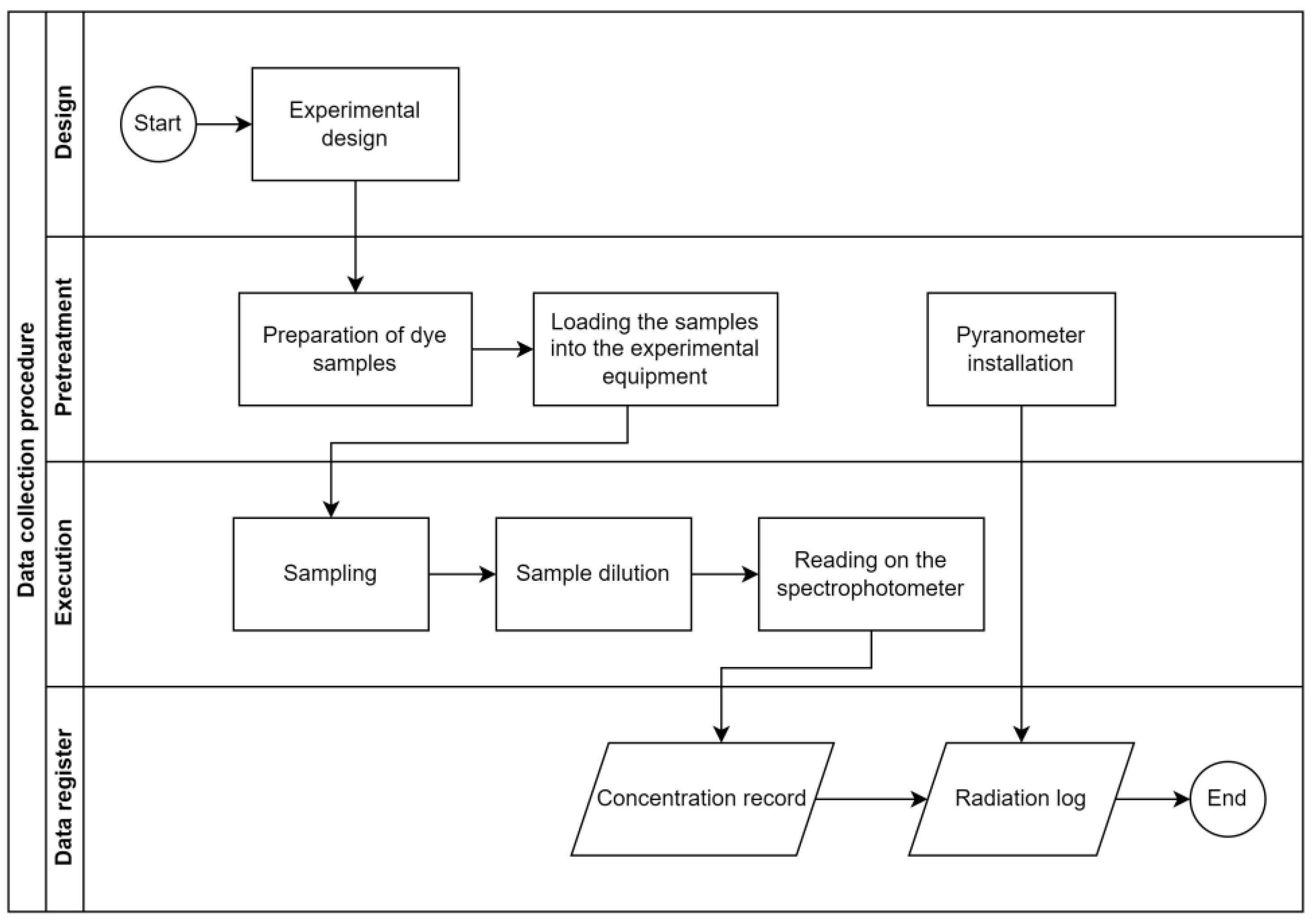
Figure 7 details the procedure carried out in the research, from the experimental design to data collection.
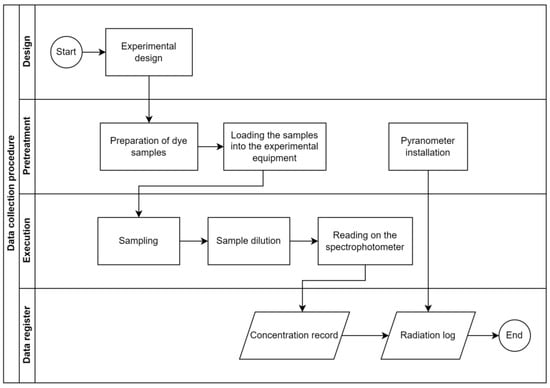
Figure 7.
Data collection procedure.
2.5. Analysis and Data Treatment
The previously taken samples underwent dilution; 2 mL of each sample was adjusted to a total volume of 50 mL by adding distilled water. The absorbance reading was taken, and the data were calculated using a standard curve and dilution factors. The results are shown in Table 2 and Table 3.

Table 2.
Results of the sumireact supra yellow s-hb solutions.

Table 3.
Results of the sumifix supra lemon-yellow e-xf solutions.
The kinetics of discoloration considering a heterogeneous process was adjusted to the Langmuir-Hinshelwood model and pseudo-homogeneous models for different reaction orders: order ½, order 1, order ³⁄₂, and order 2. The expressions for the calculation of the reaction and adsorption rate constant, depending on the case, are detailed below Langmuir-Hinshelwood model:
Plotting the inverse of the reaction rate of the concentration facilitates the determination of the reaction and adsorption rate constants.
- : Reaction rate constant (ppm/s)
- : Adsorption rate constant (ppm−1)
- : Dye concentration (ppm)
- r: Reaction speed (ppm/s)
Pseudo-homogeneous models: Integrated models for the kinetics of order ½, 1, ³⁄₂, and 2, respectively, were used, as depicted in the following equations:
The experimental data on the discoloration of the sumireact supra yellow s-hbu dye are shown in Table 2. The same data for the sumifix supra lemon-yellow e-xf dye are illustrated in Table 3. The statistical treatment of Equations (25)–(29) allows the determination of the reaction order as well as the reaction rate constants whose units depend on the model used. The model choice is influenced by the concentration and time units, with concentration expressed in ppm and time in h. The regression results are presented in Table 4 and Table 5.

Table 4.
Coefficients of correlations for red dye.

Table 5.
Coefficients of correlations for yellow dye.
3. Results
3.1. Data Collection
3.1.1. Solar Radiation Record
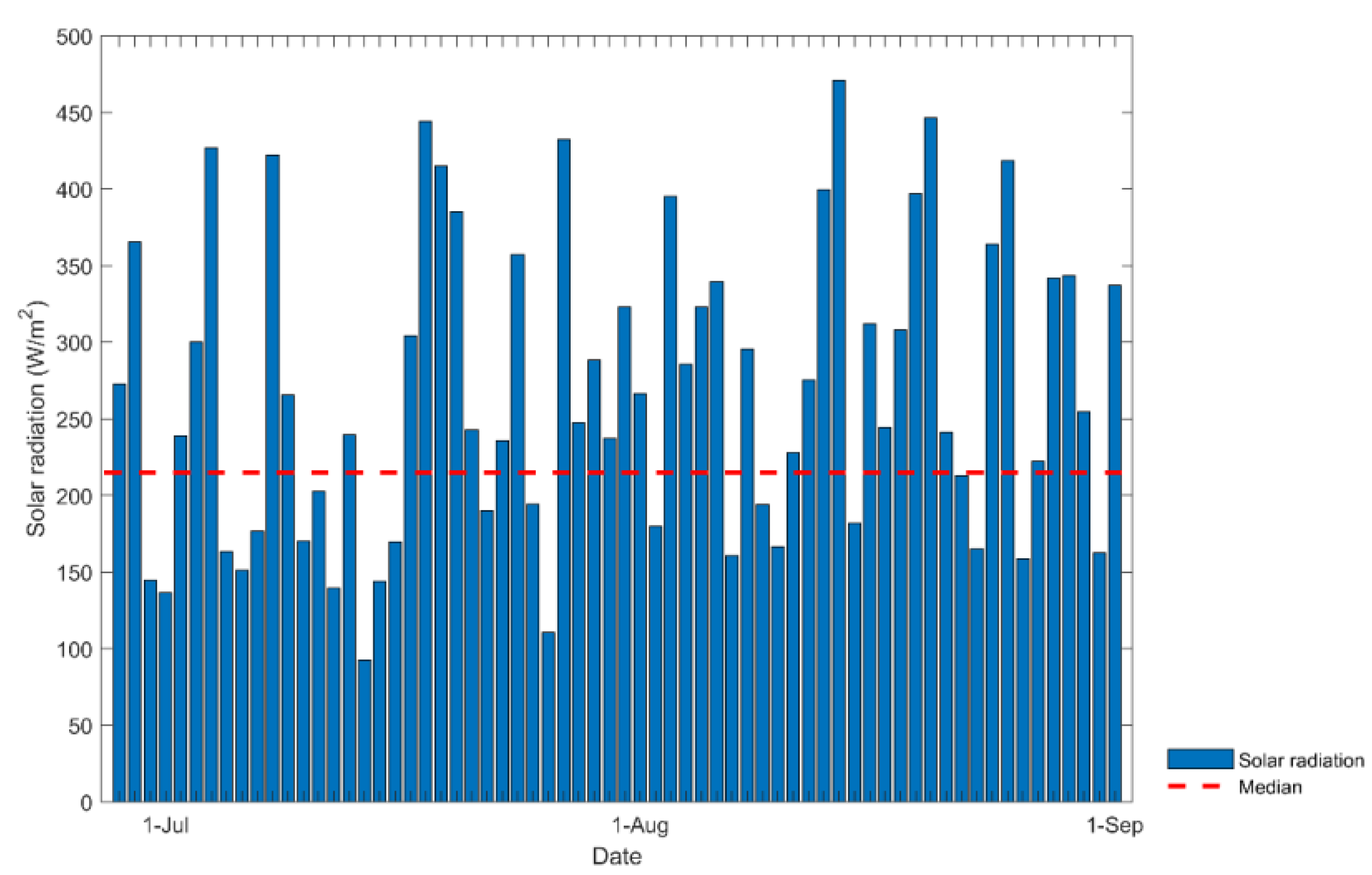
The experiment was carried out during July and August 2023, when visible solar radiation levels are usually low due to high cloud cover; these data are shown in Figure 8. The analysis of the data indicates that the intensity of the solar radiation did not present a normal distribution, so the mean value was taken as the median, which was 215 W/m2.
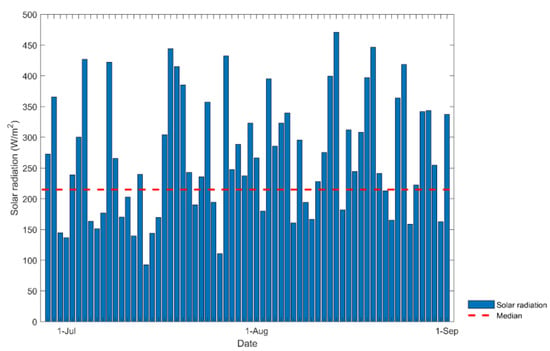
Figure 8.
Solar radiation during July and August in Callao Lima in 2023.
3.1.2. Data and Analysis of the Dye Solutions
Upon visual analysis of the data, it was observed that the solutions lacking nanoparticles (the R10, R11, R12, R13, A10, A11, A12, A13 reactors) maintained a consistent appearance and color intensity throughout the entire exposure period. The destruction of a dye should lead to complete mineralization; however, these levels of destruction are not universal for all compounds since it was shown that the mechanisms can vary. Some compounds also have slow kinetics, meaning that they can be classified as non-photoactive compounds [46]. This phenomenon of a low destruction rate was corroborated by the absorbance reading in the spectrophotometer; therefore, the data analysis focused on reactors R1 to R9 and A1 to A9, the same ones shown in the corresponding tables and figures.
3.1.3. Analysis Data of the Sumireact Supra Yellow s-hbu Solutions
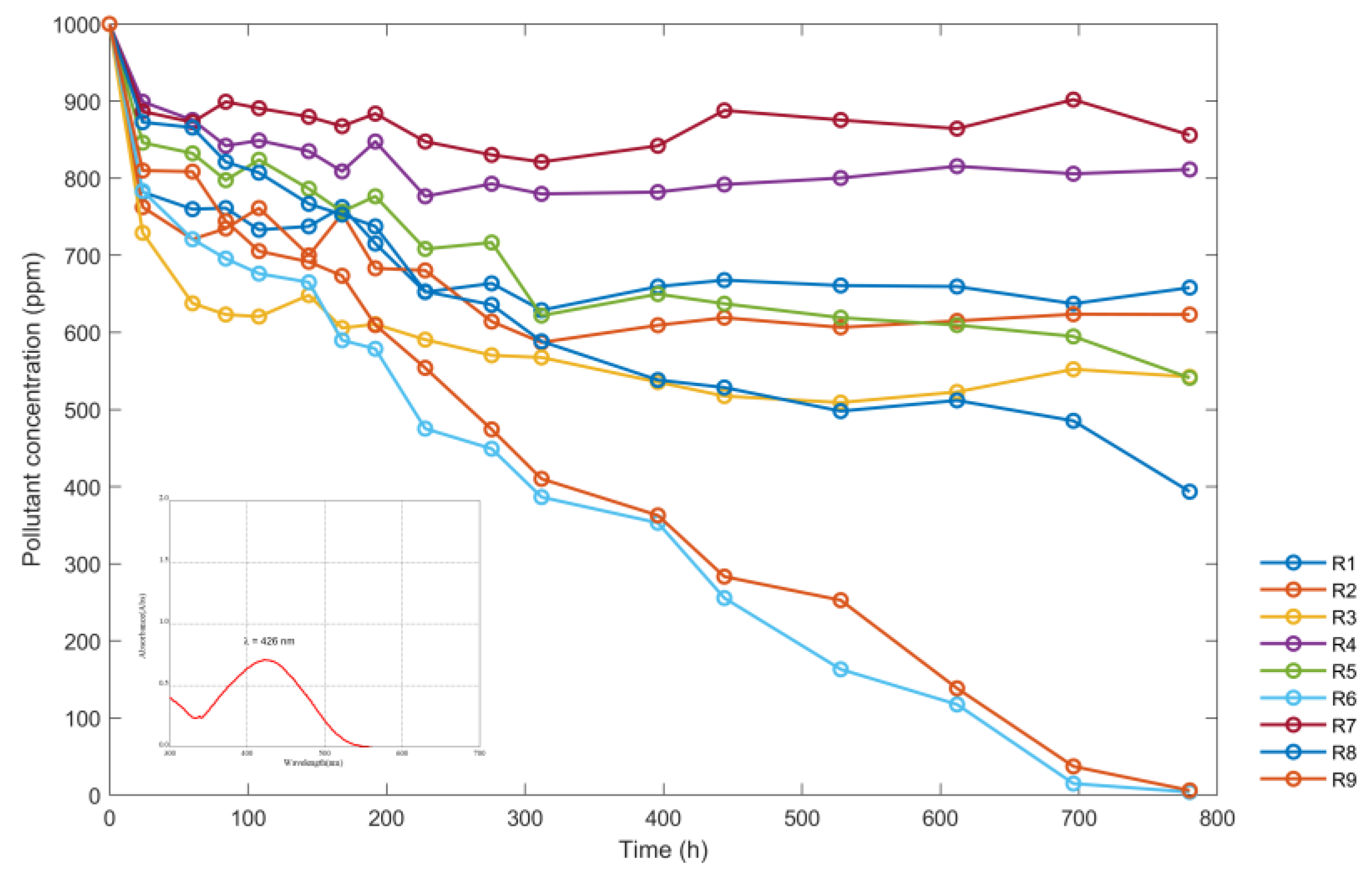
The data were collected on the concentration of “red” dye in the reactors exposed to solar radiation. The results, expressed in ppm, are shown in Table 2. Only the R6 and R9 samples were completely discolored.
Figure 9 shows the variation of the concentration of nine reactors over time (in hours) using the provided data in Table 2. The maximum wavelength at which the absorbance data were read is shown in the lower left corner.
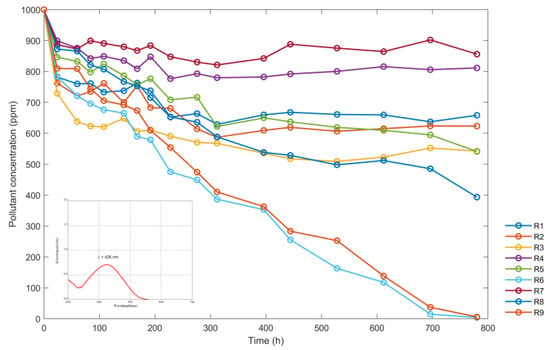
Figure 9.
Variations of the sumireact supra yellow s-hbu solution concentrations (ppm) with time (h).
Based on the variations depicted in Figure 9, the discoloration of the red dye contained in reactors R6 and R9 was predominantly caused by the highest concentration of TiO2 utilized during the process (800 ppm). Secondly, the pH levels between 3 and 4 also contributed to discoloration. Lower concentrations at 800 ppm and a pH lower than 3 did not have a significant effect on discoloration, even when exposed to radiant energy for extended periods. This was because of the low levels of radiation enhanced by the recombination of the electron of the band of conduction and the valence band, and secondly to the limited contact of the solution with ambient oxygen. Based on the data in Table 2 and Figure 9, the color removal percentage was 99.61% for the sample contained in reactor R9 and 99.40% for the sample contained in reactor R6.
3.1.4. Data Analysis for the Sumifix Supra Lemon-Yellow e-xf Solutions
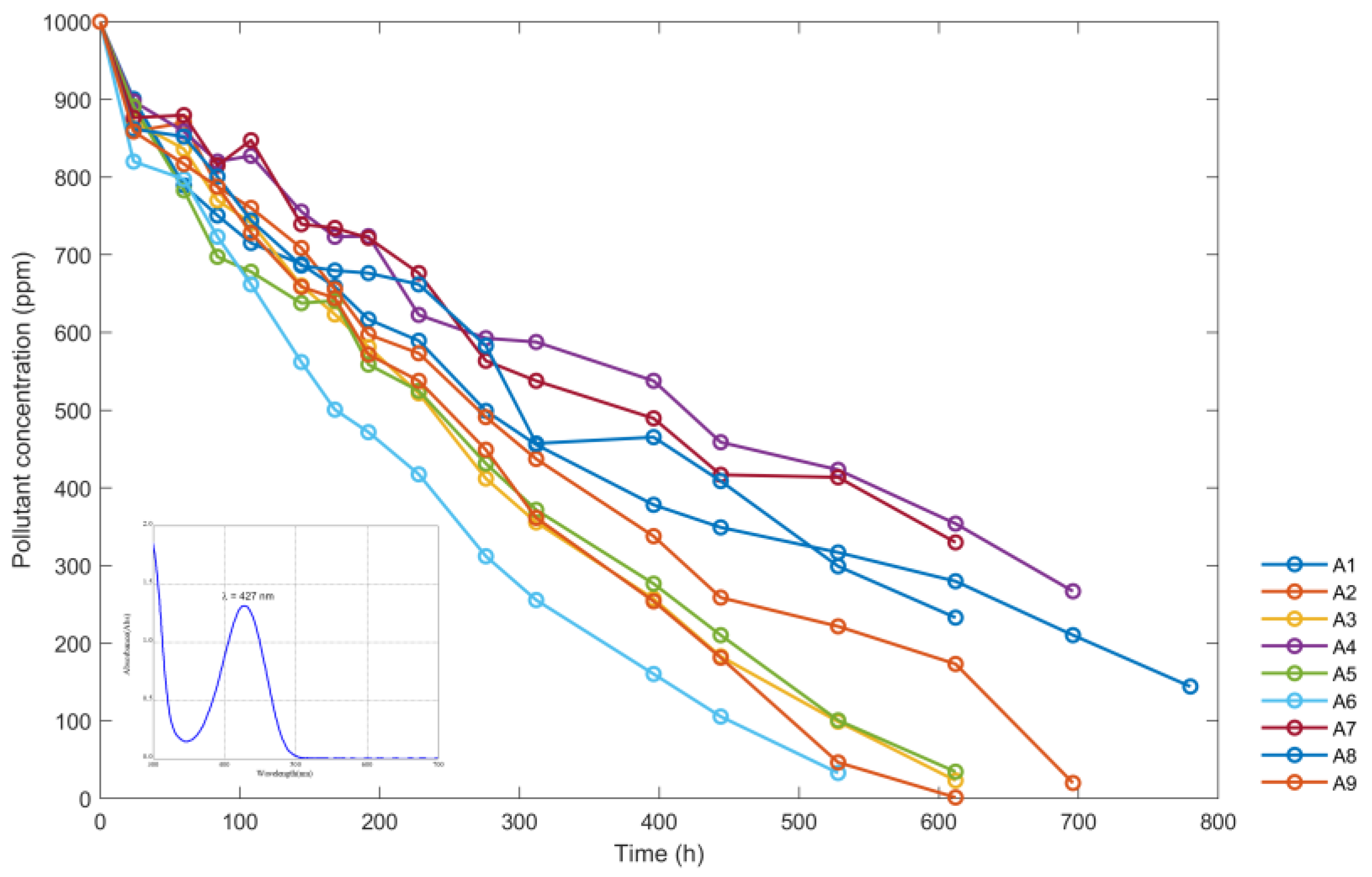
During the process of exposure to solar radiation of the reactors containing the yellow dye, the concentration data of the dye were obtained, expressed in ppm (the same data that are shown in Table 3). The A2, A3, A5, A6, A8, and A9 samples became completely discolored.
Figure 10 shows variations of sumifix supra lemon-yellow e-xf (ppm) with time using the data from nine reactors from Table 3. The maximum wavelength at which the absorbance data were read is shown in the lower left corner.
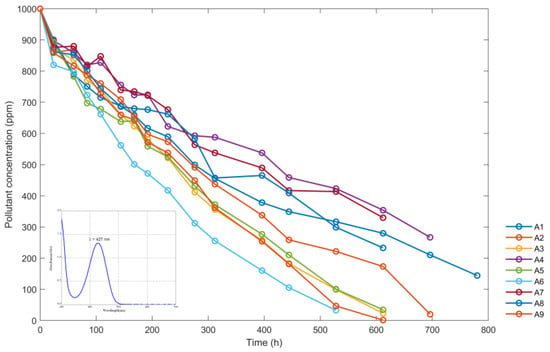
Figure 10.
Variations of sumifix supra lemon-yellow e-xf concentration (ppm) with time in hours (h).
Figure 10 shows the results of the experiments with different nanoparticle concentrations and pH levels. The solutions in reactors A6, A9, and A3, with nanoparticle concentrations equivalent to 800 ppm and pH levels of 3.15, 4.13, and 2.25, respectively, showed the greatest reduction in sumifix supra lemon-yellow e-xf concentration. The pH level of 3.15 was found to be the most effective. The solutions in reactors A2, A5, and A8, with nanoparticle concentrations of 400 ppm also showed some discoloration over a longer period. Among these, the solution in reactor A5 with a pH of 3.15 showed the most significant improvement in discoloration compared to the solutions in the A2 and A8 reactors. During the treatment period, the A1, A4, and A7 reactors, with nanoparticle concentrations of 200 ppm, were unable to decolorize the solutions. However, Figure 10 shows a tendency towards discoloration, and the final concentration levels reached during this period were quite similar between all three reactors. Therefore, it was not possible to determine the significance of pH.
Figure 11 shows the results of treating 18 samples with varying concentrations of TiO2 nanoparticles exposed to solar radiation.

Figure 11.
Final discoloration of the samples using reactors with an Eolic mini turbine.
The solutions in the R10, R11, R12, and A10, A11, and A12 reactors were studied, with the concentration of TiO2 based on the information presented in Table 1. The solutions contained in the R13 and A13 reactors only contained the aqueous solution of both dyes at a concentration of 1000 ppm of TiO2 in solution. Figure 12 shows the reactors.

Figure 12.
The samples in the R13 and A13 reactors had a dye concentration, while the samples in the R10 to R12 and A10 to A12 reactors displayed pH modifications.
Upon analyzing the absorbance readings over time for each sample within the bottles, it became apparent that there were no noticeable changes in absorbance. This led to the conclusion that modifying the pH and exposing the samples to solar radiation alone could not bleach both dyes. The conditions required to generate OH* radicals, which are one of the precursors of oxidation reactions, were not present, and therefore, the reaction of degradation did not occur.
3.2. Discoloration Kinetics
3.2.1. Order of Reaction
Data shown in Table 2 and Table 3 were subjected to the respective treatment, considering a heterogeneous process (the Langmuir Hinshelwood model) and the pseudo-homogeneous process of reaction orders ½, 1, ³⁄₂, and 2; the results are shown in Table 4 and Table 5.
The coefficients of correlation applied to the data from the R6 and R9 reactors revealed pseudo-homogeneous behavior with ½ order kinetics.
The kinetics of the degradation of organic compounds in the presence of a heterogeneous catalyst follows the Langmuir-Hinshelwood scheme [2,47,48], with oxygen adsorption on the catalyst’s surface. However, in the experiment, the reactors had limited contact with ambient oxygen, such that the adsorption rate was low, a fact that led to little formation of according to the scheme of Equation (23) in such a way that the parallel reactions did not develop at an adequate speed. When the product of the Langmuir-Hinshelwood model is less than unity, then this equation is reduced to the first-order model with an apparent constant that is the product of the reaction rate constant and the reaction rate constant adsorption [31,49]. Under these facts, the degradation kinetics do not correspond to the Langmuir-Hinshelwood model for both the red and yellow dyes, and the corresponding analysis is carried out considering the kinetics of a pseudo-homogeneous process.
3.2.2. Reaction Rate Constant
The rate constants for the reaction were measured at an average temperature of 30 °C. The temperature inside the reactors was higher than the ambient temperature due to the conversion of solar radiation into thermal energy, which was amplified by mirrors reflecting the radiation.
- ✓
- Sumireact supra yellow s-hbu dye
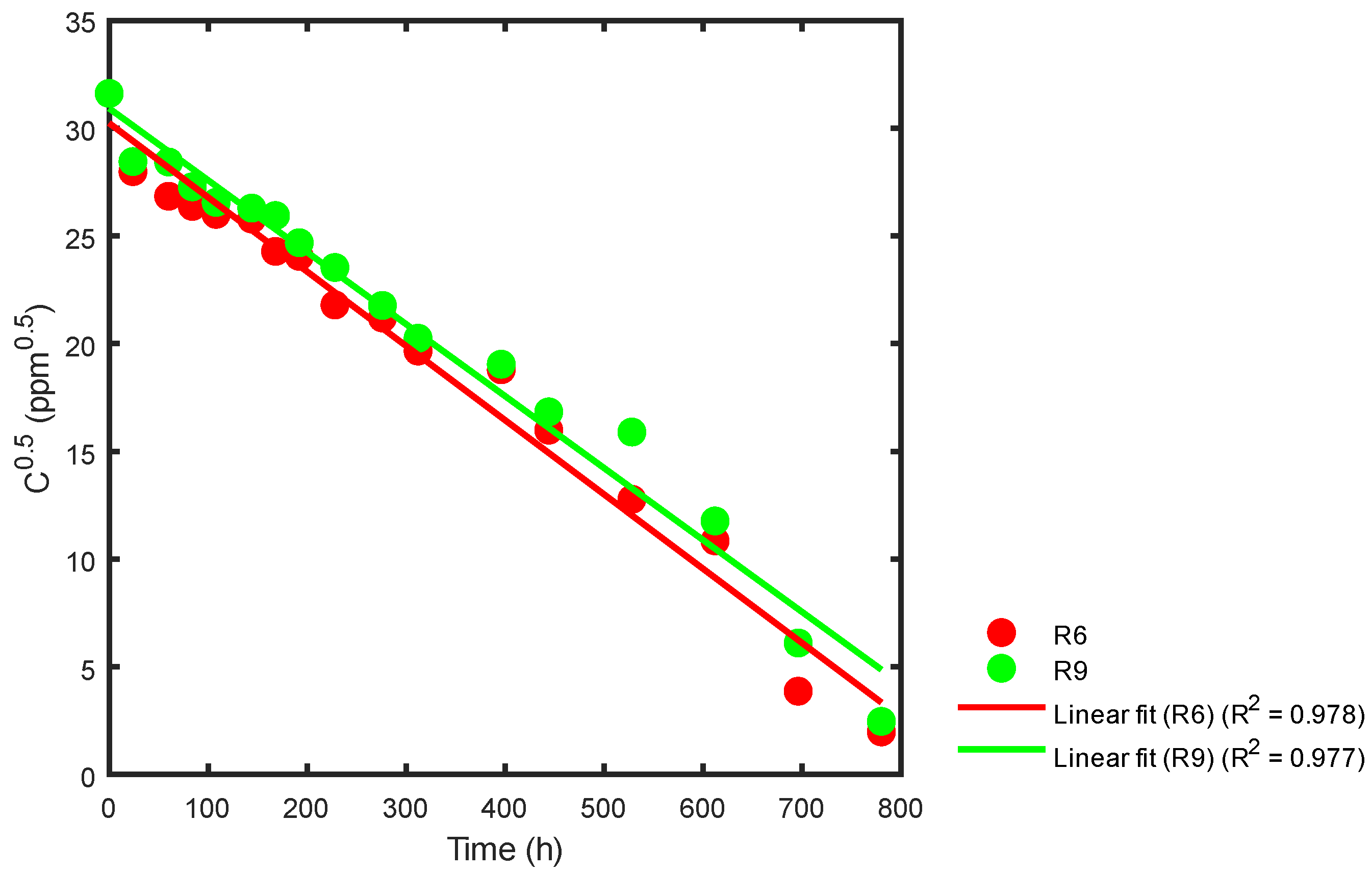
Data from Table 2 and Figure 9 were used with Equation (26) to obtain the kinetic constant for the samples from the R6 and R9 reactors after respective statistical adjustments. The results are presented in Figure 13.
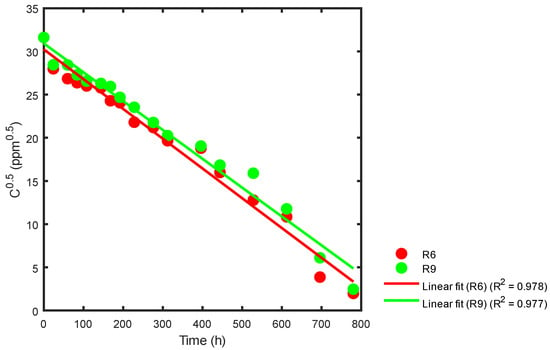
Figure 13.
Kinetic constant using statistical analysis for the R6 and R9 reactors.
In Figure 13, the slope for reactor R6 is −0.03449477, where the corresponding kinetic constant obtained is k = 0.06898954 ppm0.5 h−1. Similarly, for reactor R9, the slope is −0.03341687, and the kinetic constant is k = 0.06683374 ppm0.5 h−1. The correlation coefficients for these values can be found in Table 4.
Based on the data presented in Table 1, it appears that higher concentrations of TiO2 (800 ppm) and a pH in the range of 3.15 < pH < 4.13 favored the decomposition of the sumireact supra yellow s-hbu dye; otherwise, when the pH is low (2.25), and there is a low nanoparticle concentration (<400 ppm), the conditions are unfavorable for generating discoloration. While the discoloration constant of the sample contained in reactor R6 was slightly higher than that of reactor R9; all the samples were subjected to the same treatments, except for pH. The most efficient treatment corresponds to a pH of 3.15 and an initial TiO2 concentration of 800 ppm.
- ✓
- Sumifix supra lemon-yellow e-xf dye
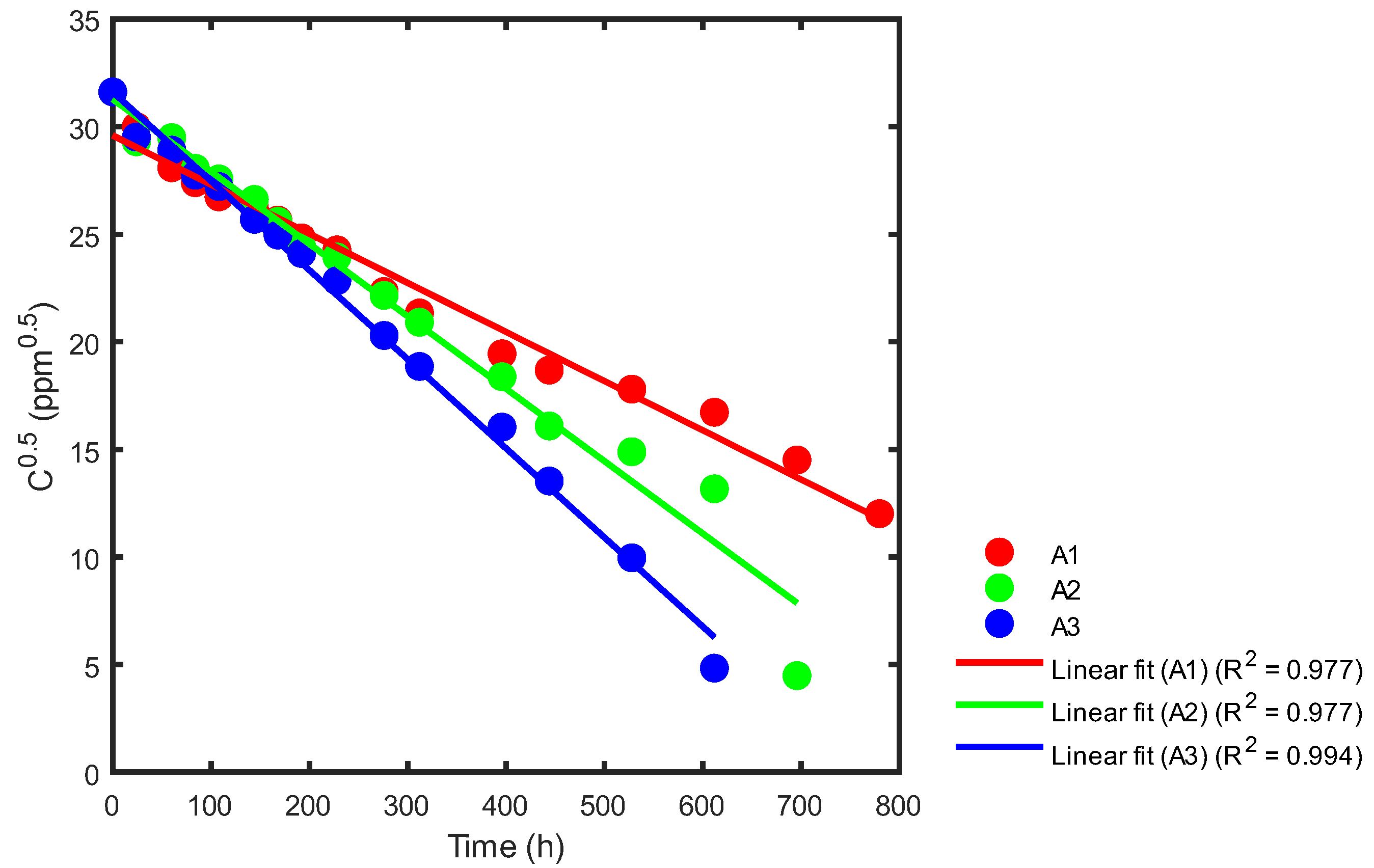
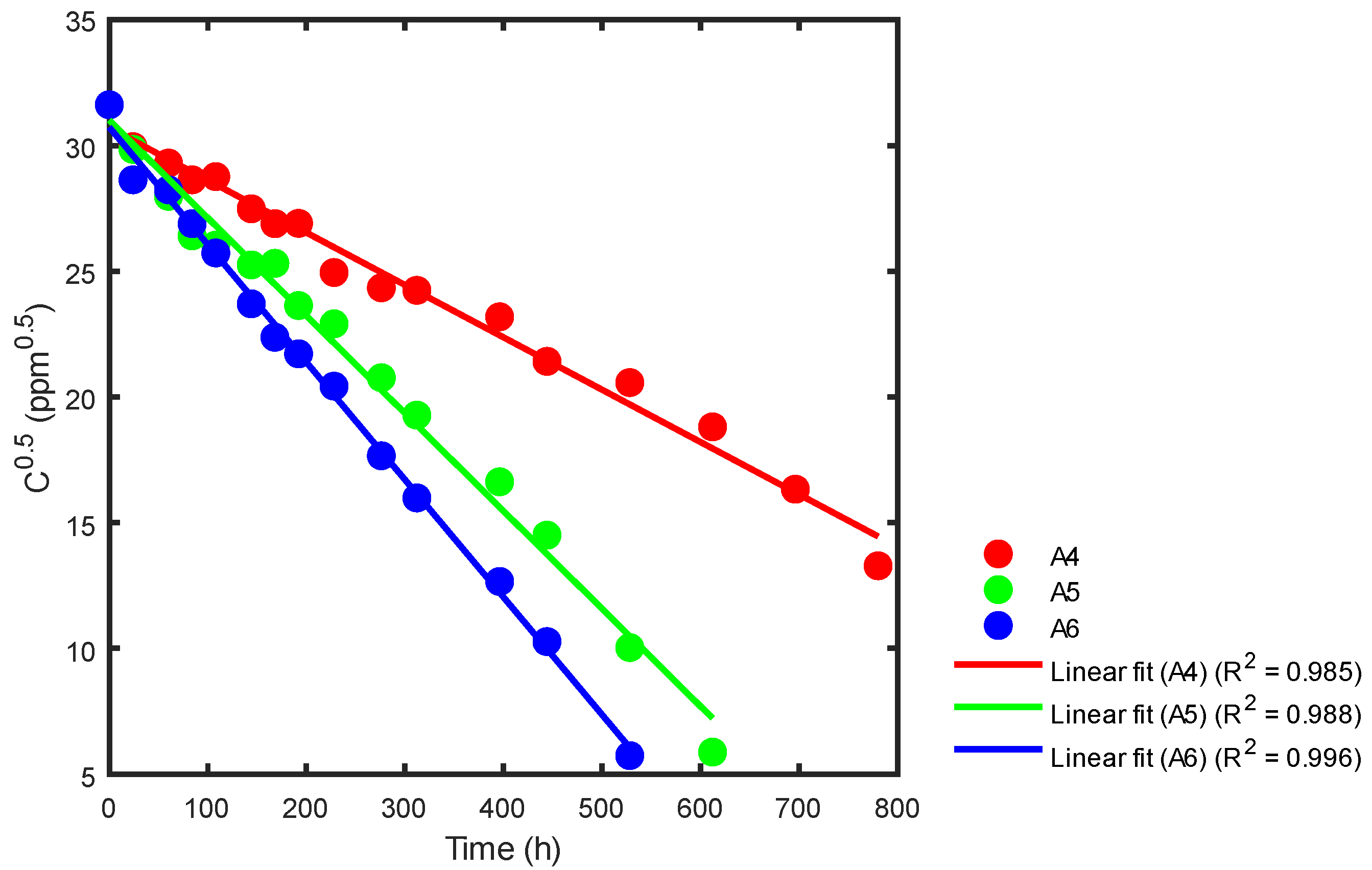
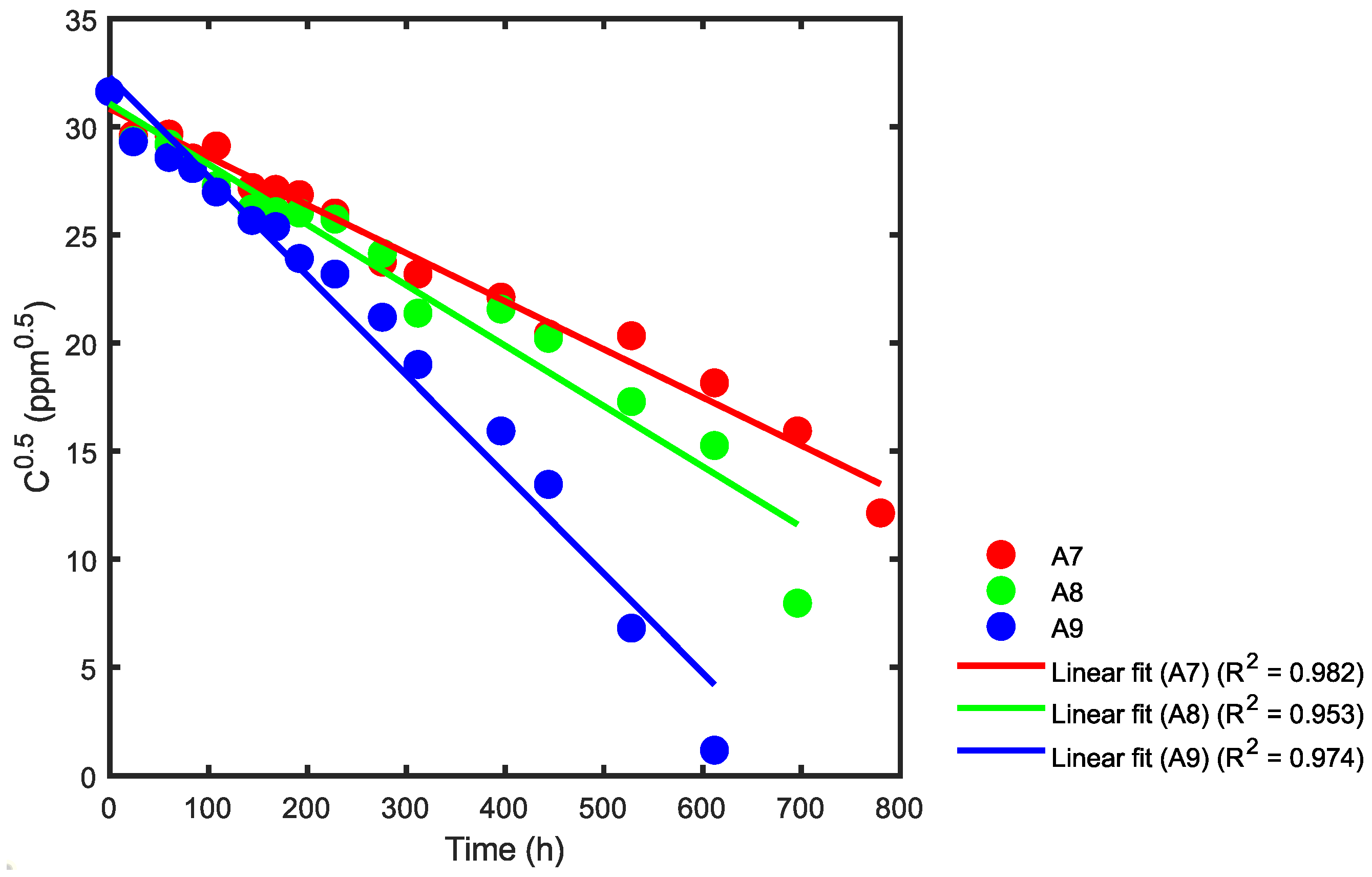
Based on Table 3 and Figure 10 and utilizing Equation (26), statistical adjustments were applied to the reported data from the respective reactors. This process enabled the determination of the corresponding kinetic constants, as illustrated in Figure 14, Figure 15 and Figure 16.
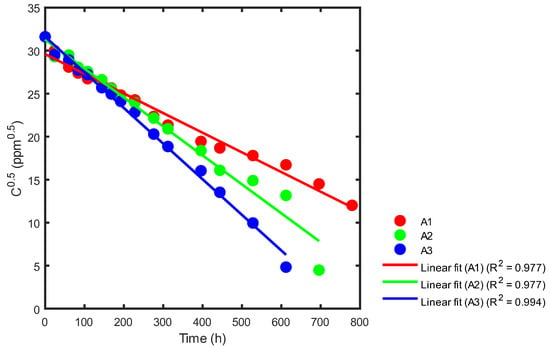
Figure 14.
Kinetic rate constant using statistical analysis for reactors A1, A2 and A3.
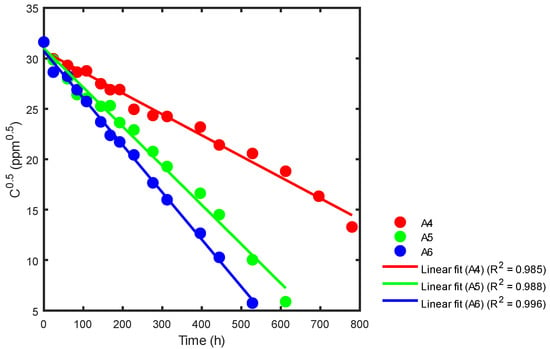
Figure 15.
Kinetic rate constant using statistical analysis for reactors A4, A5 and A6.
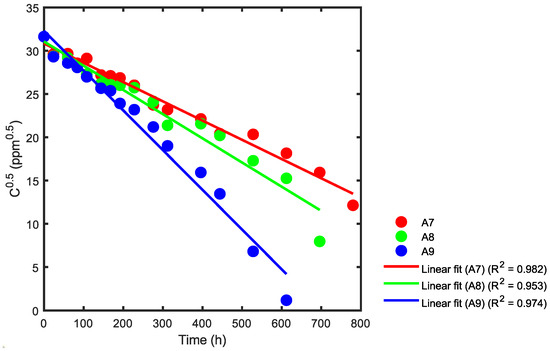
Figure 16.
Kinetic rate constant using statistical analysis for reactors A7, A8 and A9.
In Figure 14, we can see that the slope of reactor A1 (red) is −0.0229, with a reaction rate constant of k = 0.0457 ppm0.5h−1. For reactor A2, the slope (green) is −0.0337, with a reaction rate constant of k = 0.0673 ppm0.5h−1. Finally, for reactor A3, the slope (blue) is −0.0414, with a reaction rate constant of k = 0.0829 ppm0.5h−1. A summary of the correlation coefficients is shown in Table 5.
In Figure 15, we can see that reactor A4 has a slope (red) of −0.0208 and a reaction rate constant of k = 0.0417 ppm0.5h−1. Similarly, reactor A5 has a slope (green) of −0.0389 and a reaction rate constant of k = 0.0777 ppm0.5h−1. Lastly, reactor A6 has a slope (blue) of −0.0467, resulting in a reaction rate constant of k = 0.0935 ppm0.5h−1. Table 5 provides a summary of the correlation coefficient constants for all three reactors, A4, A5, and A6.
In Figure 16, the slope for reactor A7 is −0.0222 and the reaction rate constant is k = 0.0445 ppm0.5h−1. For reactor A8, the slope (green) is −0.0280 and the reaction rate constant k is 0.0559 ppm0.5h−1. Finally, for reactor A9, the slope (blue) is −0.0459 and the reaction rate constant k is 0.0918 ppm0.5h−1. Table 5 provides a summary of the correlation coefficients.
4. Discussion
Solar water treatment systems are based on two fundamental factors. Firstly, the high percentage of UV photons in the diffuse component of solar radiation and, secondly, the dependence on the low rate of light intensity. Experimental measurements indicate that, beyond a certain UV photon flux, the dependence of reaction rates on intensity shifts from order 1 to order ½ [50,51]. However, this transition does not occur at a specific radiation intensity, as various researchers have obtained different results under different experimental conditions [46]. According to some investigators, the shift from reaction order 1 to ½ is caused by an excess of photogenerated species, which includes electrons, holes, and hydroxyl radicals (e−, h+, and ) [52]. At higher radiation intensities, another shift occurs from order ½ to order 1, where the photocatalytic reaction becomes independent of the received radiation and solely depends on the mass transfer within the reaction. This means that even if the radiation increases, the rate of reaction remains constant. Several factors may contribute to this phenomenon, such as a lack of electron attractors like oxygen (i.e., O2), organic molecules in the vicinity of the TiO2 surface, and/or excess products occupying the active centers of the catalyst. These occurrences are more pronounced when using supported catalysts or employing slow stirring, indicating a limited surface area of the catalyst in contact with the liquid and reduced turbulence. In our research, empirical evidence shows that the reaction order is ½ in all cases. The statistical analysis presented in Table 4 and Table 5 confirms the absence of a transition in the reaction order, indicating the recombination of photogenerated species. This hinders the contact between the reactant and the catalyst, as well as the diffusion of products from the vicinity of the catalyst to the liquid [46]. This is evident in Figure 6, where there are periods during the day with no air currents, causing the agitation to cease. The sumireact supra yellow s-hbu red dye exhibits a strong dependence on the concentration of NPs (800 ppm) in the solution and the pH between 3 and 4, which correspond to the R6 and R9 reactors. This range favored contact between the dye and the nanoparticles as compared to the concentrations of 400 and 200 ppm. In contrast, low contact with oxygen (an electron acceptor) negatively affected the dye’s degradation. This suggests that in the R1, R2, R3, R4, R5, R7, and R8 reactors, neither the radiation intensity nor the mass transfer had any influence. This could be due to the more stable chemical structure of this red dye compared to the yellow dye because all the solutions were subjected to the same experimental conditions.
Based on the results of Table 5, the kinetics of order ½ were observed for sumifix supra lemon-yellow e-xf yellow dye, which indicates a high rate of recombination between the electrons of the conduction band and the valence band. As a result, the net flow of electrons for discoloration was low, which indicates that solar radiation controls the process during the experimental testing period at 215 W/m2. The low discoloration rate can also be attributed to the low oxygen levels in the solution and low agitation rates; however, the highest concentrations of nanoparticles and a pH between 3 and 4, compared to the other doses, were found to be favorable conditions for discoloration.
The red dye did not produce enough data, so the inferential analysis focused on the yellow dye. The analysis revealed the reaction rate constants for all the solutions in the nine reactors. Table 6 shows the summary of the experimental design, which indicates that the highest value of the reaction rate constant corresponds to a NP concentration of 800 ppm and a pH of 3.15; meanwhile, the lowest values of the constant correspond to a NP concentration of 200 ppm and a pH whose influence is inconclusive.

Table 6.
Summary of the experimental design for the treatment of yellow dye in terms of the reaction rate constant.
The results of the analysis of variance for the experimental design are presented in Table 7. The tests were conducted with various concentrations of nanoparticles (ppm) and pH level adjustments to the value of the kinetic constant k (ppm0.5h−1). The p-value (p = 0.042 < 0.05) indicates significant differences between the tests, and the concentration of nanoparticles was identified as the factor that generated the most significant change in the response of the tests.

Table 7.
Variance analysis.
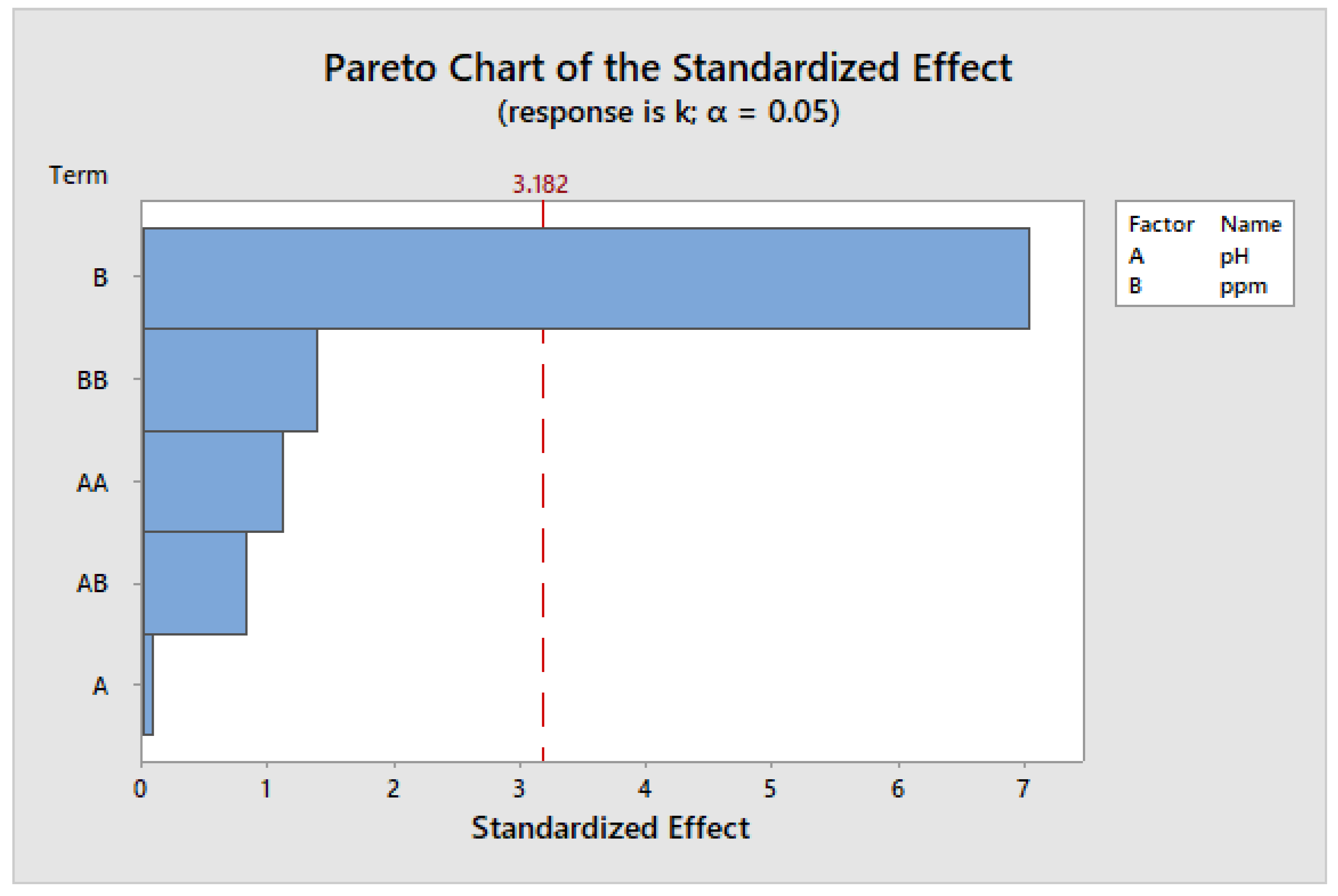
According to Figure 17, the Pareto chart of the standard effects, the concentration of TiO2 nanoparticles was the most important factor in the degradation of sumifix supra lemon-yellow e-xf textiles. The chart also suggests that pH does not have a significant influence within the experimental range. Nevertheless, it remains a crucial parameter, as observed in a separate study, where a pH of 3.15 led to a higher value of the reaction rate constant, signifying an increased degradation rate [53].
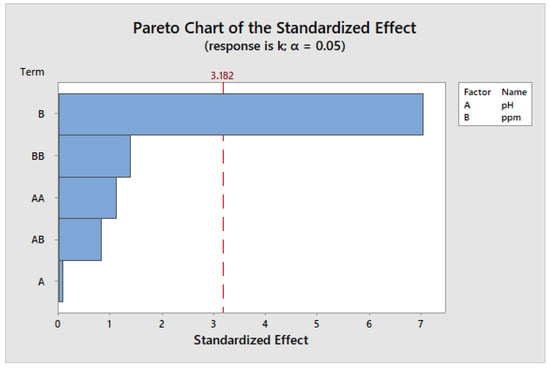
Figure 17.
Pareto chart for standard effects with the constant k.
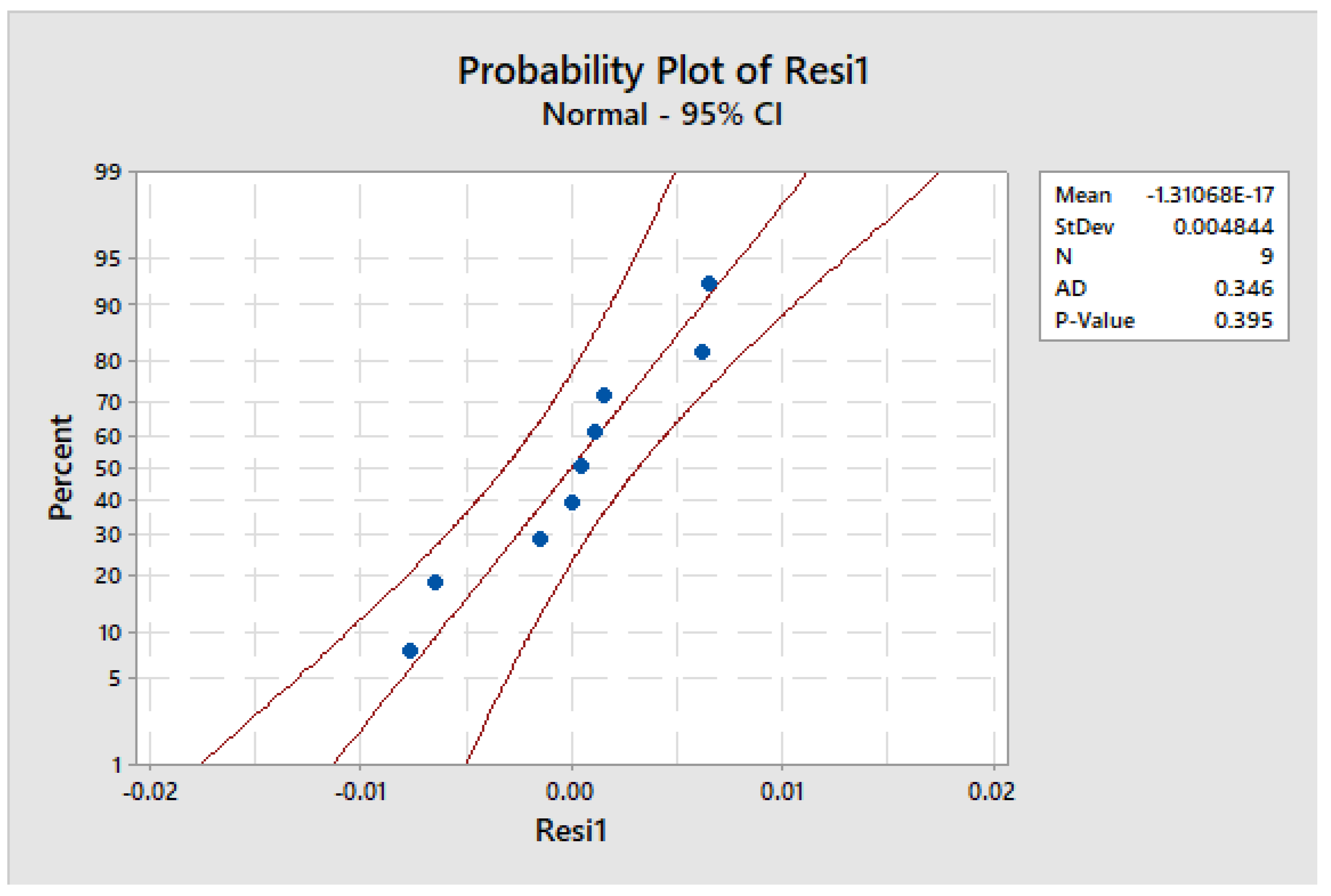
The experimental design presented in Figure 18 was subjected to a normality test to apply to the residuals. The p-value (p = 0.395 > 0.05) indicates that the test produced satisfactory results, thereby confirming that the residuals follow a normal distribution. Hence, it was concluded that the normality assumption of the analysis of variance was met and validated.
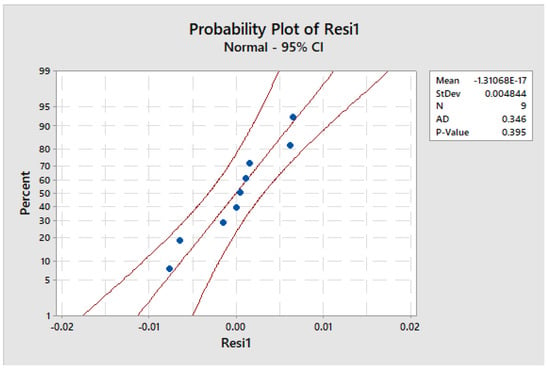
Figure 18.
Test for the normality of the residuals.
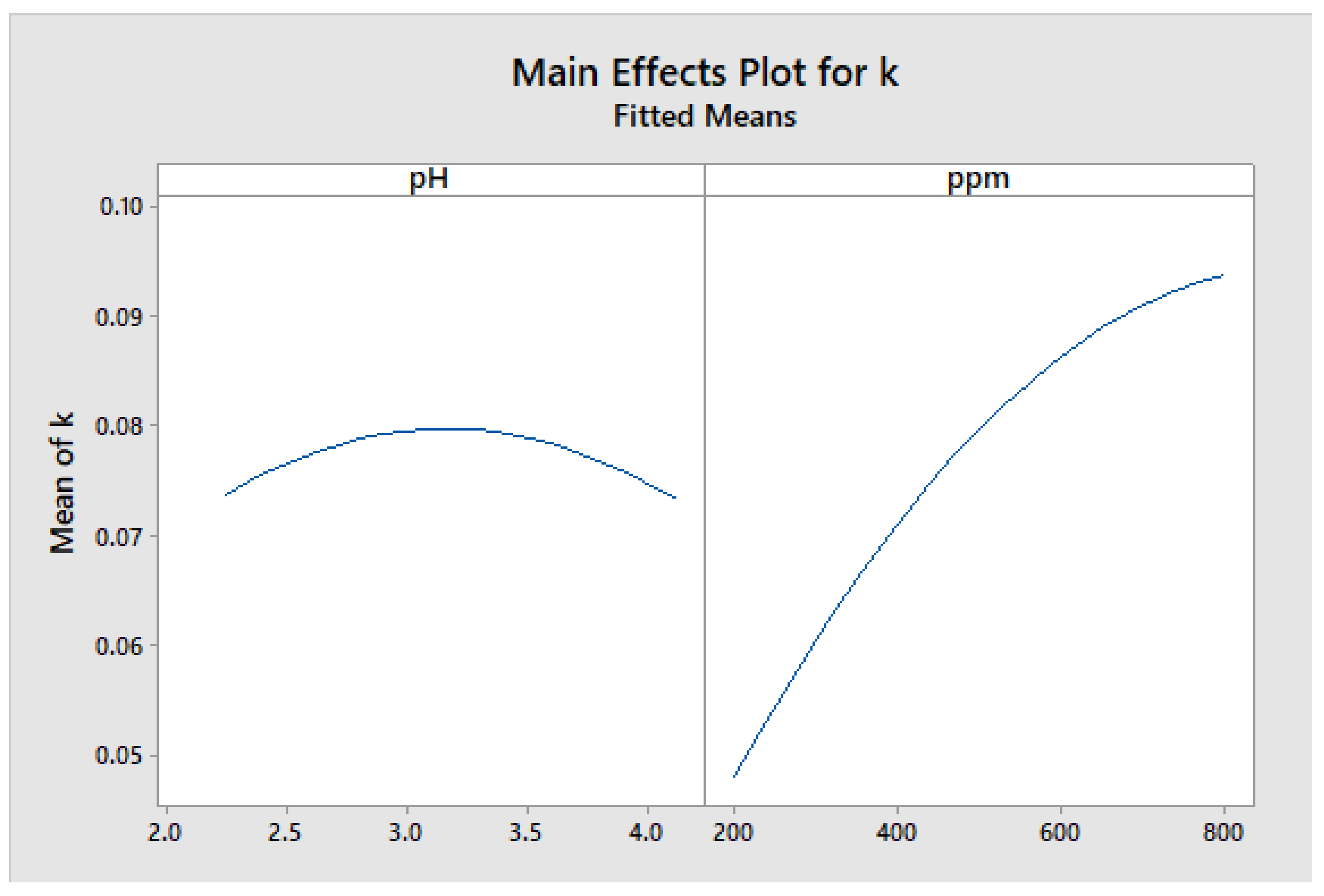
Figure 19 displays the graph of the main effects of the factors investigated on the kinetic constant (k). The results indicate that an intermediate pH value had the greatest impact on the mean response. Additionally, the concentration was found to be a significant factor, with an increase in the mean response as the concentration value, in ppm, increases.
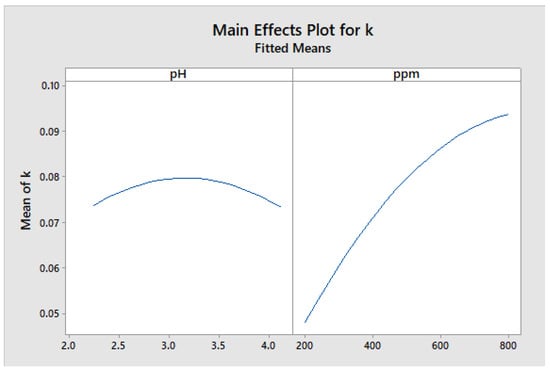
Figure 19.
The Main effects of the constant k.
Figure 19 shows that the optimal pH for use was pH 3, while the optimal concentration of TiO2 nanoparticles was 800 ppm.
5. Conclusions
It is possible to remove the color of reactive textile dyes such as Sumireact supra yellow s-hbu and Sumifix supra lemon-yellow e-xf by adjusting the pH to approximately 3, adding 800 parts per million of titanium dioxide nanoparticles, and exposing the dye to solar radiation. However, achieving this discoloration requires approximately 780 h of exposure, as observed during the winter period between July and August 2023. This timeframe coincides with the lowest intensity of solar radiation on the Peruvian coast, posing a significant limitation to the discoloration process. The promotion of electrons from the valence band of titanium dioxide to the conduction band is contingent upon radiation intensity. Another notable limitation is the intermittent agitation of the reactors, dependent on the strength of the wind.
It was observed that the dye named “red” (sumireact supra yellow s-hbu) was more resistant to discoloration compared to the yellow sumifix supra lemon-yellow e-xf dye. Only two solutions in the R6 and R9 reactors were degraded in the first case, while in the second case, six solutions in the A2, A3, A5, A6, A8, and A9 reactors were discolored, and the solutions in the A1, A4, and A7 reactors showed a tendency to discolor. It is noteworthy that the same dosage and treatment conditions were applied in both cases.
The data on concentration over time were analyzed using the Langmuir-Hinshelwood model and different pseudo-homogeneous reaction orders. Based on the correlation coefficients, it was determined that the reaction order for the analyzed solutions was ½, indicating that there was recombination between the electrons of the conduction band and the “holes” generated in the valence band. This process is considered pseudo-homogeneous.
The study used a regression line to analyze the different solutions and determine the value of the slope and reaction rate constants. The results showed that the solutions with a pH close to 3 and a nanoparticle concentration of 800 ppm had the highest discoloration rate, and the statistical analysis revealed that the concentration of nanoparticles in the solution significantly affects the discoloration treatment.
The rate depends on several factors, such as the intensity of solar radiation, concentration of titanium dioxide nanoparticles, oxygen dissolved in the solution, pH, stirring speed, and chemical structure of the substances to be treated. This last factor seemed to be the determinant in the discoloration rate between the red and yellow dyes compared to each other. Considering both dyes, the extended time required for discoloration strongly suggests dependence on solar radiation intensity, oxygen concentration in the solution, nanoparticle concentration, and pH. While it is challenging to individually discern the contributions of increased oxygen and radiation, it is evident that both factors accelerate the discoloration rate. The primary contribution of this study lies in the effective utilization of solar radiation through the incorporation of a rotating plate equipped with mirrors and the design of micro-agitators powered by wind energy for decolorizing a textile dye with an initial concentration of 1000 ppm. This approach enabled the determination of titanium dioxide NP concentration and the initial pH for efficient treatment.
Author Contributions
Conceptualization, L.A.C.-V. and J.T.M.-C.; methodology, J.T.M.-C. and A.P.N.; software, D.G.M.-H. and H.R.C.-T.; validation, H.R.C.-T. and C.G.-C.; formal analysis, A.P.N. and L.G.C.-P.; investigation, L.G.C.-P. and L.A.C.-V.; data curation, S.E.H.-S. and J.C.C.-C.; writing—original draft preparation, J.C.C.-C. and S.E.H.-S.; writing—review and editing, C.G.-C. and D.G.M.-H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by the Universidad Nacional del Callao, Lima Perú.
Data Availability Statement
The data are unavailable due to privacy.
Acknowledgments
We thank Cesar Gutiérrez Cuba for providing the facilities of the operations and unitary processes laboratory (LOPU) of the Faculty of Chemical Engineering of the National University of Callao.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Arias, P.E.Z.; Nájera, J.B.P.; Hernández, I.C.; Ayala, H.I.S. Textile industrial dyes and optimal wastewater effluents treatments: A short review. Rev. La Fac. Cienc. Quìmicas 2018, 19, 38–47. [Google Scholar]
- Gogate, P.R.; Pandit, A.B. A review of imperative technologies for wastewater treatment I: Oxidation technologies at ambient conditions. Adv. Environ. Res. 2004, 8, 501–551. [Google Scholar] [CrossRef]
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Spasiano, D.; Marotta, R.; Malato, S.; Fernandez-Ibañez, P.; Di Somma, I. Solar photocatalysis: Materials, reactors, some commercial, and pre-industrialized applications. A comprehensive approach. Appl. Catal. B Environ. 2015, 170–171, 90–123. [Google Scholar] [CrossRef]
- Augugliaro, V.; Litter, M.; Palmisano, L.; Soria, J. The combination of heterogeneous photocatalysis with chemical and physical operations: A tool for improving the photoprocess performance. J. Photochem. Photobiol. C Photochem. Rev. 2006, 7, 127–144. [Google Scholar] [CrossRef]
- Braslavsky, S.E. Glossary of terms used in photochemistry, 3rd edition (IUPAC Recommendations 2006). Pure Appl. Chem. 2007, 79, 293–465. [Google Scholar] [CrossRef]
- Santiado, A.C.B. Degradación Fotocatalítica de Fenol Mediante TiO2 Modificado Con Metales de Transición y Sulfato. Ph.D. Thesis, Universidad de Málaga, Malaga, Spain, 2001. [Google Scholar]
- Maira, A.J.; Yeung, K.L.; Lee, C.Y.; Yue, P.L.; Chan, C.K. Size Effects in Gas-Phase Photo-oxidation of Trichloroethylene Using Nanometer-Sized TiO2 Catalysts. J. Catal. 2000, 192, 185–196. [Google Scholar] [CrossRef]
- Lusvardi, G.; Barani, C.; Giubertoni, F.; Paganelli, G. Synthesis and Characterization of TiO2 Nanoparticles for the Reduction of Water Pollutants. Materials 2017, 10, 1208. [Google Scholar] [CrossRef]
- Chiva, S.; Berlanga, J.; Martínez, R.; Climent, J. Procesos de Oxidación Avanzada en el Ciclo Integral del Agua; Universitat Jaume I.: Valencia, Spain, 2017. [Google Scholar]
- Rodriguez, J.; Candal, R.J.; Solís, J.; Estrada, W.; Blesa, M.A. El fotocatalizador: Síntesis, propiedades y limitaciones. Sol. Safe Water 2005, 9, 135–152. [Google Scholar]
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Garcés, L.F.; Mejía, E.A.; Santamaría, J.J. La fotocatálisis como alternativa para el tratamiento de aguas residuales. Rev. Lasallista 2004, 1, 83–92. [Google Scholar]
- Linsebigler, A.L.; Lu, G.; Yates, J.T. Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Pandiyaraj, K.N.; Vasu, D.; Ghobeira, R.; Tabaei, P.S.E.; De Geyter, N.; Morent, R.; Pichumani, M.; Padmanabhanan, P.V.A.; Deshmukh, R.R. Dye wastewater degradation by the synergetic effect of an atmospheric pressure plasma treatment and the photocatalytic activity of plasma-functionalized Cu-TiO2 nanoparticles. J. Hazard. Mater. 2021, 405, 124264. [Google Scholar] [CrossRef]
- Bakar, F.A.; Foad, N.S.I.M. Synthesis of TiO2 photocatalyst with tunable optical properties and exposed facet for textile wastewater treatment. Results Opt. 2023, 13, 100545. [Google Scholar] [CrossRef]
- Suhan, M.B.K.; Al-Mamun, M.R.; Farzana, N.; Aishee, S.M.; Islam, M.S.; Marwani, H.M.; Hasan, M.; Asiri, A.M.; Rahman, M.M.; Islam, A.; et al. Sustainable pollutant removal and wastewater remediation using TiO2-based nanocomposites: A critical review. Nano-Struct. Nano-Objects 2023, 36, 101050. [Google Scholar] [CrossRef]
- Al-Mamun, M.R.; Kader, S.; Islam, M.S.; Khan, M.Z.H. Photocatalytic activity improvement and application of UV-TiO2 photocatalysis in textile wastewater treatment: A review. J. Environ. Chem. Eng. 2019, 7, 103248. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Bagwasi, S.; Tian, B.; Zhang, J.; Nasir, M. Synthesis, characterization and application of bismuth and boron Co-doped TiO2: A visible light active photocatalyst. Chem. Eng. J. 2013, 217, 108–118. [Google Scholar] [CrossRef]
- Augugliaro, V.; Baiocchi, C.; Prevot, A.B.; García-López, E.; Loddo, V.; Malato, S.; Marci, G.; Palmisano, L.; Pazzi, M.; Pramauro, E. Azo-dyes photocatalytic degradation in aqueous suspension of TiO2 under solar irradiation. Chemosphere 2002, 49, 1223–1230. [Google Scholar] [CrossRef]
- Bandara, J.; Mielczarski, J.A.; Kiwi, J. 1. Molecular Mechanism of Surface Recognition. Azo Dyes Degradation on Fe, Ti, and Al Oxides through Metal Sulfonate Complexes. Langmuir 1999, 15, 7670–7679. [Google Scholar] [CrossRef]
- Daneshvar, N.; Salari, D.; Khataee, A.R. Photocatalytic degradation of azo dye acid red 14 in water: Investigation of the effect of operational parameters. J. Photochem. Photobiol. A Chem. 2003, 157, 111–116. [Google Scholar] [CrossRef]
- Epling, G.A.; Lin, C. Photoassisted bleaching of dyes utilizing TiO2 and visible light. Chemosphere 2002, 46, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, J.; Huang, L.; Lu, G. Photoelectrocatalytic degradation of methyl orange over mesoporous film electrodes. Photochem. Photobiol. Sci. 2010, 9, 39–46. [Google Scholar] [CrossRef]
- Mahmood, T.; Chen, C.; Liu, L.; Zhao, D.; Ma, W.; Lin, J.; Zhao, J. Effect of dye-metal complexation on photocatalytic decomposition of the dyes on TiO2 under visible irradiation. J. Environ. Sci. 2009, 21, 263–267. [Google Scholar] [CrossRef]
- Rao, N.N.; Dube, S. Photocatalytic degradation of reactive orange 84(RO 84) in dye-house effluent using single pass reactor. Stud. Surf. Sci. Catal. 1998, 113, 1045–1050. [Google Scholar]
- Vinodgopal, K.; Wynkoop, D.E.; Kamat, P.V. Environmental photochemistry on semiconductor surfaces: Photosensitized degradation of a textile Azo Dye, Acid Orange 7, on TiO2 particles using visible light. Environ. Sci. Technol. 1996, 30, 1660–1666. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, J.; Shen, T.; Hidaka, H.; Pelizzetti, E.; Serpone, N. TiO2-assisted photodegradation of dye pollutants II. Adsorption and degradation kinetics of eosin in TiO2 dispersions under visible light irradiation. Appl. Catal. B Environ. 1998, 15, 147–156. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Kisch, H. Semiconductor Photocatalysis—Mechanistic and Synthetic Aspects. Angew. Chemie Int. Ed. 2013, 52, 812–847. [Google Scholar] [CrossRef]
- Kumar, K.; Chowdhury, A. Use of novel nanostructured photocatalysts for the environmental sustainability of wastewater treatments. In Encyclopedia of Renewable and Sustainable Materials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 949–964. [Google Scholar]
- Michal, R.; Sfaelou, S.; Lianos, P. Photocatalysis for Renewable Energy Production Using PhotoFuelCells. Molecules 2014, 19, 19732–19750. [Google Scholar] [CrossRef] [PubMed]
- Olivarez, G.H. Degradación de Contaminantes Presentes en Agua Mediante Fotocatálisis Solar. Master’s Thesis, Instituto de Ingeniería de la UNAM, Ciudad de México, Mexico, 2014. [Google Scholar]
- Cantarella, M.; Mangano, M.; Zimbone, M.; Sfuncia, G.; Nicotra, G.; Scalisi, E.M.; Brundo, M.V.; Pellegrino, A.L.; Guifridda, F.; Privitera, V.; et al. Green synthesis of photocatalytic TiO2/Ag nanoparticles for an efficient water remediation. J. Photochem. Photobiol. A Chem. 2023, 443, 114838. [Google Scholar] [CrossRef]
- Yao, W.; Luo, C.; Wu, J.; Hou, G. Degradation of Acid Yellow 36 Azo Dye from Textile Wastewater using Vanadium-doped TiO2 Photonanocatalyst. Int. J. Electrochem. Sci. 2022, 17, 220916. [Google Scholar] [CrossRef]
- He, X.; Meng, X.; Sun, J.; Yuan, Z.; He, Y.; Chen, S. Synthesis of TiO2@Fe2O3 Nanocomposites as effective Photocatalyst for degradation of p-nitophenol in oilfield wastewater. Int. J. Electrochem. Sci. 2022, 17, 221179. [Google Scholar] [CrossRef]
- Wang, R.; Cao, J.; Liu, J.; Zhang, Y. Synthesis of CuO@TiO2 nanocomposite and its photocatalytic and electrochemical properties. Application for treatment of azo dyes in industrial wastewater. Int. J. Electrochem. Sci. 2023, 18, 100316. [Google Scholar] [CrossRef]
- Song, C. Enhancing photocatalytic degradation of hydrolyzed polyacrylamide in oilfield wastewater using BiVO4/TiO2 heterostructure nano-photocatalyst under visible light irradiation. Int. J. Electrochem. Sci. 2023, 18, 100363. [Google Scholar] [CrossRef]
- Sutisna; Wibowo, E.; Rokhmat, M.; Rahman, D.Y.; Murniati, R.; Abdullah, M. Batik wastewater treatment using TiO2 nanoparticles coated on the surface of plastic sheet. Procedia Eng. 2017, 170, 78–83. [Google Scholar] [CrossRef]
- Dzinun, H.; Khalid, N.H.A.; Hairom, N.H.H. Photocatalytic performance of TiO2/Eggshell composite for wastewater treatment. Mater. Today Proc. 2022, 65, 3000–3006. [Google Scholar] [CrossRef]
- Ohtani, B. Principle of Photocatalysis and Design of Active Photocatalysts. In New and Future Developments in Catalysis; Elsevier: Amsterdam, The Netherlands, 2013; pp. 121–144. [Google Scholar]
- Talaiekhozani, A.; Mosayebi, M.R.; Fulazzaky, M.A.; Eskandari, Z.; Sanayee, R. Combination of TiO2 microreactor and electroflotation for organic pollutant removal from textile dyeing industry wastewater. Alex. Eng. J. 2020, 59, 549–563. [Google Scholar] [CrossRef]
- García, S.N. Descontaminación de Agua Mediante Energía Solar Utilizando un Fotocatalizador. Bachelor’s Thesis, Universidad de la Laguna, Santa Cruz de Tenerife, Spain, 2015. [Google Scholar]
- Romero, M.; Blanco, J.; Sánchez, B.; Vidal, A.; Malato, S.; Cardona, A.I.; Garcia, E. Solar photocatalytic degradation of water and air pollutants: Challenges and perspectives. Sol. Energy 1999, 66, 169–182. [Google Scholar] [CrossRef]
- Dijkstra, M.F.; Michorius, A.; Buwalda, H.; Panneman, H.; Winkelman, J.G.; Beenackers, A.A.C. Comparison of the efficiency of immobilized and suspended systems in photocatalytic degradation. Catal. Today 2001, 66, 487–494. [Google Scholar] [CrossRef]
- Mills, A.; Le Hunte, S. An overview of semiconductor photocatalysis. J. Photochem. Photobiol. A Chem. 1997, 108, 1–35. [Google Scholar] [CrossRef]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Sun, L.; Bolton, J.R. Determination of the quantum yield for the photochemical generation of hydroxyl radicals in TiO2 suspensions. J. Phys. Chem. 1996, 100, 4127–4134. [Google Scholar] [CrossRef]
- Pichat, P. Photocatalytic degradation of pollutants in water and air: Basic concepts and applications. In Environmental Science and Pollution Control Series; Marcel-Dekker, Inc.: New York, NY, USA, 2003; pp. 77–120. [Google Scholar]
- Kormann, C.; Bahnemann, D.W.; Hoffmann, M.R. Photolysis of chloroform and other organic molecules in aqueous titanium dioxide suspensions. Environ. Sci. Technol. 1991, 25, 494–500. [Google Scholar] [CrossRef]
- Pekakis, P.A.; Xekoukoulotakis, N.P.; Mantzavinos, D. Treatment of textile dyehouse wastewater by TiO2 photocatalysis. Water Res. 2006, 40, 1276–1286. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).